Abstract
The use of exosomes in clinical settings is progressively becoming a reality, as clinical trials testing exosomes for diagnostic and therapeutic applications are generating remarkable interest from the scientific community and investors. Exosomes are small extracellular vesicles secreted by all cell types playing intercellular communication roles in health and disease by transferring cellular cargoes such as functional proteins, metabolites and nucleic acids to recipient cells. An in-depth understanding of exosome biology is therefore essential to ensure clinical development of exosome based investigational therapeutic products. Here we summarise the most up-to-date knowkedge about the complex biological journey of exosomes from biogenesis and secretion, transport and uptake to their intracellular signalling. We delineate the major pathways and molecular players that influence each step of exosome physiology, highlighting the routes of interest, which will be of benefit to exosome manipulation and engineering. We highlight the main controversies in the field of exosome research: their adequate definition, characterisation and biogenesis at plasma membrane. We also delineate the most common identified pitfalls affecting exosome research and development. Unravelling exosome physiology is key to their ultimate progression towards clinical applications.
Video Abstract
Similar content being viewed by others
Background
Extracellular vesicles (EVs) are released by all cells, prokaryotes and eukaryotes, and regulate intercellular communication in health and disease [1, 2]. Exosomes are a subset of EVs that were initially identified as a cellular mechanism to excrete unwanted cellular products [3]. They are now known to play significant roles in cellular communication by transferring functional proteins, metabolites and nucleic acids to recipient cells [4,5,6]. They influence a broad range of physiological processes such as immune responses [7], tissue repair [8, 9], stem cell maintenance [10], central nervous system (CNS) communication [6] and pathological processes in cardiovascular diseases [11, 12], neurodegeneration [13], cancer [14] and inflammation [15].
Exosomes have generated considerable interest for clinical application as diagnostic biomarkers and therapeutic cargo carriers [16]. Reduced immunogenicity due to their biocompatibility and a bi-layered lipid structure, which protects the genetic cargo from degradation, makes them attractive as therapeutic vectors. Their small size and membrane composition allow them to cross major biological membranes including the blood brain barrier. Production of engineered exosomes is an active research field, which fosters assessment of various therapeutic cargoes, enhancement of target selectivity and optimisation of manufacturing [17, 18]. A major limitation for successful translation remains the difficulty to precisely target the cell type or organ of interest whilst limiting off-target biodistribution. Another concern is the presence of naturally incorporated cellular genetic impurities with potential immunogenicity [18,19,20]. To circumvent these difficulties, a better understanding of exosome biology in order to improve therapeutic exosome engineering is key.
In this review, we present the most up-to-date knowledge of exosome biology detailing their biogenesis and secretion mechanisms, targeting of recipient cell, uptake and intracellular signalling. Although we acknowledge the complexity of multiple biological mechanisms for secretion and uptake, we highlight the main mechanisms, which could be relevant to exosome engineering for therapeutic applications. We address as well current controversies and common pitfalls impeding exosome research.
EV classification
Extracellular vesicles (EVs) are classified into three groups typically based on their size and biogenesis: exosomes (30–200 nm), microvesicles (MVs) (100–1000 nm) and apoptotic bodies (> 1000 nm) [5, 21,22,23,24] (Fig. 1). Exosomes are considered to be of endocytic origin, MVs are produced by budding and blebbing from the plasma membrane and apoptotic bodies are released by cells undergoing programmed cell death and signal cell engulfment [25]. EVs are further differentiated based on their density, composition and function [25, 26] (Table 1). While all EVs have complex composition of proteins, nucleic acids, lipids and metabolites (Table 1, Fig. 1), sizes and marker overlaps between heterogeneous EVs can make their differentiation harder [2, 27]. In this review, the exosome terminology is used if clarified in the referenced publication and the term EV is used if the differentiation is unclear.
Extracellular vesicles (EVs) classification. The three different classes of EVs are depicted. a Exosomes are generated through the endocytic pathway and are released via exocytosis, are spherical in shape and have size range of 30–200 nm of diameter. b Microvesicles (MVs) are released through budding from plasma membrane, are irregular in shape and range in size between 100–1000 nm of diameter. c Apoptotic bodies are released through blebbing by cells undergoing apoptosis and are > 1000 nm in size
Biological composition of exosomes
Exosomes are membrane bound carriers. Their cargoes can include proteins, nucleic acids and metabolites (Fig. 2) [28], reflecting the nature of donor cell and its physiological state [12]. Exosomes have a spheroid shape in solution but appear bi-concave or cup-shaped when produced by artificial drying during preparation [29]. The main membrane bound and cytosolic proteins incorporated in exosomes are members of the tetraspanin family (CD9, CD63 and CD81), endosomal sorting complex required for transport (ESCRT) proteins (Alix, TSG101), integrins, heat shock proteins (Hsp), actin and flotillins [16, 30] (Table 2). While proteins such as heat shock proteins, CD63, ESCRT and cytoskeletal components are common among all exosomes, other proteins such as MHC Class I and II are specific to the donor cell type [31]. The rigid bilayer membrane of exosomes also consist of lipid components such as sphingomyelin, cholesterol and ceramides, which influence cargo sorting, exosome secretion, structure and signalling [32, 33] (Table 2). A complex of nucleic acids such as DNA, mRNA and noncoding RNA species as well form part of the exosome composition [2, 31]. MicroRNAs (miRs) are one of the most abundant RNA species in exosomes [16, 34]. MiRs, which play roles in multiple biological processes such as exocytosis, hematopoesis and angiogenesis, participate in exosome mediated cellular communication [16]. Other exosomal RNA species include ribosomal RNA (rRNA), long non-coding RNA (lncRNA), transfer RNA (tRNA), small nuclear RNA (snRNA), small nucleolar RNA and p-element-induced wimpy testis (piwi)-interacting RNA, all of which impact biological processes, particularly influencing tumor development [16, 35]. Studies have thus explored their potential for use as non-invasive disease diagnostic and prognostic tool.
Composition of exosomes. Exosomes are composed of various proteins: transmembrane proteins such as tetraspanins, antigen presenting molecules, glycoproteins and adhesion molecules; proteins in exosome lumen such as heat shock proteins (Hsp), cytoskeletal proteins, ESCRT components, membrane transport, fusion proteins, growth factors and cytokines. Exosomes also comprise of multiple lipids such as cholesterol, ceramides, sphingomyelin, phosphatidylinostol (PI), phosphatidylserine (PS), phosphatidylcholine (PC), phosphatidylethanolamine (PE) and gangliosides (GM) along with nucleic acids such as mRNA, miRNA, non-coding RNA and DNA in their lumen. Hsc = Heat shock cognate; TSG = tumor suspectibility gene; TNF = tumor necrosis factor; TGF = Transforming growth factor; TRAIL = TNF-related apoptosis-inducing ligand; FasL = Fas ligand; TfR = Transferrin receptor
Exosome biogenesis in multivesicular bodies
Multivesicular bodies (MVBs) and late endosomes are a subset of specialised endosomal compartments rich in intraluminal vesicles (ILVs), which sequester specific proteins, lipids and cytosolic components. Secreted ILVs become exosomes. ILVs are generated by the inward budding of endosomal membranes, first discovered through the study of vesicular secretion of transferrin receptor (TfR) by mature reticulocytes [36]. MVBs get transported to plasma membrane via cytoskeletal and microtubule network and undergo exocytosis post fusion with the cell surface whereby the ILVs get secreted as exosomes [25]. Other MVBs follow a degradation pathway either by direct fusion with lysosomes or by fusion with autophagosomes followed by lysosomes [37]. MVBs are a heterogeneous population [38] and speculation remains whether the secretory and degradatory MVB pathways are distinct. It is also unknown if some specific markers or cargoes influence these pathways. To date, multiple mechanisms involved in exosome biogenesis have been identified. ESCRT machinery plays a prominent role in this process, with SNARE proteins and their effectors such as RAB GTPases playing important role in their secretion alongside [5, 31]. Furthermore, the importance of mechanisms relying on tetraspanins and lipids cannot be underestimated and have helped improve our understanding of dynamics of exosome generation and release (Fig. 3).
Exosome biogenesis. Within the endosomal system, [1] internalised cargoes are [2] sorted into early endosomes, [3] which then mature into late endosomes or multivesicular bodies. Late endosomes/multivesicular bodies are specialised endosomal compartments rich in intraluminal vesicles (ILVs), which sequester proteins, lipids, and cytosolic compartments and potential exosome cargoes. [4] Cargoes are also delivered from trans-Golgi network and possibly from cytosol. [5] Multivesicular bodies containing exosome cargoes get [5] transported to the plasma membrane, [6] fuse with the cell surface and [7] the ILVs then get secreted as exosomes. ER: Endoplasmic Reticulum; MVB: Multivesicular Bodies; PM: Plasma membrane
ILV biogenesis and secretion are mainly driven by the ESCRT machinery, a cytoplasmic multi-subunit system essential for membrane remodelling, which enables vesicle budding and cargo sorting in MVBs and relies on five core ESCRT complexes: ESCRT-0, -I, -II, -III and Vps4 [39]. Ubiquitinated cargoes are recognised and sorted by the key subunit hepatocyte growth factor-regulated tyrosine kinase substrate (Hrs) of ESCRT-0 to phosphatidylinositol-3-phosphate (PI3P) enriched endosomal compartments [39]. PI3P is a phospholipid found predominantly in early and late endosomes regulating cell signalling and membrane trafficking [40]. In the ESCRT pathway, PI3P promotes cargo organisation through Hrs interaction. Subsequently ESCRT-0 recruits ESCRT-I by interacting with the ESCRT-subunit tumor susceptibility gene 101 (Tsg101). ESCRT-I along with ESCRT-II promotes endosomal inward budding around clusters of ubiquitinated proteins. The charged multivesicular body protein-6 (CHMP6) subunit from ESCRT-III then binds to ESCRT-II and recruits CHMP4 which polymerises as a coil around the neck of the budding ILV pouch. Upon addition of CHMP3, the bud is cleaved forming ILVs, followed by ESCRT-III disassembly using ATP catalysed by Vps4 [39].
Multiple evidences support the critical role of ESCRT in exosome biogenesis through ILV formation. Loss of Hrs, ESCRT-0 subunit STAM1 (Signal Transducing Adaptor Molecule) and Tsg-101 all reduce exosome secretion in multiple cell types such as tumor and dendritic cells [41, 42]. Leptin, a hormone which regulates energy balance and hunger, enhances exosome release by increasing TSG-101 expression [43]. Hepatitis C virus (HCV) infected cells are dependent on CHMP4B (ESCRT-III component) for exosome-mediated transfer of viral RNA [44]. ESCRT components, TSG101 and ALIX, are commonly occuring exosome constituent proteins [16]. ALIX is an accessory ESCRT protein which binds ESCRT-III subunits and aid the budding and abscission process for ILV formation, and is shown to have prominent role in exosome formation particularly in tumor cells. ALIX interacts with syndecan heparan sulfate proteoglycan through its cytoplasmic adaptor, syntenin to drive ILV formation and hence exosome production [45]. The ALIX-syndecan interaction also influences the sorting of syndecan interactor cargoes into ILVs [45,46,47,48]. This syndecan-syntenin interaction is also exploited by oncogenic Src kinase in tumor environment to induce exosomal promigratory activity [48]. ALIX also facilitates incorporation and secretion of tetraspanins into exosomal membrane by directly recruiting ESCRT-III to late endosomes [49]. ESCRT-III is recruited through direct interaction with lysobiphosphatidic acid (LBPA), independent of the classic ESCRT pathway [49]. However, in non-tumor cells such as dendritic cells, ALIX silencing increased MHC-II exosomal secretion but reduced CD63 presence in exosomes [41].
ESCRT is a ubiquitination dependent process and ubiquitin binding ESCRT proteins like Hrs, STAM1 and TSG101 all play important roles in exosome biogenesis. However the role of ubiquitination in exosome cargo sorting is unclear. Although ubiquitinated soluble cargoes are enriched in exosomes [50, 51] and presence of ubiquitination sequence in cargoes such as Major Histocompatibility Complex (MHC)-II enhances their ILV incorporation [52], non-ubiquitinated MHC-II are still recovered in exosomes [53] suggesting ubiquitination independent exosome incorporation to also occur.
Alongside ESCRT dependent processes, the roles of complex lipids and other protein related pathways in exosome generation have also been highlighted [33]. Complex lipids such as ceramide can self-associate to form raft-like structures and contribute to the initial membrane curvature for inward budding to form intraluminal vesicles [54]. Loss of sphingomyelinase, an enzyme which breaks down sphingolipid to ceramide, impairs exosome secretion of Aβ- peptides in neurons [55] and exosomes containing CD63, CD81, Tsg101 and miRNAs in multiple tumor models [42, 56, 57]. Sphingomyelinase inhibition also reduces exosomal viral RNA transfer from hepatitis C infected cells [44]. Similarly, Zika virus relies on sphingomyelinase activity in cortical neurons to mediate infection and viral transmission through exosomes [58]. Curcumin, a hydrophobic polyphenol found in the plant Curcuma longa and the main compound of turmeric, also drives exosome secretion by increasing intracellular concentration of ceramide and reducing lipid concentration within endolysosomal compartments [59]. ESCRT-dependent and ESCRT-independent lipid-mediated pathways co-exist in numerous biological processes like in the viral RNA transfer and invasive process of carcinoma cells [42, 44]. However, lipid dependent regulation of exosome biogenesis is cell type dependent, for instance in melanoma cells where exosome production is unaffected by the loss of ceramide production [60].
Tetraspanins are highly conserved membrane integral proteins, which play important roles in protein scaffolding and anchoring in cellular membranes [61]. Tetraspanins CD9, CD63 and CD81 are highly present in exosomes, are often used as exosome biomarkers and can influence exosome biogenesis and composition [61,62,63]. CD63 (LAMP-3) regulates exosome loading of the latent membrane protein 1 (LMP1), the main Epstein Barr Virus (EBV) related oncoprotein, which enables escape from lysosomal degradation [64]. CD63 interacts with apolipoprotein E to regulate loading of premelanosomes and ILV sorting during melanogenesis independently of ESCRT [60, 65]. Interestingly, tetraspanins influences cargo sorting for release or degradation as CD63 loss results in ESCRT-dependent lysosomal degradation of premelanosomes [60]. CD63 is one of the main proteins used in engineered exosomes to faciliate increased loading of cargoes and reporters [6, 66,67,68,69]. Other tetraspanins that play roles in exosome biogenesis include CD9 which interacts with metalloproteinase CD10, a common leukemia antigen, to enhance exosomal loading of CD10 [70] and CD81-enriched microdomains provide platform for cargo sorting [63]. Tetraspanin-mediated exosome biogenesis closely interact with complex lipids like the interplay of CD9 and CD82 with ceramide to secrete in exosomes β-catenin, a key protein in cell–cell adhesion and gene expression [56]. Contrastingly, exosome production is negatively regulated by tetraspanin-6, which through its interacting partner syntenin influences ALIX-syndecan-syntenin function and directs MVB cargoes for lysosomal degradation [71].
In terms of exosome secretion, Rab GTPases, the most abundant family of proteins in Ras superfamily of GTPases, play a crucial role in intracellular vesicle transport including endosome recycling and MVBs trafficking to lysosomes [72]. Rab GTPase modulation of exosome secretion is heteregenous, depending on cell-type and cargoes. Rab GTPases and SNARE proteins interact to induce exosome secretion [5]. Rab27a is involved in the MVB docking at plasma membrane in Hela cells [73], neurons and podocytes [74] and in exosome-mediated invasiveness of carcinoma cells [42]. Rab27a also determines exosome size while Rab27b, which shares common function with Rab27a in endosomal trafficking, instead influences the intracellular distribution of MVBs in exosomal trafficking [73]. Rab11 and Rab35, which typically acts in the endosome recycling pathway [75, 76], also influence exosome cargo secretion [76]. Rab11 is required for the exosomal secretion of evenness interrupted (Evi) within the neuromuscular junction of drosophila, which facilitates synaptic development and plasticity [77]. Rab35 is required for the exosomal secretion of myelin protein proteolipid (PLP) in oligodendrocytes [78, 79] by docking MVBs to plasma membrane. Loss of both Rab11 and Rab35 results in enhancement of intracellular accumulation of endosomal cargoes, highlighting their important roles in the cargo secretion and also in recycling of late endosomal compartments [76]. Rab7, which regulates endosomal trafficking of MVBs to lysosomes [78], display contrasting role in exosome release dependent on the cell-type [45, 73, 80]. Additionally Rab2b, Rab5a and Rab9a enhance exosome secretion [73]. Hence, modulation of exosome secretion by Rab GTPases depends on both their distinctive trafficking functions and the cell-type. RAL1 (Ras related GTPases homolog) also mediates ILV budding and tethering of MVB to plasma membrane in breast cancer cells and Candida elegans [81]. RAL1 regulates exosome secretion in co-ordination with T-SNARE syntaxin 5 and an unidentified V-SNARE [81]. R-SNARE Ytk6 interacts with ESCRT-dependent exosome secretion of active Wnt and hedgehog signalling proteins in drosophila and vertebrate cells [82, 83]. Study in HeLa cells also show MVB fusion to plasma membrane and subsequent secretion of exosomes regulated by T-SNARE SNAP23 and syntaxin-4 [66]. As evident, the mechanisms of exosome biogenesis and secretion are heterogeneous with the main ESCRT pathway being seconded by other mechanisms involving lipid rafts and tetraspanins. Rab proteins further aid the cargo sorting and exosome secretion.
Finally, autophagy related protein-5 (Atg5) and autophagy-related-16 like-1 (Atg16L1) also regulate exosome biogenesis in breast cancer cells [84] and mediate exosome secretion of prion proteins in central and peripheral neuronal cells [85]. Autophagy is a regulated self-degradative process that removes unnecessary and dysfunctional cellular components for recycling [86]. Interestingly mechanistic target of rapamycin complex 1 (mTORC1), a highly conserved Ser/Thr kinase and master regulator of autophagy, also negatively regulates exosome release in response to changes in nutrient and growth factors, in a manner similar to autophagy [67]. The concurrent regulation of these two processes likely allows cellular waste management and recycling, particularly under stress conditions. Such regulation of exosome release possibly occurs in stressful environment of glucose starvation and hypoxia [30].
Hence exosome biogenesis is a finely tuned and reactive pathway with multiple molecular players which are involved in other key cellular functions or vesicle related physiology.
Transport and biodistribution
Exosomes mediate cell–cell communication locally and systemically and are secreted by most cell types including dendritic cells, macrophages, cancer cells and mesenchymal stem cells [87]. Exosomes are present in various biological fluids such as breast milk, blood, serum, urine, saliva, amniotic and synovial fluids [88]. Moreover exosomes might undergo multiple cell uptake and release cycles to allow access to several layers of tissues [89]. Biodistribution studies are commonly performed using heterologous exosomes delivered through various routes of administration, which are likely to behave differently than autologous exosomes, thereby influencing their kinetics and biodistribution [87]. Study using both cell-derived and body fluid-derived exosomes (like bovine milk-derived exosomes) show biodistribution to most organs including liver, lung, kidney, pancreas, spleen, ovaries, colon, and brain after oral administration but an intravenous administration causes a predominant sequestration in the liver followed by spleen, lungs and the gastrointestinal tract [87, 90, 91]. Intravenous injection results in rapid clearance of exosomes in the bloodstream while intratumoral injection allows longer exosome detection in tumors [92] and intranasal administration favors delivery to the brain [17, 93]. Macrophages commonly mediate the uptake in most tissues, while endothelial cells preferentially mediate the uptake in lungs [94,95,96]. Exosome size also influences transport and biodistribution as larger EVs preferentially accumulate in bones, lymph nodes and liver [97].
Although a non-specific uptake is shared by all cell types [98], specific targeting to recipient cells is paramount to deliver exosome content and exert its function [99]. This is mediated by the surface composition of the exosome (Table 2). For instance, integrating central nervous system-specific rabies viral glycoprotein (RVG) [100], which specifically interacts with acetylcholine receptor enables exosome delivery to the brain [101, 102]. Another exosome targeting specificity is the conservation of tropism between donor and recipient cells. This cellular signature conserved in the secreted exosomes acts as recognition motifs for uptake by the same recipient cell types in vitro and in vivo [103, 104]. For instance cancer cells target cell types by harbouring mannose- and sialic acid- enriched glycoproteins on exosome surface like ovarian cancer cells [105]. Integrins α6β4 and α6β1 target lung metastasis and integrins αvβ5 target liver metastasis [106, 107]. Neuroblastoma cells release CD63 positive exosomes targeting neuronal dendrites and CD63 negative exosomes targeting whole neurons and glial cells simultaneously [108]. Equally, the presence of certain receptors facilitates evasion from the host immune system. For instance, CD47 at the surface of engineered exosomes contributes to evasion from host immune cells during circulation in vivo [109]. Complex lipids also influence exosome targeting as observed in cancer cells. Glioblastoma-derived exosomes enriched with phosphatidylethanolamine preferentially target glioblastoma cells along with fibrosarcoma and breast cancer cells [110]. Sphingomyelin enriched melanoma derived exosomes show enhanced targeting in the tumor microenvironment [111]. Lipid targeting is also used by other cell types such as dendritic cells where reduced sphingolipid composition negatively regulates their exosomes uptake ability [96]. Phosphatidylinositol-enriched exosomes decrease macrophage targeting [112]. Therefore cell origin, route of administration and exosome composition are all important factors influencing exosome biodistribution.
Reaching the recipient cell
When reaching the target cell, exosomes can either trigger signalling by directly interacting with extracellular receptors or be uptaken by direct fusion with the plasma membrane or get internalised.
Direct interaction
The transmembrane ligands on exosome surface can bind directly with the surface receptors on the recipient cell and generate downstream signalling cascade to activate the target cell (Fig. 4a). This is a common route to mediate immunomodulatory and apoptotic functions. Exosomes released from dendritic cells activate T lymphocytes through MHC-peptide complex [113] and bind Toll-like receptor ligands on bacterial surface to activate bystander dendritic cells and enhance immune responses [114]. Umbilical cord blood-derived exosomes expressing tumor antigens such as MHC-I, MHC-II and tetraspanins (CD34, CD80) also stimultate T cell proliferation to produce antitumor activity [115]. Ligands including tumor necrosis factor (TNF), Fas ligand (FasL) and TNF related apoptosis inducing ligand (TRAIL) expressed on exosome surface released by dendritic cells can bind to TNF receptors on tumor cells and trigger caspase activation for apoptosis [116].
Exosome signalling by direct interaction or membrane fusion. Upon reaching the target cells, a membrane receptors within the exosome surface and plasma membrane of target cells can interact inducing downstream signalling cascade in the recipient cell. b Exosomes membrane can also fuse with the plasma membrane and release their contents into the cytosol directly
Fusion with plasma membrane
Exosomes can also fuse with the plasma membrane and release their content directly into the cytosol of target cells (Fig. 4b). This includes hemi-fusion stalk formation between hydrophobic lipid bilayers of the exosome and plasma membrane followed by expansion forming one consistent structure. Families of SNAREs and Rab proteins likely mediate this fusion [117, 118] as shown in studies from cell membrane fusion [119]. Lipid raft like domains, integrins and adhesion molecules present on the exosome surface also mediate interaction, attachment and membrane fusion with the target cell [120,121,122]. Studies using exosomes incorporating lipophilic dye octadecyl rhodamine B (R18) help distinguishing endocytosis from fusion. R18 is typically introduced into the exosome bilayer at self-quenching concentrations which is diluted upon fusion with unlabelled recipient membranes resulting in concomitant fluorescence, thus allowing to monitor membrane fusion [123]. This process has been observed in dendritic and tumoral cells [124, 125]. Although evidences to support this mechanism remain weak, some authors have speculated that the low pH of tumor microenvironment resulting in higher rigidity and increased sphingomyelin, could facilitate exosome fusion [111], thus making it a likely route to be adopted by tumor cells.
Internalisation
Exosomes are primarily internalised by the recipient cell followed by cargo release [67, 126, 127]. This uptake process is rapid and temperature-sensitive, decreased by low temperature [105]. The common endocytic pathways are involved in exosome internalisation.
Clathrin-mediated endocytosis is a stepwise assembly of various transmembrane receptors and ligands, characterised by the involvement of triskelion scaffold (clathrin), forming clathrin-coated vesicles (Fig. 5a). The internalised vesicles undergo uncoating and fuse with endosomes [128]. This mode of exosome entry occurs in most cell types such as ovarian and colon tumor cells [66, 99, 105], cardiomyocytes [129], macrophages [130, 131], hepatocytes [131] or neural cells [53, 115], epithelial cells [132], illustrated by their dependence on factors essential for clathrin mediated endocytosis. Dynamin-2, an important player in clathrin-mediated endocytosis, forms a collar-like structure in the neck of invaginations required for scission. Dynamin-2 inhibition decreases exosome uptake by macrophages [130, 133] and microglia cells [134]. In cancer cells, the overexpression of transferrin receptor, a major cargo for clathrin-mediated endocytosis, facilitates enhanced exosome uptake [135]. Cumulative evidence suggests clathrin-mediated endocytosis to be one of the canonical exosome uptake pathway. This highly regulated process can also be influenced by the cargo and exosome composition [128].
Exosome internalisation. Exosomes are internalised by the recipient cells and fuse with the intracellular compartments/endosomal pathway for cargo release. Exosomes can be internalised by a clathrin-mediated endocytosis, b lipid-raft mediated, c caveolin-mediated endocytosis, d phagocytosis or e micropinocytosis. These pathways are not always mutually exclusive and can co-exist for the internalisation of a same set of exosomes
Lipid raft-associated membrane invagination is a major endocytic mechanism to shift cargo into early endosome (Fig. 5b) and influence exosome uptake [136]. Lipid rafts are detergent-resistant membrane microdomains enriched in cholesterol, sphingolipids and glycosylphosphatidylinositol (GPI)-anchored proteins [121]. Metabolic inhibition of complex lipids alters exosome uptake by lipid rafts. Methyl-β-cyclodextrin, which interferes with intracellular cholesterol transport reduces exosome uptake in breast cancer cells [137]. Exosome uptake by dendritic cells is impaired when the exosome producing cell is pre-treated with a sphingolipid synthesis inhibitor [138]. Pre-treating tumor cells with filipin, which binds to cholesterol and forms ultrastructural aggregates, results in reduced exosome uptake [111, 139]. Annexin AnxA2 promotes lipid raft-mediated endocytosis by immobilising exosomes on the cell surface at specific adherent sites [137]. Flotillin, a component of lipid rafts, also positively regulates lipid raft-mediated endocytosis [140].
Conflicting reports have involved caveolin-dependent endocytosis as another potential exosome uptake route. Caveolin-dependent endocytosis is mediated by integral membrane proteins named caveolins, which create a small flask or omega shaped plasma membrane invaginations called caveolae [141]. Caveolae enable internalisation of caveosomes, large vesicles enriched by highly hydrophobic and detergent-resistant membrane lipids containing cholesterol and sphingolipids (Fig. 5c) [141]. Caveolin-1, 2 and 3 are the main structural proteins of caveolae [141]. Caveolin-1 positively regulates exosome uptake in epithelial cells [142] but negatively regulates exosome uptake in fibroblasts and glioma cells [143]. Both clathrin- and caveolin-mediated endocytosis share molecular players such as dynamin-2, which hinder their differentiation [135] and warrant further studies. This is for instance the case in macrophage activation mediated by exosomal Wnt5a for invasion of breast tumor cells [128, 138]. However, using specific clathrin inhibitors can help differentiate between the two uptake pathways [125].
Phagocytosis typically engulfs large particles like bacteria and dead cells but can also internalise small particles like exosomes. Phagocytosis is a stepwise process where cell membrane deformations encircle the bulk extracellular particles forming phagosomes eventually directing internalised cargo to lysosomes [144] (Fig. 5d). Phosphatidylinositol-3-kinase (PI3K) and phospholipase C (PLC) enzymes are necessary for the phagosome closure. Unsurprisingly, this route of exosome uptake is predominantly used by immune cells such as macrophages and dendritic cells, demonstrated by their dependence on PI3K and actin cytoskeleton activity [124, 130].
Macropinocytosis uses actin-driven lamellopodia to induce inwards plasma membrane invagination that get pinched off to form intracellular compartments called macropinosomes (Fig. 5e). They are growth factors dependent and result in non-specific uptake of extracellular soluble molecules, nutrients and antigens [145]. Cholesterol-mediated Rac1 GTPase recruitment, Na+/H+ exchanger function and in some cases dynamin regulate macropinocytosis [146]. The subsequent macropinosome matures and is then internalised by fusion with the lysosome for degradation or recycling back to the plasma membrane [147, 148]. Exosome uptake can rely on macropinocytosis in HeLa cells [149], subsets of microglial cells [134] highlighted by an Na + /H + exchanger activity dependent uptake [134] and partially in epithelial cells [32]. Anecdotical micropinocytosis, dependent on growth factors, has been reported in Ras-expressing carcinoma cells. The secretion of growth factors could induce micropinocytosis by the use of EGFR stimulation [150]. Uptake of engineered exosomes targeting oncogenic KRAS was also facilitated by RAS-mediated macropinocytosis [151].
These different modes of exosome entry can co-exist. Exosome uptake in ovarian tumor and melanoma cells occurs mainly through-cholesterol associated lipid rafts but clathrin-mediated endocytosis, phagocytosis and micropinocytosis are concomitantly used [105, 152, 153]. Macrophage-derived exosomes use both macropinocytosis and clathrin-mediated endocytosis to penetrate hepatocytes and transfer interferon (IFNƴ) induced resistance to Hepatitis A virus [154]. Clathrin-mediated endocytosis and macropinocytosis are used concomitantly for the uptake of PC12-derived exosomes by bone marrow-derived MSCs [155]. Phagocytosis of exosome by macrophages is also lipid-raft dependent [130]. Similarly caveolin-dependent uptake of exosomes by bone marrow stem cells is also partially mediated by macropinocytosis and membrane fusion [125]. Rarely these routes can play opposite roles as observed in glioblastoma cells, which stimulate and inhibit exosome uptake by lipid rafts and caveolin mediated endocytosis, respectively [143].
Recently a specific filopodial mode of entry has been described in fibroblasts [156]. Filopodia are thin, actin-rich cytoplasmic protrusions that allow cells to probe their environment by increasing cellular surface area and interaction with the extracellular ligands [157]. They can influence various cellular processes including exosome uptake in a manner similar to the uptake of pathogenic bacteria and viruses [158]. Exosomes surf on filopodia at constant speed preceding their internalisation as intact vesicles, while some exosomes encounter laterally moving filopodia with grabbing or pulling motions. This actin-dependent process relies on F-actin dependent retrograde flow [156]. The filopodial motion might happen immediately upstream of the endocytic uptake to facilitate exosome internalisation and adhesion via transmembrane molecules such as integrins possibly acting as coupling receptors [158]. However, whether this filopodial base acting as endocytic hotspot for exosome uptake is specific to fibroblasts, what mediates these filopodial surfing motions and whether they precede or replace other uptake routes are not known.
Exosomes intracellular signalling
Exosomes which fuse with the plasma membrane release their contents into the cytosol [121] while direct interaction of exosomes with the surface receptors of recipient cells induces downstream signalling cascades [115]. The intracellular fate of exosomes post internalisation follows the typical endosomal pathway, from early endosomes as sorting compartments to acidic vesicles i.e. late endosomes and MVBs, which fuses with lysosomes [111, 159], eventually undergoing degradation. Lysosome targeting requires active transport along the cytoskeleton, a process mediated by the lipid composition [160], SNARE proteins [20] and intracellular pH. Supporting this, motion of exosomes and their cargo along intracellular filamentous structures has been recently confirmed [161]. Exosome membrane lipids are directed to other cellular locations for supposed recycling while transmembrane exosome proteins remain in the perinuclear space suggesting degradation [126, 127].
However, exosome cargoes likely bypasses degradation as various studies demonstrate exosome-mediated functional changes in recipient cells [15, 19]. The gradual acidification through the endosomal compartments can facilitate the exosome cargo function. For instance, exosomes incorporating the pH sensitive latent transforming growth factor (TGF) β-1 are activated in the acidic endosomal environment and induce phenotypic changes in the recipient cell [162]. TGF β-1 cargo is retained in the endosomal compartments during signalling, allowing sustained cellular signalling compared to free TGF β-1 [162] The fusion of endosome and lysosome compartments also allows cytosolic cargo exposure through acidification and in a cholesterol-dependent manner [67]. Some exosome content can also passively diffuse across the cytoplasm, potentially creating an exosome leakage [126, 127].
Endoplasmic reticulum (ER) which is a nucleation site for translation [163], could be a route for lysosomal escape enabling cargo release as ER scanning can occur after exosome sorting into the endosome trafficking circuit [156]. This would be a route of choice for exosomes carrying mRNA and miRNAs to release their cargoes in ER for rapid translation and mediation of altered gene expression. Rab5/Rab7 positive endosomal vesicles interact with ER, highlighting the coupling between endosomal maturation and trafficking [164]. Ultimately exosomes fuse with lysosomes possibly degrading excess cargoes.
Nucleoplasmic reticulum is a sub-nuclear compartment consisting of nuclear associated invaginations penetrating into the nucleoplasm, where the nuclear transfer of exosomes can occur. The nuclear envelope associated invaginations linked with the late endosomes can allow delivery of exosome components into the nucleoplasm and is likely a route for nuclear cargoes [165, 166].
Exosomes are also able to use pathways similar to viruses to avoid lysosomal degradation. In dendritic cells internalised exosomes can bypass lysosomal degradation by being routed to a specialised, surface-accessible CD81 positive LAMP-1 negative intracellular compartment contiguous with the plasma membrane, in a manner similar to HIV-1 particles [138, 167]. However whether this property is specific to a cell type or to the exosomes themselves is not known. Fusion with late endosomes also provide an optimal environment for cargo uncoating and release into cytosol via endosome penetration aided by the high concentrations of anionic lipids in late endosomes. Notably the anionic lipid LBPA, which facilitates the cytosolic entry of viruses and viral vectors [168, 169], also allows exosome fusion with the late endosomes in macrophages, followed by cargo uncoating and potential cytosolic release of contents [154, 169].
Other possible routes that allow exosomal escape from lysosomal degradation include redirection of exosome cargoes from endosomal pathway to trans-Golgi network through retrograde trafficking [170], cargo release into the cytosol through release of partially degraded materials from ruptured endosomal or lysosomal compartments [90] or membrane fusion between exosomes and endosomal membrane [67]. Exosomes can also be redirected back to the plasma membrane from early endosomes via recycling endosomes [171]. This uptake and release cycle possibly allows dissemination to multiple cellular layers and paracrine effect [89].
Hence, exosome cargoes can undertake multiple routes to bypass direct lysosomal degradation to fulfil their signalling functions and the routes can be determined by the cell type, exosome composition and/or the cargoes. It is equally plausible that some exosomes are fated for direct degradation. This seems to be the case in the constitutive macropinocytotic internalisation of oligodendroglial exosomes by subset of microglia lacking antigen-presentation capacity, thereby acting as a mechanism for oligodendroglial membrane clearance in a ‘silent’ manner [134]. Of note, most of the studies determining intracellular exosome fate use labelled exosome membrane lipids and proteins making it challenging to study the cargo fate itself. Despite progress in cargo loading efficacy, direct evidence of cargo release is limited. Labelled membrane bound cargoes combined with high resolution imaging have allowed the detection of cargo exposure in the cytosol of acceptor cells [67]. However, development of better tools to understand the intracellular pathways of exosomes and their cargoes is key to improve our understanding of how exosomes deliver their signalling function.
Controversies in exosome research
Exosome biogenesis at plasma membrane
EVs of endosomal origin are identified as exosomes. EVs produced directly from outward plasma membrane budding are classified as ectosomes/MVs and display a size range from 50 nm to 1 µm [2, 37]. Some controversial studies however have suggested that exosome formation can happen directly at the plasma membrane within discrete domains. Plasma membrane of Jurkat T cells have domains enriched in exosome proteins and lipids, referred to as “endosome-like”, potentially to allow rapid and direct exosome biogenesis [172]. Outward vesicle budding from plasma membrane rich in exosomal proteins like CD63 and CD81 can also be observed within these domains [172, 173]. Another study demonstrated exosome markers CD9 and CD81 to bud out fivefold more efficiently from plasma membrane than from endosomal compartments [174]. Still debated, further evidences are necessary to support exosome biogenesis at plasma membrane. Whether this is due to limited characterisation of vesicles studied and/or lack of definite markers to differentiate between different vesicles is also arguable.
Exosomes heterogeneity and characterisation
Heterogeneity of exosomes due to their varied size, composition, function and cellular origin adds complexity to their characterisation. Distinct exosome subpopulations have been identified, differentiated by their sizes and density [97, 175]. Advanced fractionation separated exosomes by their size, classifying them as large exosomes (90–120 nm) or small exosomes (60–80 nm) [97], while additional density centrifugation separated high and low density exosomes [175]. It is likely that the limiting membrane of MVBs during ILV formation or differences in molecular routes uptaken for exosome biogenesis contribute to these differences [37]. Such heterogeneity can result in differential exosome contents as such the exosome subpopulations are distinct in both their biophysical properties and in their composition [97, 175]. Overall > 4400 proteins, ~ 200 lipids, > 1600 mRNA and > 750 miRNA have been identified from exosomes [176]. Proteomic analysis further reveal that not all exosome proteins are shared among all exosomes regardless of parent cells. Only a small fraction is cell-specific reflecting cell type and physiological condition [177]. Exosome loading varies as reflected by differential qualitative and quantitative content of cargoes [178] influenced by cellular biology and microenvironment [179]. Supporting this, study on cancer cells show differential miRNA packaging by selectively packing tumour inducing miRNAs within exosomes [180]. Cancer cells also secrete higher quantities of exosomes compared to normal cells [181]. Such heterogeneity result in diverse organ biodistribution and distinct biological functions [97, 175, 181].
Recognising exosome heterogeneity is essential to determine their content, functional role and to allow better EV differentiation. Currently isolation methods such as ultracentrifugation, size exclusion, immunoaffinity isolation coupled with analysis methods such as nanoparticle tracking, electron microscopy, flow cytometry and western blots are employed for exosome generation and characterisation [2]. Employment of global and targeted proteomics further aids this process [2]. However, lack of standardisation of these methods has led to substantial overlap in protein profiles of isolated EVs. Lack of specific or universal markers for EVs particularly for MVs and exosomes also complicates their differentiation [2]. Characterisation guidelines placed by the International Society for extracellular vesicles (ISEV) board are being continuously reviewed owing to the evolving nature of EVs and exosome research [182]. Nevertheless to help standardise the field, categories of markers to be analysed in all bulk EV preparations are listed in the Minimal Information for Studies of Extracellular Vesicles (MISEV) guidelines along with recommended changes in reporting of EV terminology [182]. This include classifications based on size (small, medium/large), densities (low, middle, high), biochemical compositions (surface markers) and/or cellular origin [182]. The constant improvement of isolation and purification methods along with continuous research advancements in EV biology is providing increasing support. A recent study highlighted annexin A1, a membrane-associated protein, as exclusive marker for MVs and lack of glycolytic enzymes and cytoskeletal proteins as potential negative markers for exosomes [183]. Having a standard set of markers unique either to the isolation method used or the parental cell is also proposed [2].
Pitfalls in exosome research
Deciphering exosome biology has been challenged by some pitfalls that the research field aims to address. For instance, molecular players in exosome biogenesis are also involved in other cellular trafficking pathways. Loss and gain of function experiments implemented to study their roles can be exerting direct or indirect effects e.g. altering their function in another cellular vesicular pathways including Golgi, lysosomes and autophagy. This can result in secondary effect on exosome production or secretion [37]. Variation in parent cell types, culture conditions, lack of standardised exosome generation and characterisation methods can all impact experimental reproducibility leading to an overlap in chemical and physical properties between EVs [2, 16]. Implementing multiple, complementary characterisation methods and tracking for any co-isolated non-EV/exosome components is key for better classification [182]. However not all studies implement such rigorous characterisation leading to mixed population of vesicles [177], inadvertently hampering studies on the effect of intended exosomes. Moreover, a survey showed that some researchers have studied the effect of exosomes from culture media rather than intended target cell derived exosomes [184].
Unintended effect of contamination from mycoplasma and other microorganisms, which alters the cellular physiology of donor cells and release their own exosomes, also need to be taken into account [182]. Effect of pre-analytical variables from biofluids and conditioned media need to be explored. In analysing tissues, exosomes either from the extracellular space or artfactual intracellular vesicles released during tissue processing can flaw experimental outcomes [182]. Other factors, such as processing and storage, also alter exosome physiology and affect exosome research [182]. Identifying and overcoming these experimental artefacts are keys for the reliable advancement of exosome research.
Conclusion
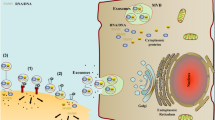
Studying exosome physiology is a novel and rapidly expanding field of cellular biology. The important role of exosomes in cell–cell communication has been highlighted in multiple studies exploring their physiological and pathophysiological functions. This is essential as these vesicles once secreted can provide key information from the cell of origin similar to a “cell biopsy”. Studies on their clinical application as biomarkers for diagnosis, disease severity and response to therapy along with engineering applications as delivery vectors for therapeutic cargoes are actively being developed and rapidly translated for human applications. These perspectives emphasize the need of a better mechanistic understanding of exosome biology. Various processes and interactions between numerous pathways highlighted in this review provide a framework, which enables delineation of the main steps and routes of interest to enhance cell targeting, exosome uptake or lysosomal escape post internalisation (Fig. 6). If the main mechanisms of exosome biology have been delineated, numerous uncertainties remain about the regulation of these processes. Exosome heterogeneity, their differing content, their properties influenced by donor and recipient cells, lack of standardised exosome characterisation in the literature add to the complexity of unravelling the regulatory processes. Ongoing progress in isolation, characterisation and purification of exosomes in parallel with development of innovative dyes will help in advancing the knowledge of exosome physiology, an essential step for clinical translation of exosome applications.
Exosome biology. [1] Exosomes are generated through the formation of ILVs in the late endocytic pathway and [2] gets secreted via exocytosis from the plasma membrane. Upon reaching the target recipient cell, [3] exosomes either interact with the surface molecules of recipient cell to induce downstream signalling or [4] fuse with the plasma membrane to release their contents into cytosol or [5] get internalised via various routes. [6] Upon internalisation, exosomes are addressed in the early endosome, then late endosomes or MVBs and undergo multiple fates. [7] The exosome contents can get released into the nucleus or ER, [8] leak into cytosol or [9] get degraded in the lysosomes. [10] Another possibility include release back to the extracellular space through the recycling endosome. ILV: Intraluminal Vesicles; EE: Early Endosomes, RE: Recycling endosomes, MVB: Multivesicular Bodies, ER: Endoplasmic Reticulum
Abbreviations
- CD:
-
Cluster of differentiation
- CHMP:
-
CHarged multivesicular body protein
- EBV:
-
Epstein barr virus
- EGF:
-
Epidermal growth factor
- ESCRT:
-
Endosomal sorting complexes required for transport
- EV:
-
Extracellular vesicles
- Evi:
-
Evenness interrupted
- FasL:
-
Fas ligand
- GM:
-
Gangliosides
- Hrs:
-
Hepatocyte growth factor-regulated tyrosine kinase substrate
- Hsc:
-
Heat shock cognate
- Hsp:
-
Heat shock protein
- ILV:
-
Intraluminal vesicle
- ISEV:
-
International society for extracellular vesicles
- LBPA:
-
LysoBisPhosphatidic acid
- MHC:
-
Major histocompatibility complex
- MISEV:
-
Minimal information for studies of extracellular vesicles
- MV:
-
Microvesicle
- MVB:
-
Multivesicular bodies
- PC:
-
PhosphatidylCholine
- PE:
-
PhosphatidylEthanolamine
- PI:
-
PhosphatidylInostol
- PS:
-
Phosphatidylserine
- PI3P:
-
PhosphatidylInositol-3-phosphate
- RVG:
-
Rabies viral glycoprotein
- STAM:
-
Signal transducing adaptor molecule
- SNAP:
-
Soluble NSF attachment proteins
- SNARE:
-
SNAP receptor
- TfR:
-
Transferrin receptor
- TGF:
-
Transforming growth factor
- TNF:
-
Tumor necrosis factor
- TRAIL:
-
TNF related apoptosis inducing ligand
- TSG:
-
Tumor susceptibility gene
References
Deatherage BL, Cookson BT. Membrane vesicle release in bacteria, eukaryotes, and archaea: a conserved yet underappreciated aspect of microbial life. Infect Immun. 2012;80(6):1948–57.
Doyle LM, Wang MZ. Overview of extracellular vesicles, their origin, composition, purpose, and methods for exosome isolation and analysis. Cells. 2019;8(7):727.
Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J Biol Chem. 1987;262(19):9412–20.
Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9(6):654–9.
Pegtel DM, Gould SJ. Exosomes. Annu Rev Biochem. 2019;88:487–514.
Men Y, Yelick J, Jin S, Tian Y, Chiang MSR, Higashimori H, et al. Exosome reporter mice reveal the involvement of exosomes in mediating neuron to astroglia communication in the CNS. Nat Commun. 2019;10(1):4136.
Nolte-’t Hoen EN, Buschow SI, Anderton SM, Stoorvogel W, Wauben MH. Activated T cells recruit exosomes secreted by dendritic cells via LFA-1. Blood. 2009;113(9):1977–81.
Zhang B, Wang M, Gong A, Zhang X, Wu X, Zhu Y, et al. HucMSC-exosome mediated-Wnt4 signaling is required for cutaneous wound healing. Stem Cells. 2015;33(7):2158–68.
Cui X, He Z, Liang Z, Chen Z, Wang H, Zhang J. Exosomes from adipose-derived mesenchymal stem cells protect the myocardium against ischemia/reperfusion injury through Wnt/β-catenin signaling pathway. J Cardiovasc Pharmacol. 2017;70(4):225–31.
Ratajczak J, Miekus K, Kucia M, Zhang J, Reca R, Dvorak P, et al. Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: evidence for horizontal transfer of mRNA and protein delivery. Leukemia. 2006;20(5):847–56.
Bang C, Batkai S, Dangwal S, Gupta SK, Foinquinos A, Holzmann A, et al. Cardiac fibroblast-derived microRNA passenger strand-enriched exosomes mediate cardiomyocyte hypertrophy. J Clin Invest. 2014;124(5):2136–46.
Zamani P, Fereydouni N, Butler AE, Navashenaq JG, Sahebkar A. The therapeutic and diagnostic role of exosomes in cardiovascular diseases. Trends Cardiovasc Med. 2019;29(6):313–23.
Howitt J, Hill AF. Exosomes in the pathology of neurodegenerative diseases. J Biol Chem. 2016;291(52):26589–97.
Osaki M, Okada F. Exosomes and their role in cancer progression. Yonago Acta Med. 2019;62(2):182–90.
Deng ZB, Liu Y, Liu C, Xiang X, Wang J, Cheng Z, et al. Immature myeloid cells induced by a high-fat diet contribute to liver inflammation. Hepatology. 2009;50(5):1412–20.
Zhang Y, Liu Y, Liu H, Tang WH. Exosomes: biogenesis, biologic function and clinical potential. Cell Biosci. 2019;9:19.
Haney MJ, Klyachko NL, Zhao Y, Gupta R, Plotnikova EG, He Z, et al. Exosomes as drug delivery vehicles for Parkinson’s disease therapy. J Control Release. 2015;207:18–30.
Bunggulawa EJ, Wang W, Yin T, Wang N, Durkan C, Wang Y, et al. Recent advancements in the use of exosomes as drug delivery systems. J Nanobiotechnol. 2018;16(1):81.
Ha D, Yang N, Nadithe V. Exosomes as therapeutic drug carriers and delivery vehicles across biological membranes: current perspectives and future challenges. Acta Pharm Sin B. 2016;6(4):287–96.
Mathieu M, Martin-Jaular L, Lavieu G, Théry C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol. 2019;21(1):9–17.
Andaloussi SEL, Mäger I, Breakefield XO, Wood MJ. Extracellular vesicles: biology and emerging therapeutic opportunities. Nat Rev Drug Discov. 2013;12(5):347–57.
Ettelaie C, Collier ME, Maraveyas A, Ettelaie R. Characterization of physical properties of tissue factor-containing microvesicles and a comparison of ultracentrifuge-based recovery procedures. J Extracell Vesicles. 2014;3:23592.
Battistelli M, Falcieri E. Apoptotic bodies: particular extracellular vesicles involved in intercellular communication. Biology (Basel). 2020;9(1):21.
Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35(4):495–516.
Colombo M, Raposo G, Théry C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30:255–89.
Crescitelli R, Lässer C, Szabó TG, Kittel A, Eldh M, Dianzani I, et al. Distinct RNA profiles in subpopulations of extracellular vesicles: apoptotic bodies, microvesicles and exosomes. J Extracell Vesicles. 2013;2:20677.
Corso G, Mäger I, Lee Y, Görgens A, Bultema J, Giebel B, et al. Reproducible and scalable purification of extracellular vesicles using combined bind-elute and size exclusion chromatography. Sci Rep. 2017;7(1):11561.
Bobrie A, Colombo M, Raposo G, Théry C. Exosome secretion: molecular mechanisms and roles in immune responses. Traffic. 2011;12(12):1659–68.
Yellon DM, Davidson SM. Exosomes: nanoparticles involved in cardioprotection? Circ Res. 2014;114(2):325–32.
Garcia NA, Ontoria-Oviedo I, González-King H, Diez-Juan A, Sepúlveda P. Glucose starvation in cardiomyocytes enhances exosome secretion and promotes angiogenesis in endothelial cells. PLoS ONE. 2015;10(9):e0138849.
Mashouri L, Yousefi H, Aref AR, Ahadi AM, Molaei F, Alahari SK. Exosomes: composition, biogenesis, and mechanisms in cancer metastasis and drug resistance. Mol Cancer. 2019;18(1):75.
Skotland T, Hessvik NP, Sandvig K, Llorente A. Exosomal lipid composition and the role of ether lipids and phosphoinositides in exosome biology. J Lipid Res. 2019;60(1):9–18.
Skryabin GO, Komelkov AV, Savelyeva EE, Tchevkina EM. Lipid rafts in exosome biogenesis. Biochemistry (Mosc). 2020;85(2):177–91.
Huang X, Yuan T, Tschannen M, Sun Z, Jacob H, Du M, et al. Characterization of human plasma-derived exosomal RNAs by deep sequencing. BMC Genomics. 2013;14:319.
Ge L, Zhang N, Li D, Wu Y, Wang H, Wang J. Circulating exosomal small RNAs are promising non-invasive diagnostic biomarkers for gastric cancer. J Cell Mol Med. 2020;24(24):14502–13.
Pan BT, Johnstone RM. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell. 1983;33(3):967–78.
Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367(6478):eaau6977.
White IJ, Bailey LM, Aghakhani MR, Moss SE, Futter CE. EGF stimulates annexin 1-dependent inward vesiculation in a multivesicular endosome subpopulation. Embo J. 2006;25(1):1–12.
Schmidt O, Teis D. The ESCRT machinery. Curr Biol. 2012;22(4):R116–20.
Marat AL, Haucke V. Phosphatidylinositol 3-phosphates-at the interface between cell signalling and membrane traffic. Embo J. 2016;35(6):561–79.
Colombo M, Moita C, van Niel G, Kowal J, Vigneron J, Benaroch P, et al. Analysis of ESCRT functions in exosome biogenesis, composition and secretion highlights the heterogeneity of extracellular vesicles. J Cell Sci. 2013;126(Pt 24):5553–65.
Hoshino D, Kirkbride KC, Costello K, Clark ES, Sinha S, Grega-Larson N, et al. Exosome secretion is enhanced by invadopodia and drives invasive behavior. Cell Rep. 2013;5(5):1159–68.
Giordano C, Gelsomino L, Barone I, Panza S, Augimeri G, Bonofiglio D, et al. Leptin modulates exosome biogenesis in breast cancer cells: an additional mechanism in cell-to-cell communication. J Clin Med. 2019;8(7):1027.
Dreux M, Garaigorta U, Boyd B, Décembre E, Chung J, Whitten-Bauer C, et al. Short-range exosomal transfer of viral RNA from infected cells to plasmacytoid dendritic cells triggers innate immunity. Cell Host Microbe. 2012;12(4):558–70.
Baietti MF, Zhang Z, Mortier E, Melchior A, Degeest G, Geeraerts A, et al. Syndecan-syntenin-ALIX regulates the biogenesis of exosomes. Nat Cell Biol. 2012;14(7):677–85.
Ghossoub R, Lembo F, Rubio A, Gaillard CB, Bouchet J, Vitale N, et al. Syntenin-ALIX exosome biogenesis and budding into multivesicular bodies are controlled by ARF6 and PLD2. Nat Commun. 2014;5:3477.
Abrami L, Brandi L, Moayeri M, Brown MJ, Krantz BA, Leppla SH, et al. Hijacking multivesicular bodies enables long-term and exosome-mediated long-distance action of anthrax toxin. Cell Rep. 2013;5(4):986–96.
Imjeti NS, Menck K, Egea-Jimenez AL, Lecointre C, Lembo F, Bouguenina H, et al. Syntenin mediates SRC function in exosomal cell-to-cell communication. Proc Natl Acad Sci U S A. 2017;114(47):12495–500.
Larios J, Mercier V, Roux A, Gruenberg J. ALIX- and ESCRT-III-dependent sorting of tetraspanins to exosomes. J Cell Biol. 2020;219(3):e201904113.
Buschow SI, Liefhebber JM, Wubbolts R, Stoorvogel W. Exosomes contain ubiquitinated proteins. Blood Cells Mol Dis. 2005;35(3):398–403.
Putz U, Howitt J, Lackovic J, Foot N, Kumar S, Silke J, et al. Nedd4 family-interacting protein 1 (Ndfip1) is required for the exosomal secretion of Nedd4 family proteins. J Biol Chem. 2008;283(47):32621–7.
van Niel G, Wubbolts R, Ten Broeke T, Buschow SI, Ossendorp FA, Melief CJ, et al. Dendritic cells regulate exposure of MHC class II at their plasma membrane by oligoubiquitination. Immunity. 2006;25(6):885–94.
Buschow SI, Nolte-’t Hoen EN, van Niel G, Pols MS, ten Broeke T, Lauwen M, et al. MHC II in dendritic cells is targeted to lysosomes or T cell-induced exosomes via distinct multivesicular body pathways. Traffic. 2009;10(10):1528–42.
Trajkovic K, Hsu C, Chiantia S, Rajendran L, Wenzel D, Wieland F, et al. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science. 2008;319(5867):1244–7.
Yuyama K, Sun H, Mitsutake S, Igarashi Y. Sphingolipid-modulated exosome secretion promotes clearance of amyloid-β by microglia. J Biol Chem. 2012;287(14):10977–89.
Chairoungdua A, Smith DL, Pochard P, Hull M, Caplan MJ. Exosome release of β-catenin: a novel mechanism that antagonizes Wnt signaling. J Cell Biol. 2010;190(6):1079–91.
Kosaka N, Iguchi H, Yoshioka Y, Takeshita F, Matsuki Y, Ochiya T. Secretory mechanisms and intercellular transfer of microRNAs in living cells. J Biol Chem. 2010;285(23):17442–52.
Zhou W, Woodson M, Sherman MB, Neelakanta G, Sultana H. Exosomes mediate Zika virus transmission through SMPD3 neutral Sphingomyelinase in cortical neurons. Emerg Microbes Infect. 2019;8(1):307–26.
García-Seisdedos D, Babiy B, Lerma M, Casado ME, Martínez-Botas J, Lasunción MA, et al. Curcumin stimulates exosome/microvesicle release in an in vitro model of intracellular lipid accumulation by increasing ceramide synthesis. Biochim Biophys Acta Mol Cell Biol Lipids. 2020;1865(5):158638.
van Niel G, Charrin S, Simoes S, Romao M, Rochin L, Saftig P, et al. The tetraspanin CD63 regulates ESCRT-independent and -dependent endosomal sorting during melanogenesis. Dev Cell. 2011;21(4):708–21.
Hemler ME. Tetraspanin proteins mediate cellular penetration, invasion, and fusion events and define a novel type of membrane microdomain. Annu Rev Cell Dev Biol. 2003;19:397–422.
Escola JM, Kleijmeer MJ, Stoorvogel W, Griffith JM, Yoshie O, Geuze HJ. Selective enrichment of tetraspan proteins on the internal vesicles of multivesicular endosomes and on exosomes secreted by human B-lymphocytes. J Biol Chem. 1998;273(32):20121–7.
Perez-Hernandez D, Gutiérrez-Vázquez C, Jorge I, López-Martín S, Ursa A, Sánchez-Madrid F, et al. The intracellular interactome of tetraspanin-enriched microdomains reveals their function as sorting machineries toward exosomes. J Biol Chem. 2013;288(17):11649–61.
Verweij FJ, van Eijndhoven MA, Hopmans ES, Vendrig T, Wurdinger T, Cahir-McFarland E, et al. LMP1 association with CD63 in endosomes and secretion via exosomes limits constitutive NF-κB activation. Embo J. 2011;30(11):2115–29.
van Niel G, Bergam P, Di Cicco A, Hurbain I, Lo Cicero A, Dingli F, et al. Apolipoprotein E regulates amyloid formation within endosomes of pigment cells. Cell Rep. 2015;13(1):43–51.
Verweij FJ, Bebelman MP, Jimenez CR, Garcia-Vallejo JJ, Janssen H, Neefjes J, et al. Correction: Quantifying exosome secretion from single cells reveals a modulatory role for GPCR signaling. J Cell Biol. 2018;217(3):1157.
Joshi BS, de Beer MA, Giepmans BNG, Zuhorn IS. Endocytosis of extracellular vesicles and release of their cargo from endosomes. ACS Nano. 2020;14(4):4444–55.
Sung BH, von Lersner A, Guerrero J, Krystofiak ES, Inman D, Pelletier R, et al. A live cell reporter of exosome secretion and uptake reveals pathfinding behavior of migrating cells. Nat Commun. 2020;11(1):2092.
Bebelman MP, Bun P, Huveneers S, van Niel G, Pegtel DM, Verweij FJ. Real-time imaging of multivesicular body-plasma membrane fusion to quantify exosome release from single cells. Nat Protoc. 2020;15(1):102–21.
Mazurov D, Barbashova L, Filatov A. Tetraspanin protein CD9 interacts with metalloprotease CD10 and enhances its release via exosomes. Febs j. 2013;280(5):1200–13.
Ghossoub R, Chéry M, Audebert S, Leblanc R, Egea-Jimenez AL, Lembo F, et al. Tetraspanin-6 negatively regulates exosome production. Proc Natl Acad Sci U S A. 2020;117(11):5913–22.
Blanc L, Vidal M. New insights into the function of Rab GTPases in the context of exosomal secretion. Small GTPases. 2018;9(1–2):95–106.
Ostrowski M, Carmo NB, Krumeich S, Fanget I, Raposo G, Savina A, et al. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat Cell Biol. 2010;12(1):19–30.
Song L, Tang S, Han X, Jiang Z, Dong L, Liu C, et al. KIBRA controls exosome secretion via inhibiting the proteasomal degradation of Rab27a. Nat Commun. 2019;10(1):1639.
Savina A, Vidal M, Colombo MI. The exosome pathway in K562 cells is regulated by Rab11. J Cell Sci. 2002;115(Pt 12):2505–15.
Sato M, Sato K, Liou W, Pant S, Harada A, Grant BD. Regulation of endocytic recycling by C. elegans Rab35 and its regulator RME-4, a coated-pit protein. Embo J. 2008;27(8):1183–96.
Koles K, Nunnari J, Korkut C, Barria R, Brewer C, Li Y, et al. Mechanism of evenness interrupted (Evi)-exosome release at synaptic boutons. J Biol Chem. 2012;287(20):16820–34.
Hsu C, Morohashi Y, Yoshimura S, Manrique-Hoyos N, Jung S, Lauterbach MA, et al. Regulation of exosome secretion by Rab35 and its GTPase-activating proteins TBC1D10A-C. J Cell Biol. 2010;189(2):223–32.
Frühbeis C, Fröhlich D, Kuo WP, Amphornrat J, Thilemann S, Saab AS, et al. Neurotransmitter-triggered transfer of exosomes mediates oligodendrocyte-neuron communication. PLoS Biol. 2013;11(7):e1001604.
Song P, Trajkovic K, Tsunemi T, Krainc D. Parkin modulates endosomal organization and function of the endo-lysosomal pathway. J Neurosci. 2016;36(8):2425–37.
Hyenne V, Apaydin A, Rodriguez D, Spiegelhalter C, Hoff-Yoessle S, Diem M, et al. RAL-1 controls multivesicular body biogenesis and exosome secretion. J Cell Biol. 2015;211(1):27–37.
Gradilla AC, González E, Seijo I, Andrés G, Bischoff M, González-Mendez L, et al. Exosomes as Hedgehog carriers in cytoneme-mediated transport and secretion. Nat Commun. 2014;5:5649.
Gross JC, Chaudhary V, Bartscherer K, Boutros M. Active Wnt proteins are secreted on exosomes. Nat Cell Biol. 2012;14(10):1036–45.
Guo H, Chitiprolu M, Roncevic L, Javalet C, Hemming FJ, Trung MT, et al. Atg5 disassociates the V(1)V(0)-ATPase to promote exosome production and tumor metastasis independent of canonical macroautophagy. Dev Cell. 2017;43(6):716-30.e7.
Abdulrahman BA, Abdelaziz DH, Schatzl HM. Autophagy regulates exosomal release of prions in neuronal cells. J Biol Chem. 2018;293(23):8956–68.
Zou W, Lai M, Zhang Y, Zheng L, Xing Z, Li T, et al. Exosome release is regulated by mTORC1. Adv Sci (Weinh). 2019;6(3):1801313.
Morishita M, Takahashi Y, Nishikawa M, Takakura Y. Pharmacokinetics of exosomes-an important factor for elucidating the biological roles of exosomes and for the development of exosome-based therapeutics. J Pharm Sci. 2017;106(9):2265–9.
Qin J, Xu Q. Functions and application of exosomes. Acta Pol Pharm. 2014;71(4):537–43.
Lakhal S, Wood MJ. Exosome nanotechnology: an emerging paradigm shift in drug delivery: exploitation of exosome nanovesicles for systemic in vivo delivery of RNAi heralds new horizons for drug delivery across biological barriers. BioEssays. 2011;33(10):737–41.
Murphy DE, de Jong OG, Brouwer M, Wood MJ, Lavieu G, Schiffelers RM, et al. Extracellular vesicle-based therapeutics: natural versus engineered targeting and trafficking. Exp Mol Med. 2019;51(3):1–12.
Munagala R, Aqil F, Jeyabalan J, Gupta RC. Bovine milk-derived exosomes for drug delivery. Cancer Lett. 2016;371(1):48–61.
Smyth T, Kullberg M, Malik N, Smith-Jones P, Graner MW, Anchordoquy TJ. Biodistribution and delivery efficiency of unmodified tumor-derived exosomes. J Control Release. 2015;199:145–55.
Zhuang X, Xiang X, Grizzle W, Sun D, Zhang S, Axtell RC, et al. Treatment of brain inflammatory diseases by delivering exosome encapsulated anti-inflammatory drugs from the nasal region to the brain. Mol Ther. 2011;19(10):1769–79.
Kim SH, Bianco NR, Shufesky WJ, Morelli AE, Robbins PD. Effective treatment of inflammatory disease models with exosomes derived from dendritic cells genetically modified to express IL-4. J Immunol. 2007;179(4):2242–9.
Chow A, Zhou W, Liu L, Fong MY, Champer J, Van Haute D, et al. Macrophage immunomodulation by breast cancer-derived exosomes requires Toll-like receptor 2-mediated activation of NF-κB. Sci Rep. 2014;4:5750.
Charoenviriyakul C, Takahashi Y, Morishita M, Matsumoto A, Nishikawa M, Takakura Y. Cell type-specific and common characteristics of exosomes derived from mouse cell lines: yield, physicochemical properties, and pharmacokinetics. Eur J Pharm Sci. 2017;96:316–22.
Zhang H, Freitas D, Kim HS, Fabijanic K, Li Z, Chen H, et al. Identification of distinct nanoparticles and subsets of extracellular vesicles by asymmetric flow field-flow fractionation. Nat Cell Biol. 2018;20(3):332–43.
Zech D, Rana S, Büchler MW, Zöller M. Tumor-exosomes and leukocyte activation: an ambivalent crosstalk. Cell Commun Signal. 2012;10(1):37.
Horibe S, Tanahashi T, Kawauchi S, Murakami Y, Rikitake Y. Mechanism of recipient cell-dependent differences in exosome uptake. BMC Cancer. 2018;18(1):47.
Lentz TL, Burrage TG, Smith AL, Crick J, Tignor GH. Is the acetylcholine receptor a rabies virus receptor? Science. 1982;215(4529):182–4.
Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJ. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol. 2011;29(4):341–5.
Cui GH, Guo HD, Li H, Zhai Y, Gong ZB, Wu J, et al. RVG-modified exosomes derived from mesenchymal stem cells rescue memory deficits by regulating inflammatory responses in a mouse model of Alzheimer’s disease. Immun Ageing. 2019;16:10.
Sancho-Albero M, Navascués N, Mendoza G, Sebastián V, Arruebo M, Martín-Duque P, et al. Exosome origin determines cell targeting and the transfer of therapeutic nanoparticles towards target cells. J Nanobiotechnol. 2019;17(1):16.
Hazan-Halevy I, Rosenblum D, Weinstein S, Bairey O, Raanani P, Peer D. Cell-specific uptake of mantle cell lymphoma-derived exosomes by malignant and non-malignant B-lymphocytes. Cancer Lett. 2015;364(1):59–69.
Escrevente C, Keller S, Altevogt P, Costa J. Interaction and uptake of exosomes by ovarian cancer cells. BMC Cancer. 2011;11:108.
Hoshino A, Costa-Silva B, Shen TL, Rodrigues G, Hashimoto A, Tesic Mark M, et al. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527(7578):329–35.
Zhao L, Gu C, Gan Y, Shao L, Chen H, Zhu H. Exosome-mediated siRNA delivery to suppress postoperative breast cancer metastasis. J Control Release. 2020;318:1–15.
Laulagnier K, Javalet C, Hemming FJ, Chivet M, Lachenal G, Blot B, et al. Amyloid precursor protein products concentrate in a subset of exosomes specifically endocytosed by neurons. Cell Mol Life Sci. 2018;75(4):757–73.
Belhadj Z, He B, Deng H, Song S, Zhang H, Wang X, et al. A combined “eat me/don’t eat me” strategy based on extracellular vesicles for anticancer nanomedicine. J Extracell Vesicles. 2020;9(1):1806444.
Toda Y, Takata K, Nakagawa Y, Kawakami H, Fujioka S, Kobayashi K, et al. Effective internalization of U251-MG-secreted exosomes into cancer cells and characterization of their lipid components. Biochem Biophys Res Commun. 2015;456(3):768–73.
Parolini I, Federici C, Raggi C, Lugini L, Palleschi S, De Milito A, et al. Microenvironmental pH is a key factor for exosome traffic in tumor cells. J Biol Chem. 2009;284(49):34211–22.
Matsumoto A, Takahashi Y, Nishikawa M, Sano K, Morishita M, Charoenviriyakul C, et al. Role of phosphatidylserine-derived negative surface charges in the recognition and uptake of intravenously injected b16bl6-derived exosomes by macrophages. J Pharm Sci. 2017;106(1):168–75.
Tkach M, Kowal J, Zucchetti AE, Enserink L, Jouve M, Lankar D, et al. Qualitative differences in T-cell activation by dendritic cell-derived extracellular vesicle subtypes. Embo J. 2017;36(20):3012–28.
Sobo-Vujanovic A, Munich S, Vujanovic NL. Dendritic-cell exosomes cross-present Toll-like receptor-ligands and activate bystander dendritic cells. Cell Immunol. 2014;289(1–2):119–27.
Guan S, Li Q, Liu P, Xuan X, Du Y. Umbilical cord blood-derived dendritic cells loaded with BGC823 tumor antigens and DC-derived exosomes stimulate efficient cytotoxic T-lymphocyte responses and antitumor immunity in vitro and in vivo. Cent Eur J Immunol. 2014;39(2):142–51.
Munich S, Sobo-Vujanovic A, Buchser WJ, Beer-Stolz D, Vujanovic NL. Dendritic cell exosomes directly kill tumor cells and activate natural killer cells via TNF superfamily ligands. Oncoimmunology. 2012;1(7):1074–83.
Chernomordik LV, Melikyan GB, Chizmadzhev YA. Biomembrane fusion: a new concept derived from model studies using two interacting planar lipid bilayers. Biochim Biophys Acta. 1987;906(3):309–52.
Jahn R, Südhof TC. Membrane fusion and exocytosis. Annu Rev Biochem. 1999;68:863–911.
Prada I, Meldolesi J. Binding and fusion of extracellular vesicles to the plasma membrane of their cell targets. Int J Mol Sci. 2016;17(8):1296.
Valapala M, Vishwanatha JK. Lipid raft endocytosis and exosomal transport facilitate extracellular trafficking of annexin A2. J Biol Chem. 2011;286(35):30911–25.
Mulcahy LA, Pink RC, Carter DR. Routes and mechanisms of extracellular vesicle uptake. J Extracell Vesicles. 2014;3:24641.
Levy S, Shoham T. Protein-protein interactions in the tetraspanin web. Physiology (Bethesda). 2005;20:218–24.
Nunes-Correia I, Eulálio A, Nir S, Düzgünes N, Ramalho-Santos J, Pedroso de Lima MC. Fluorescent probes for monitoring virus fusion kinetics: comparative evaluation of reliability. Biochim Biophys Acta. 2002;1561(1):65–75.
Montecalvo A, Larregina AT, Shufesky WJ, Stolz DB, Sullivan ML, Karlsson JM, et al. Mechanism of transfer of functional microRNAs between mouse dendritic cells via exosomes. Blood. 2012;119(3):756–66.
Zheng Y, Tu C, Zhang J, Wang J. Inhibition of multiple myeloma-derived exosomes uptake suppresses the functional response in bone marrow stromal cell. Int J Oncol. 2019;54(3):1061–70.
Tian T, Zhu YL, Hu FH, Wang YY, Huang NP, Xiao ZD. Dynamics of exosome internalization and trafficking. J Cell Physiol. 2013;228(7):1487–95.
Tian T, Wang Y, Wang H, Zhu Z, Xiao Z. Visualizing of the cellular uptake and intracellular trafficking of exosomes by live-cell microscopy. J Cell Biochem. 2010;111(2):488–96.
Mettlen M, Chen PH, Srinivasan S, Danuser G, Schmid SL. Regulation of clathrin-mediated endocytosis. Annu Rev Biochem. 2018;87:871–96.
Eguchi S, Takefuji M, Sakaguchi T, Ishihama S, Mori Y, Tsuda T, et al. Cardiomyocytes capture stem cell-derived, anti-apoptotic microRNA-214 via clathrin-mediated endocytosis in acute myocardial infarction. J Biol Chem. 2019;294(31):11665–74.
Feng D, Zhao WL, Ye YY, Bai XC, Liu RQ, Chang LF, et al. Cellular internalization of exosomes occurs through phagocytosis. Traffic. 2010;11(5):675–87.
Benmerah A, Bayrou M, Cerf-Bensussan N, Dautry-Varsat A. Inhibition of clathrin-coated pit assembly by an Eps15 mutant. J Cell Sci. 1999;112(Pt 9):1303–11.
Yoon JH, Ashktorab H, Smoot DT, Nam SW, Hur H, Park WS. Uptake and tumor-suppressive pathways of exosome-associated GKN1 protein in gastric epithelial cells. Gastric Cancer. 2020;23(5):848–62.
Barrès C, Blanc L, Bette-Bobillo P, André S, Mamoun R, Gabius HJ, et al. Galectin-5 is bound onto the surface of rat reticulocyte exosomes and modulates vesicle uptake by macrophages. Blood. 2010;115(3):696–705.
Fitzner D, Schnaars M, van Rossum D, Krishnamoorthy G, Dibaj P, Bakhti M, et al. Selective transfer of exosomes from oligodendrocytes to microglia by macropinocytosis. J Cell Sci. 2011;124(Pt 3):447–58.
Amber Gonda RM, Janviere Kabagwira, Paul A. Vallejos and Nathan R. Wall. Cellular-Defined Microenvironmental Internalization of Exosomes. In: Calderon AGDBaJAR, editor. Extracellular Vesicles and Their Importance in Human Health. www.intechopen.com. IntechOpen; 2019.
Delenclos M, Trendafilova T, Mahesh D, Baine AM, Moussaud S, Yan IK, et al. Investigation of endocytic pathways for the internalization of exosome-associated oligomeric alpha-synuclein. Front Neurosci. 2017;11:172.
Koumangoye RB, Sakwe AM, Goodwin JS, Patel T, Ochieng J. Detachment of breast tumor cells induces rapid secretion of exosomes which subsequently mediate cellular adhesion and spreading. PLoS ONE. 2011;6(9):e24234.
Izquierdo-Useros N, Naranjo-Gómez M, Archer J, Hatch SC, Erkizia I, Blanco J, et al. Capture and transfer of HIV-1 particles by mature dendritic cells converges with the exosome-dissemination pathway. Blood. 2009;113(12):2732–41.
He L, Zhu W, Chen Q, Yuan Y, Wang Y, Wang J, et al. Ovarian cancer cell-secreted exosomal miR-205 promotes metastasis by inducing angiogenesis. Theranostics. 2019;9(26):8206–20.
Phuyal S, Hessvik NP, Skotland T, Sandvig K, Llorente A. Regulation of exosome release by glycosphingolipids and flotillins. Febs J. 2014;281(9):2214–27.
Kiss AL, Botos E. Endocytosis via caveolae: alternative pathway with distinct cellular compartments to avoid lysosomal degradation? J Cell Mol Med. 2009;13(7):1228–37.
Nanbo A, Kawanishi E, Yoshida R, Yoshiyama H. Exosomes derived from Epstein-Barr virus-infected cells are internalized via caveola-dependent endocytosis and promote phenotypic modulation in target cells. J Virol. 2013;87(18):10334–47.
Svensson KJ, Christianson HC, Wittrup A, Bourseau-Guilmain E, Lindqvist E, Svensson LM, et al. Exosome uptake depends on ERK1/2-heat shock protein 27 signaling and lipid Raft-mediated endocytosis negatively regulated by caveolin-1. J Biol Chem. 2013;288(24):17713–24.
Gordon S. Phagocytosis: an immunobiologic process. Immunity. 2016;44(3):463–75.
Lim JP, Gleeson PA. Macropinocytosis: an endocytic pathway for internalising large gulps. Immunol Cell Biol. 2011;89(8):836–43.
Kerr MC, Teasdale RD. Defining macropinocytosis. Traffic. 2009;10(4):364–71.
Kerr MC, Lindsay MR, Luetterforst R, Hamilton N, Simpson F, Parton RG, et al. Visualisation of macropinosome maturation by the recruitment of sorting nexins. J Cell Sci. 2006;119(Pt 19):3967–80.
Hewlett LJ, Prescott AR, Watts C. The coated pit and macropinocytic pathways serve distinct endosome populations. J Cell Biol. 1994;124(5):689–703.
Costa Verdera H, Gitz-Francois JJ, Schiffelers RM, Vader P. Cellular uptake of extracellular vesicles is mediated by clathrin-independent endocytosis and macropinocytosis. J Control Release. 2017;266:100–8.
Nakase I, Kobayashi NB, Takatani-Nakase T, Yoshida T. Active macropinocytosis induction by stimulation of epidermal growth factor receptor and oncogenic Ras expression potentiates cellular uptake efficacy of exosomes. Sci Rep. 2015;5:10300.
Kamerkar S, LeBleu VS, Sugimoto H, Yang S, Ruivo CF, Melo SA, et al. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature. 2017;546(7659):498–503.
Plebanek MP, Mutharasan RK, Volpert O, Matov A, Gatlin JC, Thaxton CS. Nanoparticle targeting and cholesterol flux through scavenger receptor type B-1 inhibits cellular exosome uptake. Sci Rep. 2015;5:15724.
Emam SE, Ando H, Lila ASA, Shimizu T, Okuhira K, Ishima Y, et al. Liposome co-incubation with cancer cells secreted exosomes (extracellular vesicles) with different proteins expressions and different uptake pathways. Sci Rep. 2018;8(1):14493.
Yao Z, Qiao Y, Li X, Chen J, Ding J, Bai L, et al. Exosomes exploit the virus entry machinery and pathway to transmit alpha interferon-induced antiviral activity. J Virol. 2018;92(24):e01578-18.
Tian T, Zhu YL, Zhou YY, Liang GF, Wang YY, Hu FH, et al. Exosome uptake through clathrin-mediated endocytosis and macropinocytosis and mediating miR-21 delivery. J Biol Chem. 2014;289(32):22258–67.
Heusermann W, Hean J, Trojer D, Steib E, von Bueren S, Graff-Meyer A, et al. Exosomes surf on filopodia to enter cells at endocytic hot spots, traffic within endosomes, and are targeted to the ER. J Cell Biol. 2016;213(2):173–84.
Mattila PK, Lappalainen P. Filopodia: molecular architecture and cellular functions. Nat Rev Mol Cell Biol. 2008;9(6):446–54.
Lehmann MJ, Sherer NM, Marks CB, Pypaert M, Mothes W. Actin- and myosin-driven movement of viruses along filopodia precedes their entry into cells. J Cell Biol. 2005;170(2):317–25.
Mercer J, Schelhaas M, Helenius A. Virus entry by endocytosis. Annu Rev Biochem. 2010;79:803–33.
Del Conde I, Shrimpton CN, Thiagarajan P, López JA. Tissue-factor-bearing microvesicles arise from lipid rafts and fuse with activated platelets to initiate coagulation. Blood. 2005;106(5):1604–11.
Chen C, Zong S, Wang Z, Lu J, Zhu D, Zhang Y, et al. Visualization and intracellular dynamic tracking of exosomes and exosomal miRNAs using single molecule localization microscopy. Nanoscale. 2018;10(11):5154–62.
Shelke GV, Yin Y, Jang SC, Lässer C, Wennmalm S, Hoffmann HJ, et al. Endosomal signalling via exosome surface TGFβ-1. J Extracell Vesicles. 2019;8(1):1650458.
Li S, Liu L, Zhuang X, Yu Y, Liu X, Cui X, et al. MicroRNAs inhibit the translation of target mRNAs on the endoplasmic reticulum in Arabidopsis. Cell. 2013;153(3):562–74.
Friedman JR, Dibenedetto JR, West M, Rowland AA, Voeltz GK. Endoplasmic reticulum-endosome contact increases as endosomes traffic and mature. Mol Biol Cell. 2013;24(7):1030–40.
Santos MF, Rappa G, Karbanová J, Kurth T, Corbeil D, Lorico A. VAMP-associated protein-A and oxysterol-binding protein-related protein 3 promote the entry of late endosomes into the nucleoplasmic reticulum. J Biol Chem. 2018;293(36):13834–48.
Malhas A, Goulbourne C, Vaux DJ. The nucleoplasmic reticulum: form and function. Trends Cell Biol. 2011;21(6):362–73.
Yu HJ, Reuter MA, McDonald D. HIV traffics through a specialized, surface-accessible intracellular compartment during trans-infection of T cells by mature dendritic cells. PLoS Pathog. 2008;4(8):e1000134.
Wang S, Sun H, Tanowitz M, Liang XH, Crooke ST. Intra-endosomal trafficking mediated by lysobisphosphatidic acid contributes to intracellular release of phosphorothioate-modified antisense oligonucleotides. Nucleic Acids Res. 2017;45(9):5309–22.
White JM, Whittaker GR. Fusion of enveloped viruses in endosomes. Traffic. 2016;17(6):593–614.
Spooner RA, Smith DC, Easton AJ, Roberts LM, Lord JM. Retrograde transport pathways utilised by viruses and protein toxins. Virol J. 2006;3:26.
Grant BD, Donaldson JG. Pathways and mechanisms of endocytic recycling. Nat Rev Mol Cell Biol. 2009;10(9):597–608.
Booth AM, Fang Y, Fallon JK, Yang JM, Hildreth JE, Gould SJ. Exosomes and HIV Gag bud from endosome-like domains of the T cell plasma membrane. J Cell Biol. 2006;172(6):923–35.
Fang Y, Wu N, Gan X, Yan W, Morrell JC, Gould SJ. Higher-order oligomerization targets plasma membrane proteins and HIV gag to exosomes. PLoS Biol. 2007;5(6):e158.
Fordjour FK, Daaboul GG, Gould SJ. A shared pathway of exosome biogenesis operates at plasma and endosome membranes. bioRxiv. 2019;545228.
Willms E, Johansson HJ, Mäger I, Lee Y, Blomberg KE, Sadik M, et al. Cells release subpopulations of exosomes with distinct molecular and biological properties. Sci Rep. 2016;6:22519.
Mathivanan S, Fahner CJ, Reid GE, Simpson RJ. ExoCarta 2012: database of exosomal proteins, RNA and lipids. Nucleic Acids Res. 2012;40(Database issue):D1241-4.
Chiang CY, Chen C. Toward characterizing extracellular vesicles at a single-particle level. J Biomed Sci. 2019;26(1):9.
Chevillet JR, Kang Q, Ruf IK, Briggs HA, Vojtech LN, Hughes SM, et al. Quantitative and stoichiometric analysis of the microRNA content of exosomes. Proc Natl Acad Sci U S A. 2014;111(41):14888–93.
Wen SW, Lima LG, Lobb RJ, Norris EL, Hastie ML, Krumeich S, et al. Breast cancer-derived exosomes reflect the cell-of-origin phenotype. Proteomics. 2019;19(8):e1800180.
Palma J, Yaddanapudi SC, Pigati L, Havens MA, Jeong S, Weiner GA, et al. MicroRNAs are exported from malignant cells in customized particles. Nucleic Acids Res. 2012;40(18):9125–38.
Ghayad SE, Rammal G, Ghamloush F, Basma H, Nasr R, Diab-Assaf M, et al. Exosomes derived from embryonal and alveolar rhabdomyosarcoma carry differential miRNA cargo and promote invasion of recipient fibroblasts. Sci Rep. 2016;6:37088.
Théry C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7(1):1535750.
Jeppesen DK, Fenix AM, Franklin JL, Higginbotham JN, Zhang Q, Zimmerman LJ, et al. Reassessment of exosome composition. Cell. 2019;177(2):428-45.e18.
Gardiner C, Di Vizio D, Sahoo S, Théry C, Witwer KW, Wauben M, et al. Techniques used for the isolation and characterization of extracellular vesicles: results of a worldwide survey. J Extracell Vesicles. 2016;5:32945.
Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200(4):373–83.
Sluijter JP, Verhage V, Deddens JC, van den Akker F, Doevendans PA. Microvesicles and exosomes for intracardiac communication. Cardiovasc Res. 2014;102(2):302–11.
Rana S, Yue S, Stadel D, Zöller M. Toward tailored exosomes: the exosomal tetraspanin web contributes to target cell selection. Int J Biochem Cell Biol. 2012;44(9):1574–84.
Caruso Bavisotto C, Cappello F, Macario AJL, Conway de Macario E, Logozzi M, Fais S, et al. Exosomal HSP60: a potentially useful biomarker for diagnosis, assessing prognosis, and monitoring response to treatment. Expert Rev Mol Diagn. 2017;17(9):815–22.
Zhang X, Wang X, Zhu H, Kranias EG, Tang Y, Peng T, et al. Hsp20 functions as a novel cardiokine in promoting angiogenesis via activation of VEGFR2. PLoS ONE. 2012;7(3):e32765.
Lauwers E, Wang YC, Gallardo R, Van der Kant R, Michiels E, Swerts J, et al. Hsp90 mediates membrane deformation and exosome release. Mol Cell. 2018;71(5):689-702.e9.
Lynch S, Santos SG, Campbell EC, Nimmo AM, Botting C, Prescott A, et al. Novel MHC class I structures on exosomes. J Immunol. 2009;183(3):1884–91.
Gauvreau ME, Côté MH, Bourgeois-Daigneault MC, Rivard LD, Xiu F, Brunet A, et al. Sorting of MHC class II molecules into exosomes through a ubiquitin-independent pathway. Traffic. 2009;10(10):1518–27.
Wolf T, Baier SR, Zempleni J. The intestinal transport of bovine milk exosomes is mediated by endocytosis in human colon carcinoma caco-2 cells and rat small intestinal IEC-6 cells. J Nutr. 2015;145(10):2201–6.
Berenguer J, Lagerweij T, Zhao XW, Dusoswa S, van der Stoop P, Westerman B, et al. Glycosylated extracellular vesicles released by glioblastoma cells are decorated by CCL18 allowing for cellular uptake via chemokine receptor CCR8. J Extracell Vesicles. 2018;7(1):1446660.
Inuzuka T, Inokawa A, Chen C, Kizu K, Narita H, Shibata H, et al. ALG-2-interacting Tubby-like protein superfamily member PLSCR3 is secreted by an exosomal pathway and taken up by recipient cultured cells. Biosci Rep. 2013;33(2):e00026.
Subra C, Laulagnier K, Perret B, Record M. Exosome lipidomics unravels lipid sorting at the level of multivesicular bodies. Biochimie. 2007;89(2):205–12.
Menck K, Sönmezer C, Worst TS, Schulz M, Dihazi GH, Streit F, et al. Neutral sphingomyelinases control extracellular vesicles budding from the plasma membrane. J Extracell Vesicles. 2017;6(1):1378056.
Record M, Carayon K, Poirot M, Silvente-Poirot S. Exosomes as new vesicular lipid transporters involved in cell-cell communication and various pathophysiologies. Biochim Biophys Acta. 2014;1841(1):108–20.
Funding
This work was supported by funding from the United Kingdom Research Council Innovate UK Biomedical Catalyst award 14720, NIHR Great Ormond Street Hospital Biomedical Research Centre and MRC Clinician Scientist Fellowship MR/T008024/1, Nutricia Metabolic Research Grant, London Advanced Therapy / Confidence in Collaboration award 2CiC017. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
Author information
Authors and Affiliations
Contributions
SG and JB designed the work. SG wrote the manuscript. All authors revised substantially, read and approved the final manuscript. Figures created in Biorender.com. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Gurung, S., Perocheau, D., Touramanidou, L. et al. The exosome journey: from biogenesis to uptake and intracellular signalling. Cell Commun Signal 19, 47 (2021). https://doi.org/10.1186/s12964-021-00730-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12964-021-00730-1