Abstract
Amyloid beta peptide (Aβ), the main component of senile plaques of Alzheimer’s disease brains, is produced by sequential cleavage of amyloid precursor protein (APP) and of its C-terminal fragments (CTFs). An unanswered question is how amyloidogenic peptides spread throughout the brain during the course of the disease. Here, we show that small lipid vesicles called exosomes, secreted in the extracellular milieu by cortical neurons, carry endogenous APP and are strikingly enriched in CTF-α and the newly characterized CTF-η. Exosomes from N2a cells expressing human APP with the autosomal dominant Swedish mutation contain Aβ peptides as well as CTF-α and CTF-η, while those from cells expressing the non-mutated form of APP only contain CTF-α and CTF-η. APP and CTFs are sorted into a subset of exosomes which lack the tetraspanin CD63 and specifically bind to dendrites of neurons, unlike exosomes carrying CD63 which bind to both neurons and glial cells. Thus, neuroblastoma cells secrete distinct populations of exosomes carrying different cargoes and targeting specific cell types. APP-carrying exosomes can be endocytosed by receiving cells, allowing the processing of APP acquired by exosomes to give rise to the APP intracellular domain (AICD). Thus, our results show for the first time that neuronal exosomes may indeed act as vehicles for the intercellular transport of APP and its catabolites.







Similar content being viewed by others
References
Gruenberg J, Stenmark H (2004) The biogenesis of multivesicular endosomes. Nat Rev Mol Cell Biol 5:317–323. doi:10.1038/nrm1360
Denzer K, Kleijmeer MJ, Heijnen HF et al (2000) Exosome: from internal vesicle of the multivesicular body to intercellular signaling device. J Cell Sci 113:3365–3374
Laulagnier K, Schieber NL, Maritzen T et al (2011) Role of AP1 and Gadkin in the traffic of secretory endo-lysosomes. Mol Biol Cell 22:2068–2082. doi:10.1091/mbc.E11-03-0193
Edgar JR, Willén K, Gouras GK, Futter CE (2015) ESCRTs regulate amyloid precursor protein sorting in multivesicular bodies and intracellular amyloid-β accumulation. J Cell Sci 128:2520–2528. doi:10.1242/jcs.170233
White IJ, Bailey LM, Aghakhani MR et al (2006) EGF stimulates annexin 1-dependent inward vesiculation in a multivesicular endosome subpopulation. EMBO J 25:1–12. doi:10.1038/sj.emboj.7600759
Matsuo H, Chevallier J, Mayran N et al (2004) Role of LBPA and Alix in multivesicular liposome formation and endosome organization. Science 303:531–534. doi:10.1126/science.1092425
Subra C, Laulagnier K, Perret B, Record M (2007) Exosome lipidomics unravels lipid sorting at the level of multivesicular bodies. Biochimie 89:205–212. doi:10.1016/j.biochi.2006.10.014
Trajkovic K, Hsu C, Chiantia S et al (2008) Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science 319:1244–1247. doi:10.1126/science.1153124
Wollert T, Hurley JH (2010) Molecular mechanism of multivesicular body biogenesis by ESCRT complexes. Nature 464:864–869. doi:10.1038/nature08849
Colombo M, Raposo G, Théry C (2014) Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol 30:255–289. doi:10.1146/annurev-cellbio-101512-122326
Kowal J, Arras G, Colombo M et al (2016) Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc Natl Acad Sci USA 113:E968–E977. doi:10.1073/pnas.1521230113
Nanbo A, Kawanishi E, Yoshida R, Yoshiyama H (2013) Exosomes derived from Epstein-Barr virus-infected cells are internalized via caveola-dependent endocytosis and promote phenotypic modulation in target cells. J Virol 87:10334–10347. doi:10.1128/JVI.01310-13
Tian T, Zhu Y-L, Zhou Y-Y et al (2014) Exosome uptake through clathrin-mediated endocytosis and macropinocytosis and mediating miR-21 delivery. J Biol Chem 289:22258–22267. doi:10.1074/jbc.M114.588046
Valadi H, Ekström K, Bossios A et al (2007) Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 9:654–659. doi:10.1038/ncb1596
Perez-Gonzalez R, Gauthier SA, Kumar A, Levy E (2012) The exosome secretory pathway transports amyloid precursor protein carboxyl-terminal fragments from the cell into the brain extracellular space. J Biol Chem 287:43108–43115. doi:10.1074/jbc.M112.404467
Street JM, Barran PE, Mackay CL et al (2012) Identification and proteomic profiling of exosomes in human cerebrospinal fluid. J Transl Med 10:5. doi:10.1186/1479-5876-10-5
Fitzner D, Schnaars M, van Rossum D et al (2011) Selective transfer of exosomes from oligodendrocytes to microglia by macropinocytosis. J Cell Sci 124:447–458. doi:10.1242/jcs.074088
Wang G, Dinkins M, He Q et al (2012) Astrocytes secrete exosomes enriched with proapoptotic ceramide and prostate apoptosis response 4 (PAR-4): potential mechanism of apoptosis induction in Alzheimer disease (AD). J Biol Chem 287:21384–21395. doi:10.1074/jbc.M112.340513
Potolicchio I, Carven GJ, Xu X et al (2005) Proteomic analysis of microglia-derived exosomes: metabolic role of the aminopeptidase CD13 in neuropeptide catabolism. J Immunol 175:2237–2243
Fauré J, Lachenal G, Court M et al (2006) Exosomes are released by cultured cortical neurones. Mol Cell Neurosci 31:642–648. doi:10.1016/j.mcn.2005.12.003
Chivet M, Hemming F, Pernet-Gallay K et al (2012) Emerging role of neuronal exosomes in the central nervous system. Front Physiol 3:145. doi:10.3389/fphys.2012.00145
Chivet M, Javalet C, Hemming F et al (2013) Exosomes as a novel way of interneuronal communication. Biochem Soc Trans 41:241–244. doi:10.1042/BST20120266
Lachenal G, Pernet-Gallay K, Chivet M et al (2011) Release of exosomes from differentiated neurons and its regulation by synaptic glutamatergic activity. Mol Cell Neurosci 46:409–418. doi:10.1016/j.mcn.2010.11.004
Chivet M, Javalet C, Laulagnier K et al (2014) Exosomes secreted by cortical neurons upon glutamatergic synapse activation specifically interact with neurons. J Extracell Vesicles 3:24722. doi:10.3402/jev.v3.24722
Coleman BM, Hill AF (2015) Extracellular vesicles–Their role in the packaging and spread of misfolded proteins associated with neurodegenerative diseases. Semin Cell Dev Biol 40:89–96. doi:10.1016/j.semcdb.2015.02.007
Février B, Vilette D, Laude H, Raposo G (2005) Exosomes: a bubble ride for prions? Traffic 6:10–17. doi:10.1111/j.1600-0854.2004.00247.x
Emmanouilidou E, Melachroinou K, Roumeliotis T et al (2010) Cell-produced alpha-synuclein is secreted in a calcium-dependent manner by exosomes and impacts neuronal survival. J Neurosci 30:6838–6851. doi:10.1523/JNEUROSCI.5699-09.2010
Feiler MS, Strobel B, Freischmidt A et al (2015) TDP-43 is intercellularly transmitted across axon terminals. J Cell Biol 211:897–911. doi:10.1083/jcb.201504057
Jucker M, Walker LC (2013) Self-propagation of pathogenic protein aggregates in neurodegenerative diseases. Nature 501:45–51. doi:10.1038/nature12481
Haass C, Kaether C, Thinakaran G, Sisodia S (2012) Trafficking and proteolytic processing of APP. Cold Spring Harb Perspect Med 2:a006270. doi:10.1101/cshperspect.a006270
Xu W, Weissmiller AM, White JA et al (2016) Amyloid precursor protein–mediated endocytic pathway disruption induces axonal dysfunction and neurodegeneration. J Clin Invest 126:1815–1833. doi:10.1172/JCI82409
Kim S, Sato Y, Mohan PS et al (2016) Evidence that the rab5 effector APPL1 mediates APP-βCTF-induced dysfunction of endosomes in Down syndrome and Alzheimer’s disease. Mol Psychiatry 21:707–716. doi:10.1038/mp.2015.97
Willem M, Tahirovic S, Busche MA et al (2015) η-Secretase processing of APP inhibits neuronal activity in the hippocampus. Nature 526:443–447. doi:10.1038/nature14864
Baranger K, Marchalant Y, Bonnet AE et al (2016) MT5-MMP is a new pro-amyloidogenic proteinase that promotes amyloid pathology and cognitive decline in a transgenic mouse model of Alzheimer’s disease. Cell Mol Life Sci 73:217–236. doi:10.1007/s00018-015-1992-1
Das U, Scott DA, Ganguly A et al (2013) Activity-induced convergence of APP and BACE-1 in acidic microdomains via an endocytosis-dependent pathway. Neuron 79:447–460. doi:10.1016/j.neuron.2013.05.035
Takahashi RH, Milner TA, Li F et al (2002) Intraneuronal Alzheimer abeta42 accumulates in multivesicular bodies and is associated with synaptic pathology. Am J Pathol 161:1869–1879
Sannerud R, Esselens C, Ejsmont P et al (2016) Restricted location of PSEN2/γ-secretase determines substrate specificity and generates an intracellular Aβ pool. Cell 166:193–208. doi:10.1016/j.cell.2016.05.020
Rajendran L, Honsho M, Zahn TR et al (2006) Alzheimer’s disease beta-amyloid peptides are released in association with exosomes. Proc Natl Acad Sci USA 103:11172–11177. doi:10.1073/pnas.0603838103
Vingtdeux V, Hamdane M, Loyens A et al (2007) Alkalizing drugs induce accumulation of amyloid precursor protein by-products in luminal vesicles of multivesicular bodies. J Biol Chem 282:18197–18205. doi:10.1074/jbc.M609475200
Sharples RA, Vella LJ, Nisbet RM et al (2008) Inhibition of gamma-secretase causes increased secretion of amyloid precursor protein C-terminal fragments in association with exosomes. FASEB J 22:1469–1478. doi:10.1096/fj.07-9357com
Citron M, Oltersdorf T, Haass C et al (1992) Mutation of the beta-amyloid precursor protein in familial Alzheimer’s disease increases beta-protein production. Nature 360:672–674. doi:10.1038/360672a0
Belly A, Bodon G, Blot B et al (2010) CHMP2B mutants linked to frontotemporal dementia impair maturation of dendritic spines. J Cell Sci 123:2943–2954. doi:10.1242/jcs.068817
Chatellard-Causse C, Blot B, Cristina N et al (2002) Alix (ALG-2-interacting protein X), a protein involved in apoptosis, binds to endophilins and induces cytoplasmic vacuolization. J Biol Chem 277:29108–29115. doi:10.1074/jbc.M204019200
Laulagnier K, Javalet C, Hemming FJ, Sadoul R (2017) Purification and analysis of exosomes released by mature cortical neurons following synaptic activation. Methods Mol Biol 1545:129–138. doi:10.1007/978-1-4939-6728-5_9
Schagger H (2006) Tricine-SDS-PAGE. Nat Protoc 1:16–22. doi:10.1038/nprot.2006.4
Bolte S, Cordelières FP (2006) A guided tour into subcellular colocalization analysis in light microscopy. J Microsc 224:213–232. doi:10.1111/j.1365-2818.2006.01706.x
Hoey SE, Williams RJ, Perkinton MS (2009) Synaptic NMDA receptor activation stimulates alpha-secretase amyloid precursor protein processing and inhibits amyloid-beta production. J Neurosci 29:4442–4460. doi:10.1523/JNEUROSCI.6017-08.2009
Cai XD, Golde TE, Younkin SG (1993) Release of excess amyloid beta protein from a mutant amyloid beta protein precursor. Science 259:514–516
Forman MS, Cook DG, Leight S et al (1997) Differential effects of the swedish mutant amyloid precursor protein on β-amyloid accumulation and secretion in neurons and nonneuronal cells. J Biol Chem 272:32247–32253. doi:10.1074/jbc.272.51.32247
Huotari J, Helenius A (2011) Endosome maturation. EMBO J 30:3481–3500. doi:10.1038/emboj.2011.286
Stenmark H, Parton RG, Steele-Mortimer O et al (1994) Inhibition of rab5 GTPase activity stimulates membrane fusion in endocytosis. EMBO J 13:1287–1296
Cao X, Südhof TC (2004) Dissection of amyloid-beta precursor protein-dependent transcriptional transactivation. J Biol Chem 279:24601–24611. doi:10.1074/jbc.M402248200
Sullivan CP, Jay AG, Stack EC et al (2011) Retromer disruption promotes amyloidogenic APP processing. Neurobiol Dis 43:338–345. doi:10.1016/j.nbd.2011.04.002
Langui D, Girardot N, El Hachimi KH et al (2004) Subcellular topography of neuronal Abeta peptide in APPxPS1 transgenic mice. Am J Pathol 165:1465–1477
Hoey SE, Buonocore F, Cox CJ et al (2013) AMPA receptor activation promotes non-amyloidogenic amyloid precursor protein processing and suppresses neuronal amyloid-β production. PLoS One 8:e78155. doi:10.1371/journal.pone.0078155
Lesné S, Ali C, Gabriel C et al (2005) NMDA receptor activation inhibits alpha-secretase and promotes neuronal amyloid-beta production. J Neurosci 25:9367–9377. doi:10.1523/JNEUROSCI.0849-05.2005
Stoeck A, Keller S, Riedle S et al (2006) A role for exosomes in the constitutive and stimulus-induced ectodomain cleavage of L1 and CD44. Biochem J 393:609–618. doi:10.1042/BJ20051013
Jämsä A, Belda O, Edlund M, Lindström E (2011) BACE-1 inhibition prevents the γ-secretase inhibitor evoked Aβ rise in human neuroblastoma SH-SY5Y cells. J Biomed Sci 18:76. doi:10.1186/1423-0127-18-76
Lu X, Deng Y, Yu D et al (2014) Histone acetyltransferase p300 mediates histone acetylation of PS1 and BACE1 in a cellular model of Alzheimer’s disease. PLoS One 9:e103067. doi:10.1371/journal.pone.0103067
Mucke L, Masliah E, Yu GQ et al (2000) High-level neuronal expression of abeta 1-42 in wild-type human amyloid protein precursor transgenic mice: synaptotoxicity without plaque formation. J Neurosci 20:4050–4058
Falker C, Hartmann A, Guett I et al (2016) Exosomal cellular prion protein drives fibrillization of amyloid beta and counteracts amyloid beta-mediated neurotoxicity. J Neurochem 137:88–100. doi:10.1111/jnc.13514
An K, Klyubin I, Kim Y et al (2013) Exosomes neutralize synaptic-plasticity-disrupting activity of Aβ assemblies in vivo. Mol Brain 6:47. doi:10.1186/1756-6606-6-47
Cataldo AM, Peterhoff CM, Troncoso JC et al (2000) Endocytic pathway abnormalities precede amyloid beta deposition in sporadic Alzheimer’s disease and Down syndrome: differential effects of APOE genotype and presenilin mutations. Am J Pathol 157:277–286
Almeida CG, Takahashi RH, Gouras GK (2006) β-amyloid accumulation impairs multivesicular body sorting by inhibiting the ubiquitin-proteasome system. J Neurosci 26:4277–4288. doi:10.1523/JNEUROSCI.5078-05.2006
Hollingworth P, Harold D, Sims R et al (2011) Common variants at ABCA7, MS4A6A/MS4A4E, EPHA1, CD33 and CD2AP are associated with Alzheimer’s disease. Nat Genet 43:429–435. doi:10.1038/ng.803
Naj AC, Jun G, Beecham GW et al (2011) Common variants at MS4A4/MS4A6E, CD2AP, CD33 and EPHA1 are associated with late-onset Alzheimer’s disease. Nat Genet 43:436–441. doi:10.1038/ng.801
Ubelmann F, Burrinha T, Salavessa L et al (2017) Bin1 and CD2AP polarise the endocytic generation of beta-amyloid. EMBO Rep 18:102–122. doi:10.15252/embr.201642738
van Niel G, Charrin S, Simoes S et al (2011) The tetraspanin CD63 regulates ESCRT-independent and -dependent endosomal sorting during melanogenesis. Dev Cell 21:708–721. doi:10.1016/j.devcel.2011.08.019
Deyts C, Thinakaran G, Parent AT (2016) APP Receptor. To Be or Not To Be. Trends Pharmacol Sci. doi:10.1016/j.tips.2016.01.005
Sisodia SS, Koo EH, Hoffman PN et al (1993) Identification and transport of full-length amyloid precursor proteins in rat peripheral nervous system. J Neurosci 13:3136–3142
Wang Y, Ha Y (2004) The X-ray structure of an antiparallel dimer of the human amyloid precursor protein E2 domain. Mol Cell 15:343–353. doi:10.1016/j.molcel.2004.06.037
Le Blanc I, Luyet P-P, Pons V et al (2005) Endosome-to-cytosol transport of viral nucleocapsids. Nat Cell Biol 7:653–664. doi:10.1038/ncb1269
Acknowledgements
We thank M. Jacquier-Sarlin, S. Fraboulet, M. Laporte, and K. Sadoul for critically reading the manuscript. This work was funded by Grants from the Agence Nationale de la Recherche (ANR MALZ program) and from Association France Alzheimer. K. Laulagnier was supported by the Fondation Plan Alzheimer. C. Javalet was supported by the Ministère de l’Enseignement Supérieur et de la Recherche.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
18_2017_2664_MOESM1_ESM.tif
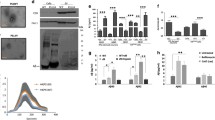
Supplementary Fig. 1 Nanoparticle Tracking Analysis shows that APPwt or APPswe expression affect neither the number nor the size of secreted exosomes. Exosomes purified from supernatants of control N2a, APPwt N2a, and APPswe N2a were resuspended and analyzed using Nanosight. a Number of particles secreted during 24 h by 9 x 106 cells per dish. Averages of five measurements +/- SEM are shown. b Distribution of sizes for each type of exosomes. Average of five measurements is shown with SEM drawn in gray. The mode size is indicated for each population. No significant differences were observed between each type of exosomes. (TIFF 765 kb)
18_2017_2664_MOESM2_ESM.tif
Supplementary Fig. 2 N2a cells were transfected with UAS-Firefly luciferase reporter construct alone (Mock) or together with NLS-Gal4 construct (NLS-Gal4). Renilla luciferase was co-expressed to normalize Firefly activity and luciferase activities measured 48 h after transfection. When indicated, 10 μM of the γ-secretase inhibitor (DAPT) was added 20 h before measurement. In contrast with experiments using APP-Gal4 in Fig. 7, DAPT does not inhibit Firefly luciferase activity. The result represents the mean of two independent cultures. (TIFF 159 kb)
Rights and permissions
About this article
Cite this article
Laulagnier, K., Javalet, C., Hemming, F.J. et al. Amyloid precursor protein products concentrate in a subset of exosomes specifically endocytosed by neurons. Cell. Mol. Life Sci. 75, 757–773 (2018). https://doi.org/10.1007/s00018-017-2664-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-017-2664-0