Abstract
The assessment of hemostasis is necessary to make suitable decisions on the management of patients with thrombotic disorders. In some clinical situations, for example, during thrombophilia screening, the presence of anticoagulants in sample makes diagnosis impossible. Various elimination methods may overcome anticoagulant interference. DOAC-Stop, DOAC-Remove and DOAC Filter are available methods to remove direct oral anticoagulants in diagnostic tests, although there are still reports on their incomplete efficacy in several assays. The new antidotes for direct oral anticoagulants – idarucizumab and andexanet alfa – could be potentially useful, but have their drawbacks. The necessity to remove heparins is also arising as heparin contamination from central venous catheter or therapy with heparin disturbs the appropriate hemostasis assessment. Heparinase and polybrene are already present in commercial reagents but a fully-effective neutralizer is still a challenge for researchers, thus promising candidates remain in the research phase.
Similar content being viewed by others
Introduction
Treatment with anticoagulants is associated with the risk of bleeding that increases with the dose, ageing, and concomitant administration of drugs affecting hemostasis, for example, antiplatelet agents [1,2,3,4,5]. Anticoagulant therapy is based on the administration of heparins, including unfractionated heparin (UFH) and low-molecular-weight heparins (LMWHs), and oral anticoagulants, such as vitamin K antagonists (VKAs) and direct oral anticoagulants (DOACs). VKAs may affect diagnostic tests and the reversal of their activity could be achieved by e.g., discontinuation of therapy or administration of vitamin K [6]. However, there are no reports the neutralization of warfarin in assays. The effect of UFH varies between patients, the adjustment of UFH dose and the monitoring of its effect are required [7]. The therapy with newer anticoagulants such as LMWHs and DOACs does not require routine monitoring. The assessment of LMWHs plasma concentration may be indicated in obese or pediatric patients, during pregnancy, or in those with impaired renal function [8, 9]. There are also many situations in which the measurement of DOACs activity is still necessary to make suitable decisions on patient management. The update of the International Council for Standardization in Haematology (ICSH) Recommendations from 2021 divides indications for monitoring DOACs into non-urgent and urgent situations [5]. DOAC therapy should be stopped for 2 to 3 days prior drawing of blood to minimize anticoagulant interference during testing. However, it is not always possible because of the risk of thrombosis [10]. Heparins and DOACs can affect the result of almost every coagulation test, thus precise monitoring of hemostasis in heparinized samples is not possible [10,11,12,13,14,15].
Accordingly, this narrative literature review examines the clinical and experimental literature and current guidelines regarding the removal or neutralization of DOACs and heparins in laboratory tests indicated in different clinical situations, for example during thrombophilia screening.
Assays affected by anticoagulants and clinical necessity of their removal in diagnostic tests
Routine coagulation tests, such as activated partial thromboplastin time (aPTT), prothrombin time (PT), and thrombin time (TT) are clot-based activity assays used for the general assessment of coagulation function. The prolonged coagulation time may be associated with clotting factors deficiencies and/or the presence of their inhibitors. The influence of anticoagulants on routine assays is widely known. The aPTT and TT are sensitive to the presence of UFH, whereas PT is not affected by UFH and LMWHs. The LMWHs influence on aPTT depends on reagent sensitivity and plasma concentration of LMHWs. Among DOACs, dabigatran shows the strongest effect on aPTT and TT, while rivaroxaban on PT. The anti-factor Xa (anti-FXa) assay is very sensitive to the presence of direct FXa inhibitors and LMWHs [8]. Therefore, some of these tests are used to monitor anticoagulant therapy, such as aPTT for UFH, anti-FXa assay for LMWHs and direct FXa inhibitors [15,16,17,18,19]. However, when hemostasis disorders are diagnosed in patients, the presence of anticoagulant in blood samples may lead to inaccurate results. Viscoelastic tests, thromboelastography (TEG) and rotational thromboelastometry (ROTEM) provide an assessment of coagulation and fibrinolysis in whole blood. Compared to standard coagulation assays, they may detect cellular interactions to better reflect in vivo hemostasis [20]. High or pathologically low results of thrombin generation (TG) assay may inform about the risk of thrombosis or bleeding, respectively [21, 22] The presence of any anticoagulant inhibits TG [23].
Thrombophilia is hereditary or acquired condition characterized by an increased tendency to blood clotting which is associated with the occurrence of venous thromboembolism (VTE) [24]. Many guidelines do not recommend thrombophilia testing because of its limited clinical utility [25, 26]. However, recent National Institute for Health and Care Excellence (NICE) VTE guidelines suggest considering testing when it is planned to stop anticoagulation treatment in patients who have had unprovoked VTE and/or have a first-degree relative with an unprovoked VTE [27]. Screening for inherited thrombophilia is based on assays estimating deficiencies of the natural anticoagulant activity – antithrombin (AT), protein C and protein S [28, 29]. AT is an endogenous anticoagulant which inhibits several clotting factors. The deficiency of AT may be detected with AT assays, functional or immunological. Functional AT assays are based on the inhibition of factor IIa (FIIa) or FXa by AT in the presence of heparin. However, heparin therapy can lead to reduction (up to 30%) in AT levels [30]. Direct FIIa or FXa inhibitors, may falsely elevate the results of anti-FIIa and anti-FXa assays, respectively [8, 31]. Activated protein C resistance (APC-R) induced primarily by the factor V Leiden mutation increases the risk of thrombosis [28, 32, 33]. APC-R may be detected when a ratio between a baseline aPTT and aPTT after the addition of exogenous activated protein C is not prolonged/less than 2.0 [32,33,34]. Genetic determination is recommended after the positive results of APC-R which may be caused by all DOACs [35, 36]. Antiphospholipid syndrome (APS) is an acquired thrombophilia characterized by venous and/or arterial thromboses or pregnancy morbidities such as miscarriages and late intrauterine fetal demise [37, 38]. The laboratory criterium of APS is the presence of at least one of the antiphospholipid antibodies: lupus anticoagulant (LA), anticardiolipin (aCL) or anti-β2-glycoprotein I (aβ2GPI) [39, 40]. LA is detected by prolonged coagulation times. The aCL and aβ2GPI antibodies are identified by measuring immunologic reactivity to a phospholipid (cardiolipin) or a phospholipid-binding protein (β2-glycoprotein I) in immunoassays [41]. Because the presence of LA strongly correlates with clinical symptoms, the assessment of LA is useful for the diagnosis and management of APS patients [42]. According to the International Society on Thrombosis and Haemostasis (ISTH) guidelines, the detection of LA requires performing two tests based on different principles – the dilute Russell Viper Venom time (dRVVT) and an LA-sensitive aPTT (aPTT-LA) [42, 43]. The dRVVT relies on the ability of Russell’s venom to directly activate FX, while the aPTT-LA is based on the activation of the intrinsic pathway. The dRVVT appeared to be more sensitive to interference by DOACs than aPTT-based assays [44,45,46]. LA-positive patients should immediately start anticoagulation therapy, but the results of LA tests repeated after 12 weeks may be hampered by the anticoagulation treatment [26, 47, 48]. Seheult et al. in a large, retrospective study showed higher positivity rates of LA assays in patients treated with DOACs (> 50%) compared to patients treated with heparin (30–36%) [49]. Heparins may be administered in some cases, such as pregnant women with a history of obstetric APS or catastrophic APS [50, 51]. Both UFH and enoxaparin interfere with dRVVT and aPTT-LA assays [42, 51]. The 3-step procedure (screen-mix-confirm) allows for avoiding false-positive LA results in heparinized plasma. However, it is necessary to assess heparin levels before testing [42]. The neutralization of anticoagulants in patient’s blood samples may improve test results, and consequently allow correct diagnosis of thrombophilia.
The surgical interventions due to a bleeding risk often require the temporary discontinuation of DOACs, which may increase the risk of a thromboembolic event. Parenteral bridging relies on the use of short-acting anticoagulants such as heparin, although it is not usually recommended and reserved for patients with a high risk of thromboembolism [52,53,54]. A prophylactic dose of heparin may be helpful when reinitiating of DOACs needs to be delayed because of additional procedures or a postoperative patient’s oral medications intolerance [55, 56]. The ICSH guidelines recommend monitoring heparin bridging in DOAC-treated patients. However, the measuring of heparin activity by the assay e.g., aPTT, modified by both anticoagulants seems to be impossible but could be achieved after the removal of DOAC during assay performance.
Blood collection from a central venous catheter may yield plasma contamination of heparin, which is used to prevent catheter occlusion and infection [12, 57]. As Jeon et al. showed that even discarding a higher volume of blood than is recommended cannot avoid heparin contamination while blood collection from catheters [58]. We and others showed that the assessment of DOACs anticoagulant activity becomes impaired in heparinized plasma [13, 59]. The appropriate assessment of anticoagulant concentration or activity in plasma may be possible after the neutralization of coexisted heparin.
Commercially available methods for DOACs removal in diagnostic tests
DOAC-Stop and DOAC-Remove
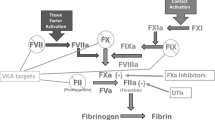
The ability of activated charcoal to absorb contaminations or drugs has been used in binding agents – DOAC-Stop (Haematex Research) and DOAC-Remove (5-Diagnostics AG). According to the manufacturer, one tablet of DOAC-Stop or DOAC-Remove is sufficient to obtain plasma deprived of all DOACs (Fig. 1). Their absorbent properties have been reported in routine coagulation assays, such as aPTT, PT, TT, diluted thrombin time and fibrinogen Clauss [60,61,62,63]. However, Cox-Morton et al. noticed statistically significant removal of rivaroxaban and apixaban in PT in 17/20 and 13/20 samples, respectively. Besides, DOAC-Stop fully neutralized dabigatran in the factor VII (FVII), factor VIII (FVIII), and FX assays [60]. The efficacy of DOAC-Remove was confirmed in an anti-FXa assay [61, 62]. In the Jourdi et al. study, the total neutralization by DOAC-Remove reached 82% for apixaban and 98% for rivaroxaban in an anti-FXa assay [62].
Recent studies have proved the ability of DOAC-Stop and DOAC-Remove to eliminate protein C and S overestimation in the presence of DOACs [36, 46, 61]. Favresse et al. have demonstrated a significant decrease of APC-R after adding DOAC-Stop to samples with dabigatran and a smaller decrease in samples with edoxaban, while no decrease was observed in samples containing rivaroxaban and apixaban [36]. The lack of effect against rivaroxaban was probably due to using Pefakit APC-R factor V Leiden, which does not interfere which rivaroxaban [15, 64]. DOAC-Remove almost fully reduced the concentration of all tested DOACs allowing for the measurement of APC-R [35].
The latest 2020 updates of the ISTH guidelines for LA detection and interpretation recommended the use of DOACs neutralizers or adsorbents in DOAC-treated patients if suspending treatment is impossible [42]. However, it has been suggested that DOAC-Stop may prolong dRVVT and aPTT in patient samples without anticoagulants or with heparin [48]. Some studies reported incomplete DOACs removal by DOAC-Stop in aPTT-LA and dRVVT tests [65, 66]. Similar results were obtained in studies using DOAC-Remove [61, 62]. In the tandem mass spectrometry (HPLC-MS/MS), Slavik et al. observed that DOAC-Stop near-total eliminates DOACs from patient plasma [67]. The concentrations of dabigatran, rivaroxaban and apixaban achieved a maximum of 2.7, 10.97 and 13.03 ng/ml, respectively. These residual amounts of DOACs did not interfere with LA testing. The gap between these studies may come from interlaboratory variation or the used protocol of binding agents. For DOAC-Remove, HPLC-MS/MS revealed almost complete elimination of dabigatran and rivaroxaban; residual concentration below lower limit of quantification was reached in 7/8 and 8/10 samples, respectively. Removal effectiveness was lower in apixaban samples and reached 5/10 samples [62].
The ability of DOAC-Stop and DOAC-Remove to neutralize apixaban, dabigatran, edoxaban and rivaroxaban was also confirmed in TG assay measured using calibrated automated thrombography (CAT) or TEG [63, 68]. DOAC-Stop induced a slight procoagulant effect, probably due to a small inhibition of tissue factor pathway inhibitor (TFPI).
DOAC Filter
The DOAC Filter (Diagnostica Stago) has been recently developed as a ready-to-use device with a filtration cartridge containing chemical hydrophobic-hydrophilic polymers to remove DOACs. It applies a solid phase extraction based on a noncovalent binding mechanism [69]. The sample of plasma is filtered and centrifuged (Fig. 2). Levels of DOAC are below the limit of detection after using DOAC Filter. The first studies showed sufficient removal efficiency in samples with rivaroxaban and dabigatran, while the absorption of apixaban-containing plasma was not complete [69,70,71,72]. A similar effect was observed in LA testing samples. In non-anticoagulated plasmas, some positive LA results changed into negative after DOAC Filter. During dRVVT and silica clotting time (SCT), interference of apixaban was removed in 50 and 60%, while for rivaroxaban in 84 and 83%, respectively. Accordingly to the ISTH guidance, using DOAC Filter should be limited to samples with DOAC [42, 72, 73]. DOAC Filter is a fairly new product, which requires further investigation to confirm its usefulness.
Potential candidates for DOACs neutralization in diagnostic tests
Ciraparantag (PER977)
Ciraparantag (PER977; Perosphere Inc.) is a new small synthetic water-soluble molecule that is developed for the reversal of anticoagulants, including UFH, enoxaparin and DOACs (dabigatran, rivaroxaban, apixaban, and edoxaban), still under clinical trials [74]. Bonding between ciraparantag and anticoagulant relies on noncovalent hydrogen bonds and charge-charge interaction. No binding to FIIa and FXa, plasma proteins, or other drugs was found [75]. Lu et al. suggested a possible increase in human platelet activation by ciraparantag using P-selectin expression induced by 10 µM adenosine diphosphate [76]. In this study, ciraparantag did not bind DOACs in vitro, which may depend on the assay. Ansell et al. showed that the DOACs reversal effect of ciraparantag was dose-related and better against apixaban while the whole blood clotting time [77]. Because of high cationic charge and low molecular weight, ciraparantag could complex with anionic chemicals used in blood tubes, such as sodium citrate, ethylenediaminetetraacetic acid or activators used in coagulation assays (aPTT, PT, anti-FXa assay), as kaolin and celite. Thus, the investigation aimed to test its usefulness in diagnostic assays is required [75, 78].
Idarucizumab
Idarucizumab, a humanized monoclonal antibody fragment, was approved in 2015 as a reversal agent for dabigatran in patients with life-threatening or serious bleeding or requiring urgent invasive procedures [79]. The mechanism of action relies on the binding of free and thrombin-bound dabigatran and its active glucuronide metabolites by hydrophobic interactions, hydrogen bonds, and a salt bridge [79, 80]. Only few studies indicated the use of idarucizumab as in vitro neutralizer. Jacquemin et al. demonstrated that idarucizumab did not interfere with routine clotting times (aPTT, TT, and PT) and other coagulation assays (FVII, FVIII, FX assays, dRVVT, aPTT-LA) [81]. Recently, Mijovski et al. found that idarucizumab increased TG in samples without dabigatran [82]. Nonetheless, this antibody seems to be potentially useful in neutralizing dabigatran in assays. However, low stability, storage conditions and costs make routine use of idarucizumab in diagnostic tests questionable.
Andexanet alfa
Andexanet alfa, a recombinant modified inactive human coagulation FXa, is approved by the FDA in 2018 for the reversal of rivaroxaban or apixaban in life-threatening or uncontrolled bleeding [83, 84]. Andexanet alfa acts as a decoy molecule that competitively binds anti-FXa inhibitors, neutralizing their anticoagulant activity [84, 85]. A few studies proved that andexanet alfa did not have a procoagulant or anticoagulant activity in clotting assays, such as aPTT, PT and prothrombinase-induced clotting time [85,86,87]. Interestingly, Siddiqui et al. observed that in the whole blood clotting time only betrixaban from among all FXa-tested inhibitors was completely neutralized by andexanet alfa [87]. Although the inhibition of TG induced by FXa inhibitors was neutralized, adding andexanet alfa alone to plasma increased the TG. Favaloro et al. noticed that during LA-testing andexanet alfa was not able to fully correct aPTT and dRVVT in rivaroxaban-spiked samples [88]. The rivaroxaban-neutralizing effect by andexanet alfa was reagent-dependent and showed a higher spread of test data in comparison to DOAC-Stop during FVIII and factor IX (FIX) testing [89]. However, andexanet alfa does not impact coagulation proteins except transiently decreased activity of TFPI [84, 86]. In the presence of rivaroxaban, the andexanet-TFPI bond intensified TG [86]. Because of high-affinity binding to the drug-bound AT, andexanet alfa also effectively reverses the anticoagulant effects of ATIII-dependent FXa inhibitors, like heparins, which was confirmed by the results of coagulation tests [84,85,86, 90, 91]. Further investigation is needed due to limited clinical evidence [91]. The high price and mentioned impact on some assays hold andexanet alfa back for use in laboratory diagnostics.
Commercially available agents for heparins removal in diagnostic tests
Heparinase
Heparinase is an enzyme obtained from the bacterium Flavobacterium heparinum. Its heparin-neutralizing abilities rely on the cleavage of alpha-glycosidic linkages at the ATIII binding site and prevent a heparin-AT-thrombin complexation [92, 93]. Lots of studies confirmed the reversal of the heparin effect by heparinase at aPTT, PT and TT tests [14, 94]. Heparinase restores the thrombin activity, decreases coagulation time and has a minimal effect on platelets [93, 95]. Current guidelines suggest the use of heparinase to quench the activity of both UFH and LMWH in LA detection [73]. There are commercially available TEG cuvettes with heparinase, which can be used in patients who received heparin [96]. Coppell et al. demonstrated effective neutralization of UFH, LMWH and danaparoid by heparinase [96]. HEP-TEM is a standardized, validated laboratory reagent containing heparinase. The addition of HEP-TEM to the TG assay can neutralize prophylactic and therapeutic doses of UFH and LMWH [97]. Strickland et al. have developed a new 3-step laboratory test to monitor UFH dosing using heparinase in patients taking apixaban. The difference between the first and second results of anti-FXa activity measurement indicates only the contribution of DOAC because of the removal of heparin by heparinase [59]. This assay may help in quantifying heparin in the presence of DOACs. Unfortunately, this approach requires longer analytical time than standard tests.
Polybrene
Polybrene (hexadimethrine bromide), a stable quaternary ammonium salt, has been known as a heparin-neutralizer agent since 1953 [98]. The mechanism of action is similar to that of protamine and depends on the interaction of cationic groups with anionic heparin chains. This synthetic polycation reverses the effects of heparin in vitro and in vivo. In first studies with polybrene demonstrated its superiority over protamine in the neutralizing of heparin with less severe side effects [99]. However, the clinical development of polybrene was stopped following reports of acute renal failure, proteinuria, hypotension and increased pulmonary artery pressure found in patients [92]. Kikura et al. demonstrated the polybrene ability to neutralize heparin in activated clotting time in comparison to another neutralizer available then [92]. Currently, polybrene is commonly used in vitro and can bind heparins in blood samples during routine tests such as PT and aPTT [100]. Cumming et al. proved that 100 µg/ml of polybrene was able to completely neutralize 10 IU/ml of heparin [101]. We also confirmed the usefulness of polybrene in the monitoring of dabigatran activity by TT test in the presence of heparin [13]. A commercially available reagent from Haematex - the Heparin Resistant Recalcyfying Solution (HRRS) – contains the calcium salt solution with polybrene, which may be used in aPTT, surface active clotting test and kaolin clotting test. Polybrene as a heparin neutralizer is also used in commercial kits for LA detection. Jacobsen et al. confirmed that adding polybrene to a sample containing heparin in a concentration of up to 1.3 IU/ml enables assessment of the lupus ratio regardless of heparin presence [102]. The guidelines recommend the use of polybrene or other neutralizers while LA testing [73]. Schäfer et al. conducted thromboelastometric tests using ROTEM delta analyzers for the detection and differentiation of DOACs and VKAs. Polybrene was included in EXTEM and FIBTEM tests for heparins removal [103]. During the TG study, polybrene (0.025 mg/ml) was able to bind UFH and enoxaparin up to 1.0 and 1.2 IU/ml, respectively, and completely restore TG in a concentration-dependent manner [11]. However, higher concentrations of polybrene may inhibit TG [97]. Furthermore, the prolongation of TG lag time and time to peak observed after the addition of polybrene may result from its inhibition of tissue factor-dependent FVII activation [11].
Potential candidates for heparins removal in diagnostic tests
Protamine sulfate
The oldest heparin reversal agent is protamine sulfate (PS), approved in 1939. PS is an arginine-rich protein sourced from the salmon fish sperm or produced through recombinant biotechnology [104]. The mechanism relies on the interaction between positive charged PS and polyanionic heparin, which create stable complex in a 1:1 ratio and thus PS displace ATIII from heparin complex [105]. One milligram of PS neutralizes 100 IU of heparin. PS normalized TT and anti-FXa activity (to 0 IU/ml) which was achieved at 0.6:1 ratios of PS to UFH [106]. Increasing doses of PS prolong clotting time tests, such as activated clotting time, PT and aPTT because of interference with coagulation factors and platelet function [107, 108]. Because neutralization by PS depends on the molecular weight of heparin, the reversal effect of LMWH is only partial [109]. A lower concentration of PS restored TG prolonged by UFH or LMWH up to 0.4 IU/ml [97]. Zmuda et al. found that PS incompletely reversed the prolongation of reaction time induced by heparin in the TEG method [110]. Although PS is still a neutralizer of heparin effects in clinical practice, its use in routine diagnostic tests seems to be inappropriate due to the activation of coagulation [111, 112].
Heparin-binding copolymer
A synthetic macromolecule named heparin-binding copolymer (HBC), a diblock polymer containing a neutral poly(ethylene glycol) block and a cationic poly(3-(methacryloylamino) propyl trimethylammonium chloride) block was developed previously by us for heparin and heparin mimetics complexation [113,114,115]. The ability of HBC to bind UFH was presented by colorimetric and optical methods. In rats, HBC neutralized the anticoagulant activity of UFH during aPTT testing with a ratio of 0.65 mg of HBC for 100 IU of UFH [113]. Full neutralization of enoxaparin, nadroparin, dalteparin, tinzaparin, and fondaparinux required different concentrations of HBC during the measurement of the anti-FXa activity [114]. Recently, we showed the effectiveness of HBC in heparin neutralization in vitro during DOACs measurement. Prolonged TT by both UFH and enoxaparin was restored in samples with dabigatran. We also proved the ability of HBC for heparin neutralization while measuring rivaroxaban activity by anti-FXa activity assay, although the approach still requires validation [13, 114].
Other cationic polymers
We previously found that modified dextran and chitosan can neutralize UFH and normalize aPTT and bleeding time in rats and mice models of thrombosis [116,117,118]. Among different polymers, the most active and safe reversal agent was Dex40-GTMAC3 [119, 120]. However, in vitro study showed that Dex40-GTMAC3 prolonged the aPTT above the concentration of 50 mg/ml [119].
There are also cationic polymers such as universal heparin reversal agent, dynamic covalent polymers, and others described by Bromfield et al., which could be useful as neutralizers in diagnostic tests, although further research is needed to check if their efficacy profile could be better than protamine [121,122,123].
Perspectives
Our review describes the methods of anticoagulant neutralization in diagnostic tests. Other options are methods that are not sensitive to anticoagulant activity. For example, even the concomitant presence of heparin in blood samples allows for the determination of DOACs activity using liquid chromatography-mass spectrometry. However, it has limited usage in routine diagnostics because of its low availability, time-consuming process and expensive equipment requirement [124,125,126,127]. The new rapid test – DOAC Dipstick – provides DOACs detection in the urine. Heparins cannot interact with DOAC Dipstick’s test pads because of a lack of antithrombin in urine [128,129,130]. This method for DOACs measurement could be helpful if urgent administration of antidotes is needed. For heparin, no gold-standard methods were established. The implementation of assay-neutral methods in routine diagnostics could greatly improve the performance of not only coagulation assays but also many other diagnostic tests.
Limitations
Differences in used protocol between laboratories may affect the results of described methods. The current approach focuses on neutralizing heparins or DOACs in laboratory assays which is not a problem in the case of VKAs. The monitoring of acute clinical settings requires quick and accurate assays. The guidelines suggest the use of DOAC neutralizers in standard practice. Commercially available methods, such as DOAC-Stop, DOAC-Remove, heparinase and polybrene, seem to be useful in standard laboratory tests. However, it will make the diagnosis of some disorders such as APS even more complex in urgent cases. Additionally, pretreatment with neutralizers can be advised only in anticoagulant-treated patients. More specific assays can accurately quantify drug levels, making them useful in important clinical situations. However, they are not available in all laboratories and require high level of expertise. Furthermore, if a neutralizer was added, some disturbances were described in specific assays like thrombin generation assays. Experimental methods, like idarucizumab, andexanet alfa, protamine sulfate and HBC have few studies confirming their usefulness in diagnostic assays.
Conclusions
The interfering with diagnostic tests by anticoagulants is a well-known issue and depends on the type of drug and its concentration, type of assay, reagents and analyzer used [72]. In this review we summarized different methods for removal of DOACs and heparins in diagnostic assays, both commercially available (Table 1), and in the development (Table 2). DOAC-Stop, DOAC-Remove and DOAC Filter were developed to neutralize DOACs. However, the incomplete reversal effect was observed, especially during LA testing. Idarucizumab and andexanet alfa, the antidotes for DOACs, are administered to patients with life-threatening bleeding, although their effectiveness as neutralizers in diagnostic tests has not been confirmed. Synthetic compounds, like polymers, or based on activated charcoal, seem to be the most promising in the neutralization of DOACs. Heparin may change the test results which could lead to incorrect patient diagnosis and therapy [131, 132]. Despite lengthy preparation of a sample or interference with some tests, heparinase and polybrene are present for removal of heparin in commercially available reagents.
Whenever hemostasis is monitored, the results can be affected by the presence of anticoagulants in the blood sample. Particularly, the situations when the contamination of sample is unknown or not expected, for example in unconscious patient, are the most vulnerable to misinterpretations. Thus, the elimination of anticoagulants from sample could improve the reliability of assay, and have potentially broader application (Fig. 3). The incomplete reversal action, interference with reagents/assays or neutralization of only one type of anticoagulant are drawbacks of currently available methods. A whole lot of potential candidates have not been studied yet.
Data Availability
Not applicable.
References
Levine MN, Raskob G, Beyth RJ, Kearon C, Schulman S. Hemorrhagic complications of anticoagulant treatment: the Seventh ACCP Conference on Antithrombotic and thrombolytic therapy. Chest. 2004;126(Suppl 3):287S–310S. https://doi.org/10.1378/chest.126.3_suppl.287S.
Tompkins C, Cheng A, Dalal D, Brinker JA, Leng CT, Marine JE, et al. Dual antiplatelet therapy and heparin “bridging” significantly increase the risk of bleeding complications after pacemaker or implantable cardioverter-defibrillator device implantation. J Am Coll Cardiol. 2010;55:2376–82. https://doi.org/10.1016/j.jacc.2009.12.056.
Campbell NR, Hull RD, Brant R, Hogan DB, Pineo GF, Raskob GE. Aging and heparin-related bleeding. Arch Intern Med. 1996;156:857–60.
Rose DK, Bar B. Direct oral anticoagulant agents: Pharmacologic Profile, indications, Coagulation Monitoring, and reversal agents. J Stroke Cerebrovasc Dis. 2018;27:2049–58. https://doi.org/10.1016/j.jstrokecerebrovasdis.2018.04.004.
Douxfils J, Adcock DM, Bates SM, Favaloro EJ, Gouin-Thibault I, Guillermo C, et al. 2021 update of the International Council for standardization in Haematology Recommendations for Laboratory Measurement of direct oral anticoagulants. Thromb Haemost. 2021;121:1008–20. https://doi.org/10.1055/a-1450-8178.
McRae HL, Militello L, Refaai MA. Updates in anticoagulation therapy monitoring. Biomedicines. 2021;9(3):262. https://doi.org/10.3390/biomedicines9030262.
Garcia DA, Baglin TP, Weitz JI, Samama MM, Parenteral Anticoagulants. Chest. 2012;141. https://doi.org/10.1378/chest.11-2291. Suppl 2:e24S-e43S.
Funk DM (Adcock), editor. Coagulation assays and anticoagulant monitoring. Hematology 2012;2012:460–5. https://doi.org/10.1182/asheducation.V2012.1.460.3798662.
Babin JL, Traylor KL, Witt DM. Laboratory Monitoring of Low-Molecular-Weight Heparin and Fondaparinux. Semin Thromb Hemostasis. 2017;43:261–9. https://doi.org/10.1055/s-0036-1581129.
Siriez R, Dogné J-M, Gosselin R, Laloy J, Mullier F, Douxfils J. Comprehensive review of the impact of direct oral anticoagulants on thrombophilia diagnostic tests: practical recommendations for the laboratory. Int J Lab Hematol. 2021;43:7–20. https://doi.org/10.1111/ijlh.13342.
Hardy M, Douxfils J, Morimont L, Didembourg M, Carlo A, de Maistre E, et al. Study of in vitro thrombin generation after neutralization of heparin. Int J Lab Hematol. 2022;44:168–76. https://doi.org/10.1111/ijlh.13703.
da Silva SR, Reichembach MT, Pontes L, Souza G, de de PESCM S. Heparin solution in the prevention of occlusions in Hickman® catheters a randomized clinical trial. Rev Lat Am Enfermagem. 2021;29:e3385. https://doi.org/10.1590/1518-8345.3310.3385.
Jakimczuk A, Kalaska B, Kamiński K, Miklosz J, Yusa S-I, Pawlak D, et al. Monitoring of anticoagulant activity of Dabigatran and Rivaroxaban in the Presence of Heparins. J Clin Med. 2022;11:2236. https://doi.org/10.3390/jcm11082236.
Harenberg J, Reichel Th, Malsch R, Hirsh J, Rustagi P. Multicentric evaluation of heparinase on aPTT, thrombin clotting time and a new PT reagent based on recombinant human tissue factor. Blood Coagul Fibrinolysis. 1996;7:453–8. https://doi.org/10.1097/00001721-199606000-00004.
Douxfils J, Ageno W, Samama C-M, Lessire S, ten Cate H, Verhamme P, et al. Laboratory testing in patients treated with direct oral anticoagulants: a practical guide for clinicians. J Thromb Haemost. 2018;16:209–19. https://doi.org/10.1111/jth.13912.
Greaves M, Control of Anticoagulation Subcommittee of the Scientific and Standardization Committee of the International Society of Thrombosis and Haemostasis. Limitations of the laboratory monitoring of heparin therapy. Scientific and Standardization Committee Communications: on behalf of the control of Anticoagulation Subcommittee of the Scientific and Standardization Committee of the International Society of thrombosis and haemostasis. Thromb Haemost. 2002;87:163–4.
Laposata M, Green D, Van Cott EM, Barrowcliffe TW, Goodnight SH, Sosolik RC. College of American Pathologists Conference XXXI on laboratory monitoring of anticoagulant therapy: the clinical use and laboratory monitoring of low-molecular-weight heparin, danaparoid, hirudin and related compounds, and argatroban. Arch Pathol Lab Med. 1998;122:799–807.
Favaloro E, Lippi G. Laboratory Testing in the era of Direct or non–vitamin K antagonist oral anticoagulants: a practical guide to measuring their activity and avoiding diagnostic errors. Semin Thromb Hemost. 2015;41:208–27. https://doi.org/10.1055/s-0035-1546827.
Douxfils J, Tamigniau A, Chatelain B, Chatelain C, Wallemacq P, Dogné J-M, et al. Comparison of calibrated chromogenic anti-xa assay and PT tests with LC-MS/MS for the therapeutic monitoring of patients treated with rivaroxaban. Thromb Haemost. 2013;110:723–31. https://doi.org/10.1160/TH13-04-0274.
Carll T, Wool GD. Basic principles of viscoelastic testing. Transfusion. 2020;60:1–9. https://doi.org/10.1111/trf.16071.
Hron G, Kollars M, Binder BR, Eichinger S, Kyrle PA. Identification of patients at low risk for recurrent venous thromboembolism by measuring thrombin generation. JAMA. 2006;296:397–402. https://doi.org/10.1001/jama.296.4.397.
Dargaud Y, Béguin S, Lienhart A, Al Dieri R, Trzeciak C, Bordet JC, et al. Evaluation of thrombin generating capacity in plasma from patients with haemophilia A and B. Thromb Haemost. 2005;93:475–80. https://doi.org/10.1160/TH04-10-0706.
Hemker HC, Al Dieri R, Béguin S. Thrombin generation assays: accruing clinical relevance. Curr Opin Hematol. 2004;11(3):170. https://doi.org/10.1097/01.moh.0000130314.33410.d7.
Favaloro EJ. Danger of false negative (exclusion) or false positive (diagnosis) for ‘congenital thrombophilia’ in the age of anticoagulants. Clin Chem Lab Med. 2019;57:873–82. https://doi.org/10.1515/cclm-2018-1041.
Stevens SM, Woller SC, Bauer KA, Kasthuri R, Cushman M, Streiff M, et al. Guidance for the evaluation and treatment of hereditary and acquired thrombophilia. J Thromb Thrombolysis. 2016;41:154–64. https://doi.org/10.1007/s11239-015-1316-1.
Darlow J, Mould H. Thrombophilia testing in the era of direct oral anticoagulants. Clin Med (Lond). 2021;21:e487–91. https://doi.org/10.7861/clinmed.2020-1008.
Venous thromboembolic diseases. Diagnosis, management and thrombophilia testing. London: National Institute for Health and Care Excellence (NICE); 2020.
Connors JM. Thrombophilia Testing and venous thrombosis. N Engl J Med. 2017;377:1177–87. https://doi.org/10.1056/NEJMra1700365.
Khider L, Gendron N, Mauge L. Inherited Thrombophilia in the era of direct oral anticoagulants. Int J Mol Sci. 2022;23:1821. https://doi.org/10.3390/ijms23031821.
Khor B, Van Cott EM. Laboratory tests for antithrombin deficiency. Am J Hematol. 2010;85:947–50. https://doi.org/10.1002/ajh.21893.
Van Cott EM, Orlando C, Moore GW, et al. Recommendations for clinical laboratory testing for antithrombin deficiency; communication from the SSC of the ISTH. J Thromb Haemost. 2020;18:17–22. https://doi.org/10.1111/jth.14648.
Kadauke S, Khor B, Van Cott EM. Activated protein C resistance testing for factor V Leiden. Am J Hematol. 2014;89:1147–50. https://doi.org/10.1002/ajh.23867.
Graf LL, Welsh CH, Qamar Z, Marlar RA. Activated protein C resistance assay detects thrombotic risk factors other than factor V Leiden. Am J Clin Pathol. 2003;119:52–60. https://doi.org/10.1309/QCUU-NRMV-JY8M-WPPL.
Van Cott EM, Soderberg BL, Laposata M, Activated Protein C. Resistance, the factor V Leiden Mutation, and a Laboratory Testing Algorithm. Arch Pathol Lab Med. 2002;126:577–82. https://doi.org/10.5858/2002-126-0577-APCRTF.
Kopytek M, Ząbczyk M, Malinowski KP, Undas A, Natorska J. DOAC-Remove abolishes the effect of direct oral anticoagulants on activated protein C resistance testing in real-life venous thromboembolism patients. Clin Chem Lab Med. 2020;58:430–7. https://doi.org/10.1515/cclm-2019-0650.
Favresse J, Lardinois B, Sabor L, Devalet B, Vandepapeliere J, Braibant M, et al. Evaluation of the DOAC-Stop® Procedure to overcome the effect of DOACs on several Thrombophilia Screening tests. TH Open. 2018;02:e202–9. https://doi.org/10.1055/s-0038-1657785.
Ortel TL. Antiphospholipid syndrome: laboratory testing and diagnostic strategies. Am J Hematol. 2012;87(Suppl 1):75–81. https://doi.org/10.1002/ajh.23196.
Devreese KMJ, Ortel TL, Pengo V, de Laat B, for the Subcommittee on Lupus Anticoagulant/Antiphospholip ID Antibodies. Laboratory criteria for antiphospholipid syndrome: communication from the SSC of the ISTH. J Thromb Haemost. 2018;16:809–13. https://doi.org/10.1111/jth.13976.
Petri M. Antiphospholipid syndrome. Transl Res. 2020;225:70–81. https://doi.org/10.1016/j.trsl.2020.04.006.
Pignatelli P, Ettorre E, Menichelli D, Pani A, Violi F, Pastori D. Seronegative antiphospholipid syndrome: refining the value of “non-criteria” antibodies for diagnosis and clinical management. Haematologica. 2020;105:562–72. https://doi.org/10.3324/haematol.2019.221945.
Levine JS, Branch DW, Rauch J. The Antiphospholipid Syndrome. N Engl J Med. 2002;346:752–63. https://doi.org/10.1056/NEJMra002974.
Devreese KMJ, de Groot PG, de Laat B, Erkan D, Favaloro EJ, Mackie I, et al. Guidance from the Scientific and Standardization Committee for lupus anticoagulant/antiphospholipid antibodies of the International Society on thrombosis and haemostasis. J Thromb Haemost. 2020;18:2828–39. https://doi.org/10.1111/jth.15047.
Pengo V, Tripodi A, Reber G, Rand JH, Orâ„¡ TL, Galli M, et al. Update of the guidelines for lupus anticoagulant detection. J Thromb Haemost. 2009;7:1737–40. https://doi.org/10.1111/j.1538-7836.2009.03555.x.
Bonar R, Favaloro EJ, Mohammed S, Ahuja M, Pasalic L, Sioufi J, et al. The effect of the direct factor xa inhibitors apixaban and rivaroxaban on haemostasis tests: a comprehensive assessment using in vitro and ex vivo samples. Pathology. 2016;48:60–71. https://doi.org/10.1016/j.pathol.2015.11.025.
Bonar R, Favaloro EJ, Mohammed S, Pasalic L, Sioufi J, Marsden K. The effect of dabigatran on haemostasis tests: a comprehensive assessment using in vitro and ex vivo samples. Pathology. 2015;47:355–64. https://doi.org/10.1097/PAT.0000000000000252.
Favre R, Zia-Chahabi S, Talb Y, de Gunzburg N, Flaujac C. Direct oral anticoagulant neutralization by activated charcoal DOAC-Remove for thrombophilia screening. Blood Coagul Fibrinolysis. 2021;32:356. https://doi.org/10.1097/MBC.0000000000001040.
Depreter B, Devreese KMJ. Dilute Russell’s viper venom time reagents in lupus anticoagulant testing: a well-considered choice. Clin Chem Lab Med. 2017;55:91–101. https://doi.org/10.1515/cclm-2016-0245.
De Kesel PM, Devreese KMJ. Direct oral anticoagulant adsorption: impact on lupus anticoagulant testing—review of the literature and evaluation on spiked and patient samples. J Thromb Haemost. 2020;18:2003–17. https://doi.org/10.1111/jth.14894.
Seheult JN, Meyer MP, Bontempo FA, Chibisov I. The Effects of Indirect- and direct-acting anticoagulants on Lupus anticoagulant assays: a large, retrospective study at a Coagulation Reference Laboratory. Am J Clin Pathol. 2017;147:632–40. https://doi.org/10.1093/ajcp/aqx035.
Tektonidou MG, Andreoli L, Limper M, Amoura Z, Cervera R, Costedoat-Chalumeau N, et al. EULAR recommendations for the management of antiphospholipid syndrome in adults. Ann Rheum Dis. 2019;78:1296–304. https://doi.org/10.1136/annrheumdis-2019-215213.
De Kesel PMM, Devreese KMJ. The effect of unfractionated heparin, enoxaparin, and danaparoid on lupus anticoagulant testing: can activated carbon eliminate false-positive results? Res Pract Thromb Haemost. 2019;4:161–8. https://doi.org/10.1002/rth2.12264.
Steffel J, Verhamme P, Potpara TS, Albaladejo P, Antz M, Desteghe L, et al. The 2018 European Heart Rhythm Association practical guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Eur Heart J. 2018;39:1330–93. https://doi.org/10.1093/eurheartj/ehy136.
Koscielny J, von Heymann C, Bauersachs R, Mouret P, Antz M. [Perioperative anticoagulation with NOAC using the example of rivaroxaban]. MMW Fortschr Med. 2017;159:18–23. https://doi.org/10.1007/s15006-017-9295-0.
Dubois V, Dincq A-S, Douxfils J, Ickx B, Samama C-M, Dogné J-M, et al. Perioperative management of patients on direct oral anticoagulants. Thromb J. 2017;15:14. https://doi.org/10.1186/s12959-017-0137-1.
Spyropoulos AC, Douketis JD. How I treat anticoagulated patients undergoing an elective procedure or surgery. Blood. 2012;120:2954–62. https://doi.org/10.1182/blood-2012-06-415943.
Doherty JU, Gluckman TJ, Hucker WJ, Januzzi JL, Ortel TL, Saxonhouse SJ, et al. 2017 ACC Expert Consensus decision pathway for Periprocedural Management of Anticoagulation in patients with Nonvalvular Atrial Fibrillation: a report of the American College of Cardiology Clinical Expert Consensus Document Task Force. J Am Coll Cardiol. 2017;69:871–98. https://doi.org/10.1016/j.jacc.2016.11.024.
Gorski LA. The 2016 infusion Therapy Standards of Practice. Home Healthc Now. 2017;35:10–8. https://doi.org/10.1097/NHH.0000000000000481.
Jeon M, Han A, Kang H, Lee K-H, Lee J-H, Lee J-H. A comparison of coagulation test results from heparinized central venous catheter and venipuncture. Blood Coagul Fibrinolysis. 2020;31:145–51. https://doi.org/10.1097/MBC.0000000000000890.
Strickland SW, Palkimas S, Acker M, Bazydlo LAL. A Novel Laboratory Assay to monitor unfractionated heparin dosing in patients taking Apixaban Prior to Hospital Admission. J Appl Lab Med. 2021;6:378–86. https://doi.org/10.1093/jalm/jfaa084.
Jacquemin M, Toelen J, Feyen L, Schoeters J, Van Horenbeeck I, Vanlinthout I, et al. The adsorption of dabigatran is as efficient as addition of idarucizumab to neutralize the drug in routine coagulation assays. Int J Lab Hematol. 2018;40:442–7. https://doi.org/10.1111/ijlh.12807.
Cox-Morton S, MacDonald S, Thomas W. A diagnostic solution for haemostasis laboratories for patients taking direct oral anticoagulants using DOAC-Remove. Br J Haematol. 2019;187:377–85. https://doi.org/10.1111/bjh.16091.
Jourdi G, Delrue M, Stepanian A, Valaize J, Foulon-Pinto G, Demagny J, et al. Potential usefulness of activated charcoal (DOAC remove®) for dRVVT testing in patients receiving direct oral AntiCoagulants. Thromb Res. 2019;184:86–91. https://doi.org/10.1016/j.thromres.2019.11.001.
Riva N, Vella K, Hickey K, Gatt P, Grima C, Zammit D, et al. The effect of DOAC-Stop® on several oral and parenteral anticoagulants. Int J Lab Hematol. 2021;43:O171–5. https://doi.org/10.1111/ijlh.13487.
Gessoni G, Valverde S, Valle L, Gessoni F, Caruso P, Valle R. Lack of rivaroxaban influence on a prothrombinase-based assay for the detection of activated C protein resistance: an italian ex vivo and in vitro study in normal subjects and factor V Leiden carriers. Int J Lab Hematol. 2017;39:418–22. https://doi.org/10.1111/ijlh.12647.
Platton S, Hunt C. Influence of DOAC Stop on coagulation assays in samples from patients on rivaroxaban or apixaban. Int J Lab Hematol. 2019;41:227–33. https://doi.org/10.1111/ijlh.12950.
Ząbczyk M, Kopytek M, Natorska J, Undas A. The effect of DOAC-Stop on lupus anticoagulant testing in plasma samples of venous thromboembolism patients receiving direct oral anticoagulants. Clin Chem Lab Med. 2019;57:1374–81. https://doi.org/10.1515/cclm-2018-1197.
Slavik L, Jacova J, Friedecky D, Ulehlova J, Tauber Z, Prochazkova J, et al. Evaluation of the DOAC-Stop Procedure by LC-MS/MS assays for determining the residual activity of Dabigatran, Rivaroxaban, and Apixaban. Clin Appl Thromb Hemost. 2019;25:1076029619872556. https://doi.org/10.1177/1076029619872556.
Monteyne T, Kesel PD, Devreese KMJ. Interference of DOAC stop and DOAC remove in the thrombin generation assay and coagulation assays. Thromb Res. 2020;192:96–9. https://doi.org/10.1016/j.thromres.2020.04.044.
Sevenet P-O, Cucini V, Hervé T, Depasse F, Carlo A, Contant G, et al. Evaluation of DOAC Filter, a new device to remove direct oral anticoagulants from plasma samples. Int J Lab Hematol. 2020;42:636–42. https://doi.org/10.1111/ijlh.13267.
Farkh C, Ellouze S, Gounelle L, Sad Houari M, Duchemin J, Proulle V, et al. A diagnostic solution for Lupus Anticoagulant Testing in Patients taking direct oral FXa inhibitors using DOAC filter. Front Med (Lausanne). 2021;8:683357. https://doi.org/10.3389/fmed.2021.683357.
Bouvy C, Evrard J, Siriez R, Mullier F, Douxfils J, Gheldof D. Removal of DOACs from plasma: performance comparison and pre-analytical considerations of three different devices. Marseille, France: Poster session presented at European Congress on Thrombosis and Haemostasis; 2018.
Linskens EA, De Kesel P, Devreese KMJ. Direct oral anticoagulant removal by a DOAC filter: impact on lupus anticoagulant testing – evaluation on spiked and patient samples. Res Pract Thromb Haemost. 2022;6. https://doi.org/10.1002/rth2.12633.
Tripodi A, Cohen H, Devreese KMJ. Lupus anticoagulant detection in anticoagulated patients. Guidance from the Scientific and Standardization Committee for lupus anticoagulant/antiphospholipid antibodies of the International Society on thrombosis and haemostasis. J Thromb Haemost. 2020;18:1569–75. https://doi.org/10.1111/jth.14846.
Hafer A, McCann L. Direct oral anticoagulant reversal: an update. Nursing2020 Crit Care. 2020;15:18–29. https://doi.org/10.1097/01.CCN.0000718332.38919.36.
Ansell J, Laulicht BE, Bakhru SH, Burnett A, Jiang X, Chen L, et al. Ciraparantag, an anticoagulant reversal drug: mechanism of action, pharmacokinetics, and reversal of anticoagulants. Blood. 2021;137:115–25. https://doi.org/10.1182/blood.2020007116.
Lu G, Kotha J, Cardenas JM, Herr MJ, Pandey A, Curnutte J, et al. Abstract 18218: in Vitro characterization of Andexanet Alfa (PRT064445), a specific fXa inhibitor antidote versus aripazine (PER977), a non-specific reversal Agent. Circulation. 2014;130:A18218–8. https://doi.org/10.1161/circ.130.suppl_2.18218.
Ansell J, Bakhru S, Laulicht BE, Tracey G, Villano S, Freedman D. Ciraparantag reverses the anticoagulant activity of apixaban and rivaroxaban in healthy elderly subjects. Eur Heart J. 2022;43:985–92. https://doi.org/10.1093/eurheartj/ehab637.
Ansell JE, Bakhru SH, Laulicht BE, Steiner SS, Grosso M, Brown K, et al. Use of PER977 to Reverse the Anticoagulant Effect of Edoxaban. N Engl J Med. 2014;371:2141–2. https://doi.org/10.1056/NEJMc1411800.
Eikelboom JW, Quinlan DJ, van Ryn J, Weitz JI. Idarucizumab: the antidote for reversal of Dabigatran. Circulation. 2015;132:2412–22. https://doi.org/10.1161/CIRCULATIONAHA.115.019628.
Schiele F, van Ryn J, Canada K, Newsome C, Sepulveda E, Park J, et al. A specific antidote for dabigatran: functional and structural characterization. Blood. 2013;121:3554–62. https://doi.org/10.1182/blood-2012-11-468207.
Jacquemin M, Toelen J, Schoeters J, van Horenbeeck I, Vanlinthout I, Debasse M, et al. The addition of idarucizumab to plasma samples containing dabigatran allows the use of routine coagulation assays for the diagnosis of hemostasis disorders. J Thromb Haemost. 2015;13:2087–92. https://doi.org/10.1111/jth.13138.
Božič Mijovski M, Malmström RE, Vene N, Antovic JP, Mavri A. The in vitro addition of idarucizumab to plasma samples from patients increases thrombin generation. Sci Rep. 2021;11:5920. https://doi.org/10.1038/s41598-021-85318-y.
Portola, Pharmaceuticals. U.S. FDA approves Portola Pharmaceuticals Andexxa®, the first and only antidote for the reversal of factor Xa inhibitors [media release]. 3 May 2018.
Favresse J, Hardy M, van Dievoet M, Sennesael A, Douxfils J, Samama C, et al. Andexanet alfa for the reversal of factor xa inhibitors. Expert Opin Biol Ther. 2019;19:387–97. https://doi.org/10.1080/14712598.2019.1599355.
Lu G, DeGuzman FR, Hollenbach SJ, Karbarz MJ, Abe K, Lee G, et al. A specific antidote for reversal of anticoagulation by direct and indirect inhibitors of coagulation factor xa. Nat Med. 2013;19:446–51. https://doi.org/10.1038/nm.3102.
Hu TY, Vaidya VR, Asirvatham SJ. Reversing anticoagulant effects of novel oral anticoagulants: role of ciraparantag, andexanet alfa, and idarucizumab. Vasc Health Risk Manag. 2016;12:35–44. https://doi.org/10.2147/VHRM.S89130.
Siddiqui F, Tafur A, Ramacciotti LS, Jeske W, Hoppensteadt D, Ramacciotti E, et al. Reversal of factor xa inhibitors by Andexanet Alfa May increase thrombogenesis compared to pretreatment values. Clin Appl Thromb Hemost. 2019;25:1076029619863493. https://doi.org/10.1177/1076029619863493.
Favaloro EJ, Gilmore G, Arunachalam S, Mohammed S, Baker R. Neutralising rivaroxaban induced interference in laboratory testing for lupus anticoagulant (LA): a comparative study using DOAC stop and andexanet alfa. Thromb Res. 2019;180:10–9. https://doi.org/10.1016/j.thromres.2019.05.013.
Favaloro EJ, Gilmore G, Bonar R, Dean E, Arunachalam S, Mohammed S, et al. Reducing the effect of DOAC interference in laboratory testing for factor VIII and factor IX: a comparative study using DOAC stop and andexanet alfa to neutralize rivaroxaban effects. Haemophilia. 2020;26:354–62. https://doi.org/10.1111/hae.13930.
Yeh CH, Fredenburgh JC, Weitz JI. The real decoy. Circ Res. 2013;113:954–7. https://doi.org/10.1161/CIRCRESAHA.113.302297.
Apostel HJCL, Winckers K, Bidar E, Schreiber J-U. Successful Antithrombin Administration in Andexanet Alfa-Associated Heparin Resistance. J Cardiothorac Vasc Anesth. 2021;35:904–7. https://doi.org/10.1053/j.jvca.2020.10.042.
Kikura M, Lee MK, Levy JH. Heparin neutralization with methylene blue, hexadimethrine, or vancomycin after cardiopulmonary bypass. Anesth Analg. 1996;83:223–7. https://doi.org/10.1097/00000539-199608000-00004.
Zheng A, Zhang W, Li C, Guo Z, Li C, Zhang C, et al. The heparinase-linked differential time method allows detection of heparin potency in whole blood with high sensitivity and dynamic range. Biosens Bioelectron. 2022;198:113856. https://doi.org/10.1016/j.bios.2021.113856.
Despotis GJ, Summerfield AL, Joist JH, Goodnough LT, Santoro SA, Zimmermann JJ, et al. In Vitro reversal of Heparin Effect with Heparinas: evaluation with whole blood Prothrombin Time and activated partial Thromboplastin Time in Cardiac Surgical Patients. Anesth Analg. 1994;79:670–4. https://doi.org/10.1213/00000539-199410000-00009.
Boyce A, Walsh G. Production, characteristics and applications of microbial heparinases. Biochimie. 2022;198:109–40. https://doi.org/10.1016/j.biochi.2022.03.011.
Coppell JA, Thalheimer U, Zambruni A, Triantos CK, Riddell AF, Burroughs AK, et al. The effects of unfractionated heparin, low molecular weight heparin and danaparoid on the thromboelastogram (TEG): an in-vitro comparison of standard and heparinase-modified TEGs with conventional coagulation assays. Blood Coagul Fibrinolysis. 2006;17:97–104. https://doi.org/10.1097/01.mbc.0000203859.62739.25.
Benoit R, Nougier C, Desmurs-Clavel H, Simon M, Dargaud Y. The modification of the thrombin generation assay for the clinical assessment of hypercoagulability in patients receiving heparin therapy. Int J Lab Hematol. 2022;44:371–8. https://doi.org/10.1111/ijlh.13735.
Preston FW, Parker RP. New antiheparin agent: polybrene; effect in peptone shock and in experimental radiation injury. AMA Arch Surg. 1953;66:545–51.
Cooney A, Mann TJ. Recent experiences with hexadimethrine for neutralizing heparin after cardiopulmonary bypass. Anaesth Intensive Care. 1999;27:298–300. https://doi.org/10.1177/0310057X9902700314.
Tientadakul P, Kongkan C, Chinswangwatanakul W. Use of an automated coagulation analyzer to perform heparin neutralization with polybrene in blood samples for routine coagulation testing: practical, rapid, and inexpensive. Arch Pathol Lab Med. 2013;137:1641–7. https://doi.org/10.5858/arpa.2012-0599-OA.
Cumming AM, Jones GR, Wensley RT, Cundall RB. In vitro neutralization of heparin in plasma prior to the activated partial thromboplastin time test: an assessment of four heparin antagonists and two anion exchange resins. Thromb Res. 1986;41:43–56. https://doi.org/10.1016/0049-3848(86)90278-1.
Jacobsen EM, Trettenes EJ, Wisløff F, Abildgaard U. Detection and quantification of lupus anticoagulants in plasma from heparin treated patients, using addition of polybrene. Thromb J. 2006;4:3. https://doi.org/10.1186/1477-9560-4-3.
Schäfer ST, Otto A-C, Acevedo A-C, Görlinger K, Massberg S, Kammerer T, et al. Point-of-care detection and differentiation of anticoagulant therapy - development of thromboelastometry-guided decision-making support algorithms. Thromb J. 2021;19:63. https://doi.org/10.1186/s12959-021-00313-7.
Horrow JC, Protamine. A review of its toxicity. Anesth Analg. 1985;64:348–61.
Byun Y, Singh VK, Yang VC. Low molecular weight protamine: a potential nontoxic heparin antagonist. Thromb Res. 1999;94:53–61. https://doi.org/10.1016/s0049-3848(98)00201-1.
Khandelwal A, Phua CW, Chaudhry HR, Tsui H, Rivard GE, Teitel JM, et al. Confounding effect of therapeutic protamine and heparin levels on routine and special coagulation testing. Blood Coagul Fibrinolysis. 2020;31:60–4. https://doi.org/10.1097/MBC.0000000000000882.
Griffin MJ, Rinder HM, Smith BR, Tracey JB, Kriz NS, Li CK, et al. The effects of heparin, protamine, and heparin/protamine reversal on platelet function under conditions of arterial shear stress. Anesth Analg. 2001;93:20–7. https://doi.org/10.1097/00000539-200107000-00005.
Portmann AF, Holden WD. Protamine sulphate, heparin, and blood coagulation. J Clin Invest. 1949;28:1451–8. https://doi.org/10.1172/JCI102210.
Schroeder M, Hogwood J, Gray E, Mulloy B, Hackett A-M, Johansen KB. Protamine neutralisation of low molecular weight heparins and their oligosaccharide components. Anal Bioanal Chem. 2011;399:763–71. https://doi.org/10.1007/s00216-010-4220-8.
Zmuda K, Neofotistos D, Ts’ao C. Effects of Unfractionated Heparin, low-molecular-weight heparin, and Heparinoid on Thromboelastographic Assay of Blood Coagulation. Am J Clin Pathol. 2000;113:725–31. https://doi.org/10.1309/Q4AE-BMCW-CQ7J-NUVT.
Sokolowska E, Kalaska B, Miklosz J, Mogielnicki A. The toxicology of heparin reversal with protamine: past, present and future. Expert Opin Drug Metab Toxicol. 2016;12:897–909. https://doi.org/10.1080/17425255.2016.1194395.
Miklosz J, Kalaska B, Kaminski K, Rusak M, Szczubialka K, Nowakowska M, et al. The inhibitory effect of protamine on platelets is attenuated by heparin without inducing Thrombocytopenia in rodents. Mar Drugs. 2019;17:539. https://doi.org/10.3390/md17090539.
Kalaska B, Kaminski K, Miklosz J, Yusa S-I, Sokolowska E, Blazejczyk A, et al. Heparin-binding copolymer reverses effects of unfractionated heparin, enoxaparin, and fondaparinux in rats and mice. Transl Res. 2016;177:98–112e10. https://doi.org/10.1016/j.trsl.2016.06.009.
Kalaska B, Miklosz J, Kamiński K, Swieton J, Jakimczuk A, Yusa S-I, et al. Heparin-binding Copolymer as a complete antidote for low-molecular-weight heparins in rats. J Pharmacol Exp Ther. 2020;373:51–61. https://doi.org/10.1124/jpet.119.262931.
Kalaska B, Kamiński K, Miklosz J, Nakai K, Yusa S-I, Pawlak D, et al. Anticoagulant Properties of Poly(sodium 2-(acrylamido)-2-methylpropanesulfonate)-Based Di- and triblock polymers. Biomacromolecules. 2018;19:3104–18. https://doi.org/10.1021/acs.biomac.8b00691.
Kamiński K, Szczubiałka K, Zazakowny K, Lach R, Nowakowska M. Chitosan derivatives as novel potential heparin reversal agents. J Med Chem. 2010;53:4141–7. https://doi.org/10.1021/jm1001666.
Kamiński K, Płonka M, Ciejka J, Szczubiałka K, Nowakowska M, Lorkowska B, et al. Cationic derivatives of Dextran and Hydroxypropylcellulose as Novel potential heparin antagonists. J Med Chem. 2011;54:6586–96. https://doi.org/10.1021/jm200380w.
Kalaska B, Sokolowska E, Kaminski K, Szczubialka K, Kramkowski K, Mogielnicki A, et al. Cationic derivative of dextran reverses anticoagulant activity of unfractionated heparin in animal models of arterial and venous thrombosis. Eur J Pharmacol. 2012;686:81–9. https://doi.org/10.1016/j.ejphar.2012.04.037.
Kalaska B, Kaminski K, Sokolowska E, Czaplicki D, Kujdowicz M, Stalinska K, et al. Nonclinical evaluation of Novel Cationically Modified Polysaccharide Antidotes for Unfractionated Heparin. PLoS ONE. 2015;10:e0119486. https://doi.org/10.1371/journal.pone.0119486.
Sokolowska E, Kalaska B, Kaminski K, Lewandowska A, Blazejczyk A, Wietrzyk J, et al. The Toxicokinetic Profile of Dex40-GTMAC3-a Novel Polysaccharide candidate for reversal of Unfractionated Heparin. Front Pharmacol. 2016;7:60. https://doi.org/10.3389/fphar.2016.00060.
Shenoi RA, Kalathottukaren MT, Travers RJ, Lai BFL, Creagh AL, Lange D, et al. Affinity-based design of a synthetic universal reversal agent for heparin anticoagulants. Sci Transl Med. 2014;6:260ra150. https://doi.org/10.1126/scitranslmed.3009427.
Zong Y, Xu Y-Y, Wu Y, Liu Y, Li Q, Lin F, et al. Porous dynamic covalent polymers as promising reversal agents for heparin anticoagulants. J Mater Chem B. 2022;10:3268–76. https://doi.org/10.1039/D2TB00174H.
Bromfield SM, Wilde E, Smith DK. Heparin sensing and binding – taking supramolecular chemistry towards clinical applications. Chem Soc Rev. 2013;42:9184–95. https://doi.org/10.1039/C3CS60278H.
Dunois C. Laboratory Monitoring of Direct Oral Anticoagulants (DOACs). Biomedicines., Douxfils J, Gosselin RC. Laboratory Assessment of Direct Oral Anticoagulants. Semin Thromb Hemost. 2017;43(3):277–290. doi:10.1055/s-0036-1597296.
Gous T, Couchman L, Patel JP, Paradzai C, Arya R, Flanagan RJ. Measurement of the direct oral Anticoagulants Apixaban, Dabigatran, Edoxaban, and Rivaroxaban in Human plasma using turbulent Flow Liquid Chromatography with High-Resolution Mass Spectrometry. Ther Drug Monit. 2014;36(5):597. https://doi.org/10.1097/FTD.0000000000000059.
Wiesen MHJ, Blaich C, Taubert M, et al. Residual rivaroxaban exposure after discontinuation of anticoagulant therapy in patients undergoing cardiac catheterization. Eur J Clin Pharmacol. 2018;74(5):611–8. https://doi.org/10.1007/s00228-018-2421-9.
Gosselin RC, Adcock DM, Bates SM, et al. International Council for standardization in Haematology (ICSH) recommendations for Laboratory Measurement of direct oral anticoagulants. Thromb Haemost. 2018;118(3):437–50. https://doi.org/10.1055/s-0038-1627480.
Harenberg J, Schreiner R, Hetjens S, Weiss C. Detecting Anti-IIa and anti-xa direct oral anticoagulant (DOAC) agents in urine using a DOAC dipstick. Semin Thromb Hemost. 2019;45(3):275–84. https://doi.org/10.1055/s-0038-1668098.
Örd L, Marandi T, Märk M, et al. Evaluation of DOAC Dipstick Test for detecting direct oral anticoagulants in urine compared with a clinically relevant plasma threshold concentration. Clin Appl Thromb Hemost. 2022;28:10760296221084308. https://doi.org/10.1177/10760296221084307.
Overview. | DOAC Dipstick for detecting direct oral anticoagulants | Advice | NICE. Published February 2, 2021. Accessed April 26, 2023. https://www.nice.org.uk/advice/mib248.
Bradfield JWB, Born GVR. Lymphocytosis produced by heparin and other sulphated polysaccharides in mice and rats. Cell Immunol. 1974;14:22–32. https://doi.org/10.1016/0008-8749(74)90165-8.
Brieger DB, Mak K-H, Kottke -Marchant, Kandice, Topol EJ. Heparin-Induced Thrombocytopenia. J Am Coll Cardiol. 1998;31:1449–59. https://doi.org/10.1016/S0735-1097(98)00134-X.
Acknowledgements
None.
Funding
All authors gratefully acknowledge the financial support from the Medical University of Bialystok [grant number SUB/2/DN/22/003/2211].
Author information
Authors and Affiliations
Contributions
A.F. prepared conception and wrote the manuscript. B.K. and J.M. review the manuscript. A.M. prepared conception and review the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Frackiewicz, A., Kalaska, B., Miklosz, J. et al. The methods for removal of direct oral anticoagulants and heparins to improve the monitoring of hemostasis: a narrative literature review. Thrombosis J 21, 58 (2023). https://doi.org/10.1186/s12959-023-00501-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12959-023-00501-7