Abstract
Background
An abrupt increase of thyroid cancer has been witnessed paralleling the supplemented iodine intake in formerly iodine-deficient countries. And increased iodine intake has been linked to the rising incidence rate of papillary thyroid cancer (PTC). However, the correlation between iodine and clinicopathological features of PTC has not been well-characterized. This study aimed to investigate the associations between iodine intake and the clinicopathological features of PTC patients.
Methods
Three hundred and fifty-nine PTC patients who received surgical treatment in Peking Union Medical College Hospital from May 2015 to November 2020 were retrospectively reviewed. The associations between urinary iodine (UI), urinary iodine/creatinine ratio (UI/U-Cr), and the clinicopathological features of PTC were analyzed. Univariate and multivariate analysis were performed to investigate the relationship between UI level and central lymph node metastasis (CLNM).
Results
There were no significant differences in UI in different groups according to the variables studied, except that patients with CLNM had higher UI level than CLNM(−) patients. No associations were found between UI/U-Cr and clinicopathological features except variant subtypes (classic/follicular). After dividing patients into high-iodine group and low-iodine group, more patients were found to have CLNM in the high-iodine group (p = 0.02). In addition, younger age, larger tumor size, and classic variant were positively correlated with CLNM (p < 0.05). Univariate analysis showed that insufficient iodine intake (≤ 99 μg/L) was associated with decreased CLNM risk in PTC. And after defining insufficient iodine intake as ≤ 109 μg/L and above requirements as ≥ 190 μg/L, multivariate analysis showed that lower iodine was associated with CLNM in total population of PTC (OR 0.53, 95% CI 0.31–0.91) and in PTC < 1 cm (papillary thyroid microcarcinoma, PTMC) (OR 0.43, 95% CI 0.21–0.87).
Conclusions
Low iodine was a protective factor for CLNM in papillary thyroid cancer, particularly in those < 1 cm. These results indicated that iodine may not only be an initiator of tumorigenesis, but also a promoter of the development of PTC.
Similar content being viewed by others
Background
Thyroid cancer is by far the most common endocrine cancer and ranked the ninth in new cancer cases globally [1]. During the past decades, thyroid cancer has been rapidly growing and is estimated to be the 4th most common cancer in 2030 globally [2]. The reason for the high increasing rate has been inconclusive over time. Risk factors have been revealed such as radiation exposure [3], industrialized lifestyle such as obesity [4], pollutants [5], and population aging as well as growing scrutiny of thyroid and improved diagnostics [6]. Apart from these known ones, iodine intake has been suggested as an important impact factor for the rise of thyroid cancer.
The thyroid plays an important role in human health, regulating metabolism, body temperature, growth, development, and organ functions. Meanwhile, iodine is the indispensable trace element for the synthesis of thyroid hormone, and deficiency of iodine can result in nodule goiter, cognitive impairments, and delayed physical development [7]. For the purpose of correcting iodine-deficient disorders, iodine prophylaxis was introduced in countries with deficient environmental iodine. However, paralleling the increase of population iodine intake, many countries reported the increase of thyroid cancer, particularly papillary thyroid cancer (PTC) [8,9,10,11]. The same trend of PTC also took place in China [12], after the universal salt iodization (USI) program was introduced in 1996. Although dietary iodine greatly improved endemic thyroid disorders, it has been debated whether over intake of iodine promoted thyroid cancer. However, because thyroid screening and imaging system were also advancing during the time, epidemiological studies were limited to reveal the true effects of increased iodine intake. As the excretion of urinary iodine accounted for 80–90% of daily iodine intake, urinary iodine is a convenient, economic and excellent biomarker to reflect the iodine intake of the population, and the World Health Organization (WHO) has recommended urinary iodine (UI), preferably median urinary iodine (MUI) to assess iodine intake [13]. Studies by Wang et al. [14] found higher UI in patients with PTC than benign nodules and found higher iodine level in LNM(+) PTC than in LNM(−) PTC. However, Zhao et al. [15] did not observe significant difference of UI between benign nodules and PTC nor did they find the difference of UI between LNM(+) and LNM(−) PTC [16]. The controversies between studies indicated that more variables should be considered in the analysis of iodine and thyroid cancer.
On the other hand, relating iodine to creatinine is yet another estimate of iodine intake, because the measurement of UI could be affected by urine output. As a result, urinary iodine/creatinine ratio (UI/U-Cr) has been established to correct the variations of urine output, because urinary creatinine excretion rate is very constant [17]. However, the majority of the studies have focused on UI, and limited studies have investigated the clinical value of UI/U-Cr in PTC.
In summary, the relationship between iodine intake and the characteristics of PTC has not been studied in detail. Also, the associations between UI/U-Cr and PTC are not well-characterized. The preliminary aim of this study is to analyze the associations between UI, UI/U-Cr, and the clinicopathological features of PTC, in order to explore the underlying effects of iodine on this rapidly growing malignancy.
Methods
Study population
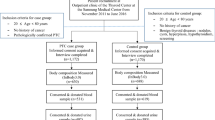
From May 2015 to November 2020, 359 consecutive patients in Peking Union Medical College Hospital were retrospectively reviewed. Inclusion criteria were patients who received total thyroidectomy or lobectomy with central lymph node dissection; postoperatively diagnosed as papillary thyroid cancer by pathological examinations. Exclusion criteria included a previous history of thyroid operation, combination of hyperthyroidism or hypothyroidism, recent use of iodine-containing medication or reagents, administration of antithyroid drugs of thyroid hormones, abnormal kidney or liver function, and history of exposure to radiation at head and neck. Basic information of the patients was collected from the electronic medical record system, including age, sex, body mass index (BMI), and previous medical history. Postoperative pathology included pathological diagnosis, variant subtypes, foci number, tumor diameter, microscopical capsular invasion, combination of other thyroid disorders (e.g., nodular goiters), and detection of central lymph node metastasis (CLNM). This study was approved by the Ethics Committee of Peking Union Medical College Hospital (No. S-K1336). The informed consent requirement was waived because individual information in this study was not accessible.
Surgical treatments
Thyroid surgery was performed in accordance with the management guidelines for thyroid cancer in China. Unilateral or bilateral thyroid resection was determined upon preoperative examination and intraoperative frozen sections as well as intraoperative surgical findings. Prophylactic central lymph node dissection was carried out as recommended by the Chinese guidelines. After surgery, the size, the number, and the pathological types of the foci were detected by the department of pathology. Tumor size was determined by its largest diameter. Multifocality was defined as the presence of ≥ 2 papillary thyroid cancer foci in the specimen, and in these cases, tumor size was represented by the largest one of the foci.
Measurement of urinary iodine and urinary creatinine
The World Health Organization has recommended using median urinary iodine concentration (MUI) as a measurement for the iodine nutritional status of the population, with urinary iodine ≤ 99 μg/L, 100–199 μg/L, 200–299 μg/L, and ≥ 300 μg/L corresponding to insufficient, adequate, above requirements, and excessive iodine intake, respectively. Given that there was an intra-individual variation of 32% for urinary iodine excretion in a previous study [18], we adopted a modest modulation of the cutoff-point of WHO criteria in our analysis, where indicated. Fasting urine samples were collected during a routine visit before surgery, and the patients were required to abstain from iodine-sufficient food for at least 3 days before sample collection. On the day of sample collection, urine was collected in a clean 5-mL white-capped VACUEET tubes with no additive. Urinary iodine concentration was measured with inductively coupled plasma mass spectrometry (ICP-MS) method. The ICP-MS analysis was performed with an iCAP-Q ICP-MS system (Thermo Scientific, Waltham, MA) coupled with an automated sampler (CETAC ASX-520; Thermo Scientific, Waltham, MA) [19]. Urinary creatinine (Maccura, China, enzymatic method) was measured using an automated chemistry analyzer (Beckman Coulter AU2700, Beckman Coulter, Brea, USA). For urinary iodine, samples were measured in triplicate, and the mean value was calculated as the iodine concentration for each sample.
Statistical analysis
Urinary iodine (UI) and urinary iodine/creatinine (UI/U-Cr) was expressed as median (upper and lower quartile) and analyzed with Wilcoxon rank-sum test because of their skewed distribution. Categorical variables were analyzed with chi-square test or Fisher’s exact test. Univariate and multivariate analyses were performed using logistic regression analysis. As UI/U-Cr was strongly associated with age and sex composition of the investigated population, propensity score matching (PSM) was applied in the analysis of UI/U-Cr to reduce the bias between groups. Age and sex were included in the algorithm using nearest-neighbor matching method with a caliper size of 0.05. The survival curve was depicted using the Kaplan-Meier method and compared using the log-rank test. A P value ≤ 0.05 was considered statistically significant. All statistical analyses were performed with SPSS software (version 26.0, IBM Corporation) and R software (version 3.6.2, R Foundation for Statistical Computing).
Results
UI and UI/U-Cr level in different clinicopathologic status among PTC patients
Among the 359 patients diagnosed with PTC, UI was not significantly different between different age, sex, or other clinicopathological features, except that UI was significantly higher in CLNM(+) patients than in N0 patients (152 μg/L vs 126 μg/L, p = 0.028). After correction with creatinine, female and patients aged ≥ 45 exhibited significantly higher UI/U-Cr. Except variant subtypes (p = 0.021), no other pathological features were significantly different regarding UI/U-Cr level. Of note, capsular invasion tended to correlate with lower UI, but the difference was not statistically significant (p = 0.066) (Table 1).
Correlations of UI, UI/U-Cr, and clinicopathological features of PTC patients
Using the median value as the cutoff, the patients were classified into an iodine-high and an iodine-low group to compare the difference between clinical and histopathological features. There was no significant difference in age, sex, BMI, smoking status, tumor size, multifocality, and capsular invasion as well as variant subtypes in the two groups. In addition, iodine was not associated with coexisted nodular goiter, chronic thyroiditis, or thyroid adenoma. However, patients in the UI-high group were more likely to be positive for CLNM compared with the UI-low group (p = 0.017) (Table 2).
As is shown in Table 3, female and patients aged ≥ 45 showed significantly higher UI/U-Cr (p < 0.001 and p < 0.01), which was due to the physiologically lower U-Cr among women and elders (Table 1). To reduce the imbalance of baseline data between the two groups (Table 3), a 1:1 PSM was performed to adjust for age and sex. After PSM, 118 age- and sex-adjusted patients in each of the two groups were identified, with no statistically differences in baseline characteristics. However, no significant differences were found in histopathological features and the distribution of CLNM (p = 0.295) (Table 3).
Risk factors of central lymph node metastasis in PTC patients
According to the results above, CLNM was closely related to a higher level of UI. However, CLNM is a pathological process affected by multiple risk factors, which could be potential confounders in the relationship of urinary iodine level and lymph node metastasis. To better understand the relationship between UI and CLNM, we further explored the risk factors that may contribute to the presence of CLNM. As Table 4 shows, age was significantly lower in patients with CLNM (p < 0.001). Tumor size was significantly larger in patients with CLNM (p < 0.001). The classic variant of PTC was more likely to present with CLNM than the follicular variant (p < 0.001). Furthermore, CLNM was marginally correlated with multiple foci (p = 0.064) (Table 4).
Univariate and multivariate logistic regression analyses on the urinary iodine level for the presence of CLMN in PTC patients
First, we investigated the association of age, tumor size, multifocality, variant subtype, and the presence of CLNM by univariate logistic regression analysis. In total PTC patients, age, tumor size, and classic variant were significantly associated with CLNM with an OR of 0.96 (95% CI 0.94–0.98), 2.01 (95% CI 1.31–3.14), and 3.57 (95% CI 1.95–6.88). Meanwhile, multifocality was marginally related to positive CLNM (OR 1.50, 95% CI 0.98–2.32).
The optimal cutoff value of UI for the diagnosis of CLNM was determined as 130.5 μg/L (Supplementary figure 1). Then, based on the WHO criteria, we found that insufficient iodine (≤ 99 μg/L) tended to be inversely associated with CLNM by univariate regression analysis (Table 5). However, no significant associations were found after adjusted for age, sex, multifocality, tumor size, and variant subtype. After we defined insufficient iodine as ≤ 109 μg/L and above requirements as ≥ 190 μg/L, we found that low UI was a protective factor for CLNM by univariate analysis (OR 0.44, 95% CI 0.26–0.72) and remained an independent protective factor in multivariate analysis (OR 0.53, 95% CI 0.31–0.91). In subgroup analysis, lower iodine was also correlated with N0 both in PTC ≥ 1 cm and PTC < 1 cm by univariate analysis. And multivariate analysis revealed that low UI remained an independent protective factor for CLNM in PTC < 1 cm (OR 0.43, 95% CI 0.21–0.87) (Table 5). As Fig. 1 shows, PTC with ≤ 109 μg/L had the smallest proportion of CLNM, while the group with ≥ 190 μg/L and 110–189 μg/L showed medium and higher proportion of CLNM. However, the recurrence-free survival was not significantly different between the three groups (p = 0.49) (Supplementary figure 2).
Discussion
It is well established that the association between iodine intake and the incidence rate of thyroid disorders follows a U-shaped pattern [20], which demonstrates that either higher or lower iodine is likely to cause thyroid pathogenesis. The major finding of this study is that low iodine was associated with less LNM in the clinical practice. Although some studies suggested high iodine promoted PTC development, whereas this only indicated the correlation of iodine overdose and cancer development. Few studies have investigated how iodine affects PTC in a normal range or below (UI < 200 μg/L). With the most control to biases, the current study found that iodine range below sufficient could be protective for CLNM. This finding added yet another layer of regulatory relation between iodine intake and thyroid. On the other hand, UI/U-Cr did not exhibit a strong correlation with characteristics of PTC like urinary iodine did. As an improved formula, UI/U-Cr was first introduced to normalize the differences of urine output when measuring UI. However, age, sex, and even protein intakes have strong influences on creatinine production [17, 21]. By adjusting for age and sex, we did not observe significant relevance of UI/U-Cr to the histopathology of PTC. Thus, this study indicated that there may be no apparent associations between UI/U-Cr and PTC, which is in agreement with the notion that variations of UI could be evened out among population and that relating UI to creatinine is to some extent an unnecessary procedure [13].
Wang et al. [14] found significantly higher MUI in positive vs negative lymph node metastasis (1584.62 μg/L vs 315.61 μg/L) (2010–2011). Zhao et al. [16] conducted a similar investigation and did not find difference of MUI between positive and negative CLNM. However, after defining CLNM as metastatic LN ≥ 2, they found those with CLNM(+) had a higher MUI (191.7 μg/L vs 176.2 μg/L) (2013–2018). It is possible that at different baseline levels, iodine could have different impacts on the tumor behavior. The different results of the studies could be attributed to different geographical environment, as the former was conducted in an iodine-sufficient coastal area and the latter conducted in an inland region. The present study, conducted inland, showed that the MUI of population was at a relatively low level. This may have caused the milder differences between groups in our analysis. Moreover, it should be noted that since 2012, the standard iodine concentration in iodized salt was reduced from 35 mg/kg to a range of 20 to 30 mg/kg in China. This further resulted in a decrease of MUI in the population. Nevertheless, by classifying iodine status into a higher and lower group, this study found more CLNM cases in the high-iodine group, suggesting that even within a lower range of iodine level, iodine may still have an impact on the pathological features of PTC.
As the risk of lymph node metastasis is multifactorial, distinguishing the risk factors for CLNM was necessary before we investigated the correlation of iodine and lymph node metastasis. Our study found CLNM was mostly correlated with age and tumor size, which was evidenced by previous meta-analyses [22,23,24]. Moreover, studies have indicated higher risk of LNM in classic PTC compared to follicular variant PTC, which resulted in poorer long term outcomes for classic PTC [25]. Thus, we have considered these important factors in the assessment of CLNM risk. Referring to the WHO criteria (≤ 99 μg/L for insufficient iodine intake, 100–199 μg/L for adequate iodine intake, and ≥ 200 μg/L for above requirements), there was a tendency that CLNM decreased with lower urinary iodine. Allowing for the natural variation of spot urinary iodine, we redefined insufficient iodine as ≤ 109 μg/L and above requirements as ≥ 190 μg/L. And we found insufficient iodine to be significantly associated with CLNM(−) for PTC < 1 cm in subgroup analysis. We thus supposed that urinary iodine may be more closely related to CLNM in PTC < 1 cm instead of PTC ≥ 1 cm. In the similar pattern, Zhao et al. [16] only observed high UI to be marginally associated with CLNM. The different observations could be explained from several aspects. First is the absence of stratification for PTC sized ≥ 1 cm and < 1 cm. As is shown in our study, iodine status may be associated with CLNM more in PTC < 1 cm than in PTC ≥ 1 cm, indicating that there are more influencing factors in PTC ≥ 1 cm. Second, we included classic and follicular variant as covariate in our model, as the classic/follicular ratio could largely affect the prevalence of CLNM. However, the classification of classic and follicular variant was often absent in previous studies. Third, we redefined and widened the range for insufficient iodine intake and above requirements, in consideration of the natural variation of urinary iodine. In clinical practice, PTC < 1 cm, or papillary thyroid microcarcinoma (PTMC) [26] are usually of good prognosis; however, 1/3 to 2/3 of PTMC could have lymph node metastasis on pathological examination [27, 28]. Therefore, it has been controversial as to whether prophylactic LN dissection should be performed on PTMC. The present study suggested that iodine should be considered a risk factor of CLNM for PTMC, and more precise stratification for iodine nutrition are needed to identify the at-risk groups for PTC patients.
In experimental researches, it was found that iodine could promote thyroid cancer growth by suppressing miR-422a and up-regulating MAPK1 [29]. Moreover, iodine may have dual roles in mediating the proliferation of thyroid cancer in vitro. At concentration below 1.0 × 10−3 mM it could promote cell growth and migration, while at concentration above 1.0 × 10−3 mM iodine exhibited an inhibitory role [30]. And within a certain range of concentration, iodine could activate Akt and Erk signaling pathway [30], which are essential for tumorigenesis, invasion, metastasis, and epithelial-mesenchymal transition [31, 32]. Furthermore, it was reported that BRAF mutation was more common in the high-water iodine region than in normal regions, while BRAF was closely related to lymph node metastases of PTC [33, 34]. These studies further confirmed iodine as a critical element during the development of thyroid cancer on the molecular basis; therefore, understanding the multi-layer mechanisms of iodine may facilitate better prevention and treatment of this malignancy.
In summary, our study showed that low UI level was a protective factor for CLNM of papillary thyroid cancer. The study also compared UI and UI/U-Cr simultaneously with the clinicopathological features of PTC and suggested that UI is a better parameter than UI/U-Cr to indicate lymph node metastasis of PTC. Our study was limited by the usual concerns related to cross-sectional studies: we cannot conclude a cause-and-effect relation between iodine and the pathological features of PTC, so further studies are still warranted to provide evidence of the regulatory mechanism between iodine and the aggressiveness of PTC. Furthermore, we did not observe significant difference of recurrence-free survival, so a longer follow up period will be required to investigate the prognosis of PTC patients with different UI levels. Although the effect of iodine on thyroid cancer is still controversial, our data further support the concept that iodine is related to lymph node metastasis of PTC, mainly through the protective effect of low iodine, which may provide novel insights into the management of iodine nutrition.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Global Cancer Observatory. https://gco.iarc.fr/today/data/factsheets/cancers/32-Thyroid-fact-sheet.pdf.
Pearce A, Bradley C, Hanly P, O'Neill C, Thomas AA, Molcho M, et al. Projecting productivity losses for cancer-related mortality 2011 - 2030. BMC Cancer. 2016;16(1):804. https://doi.org/10.1186/s12885-016-2854-4.
Sinnott B, Ron E, Schneider AB. Exposing the thyroid to radiation: a review of its current extent, risks, and implications. Endocr Rev. 2010;31(5):756–73. https://doi.org/10.1210/er.2010-0003.
Marcello MA, Cunha LL, Batista FA, Ward LS. Obesity and thyroid cancer. Endocr Relat Cancer. 2014;21(5):T255–71. https://doi.org/10.1530/ERC-14-0070.
Zhu C, Zheng T, Kilfoy BA, Han X, Ma S, Ba Y, et al. A birth cohort analysis of the incidence of papillary thyroid cancer in the United States, 1973-2004. Thyroid. 2009;19(10):1061–6. https://doi.org/10.1089/thy.2008.0342.
Li M, Dal Maso L, Vaccarella S. Global trends in thyroid cancer incidence and the impact of overdiagnosis. Lancet Diabetes Endocrinol. 2020;8(6):468–70. https://doi.org/10.1016/S2213-8587(20)30115-7.
Zimmermann MB, Boelaert K. Iodine deficiency and thyroid disorders. Lancet Diabetes Endocrinol. 2015;3(4):286–95. https://doi.org/10.1016/S2213-8587(14)70225-6.
Hofstadter F. Frequency and morphology of malignant tumours of the thyroid before and after the introduction of iodine-prophylaxis. Virchows Arch A Pathol Anat Histol. 1980;385(3):263–70. https://doi.org/10.1007/BF00432536.
Radespiel-Troger M, Batzler WU, Holleczek B, Luttmann S, Pritzkuleit R, Stabenow R, et al. Rising incidence of papillary thyroid carcinoma in Germany. Bundesgesundheitsbl Gesundheitsforsch Gesundheitsschutz. 2014;57(1):84–92. https://doi.org/10.1007/s00103-013-1884-1.
Rego-Iraeta A, Perez-Mendez LF, Mantinan B, Garcia-Mayor RV. Time trends for thyroid cancer in northwestern Spain: true rise in the incidence of micro and larger forms of papillary thyroid carcinoma. Thyroid. 2009;19(4):333–40. https://doi.org/10.1089/thy.2008.0210.
Harach HR, Ceballos GA. Thyroid cancer, thyroiditis and dietary iodine: a review based on the Salta, Argentina model. Endocr Pathol. 2008;19(4):209–20. https://doi.org/10.1007/s12022-008-9038-y.
Wang J, Yu F, Shang Y, Ping Z, Liu L. Thyroid cancer: incidence and mortality trends in China, 2005-2015. Endocrine. 2020;68(1):163–73.
WHO/UNICEF/ICCIDD: International Council for the Control of Iodine Deficiency Disorders. Assessment of iodine deficiency disorders and monitoring their elimination. 3rd ed. Geneva: World Health Organization; 2007.
Wang F, Wang Y, Wang L, Wang X, Sun C, Xing M, et al. Strong association of high urinary iodine with thyroid nodule and papillary thyroid cancer. Tumour Biol. 2014;35(11):11375–9. https://doi.org/10.1007/s13277-014-2397-8.
Zhao H, Li H, Huang T. High urinary iodine, thyroid autoantibodies, and thyroid-stimulating hormone for papillary thyroid cancer risk. Biol Trace Elem Res. 2018;184(2):317–24. https://doi.org/10.1007/s12011-017-1209-6.
Zhao H, Li H, Huang T. High iodine intake and central lymph node metastasis risk of papillary thyroid cancer. J Trace Elem Med Biol. 2019;53:16–21. https://doi.org/10.1016/j.jtemb.2019.01.015.
Knudsen N, Christiansen E, Brandt-Christensen M, Nygaard B, Perrild H. Age- and sex-adjusted iodine/creatinine ratio. A new standard in epidemiological surveys? Evaluation of three different estimates of iodine excretion based on casual urine samples and comparison to 24 h values. Eur J Clin Nutr. 2000;54(4):361–3. https://doi.org/10.1038/sj.ejcn.1600935.
Konig F, Andersson M, Hotz K, Aeberli I, Zimmermann MB. Ten repeat collections for urinary iodine from spot samples or 24-hour samples are needed to reliably estimate individual iodine status in women. J Nutr. 2011;141(11):2049–54. https://doi.org/10.3945/jn.111.144071.
Yu S, Yin Y, Cheng Q, Han J, Cheng X, Guo Y, et al. Validation of a simple inductively coupled plasma mass spectrometry method for detecting urine and serum iodine and evaluation of iodine status of pregnant women in Beijing. Scand J Clin Lab Invest. 2018;78(6):501–7. https://doi.org/10.1080/00365513.2018.1512150.
He NL, Li H, An W, et al. [Study on constructing the U-shaped response relationship of urinary iodine level and thyroid nodule prevalence based on piecewise function quantile regression method]. Zhonghua Yu Fang Yi Xue Za Zhi. 2020;54(11):1268–74. https://doi.org/10.3760/cma.j.cn112150-20200322-00403.
Konno N, Yuri K, Miura K, Kumagai M, Murakami S. Clinical evaluation of the iodide/creatinine ratio of casual urine samples as an index of daily iodide excretion in a population study. Endocr J. 1993;40(1):163–9. https://doi.org/10.1507/endocrj.40.163.
Mao J, Zhang Q, Zhang H, Zheng K, Wang R, Wang G. Risk factors for lymph node metastasis in papillary thyroid carcinoma: a systematic review and meta-analysis. Front Endocrinol. 2020;11:265.
Qu H, Sun GR, Liu Y, He QS. Clinical risk factors for central lymph node metastasis in papillary thyroid carcinoma: a systematic review and meta-analysis. Clin Endocrinol. 2015;83(1):124–32. https://doi.org/10.1111/cen.12583.
Sun W, Lan X, Zhang H, Dong W, Wang Z, He L, et al. Risk factors for central lymph node metastasis in CN0 papillary thyroid carcinoma: a systematic review and meta-analysis. PLoS One. 2015;10(10):e0139021. https://doi.org/10.1371/journal.pone.0139021.
Henke LE, Pfeifer JD, Baranski TJ, DeWees T, Grigsby PW. Long-term outcomes of follicular variant vs classic papillary thyroid carcinoma. Endocr Connect. 2018;7(12):1226–35. https://doi.org/10.1530/EC-18-0264.
Hedinger C, Williams ED, Sobin LH. The WHO histological classification of thyroid tumors: a commentary on the second edition. Cancer. 1989;63(5):908–11. https://doi.org/10.1002/1097-0142(19890301)63:5<908::AID-CNCR2820630520>3.0.CO;2-I.
Wada N, Duh QY, Sugino K, Iwasaki H, Kameyama K, Mimura T, et al. Lymph node metastasis from 259 papillary thyroid microcarcinomas: frequency, pattern of occurrence and recurrence, and optimal strategy for neck dissection. Ann Surg. 2003;237(3):399–407. https://doi.org/10.1097/01.SLA.0000055273.58908.19.
Lim YC, Choi EC, Yoon YH, Kim EH, Koo BS. Central lymph node metastases in unilateral papillary thyroid microcarcinoma. Br J Surg. 2009;96(3):253–7. https://doi.org/10.1002/bjs.6484.
Wang J, Yang H, Si Y, Hu D, Yu Y, Zhang Y, et al. Iodine promotes tumorigenesis of thyroid cancer by suppressing Mir-422a and up-regulating MAPK1. Cell Physiol Biochem. 2017;43(4):1325–36. https://doi.org/10.1159/000481844.
Xiang J, Wang X, Wang Z, Wu Y, Li D, Shen Q, et al. Effect of different iodine concentrations on well-differentiated thyroid cancer cell behavior and its inner mechanism. Cell Biochem Biophys. 2015;71(1):299–305. https://doi.org/10.1007/s12013-014-0198-8.
Jiang N, Dai Q, Su X, Fu J, Feng X, Peng J. Role of PI3K/AKT pathway in cancer: the framework of malignant behavior. Mol Biol Rep. 2020;47(6):4587–629. https://doi.org/10.1007/s11033-020-05435-1.
Navandar M, Garding A, Sahu SK, Pataskar A, Schick S, Tiwari VK. ERK signalling modulates epigenome to drive epithelial to mesenchymal transition. Oncotarget. 2017;8(17):29269–81. https://doi.org/10.18632/oncotarget.16493.
Guan H, Ji M, Bao R, Yu H, Wang Y, Hou P, et al. Association of high iodine intake with the T1799A BRAF mutation in papillary thyroid cancer. J Clin Endocrinol Metab. 2009;94(5):1612–7. https://doi.org/10.1210/jc.2008-2390.
Frasca F, Nucera C, Pellegriti G, Gangemi P, Attard M, Stella M, et al. BRAF(V600E) mutation and the biology of papillary thyroid cancer. Endocr Relat Cancer. 2008;15(1):191–205. https://doi.org/10.1677/ERC-07-0212.
Acknowledgements
Not applicable
Funding
Not applicable
Author information
Authors and Affiliations
Contributions
WK, XY, and JY conceived and designed the study. ZZ and KL analyzed the data and drafted the manuscript. XW and ZZ discussed and contributed to the data analysis. JS, SO, and ZL analyzed and interpreted the results. The authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Ethics Committees of Peking Union Medical College Hospital (No. S-K1336). Because all the data were routinely obtained and no additional investigations or procedures were carried out, informed consent was not required.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Supplementary Figure 1.
The receiver operating characteristic (ROC) curve for the diagnosis of CLNM. The ROC yielded an area under the curve (AUC) of 0.567. The sensitivity was 0.633 and the specificity was 0.523.
Additional file 2: Supplementary Figure 2.
Recurrence-free survival of the PTC patients with different UI levels.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zeng, Z., Li, K., Wang, X. et al. Low urinary iodine is a protective factor of central lymph node metastasis in papillary thyroid cancer: a cross-sectional study. World J Surg Onc 19, 208 (2021). https://doi.org/10.1186/s12957-021-02302-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12957-021-02302-6