Abstract
Background
The abnormal expression of activator protein-1(AP-1) has recently been investigated in a variety of tumors. While the relationship between AP-1 and thyroid cancer is poorly studied, our study was to evaluate the protein expression and clinical value of AP-1 in papillary thyroid carcinoma (PTC).
Methods
The expression of AP-1 was examined by immunohistochemistry on paraffin-embedded tissues obtained from PTC and correspondent paracancerous tissues of 82 patients.
Results
Compared with paracancerous tissues, AP-1 expression was significantly elevated in PTC tissues and the positive rate was 79.3% (65/82). Our study found a linear trend relationship between the expression of AP-1 and tumor size. However, the differences in AP-1 expression among gender, age, lymph node metastasis, number of lesions, location of the lesion, and extrathyroid invasion are not statistically significant.
Conclusions
The expression of AP-1 plays an important role in the proliferation process of PTC.
Similar content being viewed by others
Background
Thyroid cancer is one of the most common malignancies of the endocrine system. The incidence of thyroid cancer has continued to rise in the past several decades worldwide [1]. In 2013, there were 143,900 new cases of thyroid cancer reported and 6500 deaths in China, the national incidence of thyroid cancer was 10.58 per 100,000 (5.12 per 100,000 for men and 16.32 per 100,000 for women), and the ratio between males and females was 1:3.2 [2]. Thyroid papillary carcinoma is the most common type and contributes to more than 85% of thyroid cancer [3]. Previous studies have established that excessive activation of the MAPK pathway can drive carcinogenesis in BRAF, RAS, and RET gene mutation induced by PTC, and activator protein-1 (AP-1) is an important target of the MAPK pathway [4, 5].
AP-1 is a leucine zipper protein dimer that is composed of Jun (c-Jun, JunB, and JunD), Fos (c-Fos, FosB, Fra-1, and Fra-2), ATF (ATF-2, LRF1/ATF-3, B-ATF), JDP (JDP-1, JDP-2), and Maf (c-Maf, MafA, MafB, MafG/F/K, Nrl) [6]. Jun protein is able to form homodimer by itself; it also can form heterodimer with Fos and ATF protein family members. However, Fos protein cannot form homodimer with Jun protein. Jun-Fos dimer is the most common form of AP-1 protein in human cells. AP-1 is a downstream transcription factor of the MAPK signaling pathway that binds to specific DNA sequences on other genes, participating in a wide range of cellular processes, including cell growth, differentiation, and apoptosis [7]. Growth factors, neurotransmitters, cytokine, stress, ultraviolet, and other physiological factors can activate the transcription factor AP-1 via the MAPK signaling pathway and increase the transcriptional activity of Jun and Fos. The post-transcriptional phosphorylation and dephosphorylation of Jun and Fos can regulate the process of cell proliferation, invasion, metastasis, survival, and apoptosis [8]. When AP-1 is inappropriately expressed, it is closely related to pathological processes such as tumor cell transformation, angiogenesis, metastasis, immunity, and inflammatory diseases [9, 10]. It has been shown that nasopharyngeal cancer tissues have increased expression of AP-1 compared with normal tissues, and it was related to the progression of tumor cells [11]. Blocking the transcriptional activation of AP-1 suppresses the process of breast cancer cell invasion [12]. Some studies have also reported that the expression of AP-1 protein is upregulated in various tumor tissues such as pancreatic cancer [13] and colon cancer [14]. However, the mechanisms of AP-1 and papillary thyroid carcinoma are not well studied. The purpose of our study is to evaluate the expression and clinical significance of AP-1 in papillary thyroid carcinoma.
Methods
Tissue samples and patients
Cancer tissues and correspondent paracancerous tissues were obtained from 82 patients with PTC who underwent thyroid surgery. The histologic sections were reviewed by two expert pathologists to verify the histologic diagnosis. All samples were obtained from Shengjing Hospital of China Medical University from July 21, 2011, to July 21, 2017. Inclusion criteria were cases with complete clinical data and pathological tissue specimen. All patients in this study had signed an informed consent that their clinical and pathological data be used for research purposes. The study protocol was approved by the Clinical Research Ethics Committee of the Shengjing Hospital of China Medical University.
Immunohistochemistry
Three-micrometer-thick, formalin-fixed sections were prepared and deparaffinized, and gradient alcohol hydration, endogenous peroxidase activity was blocked with 3% hydrogen peroxide for 15 min. Antigen retrieval was performed by boiling the slides for 8 min in citric acid buffer (pH 6.0), then cooled to room temperature. Slides were incubated with the rabbit anti-human polyclonal AP-1 antibody (dilution, 1:640; proteintech, China), at 4° overnight. Slides were rinsed with PBS and incubated with secondary antibody (goat anti-rabbit, Zhongshanjinqiao, Beijing, China) at room temperature for 30 min, stained with DAB(3,3-diaminobenzidine) (Fuzhou Maixin, Fujian, China) solution for 1–2 min, counterstained with hematoxylin, dehydrated, coverslipped, and analyzed by fluorescence microscopy. AP-1-positive staining is localized in the cytoplasm and nuclear. Known positive sections were used as the positive control, and PBS buffer was used instead of primary antibody as the negative control group.
Five high-power fields were randomly collected from each slice under an optical microscope and scored by two clinical pathologists. Immunohistochemical localization of AP-1 protein was cytoplasm and nucleus. The extent of staining was estimated on a scale of 0 to 4 (0, none; 1, < 10%; 2, 10~ 50%; 3, 51~ 80%; 4, > 80%). The intensity of staining was scored on a scale from 0 to 3 (0, no staining; 1, weak; 2, moderate; 3, strong). The immunoreactive score of AP-1 was determined by the product of extent and intensity. The score ≥ 2 was considered positive expression.
Statistical analysis
Statistical analysis software SPSS 24.0 (SPSS, Inc., Chicago, IL, USA) was used for data analysis. Chi-square test was used to analyze the differences of AP-1 protein expression between PTC and paracancerous tissues. The relationship between AP-1 protein expression and clinical data characteristics in PTC was evaluated by chi-square test and Fisher’s exact probability. The relationship between tumor size and AP-1 protein expression was analyzed by assessing the linear-by-linear association results in trend chi-square test of SPSS. P < 0.05 was considered as statistically significant.
Results
The expression of AP-1 protein in thyroid tissue
The study group included 82 patients (17 males, 65 females), with an age range of 18~77 years (mean ± SD = 41.0 ± 12.5 years). There were 61 patients with lymph node metastases, and 29 patients with extrathyroid invasion. The remaining patients’ characteristics are shown in Table 1.
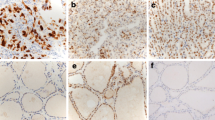
Under light microscopy, AP-1 protein was mainly expressed in the nucleus and/or cytoplasm of PTC and paracancerous normal tissues. No expression was found in the intercellular substance. The positive expression of AP-1 protein in PTC was 79.3% (65/82), which was significantly higher than paracancerous tissues (26.8%, 22/82). There was a significantly increased expression of AP-1 protein in PTC (P < 0.05) (showed as Fig. 1a and Fig. 1b).
Associations of AP-1 protein expression with clinical features in papillary thyroid carcinoma
AP-1 expression was significantly positively correlated with tumor size by using the trend test (P = 0.012). But it was negatively associated with the patient’s age, gender, number of lesions, location of lesions, lymph node metastasis, and extrathyroid invasion (P > 0.05).
As shown in Fig. 2, AP1-positive patients exhibited significantly larger tumor size than AP1-negative patients [2.0 (4.5–0.7) cm, n = 65 vs. 1.7 (2.0–1.2) cm, n = 17, P = 0.032].
Discussion
AP-1 is a leucine zipper protein that is assembled through the dimerization of a characteristic bZIP domain (basic region leucine zipper) in the Fos and Jun subunits. Numerous studies have reported that the activation of transcription factor Jun and c-fos can induce the expression of cyclinD1. CyclinD1 is a member of the cyclin protein family that is involved in increasing DNA synthesis and accelerating cell cycle progression. The synthesis of cyclinD1 drives the cell cycle progression from G0/G1 phase to S phase in tumor proliferation [15]. Ming et al. showed that IL-7 could induce cyclinD1 gene expression via an AP-1(c-Fos/c-Jun)-dependent pathway and promote lung cancer cell proliferation [16]. AP-1 activation also drives VEGF (vascular endothelial growth factor) expression and regulates the process of proliferation in blood endothelial cells and various tumor cells [17].
Previous study also reported that the expression of c-Jun, JunD, and Fra-1 protein significantly increased in human thyroid cancer tissue and played a critical role in the process of thyroid cancer cell proliferation [18]. However, Chen et al. suggested that the expression of AP-1 was negative to the tumor size [19]. We found that the tumor size of AP-1-positive group was larger than that of the negative group (P < 0.05). By using the trend test in SPSS, we found the expression of AP-1 protein increased with tumor size in PTC (P = 0.012). Some studies have reported that tumor size can predict persistence, recurrence, and death [20, 21]. PTC persistence was defined as evident structural and/or biochemical residual disease until 1 year after initial surgery. Disease detected after 1 year was considered as PTC recurrence. In a retrospective analysis with a 10-year follow-up PTC cohort study [22], the author reported that tumor size was a predictor of PTC persistence. Following the enlargement of tumor size, the risk of persistence increased. A long-term study of 1355 patients with thyroid cancers demonstrated that tumors smaller than 1.5 cm had lower 30-year recurrence and lower cancer mortality rates than those larger ones [23]. These data suggested that AP-1 may serve as a key factor in PTC cell proliferation. And it may also be used as a predictor for prognosis of PTC.
AP-1 is not only closely related to the process of tumor proliferation, but also influences the local invasion and distant metastasis of tumors by regulating VEGF and matrix metalloproteinase 9 (MMP-9). By using AP-1 transcription inhibitors, the proliferation and invasion of VEGF-dependent vascular endothelial cells in tumor cells could be blocked [24]. In addition, the transcription factor AP-1 can also regulate the expression of MMP-9 by binding to the MMP-9 promoter [25]. Upregulation of MMP-9 expression can degrade various components of the extracellular matrix and destroy the histological barrier, causing the tumor progression and invasion [26, 27]. AP-1 and NF-κB are overexpressed in esophageal cancer cells. The increasing transcriptional activity of MMP9 contributes to the invasion and metastasis of esophageal cancer [28]. In a study of 66 patients with PTC and 40 patients with benign thyroid tumors, VEGF and MMP-9-positive expression were more frequently seen in PTC and were closely correlated to tumor size and clinical stage [29]. Because of the relationship between MMP-9, VEGF, and AP-1, we guessed that expression of AP-1 contributed to the lymph node metastasis of PTC. However, our study did not find any significant difference between AP-1 expression and lymph node metastasis.
In many tumors, the oncogene Ras continuously activates and phosphorylates JNK through the MAPK signaling pathway. Activation of the transcription factor AP-1 upregulates the expression of MMP-9 and VEGF and promotes invasion and metastasis of tumor cells. On the other hand, JNK is also reported as a tumor suppressor in different types of cancer. JNK promotes apoptosis by phosphorylating c-Jun. Then, c-Jun activates the Fas apoptotic protein pathway and protease Caspase-3 to initiate apoptosis [30]. Meggiato et al. [31] found that c-jun and Caspase-3 were highly expressed on human pancreatic cancer. There was also a significant correlation between caspase3 and c-Jun. Shaulian and Karin [32] showed that JunB upregulated the expression of tumor suppressor genes and decreased cyclinD1 expression. Mitsiades et al. [33] demonstrated that bortezomib phosphorylated and activated c-Jun through the JNK signaling pathway, which initiated the Fas apoptosis pathway and improved prognosis by promoting apoptosis of medullary thyroid carcinoma and undifferentiated thyroid cancer cells. These results indicated that c-jun/AP-1 had a bidirectional effect on cell proliferation or apoptosis. In our study, there was no evident correlation between AP-1 expression and lymph node metastasis. It was consistent with Chen’s study. But the reason was still not clear.
In conclusion, our study demonstrated that the level of AP-1 protein was significantly upregulated in PTC compared with paracancerous thyroid tissue by immunohistochemistry. The expression of AP-1 is positively correlated with tumor size. Previous study identified that tumor size was a predictor of prognosis of PTC. AP-1 may serve as an outcome predictor due to the trend relationship between AP-1 and tumor size. These results showed that the immunohistochemical evaluation of the level of AP-1 in PTC might be useful as a molecular marker for the diagnosis and prognosis of PTC. And AP-1 activation may serve as a potential factor involved in the proliferation and transformation of PTC. However, the detailed mechanism of AP-1 in lymph node metastasis, extrathyroid invasion, and apoptosis regulation in PTC has not been clarified due to its complex composition. Further studies need to be focused on this promising protein.
References
La Vecchia C, Malvezzi M, Bosetti C, Garavello W, Bertuccio P, Levi F, Negri E. Thyroid cancer mortality and incidence: a global overview. Int J Cancer. 2015;136:2187–95.
Yang L, Zheng RS, Wang N, Zeng HM, Yuan YN, Zhang SW, Li HC, Liu S, Chen WQ, He J. Analysis of incidence and mortality of thyroid cancer in China, 2013. Zhonghua Zhong Liu Za Zhi. 2017;39:862–7.
Joseph KR, Edirimanne S, Eslick GD. Multifocality as a prognostic factor in thyroid cancer: a meta-analysis. Int J Surg. 2018;50:121–5.
Xing M. Molecular pathogenesis and mechanisms of thyroid cancer. Nat Rev Cancer. 2013;13:184–99.
Chen D, Qi W, Zhang P, Zhang Y, Liu Y, Guan H, Wang L. Investigation of BRAF V600E detection approaches in papillary thyroid carcinoma. Pathol Res Pract. 2017;214:303–7.
Alonso IGDLF, Liang H, Turner SD, Lagger S, Merkel O, Kenner L. The role of activator protein-1 (AP-1) family members in CD30-positive lymphomas. Cancers. 2018;10:93.
Li JK, Nie L, Zhao YP, Zhang YQ, Wang X, Wang SS, Liu Y, Zhao H, Cheng L. IL-17 mediates inflammatory reactions via p38/c-Fos and JNK/c-Jun activation in an AP-1-dependent manner in human nucleus pulposus cells. J Transl Med. 2016;14:77.
Ye N, Ding Y, Wild C, Shen Q, Zhou J. Small molecule inhibitors targeting activator protein 1 (AP-1). J Med Chem. 2014;57:6930–48.
Tewari D, Nabavi SF, Nabavi SM, Sureda A, Farooqi AA, Atanasov AG, Vacca RA, Sethi G, Bishayee A. Targeting activator protein 1 signaling pathway by bioactive natural agents: possible therapeutic strategy for cancer prevention and intervention. Pharmacol Res. 2018;128:366–75.
Trop-Steinberg S, Azar Y. AP-1 expression and its clinical relevance in immune disorders and cancer. Am J Med Sci. 2017;353:474–83.
Ma J, Xuan SH, Li Y, Zhang ZP, Li XH. Role of the TGFbeta/PDCD4/AP-1 signaling pathway in nasopharyngeal carcinoma and its relationship to prognosis. Cell Physiol Biochem. 2017;43:1392–401.
Kim JM, Noh EM, Song HK, Lee M, Lee SH, Park SH, Ahn CK, Lee GS, Byun EB, Jang BS, et al. Salvia miltiorrhiza extract inhibits TPA-induced MMP-9 expression and invasion through the MAPK/AP-1 signaling pathway in human breast cancer MCF-7 cells. Oncol Lett. 2017;14:3594–600.
Liu S, Luan J, Ding Y. MiR-144-3p targets FosB protooncogene, AP-1 transcription factor subunit (FOSB) to suppress proliferation, migration, and invasion of PANC-1 pancreatic cancer cells. Oncol Res. 2017;26(5):683–90.
Wang Y, Sun T, Sun H, Yang S, Li D, Zhou D. SCF/C-Kit/JNK/AP-1 signaling pathway promotes claudin-3 expression in colonic epithelium and colorectal carcinoma. Int J Mol Sci. 2017;18:E765.
Subramanian D, Bunjobpol W, Sabapathy K. Interplay between TAp73 protein and selected activator protein-1 (AP-1) family members promotes AP-1 target gene activation and cellular growth. J Biol Chem. 2015;290:18636–49.
Ming J, Jiang G, Zhang Q, Qiu X, Wang E. Interleukin-7 up-regulates cyclin D1 via activator protein-1 to promote proliferation of cell in lung cancer. Cancer Immunol Immunother. 2012;61:79–88.
Daft PG, Yang Y, Napierala D, Zayzafoon M. The growth and aggressive behavior of human osteosarcoma is regulated by a CaMKII-controlled autocrine VEGF signaling mechanism. PLoS One. 2015;10:e0121568.
Kim YH, Oh JH, Kim NH, Choi KM, Kim SJ, Baik SH, Choi DS, Lee ES. Fra-1 expression in malignant and benign thyroid tumor. Korean J Intern Med. 2001;16:93–7.
Chen X, Wu W, Chen X, Gong X. Roles of phosphatidylinositol 3-kinase regulatory subunit alpha, activator protein-1, and programmed cell death 4 in diagnosis of papillary thyroid carcinoma. Tumor Biol. 2016;37:6519–26.
Sophie L, Carole R, Eric B, Bernard C, Hartl DM, Jean-Michel B, Jean-Paul T, Martin S. Prognostic factors for persistent or recurrent disease of papillary thyroid carcinoma with neck lymph node metastases and/or tumor extension beyond the thyroid capsule at initial diagnosis. J Clin Endocrinol Metab. 2005;90:5723–9.
Ito Y, Kudo T, Kobayashi K, Miya A, Ichihara K, Miyauchi A. Prognostic factors for recurrence of papillary thyroid carcinoma in the lymph nodes, lung, and bone: analysis of 5,768 patients with average 10-year follow-up. World J Surg. 2012;36:1274–8.
De Castro TP, Waissmann W, Simoes TC, De Mello RCR, Carvalho DP. Predictors for papillary thyroid cancer persistence and recurrence: a retrospective analysis with a 10-year follow-up cohort study. Clin Endocrinol. 2016;85:466–74.
Mazzaferri EL, Jhiang SM. Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. Am J Med. 1994;97:418–28.
Dong W, Li Y, Gao M, Hu M, Li X, Mai S, Guo N, Yuan S, Song L. IKKα contributes to UVB-induced VEGF expression by regulating AP-1 transactivation. Nucleic Acids Res. 2012;40:2940–55.
Motomura H, Seki S, Shiozawa S, Aikawa Y, Nogami M, Kimura T. A selective c-Fos/AP-1 inhibitor prevents cartilage destruction and subsequent osteophyte formation. Biochem Biophys Res Commun. 2018;497:756–61.
Luo D, Chen H, Li X, Lu P, Long M, Peng X, Lin S, Tan L, Zhu Y, Ouyang N, Li H. Activation of the ROCK1/MMP-9 pathway is associated with the invasion and poor prognosis in papillary thyroid carcinoma. Int J Oncol. 2017;51:1209–18.
Rothhut B, Ghoneim C, Antonicelli F, Soularothhut M. Epidermal growth factor stimulates matrix metalloproteinase-9 expression and invasion in human follicular thyroid carcinoma cells through focal adhesion kinase. Biochimie. 2007;89:613–24.
Shin WS, Hong Y, Lee HW, Lee ST. Catalytically defective receptor protein tyrosine kinase PTK7 enhances invasive phenotype by inducing MMP-9 through activation of AP-1 and NF-κB in esophageal squamous cell carcinoma cells. Oncotarget. 2016;7:73242–56.
Meng XY, Zhang Q, Li Q, Lin S, Li J. Immunohistochemical levels of cyclo-oxygenase-2, matrix metalloproteinase-9 and vascular endothelial growth factor in papillary thyroid carcinoma and their clinicopathological correlations. J Int Med Res. 2014;42:619–27.
Li YS, Deng ZH, Zeng C, Lei GH. JNK pathway in osteosarcoma: pathogenesis and therapeutics. J Recept Signal Transduct Res. 2016;36:465–70.
Meggiato T, Calabrese F, De Cesare CM, Baliello E, Valente M, Del FG. C-JUN and CPP32 (CASPASE 3) in human pancreatic cancer: relation to cell proliferation and death. Pancreas. 2003;26:65.
Shaulian E, Karin M. AP-1 in cell proliferation and survival. Oncogene. 2001;20:2390–400.
Mitsiades CS, McMillin D, Kotoula V, Poulaki V, McMullan C, Negri J, Fanourakis G, Tseleni-Balafouta S, Ain KB, Mitsiades N. Antitumor effects of the proteasome inhibitor bortezomib in medullary and anaplastic thyroid carcinoma cells in vitro. J Clin Endocrinol Metab. 2006;91:4013–21.
Acknowledgements
This work was supported by National Natural Science Foundation of China (Grant No. 81672644).
Funding
This work was supported by National Natural Science Foundation of China (Grant No. 81672644).
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Contributions
FJ was responsible for the literature review, and GY designed the experiments. LQ analyzed the data. XC and HY performed the experiments and wrote the paper. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study protocol was approved by the Clinical Research Ethics Committee of Shengjing Hospital of China Medical University.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Xiao, C., Huang, Y., Gao, Q. et al. Expression of activator protein-1 in papillary thyroid carcinoma and its clinical significance. World J Surg Onc 17, 25 (2019). https://doi.org/10.1186/s12957-019-1568-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12957-019-1568-x