Abstract
Background
Brain morphometric abnormalities in schizophrenia have been extensively reported in the literature. Whole-brain volumetric reductions are almost universally reported by most studies irrespective of the characteristics of the samples studied (e.g., chronic/recent-onset; medicated/neuroleptic-naïve etc.). However, the same cannot be said of the reported regional morphometric abnormalities in schizophrenia. While certain regional morphometric abnormalities are more frequently reported than others, there are no such abnormalities that are universally reported across studies. Variability of socio-demographic and clinical characteristics across study samples as well as technical and methodological issues related to acquisition and analyses of brain structural images may contribute to inconsistency of brain morphometric findings in schizophrenia. The objective of the present study therefore was to systematically examine brain morphometry in patients with recent-onset schizophrenia to find out if there are significant whole-brain or regional volumetric differences detectable at the appropriate significance threshold, after attempting to control for various confounding factors that could impact brain volumes.
Methods
Structural magnetic resonance images of 90 subjects (schizophrenia = 45; healthy subjects = 45) were acquired using a 3 Tesla magnet. Morphometric analyses were carried out following standard analyses pipelines of three most commonly used strategies, viz., whole-brain voxel-based morphometry, whole-brain surface-based morphometry, and between-group comparisons of regional volumes generated by automated segmentation and parcellation.
Results
In our sample of patients having recent-onset schizophrenia with limited neuroleptic exposure, there were no significant whole brain or regional brain morphometric abnormalities noted at the appropriate statistical significance thresholds with or without including age, gender and intracranial volume or total brain volume in the statistical analyses.
Conclusions
In the background of the conflicting findings in the literature, our findings indicate that brain morphometric abnormalities may not be directly related to the schizophrenia phenotype. Analysis of the reasons for the inconsistent results across studies as well as consideration of alternate sources of variability of brain morphology in schizophrenia such as epistatic and epigenetic mechanisms could perhaps advance our understanding of structural brain alterations in schizophrenia.
Similar content being viewed by others
Background
Brain morphometric abnormalities have been extensively reported in schizophrenia for more than three decades. Following the initial report of brain volume reductions in schizophrenia by Johnstone et al. [1] in their computerized tomographic (CT) study, there have been an ever-increasing number of reports of brain morphometric abnormalities in schizophrenia using more powerful imaging modalities [2, 3]. These studies have used various quantitative measurements such as ventricular: brain ratio (VBR), whole brain volume, lobar volumes, volumes generated by region-of-interest (ROI)-based parcellation of cortical and subcortical structures using manual or semi-automated methods, as well as whole-brain voxel-based or surface-based analyses [3]. The majority of whole-brain morphometric studies have used Voxel-based morphometry (VBM) implemented in the Statistical Parametric Mapping (SPM) software (Wellcome Department of Imaging Neuroscience, London; http://www.fil.ion.ucl.ac.uk/spm) or surface-based analysis implemented using the FMRIB Software Library (FSL) [4]. Voxel-based morphometric studies have reported both large scale [5] as well as circumscribed volumetric reductions in regions like superior temporal gyrus [6], amygdala and cingulate [7] between patients with first episode schizophrenia and healthy comparison subjects. Similarly, surface-based studies using FreeSurfer in schizophrenia patients have also reported extensive volumetric abnormalities [8] on the one hand, to more circumscribed changes on the other [9].
The morphometric findings reported in schizophrenia using the various methods described above are summarized in reviews by Shenton et al. [10] (whole brain and regional parcellation studies); Honea et al. [11] (voxel-based morphometric studies); Steen et al. [12] (volumetric studies in first-episode psychosis); Navari and Dazzan [13] (morphometric abnormalities in relation to neuroleptic use) and Bora et al.[14] (a coordinate-based meta-analysis to assess the effects of gender, chronicity, negative symptoms and other clinical variables on regional brain metrics). It is evident from these reviews that whole brain volumetric reductions are consistently reported in almost all the studies. However, replicability of reported regional brain morphometric abnormalities using whole-brain voxel-wise analyses has been far from satisfactory [11]. This has led many researchers to even question the validity of the reported brain morphometric abnormalities in schizophrenia [15, 16]. The factors contributing to inconsistency of brain morphometric findings in schizophrenia include heterogeneity of symptom characteristics [17]; variable duration of illness (recent-onset vs. chronic) [18]; unequal gender distribution [19] and handedness of study samples [20]; age of onset (typical onset vs. late-onset) [21], medication status (drug-naïve or drug-free vs. medicated) [13] as well as life-time substance abuse [22]. Factors pertaining to Magnetic resonance imaging (MRI) acquisition (strength of the magnet, acquisition protocols etc.) [23] and analyses (hypothesis-free whole brain voxel-based analyses vs. ROI-based analyses with or without a priori hypotheses) also deserve attention while examining the issue of poor replicability of regional brain morphometric findings in schizophrenia. It has been pointed out that many of the above-mentioned confounding factors are not given adequate consideration while undertaking group comparisons [10, 12]. Perhaps the most serious methodological consideration in whole-brain voxel-based morphometric analyses is the reporting of results without specifying whether they are corrected for multiple comparisons or not [24]. We have recently reported the importance of controlling for the socio-demographic and clinical confounding factors affecting brain volumes as well as the use of statistical significance thresholds corrected for multiple comparisons in brain morphometric studies of schizophrenia [25]. Another major issue that is often not given its due consideration is the bias against publication of negative findings [16].
Therefore, as the primary objective of this study we examined brain morphometry in a sample of patients with predominant positive symptoms of schizophrenia (paranoid and undifferentiated sub-types as well as schizophreniform disorder) of recent onset (<5 years duration) (and therefore either neuroleptic-naïve or with limited neuroleptic exposure), in comparison to a healthy control sample matched for age, handedness (all right-handed) and gender distribution, using whole-brain voxel-based and surface-based analyses as well as ROI-based analyses using automated parcellation. By including only patients with recent-onset schizophrenia with limited neuroleptic exposure and by matching socio-demographic variables during recruitment phase as well as controlling for their influence on brain morphometry by including them as co-variates during statistical analyses, we expected to make reliable inferences regarding morphometric abnormalities, if any, that are hallmarks of the neurodevelopmental [26] disease process of schizophrenia. In keeping with the majority of previous reports, we expected that there would be significant whole brain volumetric reduction in patients with schizophrenia. However, in view of the lack of evidence for consistently replicated regional morphometric abnormalities from previous studies after controlling for all the above confounding factors, we assumed the null hypothesis that there would be no significant regional differences in grey matter volume in patients with schizophrenia in comparison to matched healthy control subjects.
Results
The socio-demographic and clinical characteristics of the study samples are given in Table 1. There were no significant differences in age, gender-distribution and level of education between schizophrenia and healthy control samples.
There were no significant differences in age- and intra-cranial volumetric (ICV) -adjusted total brain volume (TBV) between patients with schizophrenia (mean = 1080, S.E. = 3.242) when compared to healthy subjects (mean = 1087, S.E. = 3.570) using analysis of covariance (ANCOVA), with ICV, gender and age as co-variates (F = 2.671; p < 0.106) (Fig. 1). No significant differences in TBV were seen even on two-way ANOVA without co-variates (F = 0.659, p < 0.419). As expected there was a significant effect of gender on total brain volume in both healthy and schizophrenia subjects, but upon correction for ICV-differences, the gender effect disappeared (Additional file 1: Table S3).
Comparison of total brain volume (TBV) (in ml) between patients with recent-onset schizophrenia (ROS) (N = 45; mean = 1072.74; s.d. = 117.81) and healthy control subjects (HCS) (N = 45; 1093.16; s.d. = 120.89); The central red line represents the mean, the pink box represents the standard error of mean and the blue box, the standard deviation
A trend-level difference in age- and ICV-adjusted TBV was noted between healthy subjects, neuroleptic naïve patients and medicated patients using ANCOVA with age, gender and ICV as co-variates (F = 2.732; p < 0.071). Post-hoc pair-wise comparisons revealed that medicated patients with recent-onset schizophrenia (ROS) (n = 24) (mean = 1075, S.E. = 4.407) had significantly lower age- and ICV-adjusted TBV when compared to healthy comparison subjects (HCS) (n = 45) (mean = 1087, S.E. = 3.550) (Mean difference: Healthy-Medicated = 12.729, S.E. = 5.575; Bonferroni-adjusted p < 0.025), while no significant differences in age- and ICV-adjusted TBV emerged between neuroleptic-naïve patients with ROS (n = 21) (mean = 1086, S.E. = 4.837) and HCS (n = 45). Medicated patients had significantly longer duration of illness in comparison to neuroleptic-naïve patients [Medicated patients: mean (in months) =30.21; s.d. = 17.245; Neuroleptic-naïve patients: mean (in months) = 17.81; s.d. = 17.113] (t = 2.145, p < 0.020). Group-wise comparison of age-, ICV- and duration of illness-adjusted TBV between medicated (mean = 1066, S.E. = 4.505) and neuroleptic-naïve (1082, S.E. = 4.846) patients with ROS using ANCOVA with age, gender, ICV and duration of illness as covariates revealed significantly lower TBV in medicated patients (F = 5.532, p < 0.024).
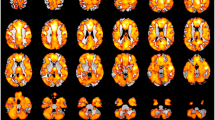
No significant regional volumetric differences emerged between schizophrenia and healthy subjects using any of the three morphometric approaches, namely, VBM (at false discovery rate (FDR) p < 0.05), FreeSurfer (Monte-Carlo (MC-Z) p < 0.05) and ROI-based analysis of FreeSurfer-generated volumes (p < 0.0007, two-tailed). The ‘uncorrected’ (P < 0.001; k = 0 voxels) results generated by VBM without including any co-variates in the design matrix are presented in Fig. 2 and Table 2. The ‘uncorrected’ (P < 0.001; k = 0 voxels) results generated by VBM when TBV, age and gender were entered as nuisance factors are presented in Fig. 3 and Table 3. As may be seen from the Tables 2 and 3, the results of VBM analyses with and without the 3 co-variates are more or less similar, except that the number of clusters identified at the statistical threshold of p < 0.001 uncorrected were marginally lesser when the 3 co-variates were included in the design matrix. The uncorrected (P < 0.001) results using FreeSurfer Qdec GUI with diagnosis (ROS vs. HCS) and gender as fixed factors and TBV and age as co-variates are given in Additional file 1: Figure S1 and Table S4. Group comparisons of regional volumes generated by FreeSurfer using ANCOVA in Statistical Package for Social Sciences (SPSS) revealed no brain regions that showed significant volumetric changes at the Bonferroni-corrected statistical threshold of p < 0.0007, nor even trend-level differences at p < 0.05.
Statistical parametric t-map of gray matter volumes shown as reduced in schizophrenia subjects (N = 45) in comparison to healthy subjects (N = 45) at a significance threshold of p < 0.001 uncorrected and an extent threshold of 0 voxels, when no co-variates were entered in the two sample random effects analysis (RFX)
Statistical parametric t-map of gray matter volumes shown as reduced in schizophrenia subjects (N = 45) in comparison to healthy subjects (N = 45) at a significance threshold of p < 0.001 uncorrected and an extent threshold of 0 voxels, with total brain volume (TBV) age and gender entered in the two sample random effects analysis (RFX) as co- variates
As detailed above, we found significant effects of medication on total brain volume. In order to examine medication effects on regional brain volumes, we carried out exploratory VBM analyses between neuroleptic-naïve patients with ROS and HCS (Additional file 1: Figure S2; Table S5); medicated patients with ROS and HCS (Additional file 1: Figure S3; Table S6) as well as medicated patients with ROS and neuroleptic-naïve patients with ROS (Additional file 1: Figures S4 and S6; Tables S7 and S8). None of the above contrasts revealed volumetric differences at the FDR p < 0.05 statistical threshold. Nevertheless, trend-level regional volumetric reductions were noted in both neuroleptic-naïve and medicated patients in comparison to healthy control subjects (Additional file 1: Figures S2 and S3; Tables S5 and S6). On the other hand, VBM comparisons between neuroleptic-naïve (n = 21) vs. medicated (n = 24) patients with age, gender, TBV and duration of illness as co-variates revealed volumetric increases in cerebellum (bilateral posterior declive and right posterior pyramis) and right inferior parietal lobule (Additional file 1: Figure S4; Table S7) as well as volumetric decreases in right pre-central gyrus and right inferior frontal gyrus in medicated patients (Additional file 1: Figure S5; Table S8).
Discussion
We aimed at examining whole- and regional-brain morphometric abnormalities in a sample of patients with recent-onset schizophrenia using three commonly used morphometric methods, controlling for the most important socio-demographic and clinical confounding factors that can potentially impact brain morphology in schizophrenia. We found no significant whole- or regional-brain volumetric differences at the appropriate statistical significance thresholds in our sample of patients having recent-onset schizophrenia with limited neuroleptic exposure, in comparison to the healthy control sample matched for age and gender distribution.
As mentioned in the Introduction, the major focus of this study was to examine whether whole- or regional-brain morphometric abnormalities are detectable in a sample of patients with schizophrenia even after controlling for possible socio-demographic and clinical confounding factors that could impact brain volumes. We have recently reported the importance of controlling for these confounding factors in brain morphometric studies of schizophrenia [25]. In the present study, we attempted to control for the effect of these confounding factors at the subject recruitment as well as data analysis stages. The effects of disease chronicity and cumulative neuroleptic exposure on brain volumes were minimized by including only patients with recent-onset schizophrenia having a maximum duration of illness of 5 years.
Approximately half (n = 21; 46.67%) of the study sample (N = 45) were neuroleptic-naïve and another 20% (n = 9) were drug-free at the time of recruitment into the study. The study samples were group-matched for age, gender distribution and educational status. Moreover, age and gender were entered as covariates in the between-group volumetric comparisons. For the whole-brain volumetric (TBV) comparisons, ICV was used as an additional covariate (to examine the differences in whole brain volume controlling for variability of intracranial volumes), while for the whole-brain voxel-wise comparisons, TBV was used as an additional covariate (to examine whether there are regional brain morphometric abnormalities even after controlling for whole brain volumetric differences).
Contrary to our a priori hypothesis, we found no significant differences in TBV between our sample of patients with recent-onset schizophrenia in comparison to matched healthy control subjects, when age, gender and ICV were entered as co-variates. A large number of previous morphometric studies in schizophrenia (chronic/recent-onset/medicated/ neuroleptic-naïve) [2, 12, 27], including a previous study on neuroleptic-naïve patients with recent-onset schizophrenia from our own group [28] have reported significant/trend-level reductions of total brain volume in patients when compared to healthy control subjects. It is quite possible that this whole brain volumetric reduction in schizophrenia might indicate the influence of aberrant neurodevelopmental processes (e.g., aberrant glutamate signaling secondary to intra-uterine or perinatal insults) on overall brain development [29] in keeping with the neurodevelopmental hypothesis of schizophrenia [26]. While whole-brain volumetric reductions may indeed be a hallmark of schizophrenia, the results of our study indicate that in carefully chosen samples of patients with recent-onset schizophrenia and limited neuroleptic exposure, significant differences in total brain volume need not necessarily be evident in comparison to age- and gender-matched healthy control samples, especially when other possible clinical confounders are minimized at the sample recruitment stage using appropriate inclusion and exclusion criteria. Figure 1 depicts the substantial overlap of brain volumes between the schizophrenia and healthy samples, indicating that whole brain volumes may not necessarily vary according to the phenotype (i.e., schizophrenia vs. healthy subjects); but perhaps could be mediated by other factors discussed later.
In the present study, whole-brain morphometric analyses using both VBM and FreeSurfer-based methods did not reveal significant regional volumetric differences between schizophrenia and healthy control samples. As noted earlier, our schizophrenia sample comprised of patients with recent-onset illness having had only minimal exposure to neuroleptics. Moreover, the socio-demographic variables (age and gender distribution) and total brain volume were entered as nuisance co-variates and the statistical significance threshold was set at FDR p < 0.05 for VBM8 and Monte-Carlo Simulation p < 0.05 for FreeSurfer, corrected for multiple comparisons. Trend-level volumetric reductions were noted at a statistical significance threshold of p < 0.001 uncorrected (extent threshold k = 0 voxels) in patients with schizophrenia; the findings being largely comparable irrespective of whether the co-variates (age, gender and TBV) were included in the design matrix or not (Figs. 2 and 3; Tables 2 and 3). This may be due to the fact that the samples were age- and gender-matched and also because the TBV was not significantly different between the two samples. However the results of VBM and FreeSurfer analyses did not show high concordance, perhaps because, at the uncorrected statistical significance threshold, the findings across two methods using different registration algorithms may not be very reliable.
The brain regions that showed a trend towards volumetric reduction in schizophrenia (at p < 0.001 uncorrected) in the present study include bilateral frontal, bilateral medial temporal, bilateral cerebellum, right anterior and posterior cingulate, right insula, right superior temporal gyrus, right lentiform nucleus, left superior parietal lobule and left middle and inferior occipital gyri. Almost all these brain regions have been reported to show volumetric reductions in previous morphometric studies of schizophrenia. However, it must be mentioned that virtually every brain region has been reported to show volumetric reductions in schizophrenia in different studies, even though, no specific brain region or a set of brain regions have been shown to be reduced in volume consistently across all or a majority of the previous studies. Given the various methodological issues related to morphometric studies in schizophrenia, we are refraining from making attempts to discuss the neurobiological significance of these trend-level regional brain morphometric observations.
There have been many reports of regional brain morphometric abnormalities in schizophrenia using Voxel-based morphometry [11] and FreeSurfer analysis [8, 9]. Such reports have emanated from studies with sample sizes ranging from 14 to 20 [5, 30, 31] to up to 173 [8] patients with schizophrenia. However, since many of these studies have not adequately controlled for the confounding factors discussed above and since many have not used statistical significance thresholds corrected for multiple comparisons, interpreting the results of such studies is challenging. Nevertheless, it is interesting to note that many studies carried out on relatively smaller samples of patients with recent-onset schizophrenia have reported significant volumetric reductions at stringent statistical thresholds (FDR or FWE-corrected) (e.g., [30, 31]). This indicates that the findings of significant morphometric differences between patients with schizophrenia and healthy control subjects may not be dependent on the sample sizes, but more likely due to other factors such as molecular genetic-, socio-demographic- and/or clinical variables that vary across the samples studied. This issue would be further handled in detail subsequently.
In the present study, regional cortical volumes generated using automated segmentation and parcellation by FreeSurfer [32] did not show significant volumetric differences between schizophrenia and healthy control subjects. Previous ROI-based analyses using manual, semi-automated and fully automated regional parcellation schemes have reported morphometric abnormalities in schizophrenia subjects [3, 10, 12]. Many of these studies are limited by small sample sizes (N = 12-22) [33, 34], variable duration of illness and clinical heterogeneity [18]. A systematic meta-review by Shepherd et al. [35] of the structural brain alterations in schizophrenia showed a large volume of conflicting low quality evidence and limited high quality evidence supporting gray or white matter changes in schizophrenia.
Therefore, the results of the present study refute our a priori hypothesis that whole brain volumetric reduction will be noted even in the early stages of schizophrenia (duration of illness ≤5 years) and will be demonstrable even after controlling for the socio-demographic and clinical confounding factors that affect brain volumes at the sample recruitment and analysis stages. On the contrary, the negative regional brain morphometric findings of the study confirm our a priori hypothesis. Previous reports of regional brain morphometric abnormalities in schizophrenia show wide variability, perhaps due to the confounding effects of the various socio-demographic and clinical variables that affect brain morphology. Having controlled for the above confounding variables at the sample recruitment and data analyses stages; and having used stringent statistical significance thresholds correcting for multiple comparisons for between-group comparisons, there were no detectable statistically significant regional brain morphometric abnormalities.
Effect of medication exposure on brain volumes
Medicated patients with recent-onset schizophrenia had significantly lower total brain volume than healthy control subjects as well as neuroleptic-naïve patients. On VBM analysis, both neuroleptic-naïve and medicated patients showed a trend (p < 0.001 uncorrected; k = 0 voxels) towards lower regional volumes in comparison to healthy subjects (Additional file 1: Figures S2 and S3 and Tables S5 and S6). However, medicated patients showed a trend towards volumetric increases in cerebellum (bilateral posterior declive and right posterior pyramis) and right inferior parietal lobule (Additional file 1: Figure S4; Table S7) as well as volumetric decreases in right pre-central gyrus and right inferior frontal gyrus in comparison to neuroleptic-naïve patients (Additional file 1: Figure S5; Table S8).
Medicated patients had significantly higher duration of illness in comparison to neuroleptic-naïve patients. This could be the reason why medicated patients showed significantly lower total brain volume than healthy control subjects as well as neuroleptic-naïve patients. However, controlling for total brain volume, medicated patients showed a trend towards predominantly increased regional volumes in cerebellum and right inferior parietal lobule in comparison to neuroleptic-naïve patients. The issue of cortical and basal ganglia volumetric changes associated with antipsychotics is a hotly debated topic with various studies reporting decrease in cortical and subcortical volumes (e.g., [36, 37] (review)); increase in volume of basal ganglia structures (e.g., [38]), as well as conflicting findings of differential effects of typical and atypical antipsychotics (e.g., [13] (review), [39–41]) on brain. Increased brain volumes in medicated vs. neuroleptic-naïve patients with schizophrenia have been previously reported [36, 42]. Many studies have also reported no significant changes associated with both typical as well as atypical antipsychotic treatment (e.g., [43]). One must also keep in mind that the methodological issues concerning brain morphometric studies discussed in the Introduction are relevant for studies that have reported effects of medication on brain volume, and may have contributed to the inconsistent reports. Therefore, it is our opinion that there is no definite evidence regarding effect of medications on brain volume in our sample, and the trend-level findings reported here may at best be considered preliminary.
Strengths and limitations of the study
The strengths of the present study include homogeneity of the patient sample with respect to clinical presentation, recent onset (≤5 years duration) of illness, and limited neuroleptic exposure. The diagnosis of schizophrenia was made with great rigor by obtaining a consensus between clinical diagnosis of an expert clinician and a research diagnosis generated by MINI-Plus interview. Moreover, diagnostic stability over 1–3 years was examined by reviewing the follow-up notes; ten subjects were excluded from the final sample following this exercise (vide Additional file 1: Table S1). The healthy and schizophrenia samples did not show significant differences in age, gender distribution and educational status. The structural images were acquired using a high resolution 3 Tesla scanner.
The results of morphometric analyses carried out using the three most commonly employed methods showed remarkable consistency in that all the three methods failed to show significant regional brain morphometric abnormalities in schizophrenia when compared to healthy comparison subjects at the appropriate significance thresholds. Confounding factors that are relevant for both the groups such as ICV/TBV, age and gender were entered during analysis as covariates. The sample sizes were adequate for whole-brain voxel-wise analyses using VBM and FreeSurfer. However, given the large number of ROIs entered in automated parcellation-based analysis (n = 68, covering both hemispheres), recruiting an adequate number of patients with recent-onset schizophrenia for group comparisons using ANCOVA was not feasible in a single-center study of this nature. Even though majority of our sample of patients with schizophrenia had no or limited previous exposure to neuroleptics (67%) (Table 1), it would have been ideal if all recruited patients were drug-naïve. Moreover, it needs to be pointed out that we have not considered many other factors such as socio-economic and nutritional status, IQ, stress levels, body weight etc. that may affect brain morphology and that could also have a potential, albeit indirect relation with the risk of developing schizophrenia. Another limitation of the present study is the absence of diffusion tensor imaging (DTI), which could have thrown light on white matter structural integrity changes in the same sample. Nevertheless, we found that there were no significant differences in total white matter volume between patients and control subjects as examined using ANCOVA controlling for age, gender and ICV (F = 0.11, p < 0.752).
It may be argued that the absence of significant regional morphometric abnormalities in the present study could be due to a Type II error owing to ‘inadequate’ sample sizes. As mentioned earlier, the studies that have reported significant morphometric abnormalities in schizophrenia using VBM and FreeSurfer analyses have had sample sizes ranging from 14 to 173 patients. On carefully reviewing these studies, there is no indication to suggest that studies with larger sample sizes have consistently reported more extensive or more specific regional morphometric abnormalities or that studies with smaller sample sizes consistently failed to find significant differences (due to Type II error). Indeed, studies with sample sizes as low as 14–20 patients with schizophrenia (neuroleptic/chronic) have reported extensive volumetric reductions in multiple brain regions [44]. In this context, the recently reported findings of the largest international multi-site mega-analysis comprising of 784 patients with schizophrenia and 986 healthy control subjects [45] are quite illuminating. VBM comparisons between these two large samples for the Control > Schizophrenia contrast yielded significant (at FDR p < 0.05) differences in gray matter volumes in regions that covered most of the brain in a single cluster. Thus the robust finding to emerge from the above mega-analysis using voxel-wise statistics was the whole-brain volumetric reduction in the schizophrenia sample. The global maxima of the above diffuse volumetric reduction was located in the MNI space in between the right insula and putamen and not in the left superior temporal gyrus or hippocampus or the prefrontal cortical regions that have been hitherto the most consistently reported regions showing brain morphometric alterations in schizophrenia [11, 46, 47]. More intriguingly, global maxima of the above mega-analysis was not found to show volumetric reductions in a recent meta-analysis of over 18,000 subjects [42]. In the above meta-analysis, medicated patients (n = 8327) were found to have diffuse volumetric reductions spanning almost the entire brain, along with reduced total brain, total gray and total white matter volumes. Neuroleptic-naïve patients, on the other hand, were shown only to have volumetric reductions in the bilateral hippocampus, thalamus and caudate, apart from the whole brain measures (total, gray and white). Moreover, thalamus and caudate were not shown to have volumetric reductions in the medicated group. This cannot be explained as the effect of neuroleptic exposure on increasing thalamic and caudate volumes, in which case, with a sample size of 8327, one would expect a significant increase in volume of these structures in the medicated group to be picked up. It must be noted that the ‘fail-safe number’ of the above meta-analysis on neuroleptic-naïve patients was quite small, while Egger’s regression test indicated publication bias for many of the brain regions reported to show volumetric reductions in medicated patients. Perhaps the most important finding of this meta-analysis is the finding of a robust association between gray matter reduction and longer duration of illness as well as higher dose of antipsychotics. This might explain the consistent whole brain volumetric reductions reported in the literature so far, since the majority of such studies were carried out in patients with longer duration of illness and having had exposure to neuroleptics over a longer period of time. The fact that the present study was carried out in a moderate-sized (n = 45) sample of patients with recent-onset schizophrenia with limited neuroleptic exposure might be one of the reasons why we did not find significant total brain volumetric reductions in our schizophrenia sample.
The present study demonstrates how a given sample of patients having recent onset schizophrenia with limited neuroleptic exposure may not show significant whole- or regional-brain morphometric alterations in comparison to a healthy control sample matched for age, gender distribution and education. However, this finding in no way suggests that a different sample of patients with recent-onset schizophrenia will not show significant volumetric differences with another healthy comparison group. Such inconsistency of findings across different samples of patients with recent-onset schizophrenia would indicate that the reported brain morphometric abnormalities in schizophrenia cannot directly be linked to the schizophrenia phenotype, but may be understood as epiphenomena that could putatively be linked to molecular genetic epistatic and epigenetic interactions as well as other socio-demographic and clinical confounding factors such as duration of illness and medication status. Moreover, given the inconsistencies of brain morphometric findings from the mega- and meta-analyses discussed above, it is amply clear that the solution to resolve the puzzle of inconsistent morphometric findings in schizophrenia is not increasing sample sizes of structural neuroimaging studies in schizophrenia. Previous studies discussed above that have reported morphometric reductions at a stringent statistical threshold (FDR- or FWE-corrected) despite small sample sizes (e.g., [30, 31]) would lend further support to this conclusion. Therefore, it is important that researchers in the field should consider giving up their ‘wishful thinking’ that consistent morphometric findings in schizophrenia would emerge simply by increasing the sample sizes. Indeed, a more logical strategy would be to examine the sources of the variability of morphometric findings across samples, most important of which are the molecular genetic factors. These factors might include genetic and epigenetic factors, including the individual and additive effects of the multiple genes responsible for schizophrenia diathesis on brain development, as recently reported from our laboratory [48, 49].
Conclusions
The present study showed that in a carefully selected sample of patients with schizophrenia having recent-onset illness (≤5 years from onset) with limited/no exposure to neuroleptics, there may not be demonstrable whole- or regional-brain morphometric alterations in comparison to age-, gender- and education-matched healthy comparison subjects at the appropriate significance thresholds. However, our finding in no way suggests that a different sample of patients with recent-onset schizophrenia will not show significant volumetric differences with another healthy comparison group. This implies that demonstrable whole- or regional-brain morphometric abnormalities are not hallmarks of the schizophrenia phenotype, but could be epiphenomena related to molecular genetic epistatic and epigenetic interactions as well as other socio-demographic and clinical confounding factors such as duration of illness and medication status. Therefore, the results of the present study indicate that the relationship between brain volumetric alterations and the schizophrenia phenotype cannot be conceptualized using a simplistic (cause-effect) framework. On the contrary, in silico models that simulate gene-gene (epistatic) and gene-environment (epigenetic) interactions affecting brain morphology might provide us with a more comprehensive understanding regarding complexities underlying the brain morphometric alterations associated with schizophrenia [50].
Methods
Ethics statement
The study was carried out at the National Institute of Mental Health and Neurosciences (NIMHANS), Bangalore, India, with due approval from the National Institute of Mental Health and Neurosciences Human Ethics Committee, thus conforming to the ethical standards laid down in the 1964 Declaration of Helsinki. Written informed consent was obtained after detailed explanation of the study protocol, from all the subjects (and their accompanying relatives in the case of individuals with schizophrenia, as required by the NIMHANS Ethics Committee) prior to enrollment into the study.
Study samples
The study samples comprised of forty five patients with ROS, recruited from those who attended the outpatient services of NIMHANS by purposive sampling and forty five HCS recruited by word of mouth from hospital staff and attendants of hospitalized patients. A total of 109 subjects (schizophrenia subjects = 58; healthy comparison subjects = 51) were recruited into the study from which the above samples were derived, with 19 subjects having to be dropped for various reasons (Additional file 1: Table S1). Only right-handed subjects (as determined by modified Annett’s inventory [51], aged between 17 and 50 years, and with an Mini-Mental Status Examination (MMSE) [52] score of ≥23 were recruited into the study. The presence of any unstable medical/neurological condition was ruled out in both groups of subjects using an unstructured clinical interview, detailed physical examination and baseline laboratory investigations. The diagnosis of schizophrenia or schizophreniform disorder was arrived at using criteria from the Diagnostic and Statistical Manual for Mental Disorders-Fourth Edition (DSM-IV) [53] based on the consensus of an experienced research psychiatrist (J.P.J.) who conducted a semi-structured interview and a trained research assistant who used the Mini International Neuropsychiatric Interview (MINI) Plus [54]. Moreover, prior to arriving at the final sample of patients with schizophrenia for morphometric analyses, we reviewed the case files of all subjects to examine for diagnostic stability, with the duration of follow-up ranging from 1 to 3 years. As given in Additional file 1: Table S1, ten subjects were removed from the schizophrenia/ schizophreniform disorder sample after this exercise. Only those patients who did not meet criteria for any other Axis I disorder, including substance dependence (other than nicotine) as per MINI-Plus, with an age of first onset of psychotic symptoms at or after 17 years of age and a duration of illness less than or equal to 5 years were recruited into the study. All patients had predominant positive symptoms and had diagnoses of paranoid (n = 30), undifferentiated (n = 8) or schizophreniform (n = 7) subtypes. The patients with schizophreniform disorder included in the morphometric analysis comprised of only those who were re-diagnosed as schizophrenia during follow-up and who retained a diagnosis of schizophrenia even after 1–3 years, as inferred from follow-up data obtained from case files. The baseline severity of schizophrenia psychopathology was evaluated using the Positive and Negative Syndrome Scale (PANSS) [55] by two trained raters who had established good inter-rater reliability. The history of exposure to antipsychotics was ascertained by interviewing the patient and relative/s, and corroborated from available medical records. Thirty of the forty five patients were not on neuroleptics, of which 21 were drug naïve at the time of recruitment into the study. The remaining patients were on antipsychotics, the cumulative doses of which were converted to ‘risperidone equivalents’ [56–58] (Table 1). The details of lifetime exposure to neuroleptics of patients who were exposed to neuroleptics are given in Additional file 1: Table S2. The healthy comparison subjects were ascertained to be free from Axis I or II psychiatric disorders using the MINI-Plus. Current use/abuse of psychotropic drugs as well as history of psychiatric illness in first-degree relatives in the healthy comparison subjects were ruled out by an unstructured clinical interview. The socio-demographic and clinical characteristics of the samples are given in Table 1.
Structural MRI
Image acquisition
Magnetic resonance imaging (MRI) structural images were acquired on a Philips Achieva 3.0 T scanner using a SENSE-8 head coil. Head movements were minimized by applying a band over the forehead during the scanning procedure. A high-resolution T1-weighted MRI volume data set of the whole brain with a resolution of 1 × 1 × 1 mm3 was acquired using an MPRAGE (Magnetization Prepared Rapid Gradient Echo) sequence: Repetition time (TR) = 8.2 ms, echo time (TE) = 3.8 ms, flip angle = 8°, sense factor: 3.5.
Image preprocessing and analyses
All scans were inspected visually for any gross structural abnormality by an expert neuroradiologist. The MR images were first converted from DICOM format to NIFTI format using dcm2nii software (http://www.mccauslandcenter.sc.edu/mricro/mricron/dcm2nii.html).
Whole-brain morphometric analyses permit hypothesis-free testing of volumetric differences between schizophrenia and healthy comparison subjects [6, 59]. Such approaches require transforming brains from different participants into a common reference frame using either volume-based registration or surface-based registration. We performed whole-brain morphometric comparisons between schizophrenia and healthy control samples using the most commonly used software utilizing volume-based registration, viz., VBM8 and surface-based registration, viz., FreeSurfer, version 5.1.
Voxel-Based Morphometry (VBM)
Voxel-based morphometry was performed using Christian Gaser’s VBM8 toolbox (http://dbm.neuro.uni-jena.de/vbm8/) running on Statistical Parametric Mapping 8 software (SPM8) (http://www.fil.ion.ucl.ac.uk/spm). Prior to VBM analysis, the images were visually inspected for artefacts or other structural anomalies and one image each from the healthy and the schizophrenia samples were omitted (refer Additional file 1: Table S1). Briefly, the image pre-processing steps using VBM8 toolbox generated normalized, segmented, modulated, and smoothed (using a Gaussian filter of kernel size 8 mm Full Width Half Maximum (FWHM)) gray matter (GM) images with a voxel size of 1 mm3, which were used for further statistical analysis. Spatial normalization was achieved by using the standard Montreal Neurological Institute (MNI) 152 template. Analysis of modulated data tests for regional differences in absolute amount (volume) of GM. We did not use any explicit or threshold masks in our whole-brain voxel-wise analysis. The total GM, white matter (WM) and ICV were generated from the VBM analysis. The TBV were calculated as sum of GM and WM volumes.
Free Surfer-based whole brain analysis
Cortical reconstruction and volumetric segmentation was performed with the Freesurfer image analysis suite, freely available online (http://surfer.nmr.mgh.harvard.edu/), the technical details of which have been described previously [32]. Briefly, this method uses both intensity and continuity information from the entire three dimensional MR volumes in segmentation and deformation procedures to produce representations of cortical thickness, calculated as the closest distance from the gray/white boundary to the gray/CSF boundary at each vertex on the tessellated surface. After a careful visual inspection for any gross anatomical abnormalities, all the scans were run as a batch on FreeSurfer v.5.1. Once the entire subject pool finished running, we made sure the reconstruction was successful by checking for Talairach registration. The adequacy of skull stripping, generation of white and pial surfaces, and segmentations were evaluated by the checking the images using TKMEDIT. Freesurfer completed the processing of the images without any need for manual interventions. The details of integration of surface- and volume-based representations are given in Makris et al. [60] and Desikan et al. [61].
ROI-based analyses using FreeSurfer-generated regional volumes
The FreeSurfer software package provides a method for complete automated parcellation of the cerebral cortex and subcortical structures [61]. The software segments the cortex and parcellates the surface into standardized regions of interest (ROIs). It allows for automated anatomical parcellation of cortex into gyral regions and subsequently surface parcellation is extended to GM volume, yielding parcellation of GM tissue sheet and regions of interest (ROIs). ROI based volumetric data were extracted and group comparisons performed using the SPSS version 16 for Windows.
Statistical analyses
Statistical analyses of demographic and clinical characteristics were performed using two-tailed Student’s t-test or Chi-square tests, as appropriate. Assumptions for normality were tested for all volumetric and demographic variables using Kolmogorov-Smirnov’s test of normality in the SPSS. Total brain volume (TBV) generated by VBM8 was compared between schizophrenia and healthy control subjects using General Linear Model (GLM) ANCOVA with ICV, age and gender as co-variates. Age, gender and intracranial volume have been reported to influence whole brain and regional brain volumes (19).
We chose to use TBV generated by VBM for our analyses, given the unreliability of calculating ICV from T1 scans by FreeSurfer. This issue has been highlighted by the authors of FreeSurfer, who suggest using other image analyses modalities for ICV correction, while computing brain volumes (http://www.freesurfer.net/fswiki/eTIV).
Voxel-wise whole-brain morphometric analysis using VBM
Whole-brain voxel-wise comparisons of gray matter between schizophrenia and healthy subjects was carried out in VBM8 using General Linear Model (GLM) Analysis of Co-variance (ANCOVA) with the ‘nuisance factors’ listed earlier as co-variates. Since SPM uses a mass univariate approach, correction for multiple comparisons was applied by employing FDR estimations with the level of significance set a priori at p < 0.05, while addressing the primary objective of the study, which was to examine group differences in brain volumes between patients with schizophrenia and matched healthy control subjects.
Surface-based whole brain morphometric analysis using FreeSurfer
The effects of diagnosis on cortical volume were evaluated for both hemispheres using GLM at each vertex, with gender as fixed factor and TBV and age as covariates using Freesurfer. The statistical significance level of the vertex-wise analysis in Freesurfer was set at p < 0.05 after Monte-Carlo (MC-Z) simulation for multiple comparisons.
ROI-based analysis of FreeSurfer-generated regional volumes using SPSS
Group comparison between schizophrenia and healthy subjects of 34 regional brain volumes on either hemisphere generated by automated parcellation using Freesurfer, was carried out using SPSS. Assumptions for normality were tested for all volumetric and demographic variables using Kolmogorov-Smirnov’s test of normality. Of the 34 volumes on either hemisphere, the following variables were not normally distributed: Left hemisphere: entorhinal, post-central and rostral middle frontal cortices; Right hemisphere: entorhinal, medial orbito frontal, lateral orbito frontal, pars orbitalis and temporal pole cortices. These variables were winsorised [62] and log10x transformed to achieve normal distribution for parametric tests. For the variables that did not achieve normal distribution despite the above method (left: post-central; right: lateral orbito frontal and pars orbitalis), Mann-Whitney U test was used for group comparisons. All the other variables were compared between the schizophrenia and healthy subjects by General Linear Model (GLM) Analysis of Co-variance (ANCOVA) with diagnosis and gender as fixed factors and age and TBV as covariates.
Abbreviations
- ANCOVA:
-
Analysis of Covariance
- CT:
-
Computerized Tomography
- DSM-IV:
-
Diagnostic and Statistical Manual for Mental Disorders–Fourth Edition
- FDR:
-
False Discovery Rate
- FSL:
-
Functional Magnetic Resonance Imaging of Brain (FMRIB) Software Library
- FWHM:
-
Full Width Half Maximum
- GLM:
-
General Linear Model
- GM:
-
Gray Matter
- HCS:
-
Healthy Comparison Subjects
- ICV:
-
Intra-cranial Volume
- MC-Z:
-
Monte-Carlo
- MINI:
-
Mini International Neuropsychiatric Interview
- MMSE:
-
Mini-Mental Status Examination
- MNI:
-
Montreal Neurological Institute
- MPRAGE:
-
Magnetization Prepared Rapid Gradient Echo
- MRI:
-
Magnetic Resonance Imaging
- NIMHANS:
-
National Institute of Mental Health and Neurosciences
- PANSS:
-
Positive and Negative Syndrome Scale
- ROI:
-
Region-of-interest
- ROS:
-
Recent-onset Schizophrenia
- SPM:
-
Statistical Parametric Mapping
- SPSS:
-
Statistical Package for Social Sciences
- TBV:
-
Total Brain Volume
- TE:
-
Echo Time
- TR:
-
Repetition Time
- VBM:
-
Voxel-based Morphometry
- VBR:
-
Ventricular-brain ratio
- WM:
-
White Matter
References
Johnstone EC, Crow TJ, Frith CD, Husband J, Kreel L. Cerebral ventricular size and cognitive impairment in chronic schizophrenia. Lancet. 1976;2:924–6.
Wright IC, Rabe-Hesketh S, Woodruff PW, David AS, Murray RM, Bullmore ET. Meta-analysis of regional brain volumes in schizophrenia. Am J Psychiatry. 2000;157:16–25.
Shenton ME, Whitford TJ, Kubicki M. Structural neuroimaging in schizophrenia: from methods to insights to treatments. Dialogues Clin Neurosci. 2010;12:317–32.
Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23:S208–19.
Jayakumar PN, Venkatasubramanian G, Gangadhar BN, Janakiramaiah N, Keshavan MS. Optimized voxel-based morphometry of gray matter volume in first-episode, antipsychotic-naive schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:587–91.
Kubicki M, Shenton ME, Salisbury DF, Hirayasu Y, Kasai K, Kikinis R, et al. Voxel-based morphometric analysis of gray matter in first episode schizophrenia. Neuroimage. 2002;17:1711–9.
Suzuki M, Zhou SY, Hagino H, Niu L, Takahashi T, Kawasaki Y, et al. Morphological brain changes associated with Schneider’s first-rank symptoms in schizophrenia: a MRI study. Psychol Med. 2005;35:549–60.
Rimol LM, Nesvag R, Hagler Jr DJ, Bergmann O, Fennema-Notestine C, Hartberg CB, et al. Cortical volume, surface area, and thickness in schizophrenia and bipolar disorder. Biol Psychiatry. 2012;71:552–60.
Murakami M, Takao H, Abe O, Yamasue H, Sasaki H, Gonoi W, et al. Cortical thickness, gray matter volume, and white matter anisotropy and diffusivity in schizophrenia. Neuroradiology. 2011;53:859–66.
Shenton ME, Dickey CC, Frumin M, McCarley RW. A review of MRI findings in schizophrenia. Schizophr Res. 2001;49:1–52.
Honea R, Crow TJ, Passingham D, Mackay CE. Regional deficits in brain volume in schizophrenia: a meta-analysis of voxel-based morphometry studies. Am J Psychiatry. 2005;162:2233–45.
Steen RG, Mull C, McClure R, Hamer RM, Lieberman JA. Brain volume in first-episode schizophrenia: systematic review and meta-analysis of magnetic resonance imaging studies. Br J Psychiatry. 2006;188:510–8.
Navari S, Dazzan P. Do antipsychotic drugs affect brain structure? A systematic and critical review of MRI findings. Psychol Med. 2009;39:1763–77.
Bora E, Fornito A, Radua J, Walterfang M, Seal M, Wood SJ, et al. Neuroanatomical abnormalities in schizophrenia: a multimodal voxelwise meta-analysis and meta-regression analysis. Schizophr Res. 2011;127:46–57.
Ioannidis JP. Why most published research findings are false. PLoS Med. 2005;2, e124.
Ioannidis JP. Excess significance bias in the literature on brain volume abnormalities. Arch Gen Psychiatry. 2011;68:773–80.
Koutsouleris N, Gaser C, Jager M, Bottlender R, Frodl T, Holzinger S, et al. Structural correlates of psychopathological symptom dimensions in schizophrenia: a voxel-based morphometric study. Neuroimage. 2008;39:1600–12.
Ellison-Wright I, Glahn DC, Laird AR, Thelen SM, Bullmore E. The anatomy of first-episode and chronic schizophrenia: an anatomical likelihood estimation meta-analysis. Am J Psychiatry. 2008;165:1015–23.
Barnes J, Ridgway GR, Bartlett J, Henley SM, Lehmann M, Hobbs N, et al. Head size, age and gender adjustment in MRI studies: a necessary nuisance? Neuroimage. 2010;53:1244–55.
Good CD, Johnsrude I, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. Cerebral asymmetry and the effects of sex and handedness on brain structure: a voxel-based morphometric analysis of 465 normal adult human brains. Neuroimage. 2001;14:685–700.
Burke L, Androutsos C, Jogia J, Byrne P, Frangou S. The Maudsley Early Onset Schizophrenia Study: the effect of age of onset and illness duration on fronto-parietal gray matter. Eur Psychiatry. 2008;23:233–6.
Ebdrup BH, Glenthoj B, Rasmussen H, Aggernaes B, Langkilde AR, Paulson OB, et al. Hippocampal and caudate volume reductions in antipsychotic-naive first-episode schizophrenia. J Psychiatry Neurosci. 2010;35:95–104.
Jovicich J, Czanner S, Han X, Salat D, van der Kouwe A, Quinn B, et al. MRI-derived measurements of human subcortical, ventricular and intracranial brain volumes: Reliability effects of scan sessions, acquisition sequences, data analyses, scanner upgrade, scanner vendors and field strengths. Neuroimage. 2009;46:177–92.
Bennett CM, Wolford GL, Miller MB. The principled control of false positives in neuroimaging. Soc Cogn Affect Neurosci. 2009;4:417–22.
Vijayakumari AA, Thirunavukkarasu P, Lukose A, Arunachalam V, Saini J, Jain S, et al. Exploration of the effect of demographic and clinical confounding variables on results of voxel-based morphometric analysis in schizophrenia. India: Springer; 2015.
Weinberger DR. On the plausibility of “the neurodevelopmental hypothesis” of schizophrenia. Neuropsychopharmacology. 1996;14:1S–1.
Olabi B, Ellison-Wright I, McIntosh AM, Wood SJ, Bullmore E, Lawrie SM. Are there progressive brain changes in schizophrenia? A meta-analysis of structural magnetic resonance imaging studies. Biol Psychiatry. 2011;70:88–96.
John JP, Burgess PW, Yashavantha BS, Shakeel MK, Halahalli HN, Jain S. Differential relationship of frontal pole and whole brain volumetric measures with age in neuroleptic-naive schizophrenia and healthy subjects. Schizophr Res. 2009;109:148–58.
Moghaddam B. Bringing order to the glutamate chaos in schizophrenia. Neuron. 2003;40:881–4.
Salgado-Pineda P, Junque C, Vendrell P, Baeza I, Bargallo N, Falcon C, et al. Decreased cerebral activation during CPT performance: structural and functional deficits in schizophrenic patients. Neuroimage. 2004;21:840–7.
Ananth H, Popescu I, Critchley HD, Good CD, Frackowiak RS, Dolan RJ. Cortical and subcortical gray matter abnormalities in schizophrenia determined through structural magnetic resonance imaging with optimized volumetric voxel-based morphometry. Am J Psychiatry. 2002;159:1497–505.
Fischl B, van der Kouwe A, Destrieux C, Halgren E, Segonne F, Salat DH, et al. Automatically parcellating the human cerebral cortex. Cereb Cortex. 2004;14:11–22.
Buchanan RW, Vladar K, Barta PE, Pearlson GD. Structural evaluation of the prefrontal cortex in schizophrenia. Am J Psychiatry. 1998;155:1049–55.
Paillere-Martinot M, Caclin A, Artiges E, Poline JB, Joliot M, Mallet L, et al. Cerebral gray and white matter reductions and clinical correlates in patients with early onset schizophrenia. Schizophr Res. 2001;50:19–26.
Shepherd AM, Laurens KR, Matheson SL, Carr VJ, Green MJ. Systematic meta-review and quality assessment of the structural brain alterations in schizophrenia. Neurosci Biobehav Rev. 2012;36:1342–56.
Ho BC, Andreasen NC, Ziebell S, Pierson R, Magnotta V. Long-term antipsychotic treatment and brain volumes: a longitudinal study of first-episode schizophrenia. Arch Gen Psychiatry. 2011;68:128–37.
Moncrieff J, Leo J. A systematic review of the effects of antipsychotic drugs on brain volume. Psychol Med. 2010;40:1409–22.
Chakos MH, Lieberman JA, Bilder RM, Borenstein M, Lerner G, Bogerts B, et al. Increase in caudate nuclei volumes of first-episode schizophrenic patients taking antipsychotic drugs. Am J Psychiatry. 1994;151:1430–6.
Scherk H, Falkai P. Effects of antipsychotics on brain structure. Curr Opin Psychiatry. 2006;19:145–50.
Lieberman JA, Tollefson GD, Charles C, Zipursky R, Sharma T, Kahn RS, et al. Antipsychotic drug effects on brain morphology in first-episode psychosis. Arch Gen Psychiatry. 2005;62:361–70.
Massana G, Salgado-Pineda P, Junque C, Perez M, Baeza I, Pons A, et al. Volume changes in gray matter in first-episode neuroleptic-naive schizophrenic patients treated with risperidone. J Clin Psychopharmacol. 2005;25:111–7.
Haijma SV, Van Haren N, Cahn W, Koolschijn PC, Hulshoff Pol HE, Kahn RS. Brain volumes in schizophrenia: a meta-analysis in over 18 000 subjects. Schizophr Bull. 2013;39:1129–38.
McClure RK, Carew K, Greeter S, Maushauer E, Steen G, Weinberger DR. Absence of regional brain volume change in schizophrenia associated with short-term atypical antipsychotic treatment. Schizophr Res. 2008;98:29–39.
Salgado-Pineda P, Landin-Romero R, Fakra E, Delaveau P, Amann BL, Blin O. Structural abnormalities in schizophrenia: further evidence on the key role of the anterior cingulate cortex. Neuropsychobiology. 2014;69:52–8.
Gupta CN, Calhoun VD, Rachakonda S, Chen J, Patel V, Liu J, et al.: Patterns of gray matter abnormalities in schizophrenia based on an international mega-analysis. Schizophr Bull 2014
Segall JM, Turner JA, van Erp TG, White T, Bockholt HJ, Gollub RL, et al. Voxel-based morphometric multisite collaborative study on schizophrenia. Schizophr Bull. 2009;35:82–95.
Fornito A, Yucel M, Patti J, Wood SJ, Pantelis C. Mapping grey matter reductions in schizophrenia: an anatomical likelihood estimation analysis of voxel-based morphometry studies. Schizophr Res. 2009;108:104–13.
Thirunavukkarasu P, Vijayakumari AA, John JP, Halahalli HN, Paul P, Sen S, et al. An exploratory association study of the influence of dysbindin and neuregulin polymorphisms on brain morphometry in patients with schizophrenia and healthy subjects from South India. Asian J Psychiatr. 2014;10:62–8.
Vijayakumari AA, John JP, Halahalli HN, Paul P, Thirunavukkarasu P, Purushottam M, et al. Effect of COMT, 5-HT2A and 5-HTTLPR polymorphisms on brain morphometry in schizophrenia and healthy subjects. Clin Psychopharmacol Neurosci. 2015;13(1):68–82.
John JP, Thirunavukkarasu P, Halahalli HN, Purushottam M, Jain S. A systematic review of the effect of genes mediating neurodevelopment and neurotransmission on brain morphology: Focus on schizophrenia. Neurol Psychiatry Brain Res. 2015;21:1–26.
Annett M. A coordination of hand preference and skill replicated. Br J Psychol. 1976;67:587–92.
Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98.
APA. Diagnostic and statistical manual of mental disorders: DSM-IV-TR. Washington, DC: American Psychiatric Association; 2000.
Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59 Suppl 20:22–33. quiz 34–57.
Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–76.
Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry. 2003;64:663–7.
Taylor D, Paton C, Kapur S. The South London and Maudsley NHS Foundation Trust and Oxleas NHS Foundation Trust Prescribing Guidelines. 10th ed. London: Informa Healthcare; 2009.
Kroken RA, Johnsen E, Ruud T, Wentzel-Larsen T, Jorgensen HA. Treatment of schizophrenia with antipsychotics in Norwegian emergency wards, a cross-sectional national study. BMC Psychiatry. 2009;9:24.
Gaser C, Volz HP, Kiebel S, Riehemann S, Sauer H. Detecting structural changes in whole brain based on nonlinear deformations-application to schizophrenia research. Neuroimage. 1999;10:107–13.
Makris N, Kaiser J, Haselgrove C, Seidman LJ, Biederman J, Boriel D, et al. Human cerebral cortex: a system for the integration of volume- and surface-based representations. Neuroimage. 2006;33:139–53.
Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–80.
Dixon WJ. Simplified estimation from censored normal samples. Ann Math Stat. 1960;31:385–91.
Acknowledgements
The authors would like to acknowledge the contributions of Prof. P.N. Jayakumar and (late) Prof. M.K. Vasudev in facilitating the acquisition of MRIs for the study. The authors thank Mr Kiran Kumar for his assistance in image acquisition and Dr Vikram Arunachalam and Dr Abish Antony for co-ordinating the recruitment of participants.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
JPJ, HNH and SJ were involved in the conception and design of the study; HNH was involved in MRI acquisition and analysis along with NSM, AL, AAV and BSB, under the supervision of JPJ; AL and NSM wrote the first draft of the manuscript. All authors reviewed and approved the final version.
Additional file
Additional file 1: Table S1.
Total number of subjects recruited into the study, reasons for dropping subjects, and final n of each sample. Table S2. Details of lifetime exposure to neuroleptics and duration of neuroleptic use (n = 24). Table S3. Comparison of total brain volume between males and females in the recent-onset schizophrenia (ROS) (N = 45) and the healthy control subject (HCS) (N = 45) samples. Between-gender analysis of covariance (ANCOVA) in both samples with age as covariate revealed significant gender differences in both samples when intracranial volume (ICV) was not used as an additional covariate; whereas there were no significant gender differences in total brain volume when ICV was used as an additional covariate. Table S4. Brain regions showing volumetric reductions in schizophrenia subjects in comparison to healthy subjects at a significance threshold of p < 0.001 uncorrected, on vertex-wise analysis using Freesurfer, with gender entered as a fixed factor and age, and total brain volume as nuisance factors. Table S5. Brain regions showing volumetric reductions in drug naïve schizophrenia subjects (N = 21) in comparison to healthy control subjects (N = 45) at a significance threshold of p < 0.001 uncorrected and an extent threshold of 0 voxels when total brain volume (TBV), age and gender were entered in the two sample random effects analysis (RFX) as co-variates. Table S6. Brain regions showing volumetric reductions in medicated schizophrenia subjects (N = 24) in comparison to healthy control subjects (N = 45) at a significance threshold of p < 0.001 uncorrected and an extent threshold of 0 voxels when total brain volume (TBV), age and gender were entered in the two sample random effects analysis (RFX) as co-variates. Table S7. Brain regions showing volumetric reductions in neuroleptic-naïve schizophrenia subjects (N = 21) in comparison to medicated schizophrenia subjects (N = 24) at a significance threshold of p < 0.001 uncorrected and an extent threshold of 0 voxels when total brain volume (TBV), age, gender and duration of illness were entered in the two sample random effects analysis (RFX) as co-variates. Table S8. Brain regions showing volumetric increases in neuroleptic-naïve schizophrenia subjects (N = 21) in comparison to medicated schizophrenia subjects (N = 24) at a significance threshold of p < 0.001 uncorrected and an extent threshold of 0 voxels when total brain volume (TBV), age, gender and duration of illness were entered in the two sample random effects analysis (RFX) as co-variates. Figure S1. Freesurfer-generated difference (t) map of brain volumes depicting grey matter volumes shown as reduced in schizophrenia subjects (N = 45) in comparison to healthy subjects (N = 45) at p < 0.001 uncorrected with gender entered as fixed factor and total brain volume and age as nuisance factors. Figure S2. Statistical parametric t-map of gray matter volumes shown as reduced in drug naïve schizophrenia subjects (N = 21) in comparison to healthy control subjects (N = 45) at a significance threshold of p < 0.001 uncorrected and an extent threshold of 0 voxels with total brain volume (TBV), age and gender were entered in the two sample random effects analysis (RFX) as co-variates. Display according to neurological convention (image left is participant’s left). Figure S3. Statistical parametric t-map of gray matter volumes shown as reduced in medicated schizophrenia subjects (N = 24) in comparison to healthy control subjects (N = 45) at a significance threshold of p < 0.001 uncorrected and an extent threshold of 0 voxels when total brain volume (TBV), age and gender were entered in the two sample random effects analysis (RFX) as co-variates. Display according to neurological convention (image left is participant’s left). Figure S4. Statistical parametric t-map of gray matter volumes shown as reduced in neuroleptic-naïve schizophrenia subjects (N = 21) in comparison to medicated schizophrenia subjects (N = 24) at a significance threshold of p < 0.001 uncorrected and an extent threshold of 0 voxels when total brain volume (TBV), age, gender and duration of illness were entered in the two sample random effects analysis (RFX) as co-variates. Display according to neurological convention (image left is participant’s left). Figure S5. Statistical parametric t-map of gray matter volumes shown as increased in neuroleptic-naïve schizophrenia subjects (N = 21) in comparison to medicated schizophrenia subjects (N = 24) at a significance threshold of p < 0.001 uncorrected and an extent threshold of 0 voxels when total brain volume (TBV), age, gender and duration of illness were entered in the two sample random effects analysis (RFX) as co-variates. Display according to neurological convention (image left is participant’s left).
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
John, J.P., Lukose, A., Bagepally, B.S. et al. A systematic examination of brain volumetric abnormalities in recent-onset schizophrenia using voxel-based, surface-based and region-of-interest-based morphometric analyses. J Negat Results BioMed 14, 11 (2015). https://doi.org/10.1186/s12952-015-0030-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12952-015-0030-z