Abstract
Colorectal cancer, the third most prevalent cancer globally, contributes significantly to mortality rates, with over 1.9 million reported cases and nearly 935,000 fatalities annually. Surgical resection is a primary approach for localized colorectal tumors, with adjunct therapies like chemotherapy, radiotherapy, and targeted/immunotherapy considered depending on the tumor stage. However, despite preferences for targeted and immunotherapy post-surgery, chemotherapy remains commonly chosen due to its lower cost and high cancer-killing efficiency. Yet, chemotherapy faces issues such as tumor resistance and severe side effects. Nanotechnology has emerged in cancer therapy by alleviating the drawbacks of current treatment approaches. In the past few decades, inorganic nanoparticles have shown promise in combating colorectal cancer, offering advantages over conventional chemotherapy. Compared to organic nanoparticles, inorganic nanoparticles exhibit properties like photosensitivity, conductivity, magnetic allure, and thermal proficiency, allowing them to function as both drug carriers and therapeutic agents. Derived primarily from carbon, silica, metals, and metal oxides, they offer superior drug-loading capacity, heightened quantum yield, and participation in advanced photothermal and photodynamic therapies. This review provides a brief overview of the pathophysiology of colorectal cancer and the pivotal role of inorganic nanoparticles in photothermal therapy photodynamic therapy, and drug delivery. Additionally, it discusses numerous inorganic nanoparticles in colorectal cancer therapy based on recent literature.
Graphical abstract

Similar content being viewed by others
Introduction
Colorectal cancer (CRC) ranks as the third most prevalent form of cancer worldwide, representing a significant contributor to mortality rates [1]. Data from Globcan (2020) reveals a staggering 1,931,50 reported cases of CRC, with 935,173 resulting in fatalities across all genders and age groups on a global scale [1, 2]. The colon and rectum, integral components of the digestive system, constitute the terminal segment of the intestinal tract. Stretching approximately one meter, their journey commences at the ileocecal valve, marking the conclusion of the small intestine, and culminates at the anus [3,4,5]. Colon cancer targets the epithelial lining of the colon, a tissue typically replenished every five days through the division of colonic epithelial stem cells. CRC emerges as the culmination of a gradual succession of progressive stages in colon neoplasia, evolving over an extended duration [6].
The approach to treating colorectal cancer is heavily influenced by the cancer’s stage, ranging from I to IV. For early-stage cancer (Stage I), surgical options such as polypectomy, endoscopic mucosal resection, or minimally invasive laparoscopic surgery are commonly used. When the cancer progresses to Stage II, infiltrating the colon wall without affecting the lymph nodes, a partial colectomy is typically performed, frequently using laparoscopic techniques, along with the removal of nearby lymph nodes. In Stage III, where cancer has spread to lymph nodes, treatment generally involves a combination of partial colectomy and chemotherapy to destroy any remaining cancer cells and lower the chances of recurrence [7]. For Stage IV cancer, which involves metastasis to distant organs like the liver or lungs, a more intensive treatment regimen is necessary, including surgery, chemotherapy, targeted therapy, and immunotherapy to control and manage the disease. Additionally, radiation therapy is employed to reduce tumor size and relieve symptoms when surgery is not an option [8, 9]. Despite the preference for immunotherapy and targeted therapy post-surgery, their limitations such as limited bioavailability and high costs often steer patients towards chemotherapy [10]. Although chemotherapy presents a notable reduction in treatment expenses, its efficacy is significantly compromised by factors such as tumor resistance, poor solubility, and permeability, limited bioavailability, lack of target specificity, and associated severe side effects [11].
Nanotechnology, an emerging field at the intersection of science and engineering, focuses on manipulating materials at the nanoscale, typically ranging from 1 to 1000 nm in size [12,13,14,15,16,17]. Within this realm, several nanomaterials exhibit tremendous potential to enhance the efficacy of cancer therapies by delivering anticancer drugs to the tumor site [18,19,20,21,22,23]. Nanoparticles (NPs) possess distinct characteristics, including their ability to passively target tumors through mechanisms like the enhanced permeability and retention (EPR) effect, evade the body’s reticuloendothelial system (RES), and facilitate improved permeation through tissues [24,25,26,27,28,29]. In recent times, the spotlight in cancer therapy research has shifted towards inorganic NPs (INPs), captivating scientists owing to their distinctive physicochemical traits, contingent upon material and size [30, 31]. Unlike organic NPs, INPs boast unparalleled properties such as exceptional photosensitivity, remarkable conductivity, optical prowess, magnetic allure, and thermal proficiency, serving dual roles as both carriers for drugs and therapeutic agents themselves (Fig. 1) [32]. Their ease of synthesis, large surface area, and mechanical and chemical stability underscore their superiority. Typically derived from metals, metal oxides, and non-metallic materials (carbon and silica), INPs offer a myriad of advantages as carriers for drugs, including heightened quantum yield and drug-loading capacity, as well as the ability to participate in PTT and PDT [33]. INPs offer significant advantages in cancer treatment through photothermal therapy (PTT) and photodynamic therapy (PDT). INPs generate localized heat in PTT and produce reactive oxygen species in PDT, enhancing precision and minimizing damage to healthy tissues. Their higher absorption coefficients, stability, and prolonged circulation improve treatment efficacy. Additionally, INPs serve as effective drug delivery systems upon surface modification/engineering, providing controlled release and multifunctional capabilities for bioimaging and therapy, making them promising candidates for advanced cancer treatments [33].
To date, there are only two INPs approved for cancer therapy. In 2010, the European Medicines Agency (EMA) approved the first INP, iron oxide NPs (Fe3O4NPs) (marketed as NanoTherm® by MagForce Nanotechnologies AG), for the treatment of glioblastoma, pancreatic, and prostate cancer through thermal ablation using a magnetic field. Subsequently, in 2019, the EMA granted its initial approval for hafnium oxide NPs (marketed as Hensify® [NBTXR3] by Nanobiotix) for radiotherapy in the treatment of locally advanced soft tissue sarcoma [34]. However, there are currently no specific INPs utilized for the treatment of CRC in the market, despite its mortality rate ranking second among all other types of cancer. This suggests that there is still potential for various other INPs to be explored, such as mesoporous silica nanoparticles (MSNs), cerium oxide NPs (CeONPs), silver NPs (AgNPs), zinc oxide NPs (ZnONPs), gold NPs (AuNPs), carbon nanotubes (CNTs), etc., for the effective treatment of CRC [32]. In this context, this review provides brief information on the pathophysiology of CRC, the role of INPs in drug delivery, photothermal therapy (PTT), and photodynamic therapy (PDT). Furthermore, it meticulously discusses the importance of several INPs in CRC therapy based on recent literature.
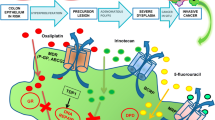
Intrinsic and synergistic anticancer effects of inorganic nanoparticles (INPs) in cancer treatment. Silver nanoparticles (AgNPs) induce apoptosis in cancer cells initially through reactive oxygen species (ROS) production and consequent reduction in the antioxidant glutathione (GSH). Subsequently, the release of Ag + ions increase the ROS level, which damages mitochondria, DNA, and the endoplasmic reticulum. Ceria nanoparticles (CeNPs) induce apoptosis in cancer cells by generating ROS through pH-induced Ce3+ ↔ Ce4 + redox, which damages mitochondria and DNA. When combined with doxorubicin (DOX), CeNPs exhibit a synergistic effect on cancer cells while protecting normal cells owing to their unique prooxidant-antioxidant activity, reproduced with permission from [32], copyright 2022, Elsevier
Pathophysiology of colorectal cancer
The progression from normal colonic epithelium to a precancerous lesion (adenoma), and eventually to invasive carcinoma, necessitates the accumulation of genetic mutations, whether acquired somatically or inherited germline (Fig. 2) [35]. The theory of colonic carcinogenesis revolves around a clonal evolution of mutations, granting cells a survival advantage and immortality, thereby facilitating the development of additional mutations that contribute to cancer hallmarks such as invasion, proliferation, metastasis, etc. Observational data in clinical settings indicate that CRC often originates from adenomatous polyps, which typically undergo dysplastic alterations for 10 to 20 years before progressing to invasive carcinoma [36]. Early identification and removal of these polyps have been demonstrated to lower the incidence of CRC. CRC entails a complex interplay of molecular pathways, among which three major pathways stand out: chromosomal instability (CIN), microsatellite instability (MSI), and CpG island methylator phenotype (CIMP). These pathways collectively contribute significantly to CRC development through a combination of genetic and epigenetic alterations [37]. These intricate molecular pathways involved in CRC can influence both tumor progression and metastatic potential, with variations observed in epidemiological patterns, mutational profiles, and immune responses depending on the specific pathway engaged. CIN stands out as the predominant feature in the majority of CRC, constituting approximately 80–85% of cases. CIN manifests through the activation of growth-promoting pathways coupled with a concurrent reduction in apoptotic pathway activity, with the latter being particularly prevalent. These tumors typically originate from adenomatous polyps, primarily triggered by mutations that deactivate the adenomatous polyposis coli (APC) gene [38]. Cells deficient in DNA mismatch repair (dMMR), often due to mutations in genes such as MLH1 or MSH2, experience a buildup of genomic errors leading to pronounced microsatellite instability (MSI-H), a characteristic feature of Lynch syndrome. CpG hypermethylation of DNA can either activate or suppress the expression of specific genes, such as BRAF and MLH1, respectively. Additionally, somatic mutations in sporadic oncogenes like RAS, SRC, and MYC have been implicated in CRC, with RAS mutations, particularly, holding significant clinical relevance. These mutations, encompassing variants such as HRAS, KRAS, and NRAS, are detected in approximately 50% of sporadic CRC cases [39]. Currently, they are being leveraged for CRC screening through stool-DNA testing and are associated with the lack of response to epidermal growth factor receptor (EGFR) targeted therapy, thus prompting the exploration of potential direct targeted agents [36].
Colorectal cancer stages and development. There are four stages in the development of colorectal cancer carcinogenesis: initiation, promotion, progression, and metastasis. The liver is the most common metastatic site, followed by the lung and bone. Although it is difficult to determine the duration required for each stage, decades will likely be required to form colorectal cancer, reproduced with permission from [35], copyright 2022, MDPI.
Inorganic nanoparticles in cancer therapy
Before delving into the specifics of each type of INP-based cancer therapy, we will first explore the potential of INPs in PTT, PDT, and as carriers for anticancer agents. These discussions will set the stage for a comprehensive understanding of how INPs can significantly enhance cancer treatment approaches [32].
Photothermal therapy
Hyperthermia-based cancer treatments involve subjecting the targeted tissue to elevated temperatures, capable of inducing cancer cell death (thermal ablation, occurring at temperatures above 45 °C) or enhancing cancer cell sensitivity to other therapeutic interventions (mild hyperthermia, temperatures ranging between 40 and 45 °C) [40]. In PTT, NIR-II wavelengths provide superior tissue penetration and enhanced safety compared to NIR-I (750–900 nm), owing to decreased scattering and absorption by biological tissues. Consequently, NIR-II (1000–1700 nm) is more effective for targeting deeper tumors and reducing harm to adjacent healthy tissue [41]. In conventional hyperthermia approaches, temperature elevation in the target tissue is typically achieved through external means, such as regional hyperthermia, superficial hyperthermia, and whole-body hyperthermia utilizing thermal baths, microwaves, or radiofrequency [42, 43]. However, this often results in a temperature gradient, peaking at the body surface and diminishing with distance from the external heat source. Consequently, healthy tissues may also be subjected to temperature elevation, leading to undesirable side effects [41, 44]. Thus, to overcome these drawbacks, researchers have directed their efforts toward developing more efficient approaches, particularly those capable of inducing localized temperature increases confined to the tumor site.
NPs exhibiting the ability to generate heat in response to external stimuli have emerged as promising candidates, addressing the limitations of conventional hyperthermia methods [33]. Notably, the size of NPs enables them to exploit the abnormal vasculature of tumors, facilitating their accumulation within the tumor tissue via mechanisms such as the EPR effect or vascular bursts [45, 46]. Subsequently, these NPs can facilitate localized thermal destruction of cancer cells upon exposure to external stimuli such as NIR radiation, thereby minimizing damage to surrounding healthy tissues. The use of NIR, particularly in the NIR-I and NIR-II ranges, is vital for NP-driven hyperthermia [42, 47]. This is because biological elements have minimal absorption in this spectrum, leading to fewer off-target interactions and increased penetration within the human body, ultimately amplifying therapeutic effectiveness [48].
Both inorganic and organic NPs have been extensively explored in PTT for cancer treatment. However, INPs offer distinct advantages over their organic counterparts. Notably, INPs tend to possess higher absorption coefficients than organic NPs, facilitating a more efficient conversion of light into heat [49, 50]. Moreover, their inherent stability and prolonged circulation in the body can enhance tumor accumulation and improve treatment efficacy. Additionally, organic NPs generally exhibit lower absorption coefficients compared to INPs, thus constraining their effectiveness in PTT [51].
Photodynamic therapy
PDT, heralded as an innovative method for tumor ablation, presents a nuanced approach with minimized long-term morbidity. By orchestrating the interplay of three essential elements such as light, molecular oxygen (O2), and a photosensitizer. PDT generates cytotoxic reactive oxygen species (ROS) that selectively exterminate cancer cells [40]. While PDT has demonstrated considerable efficacy in treating specific cancers like skin and oral cancers, its widespread adoption as a primary therapeutic option in clinical settings remains challenging due to several inherent limitations. These include the absence of an optimal photosensitizer with superior tumor selectivity and efficacy in generating ROS, as well as the lack of robust methodologies for determining appropriate light dosimetry and monitoring treatment response effectively [52,53,54]. Notably, the absence of an ideal photosensitizer stands out as the primary obstacle hindering the progress and broader application of PDT. Despite the utilization of various small organic PSs, such as porphyrin structures, synthetic dyes, and natural products in clinical settings, their efficacy against cancers is often limited by inherent drawbacks such as poor water solubility, inadequate photostability, low extinction coefficient, limited absorption in the NIR region, suboptimal generation of singlet oxygen (1O2), and inadequate cancer selectivity [55].
Recently, INPs made of metals, carbon materials, and silica, offer an innovative avenue to enhance the effectiveness of current photosensitizers and tackle obstacles in cancer PDT [56]. INP-based photosensitizers signify a substantial leap forward compared to traditional organic photosensitizers. Their benefits are multifaceted: firstly, they exhibit substantial extinction coefficients, facilitating the efficient transfer of energy for photosensitization [57]. Secondly, their surfaces are easily adaptable, allowing for the attachment of target ligands and functional groups, thus amplifying their selectivity towards tumor cells. Moreover, their diminutive size grants them expansive surface-to-volume ratios, leveraging the EPR effect to accumulate more effectively within solid tumor tissues [58]. Additionally, they seamlessly integrate with chemo drugs and imaging modalities, enabling the implementation of precision-guided treatment approaches. Remarkably, specific inorganic nanomaterials possess distinctive optical properties, enabling them to act as direct photosensitizers, and catalyzing the production of singlet oxygen (1O2) upon exposure to light, eliminating the necessity for traditional organic photosensitizers. This capability not only ensures superior 1O2 quantum yields but also confers robust resistance to photobleaching, all while maintaining significant extinction coefficients [59, 60].
Drug delivery
In cancer treatment, various organic NPs such as liposomes, micelles, and polymer-based nano-drug delivery systems have progressed to advanced developmental stages, with several even securing FDA approval [61]. Nevertheless, these conventional nanocarriers confront hurdles like drug leakage and uncontrolled release rates. Recent strides in synthesis methodologies have catalyzed the emergence of INP-based drug delivery systems, predominantly in the pre-clinical phase [62, 63]. Yet, their synthesis simplicity and modifiability empower precise regulation of size, morphology, and surface characteristics. INPs present a gamut of opportunities as integrated and versatile platforms for both bioimaging and drug delivery, harnessing their optical, electronic, and magnetic attributes [32]. Given these advantages, INPs like MSNs and CNTs serve as dedicated drug carriers. However, quantum dots (QDs), AuNPs, AgNPs, and others function not only as drug carriers but also as therapeutic agents, capitalizing on their potential in cancer therapy, making them interesting candidates in CRC treatment [64].
Inorganic nanoparticle-based approaches for colorectal cancer therapy
INPs have become the focal point of recent cancer therapy research. This shift has captivated scientists as they delve into the distinctive physicochemical properties of these particles, which are contingent upon factors such as material composition and size. These are derived from metal oxides (e.g., iron, manganese, zinc, etc.), metals (e.g., gold, silver, etc.), carbons (e.g., carbon dots, CNTs, etc.), semiconductors (QDs), etc. and extensively explored as a therapeutic tool in the treatment of various cancer conditions [65, 66]. The various inorganic NPs include MSNs, QDs, CNTs, AuNPs, Fe3O4NPs, AgNPs, and ZnONPs [67]. For instance, the QDs have unique features like high fluorescence with a higher emission range, which makes them suitable for bioimaging [68]. Metal oxide NPs exhibit greater catalytic ability for organic reactions by declining the active energy of those reactions [69, 70]. Their key features are biocompatibility, precise targeting, non-toxic, thermostability, unique optical properties, small size, enhanced surface area, controllable structure, magnificent bioavailability, and physicochemical properties [71]. Due to their distinctive properties and surface modification/engineering potential, they have been widely explored as promising candidates in CRC therapy. Additionally, comprehensive descriptions of the latest research work concerning the utilization of INPs in combating CRC are further elaborated upon in the subsequent sections.
Carbon nanotubes
CNTs are a unique type of NPs which are minute tube-shaped materials that contain carbon atoms in their nanostructure, which looks like a honeycomb lattice. Generally, based on the sheet number of carbon atoms, they are divided into single-walled CNTs (SWCNTs) and multi-walled CNTs (MWCNTs) [72]. Due to their distinctive physicochemical properties, CNTs are considerably designed for cancer therapy. These unique nanomaterials help cancer imaging and drug delivery to tumor sites [73]. Several studies reported that due to the needle-shaped architecture of CNTs, they are quickly taken up by solid tumors [74]. Over recent years CNTs have been utilized to locate the solid tumors of the colon and rectum [75]. One study disclosed that the collaboration of SWCNTs with TRAIL enhanced the cell apoptosis in colorectal cell lines around ten times greater than TRAIL alone [76]. Several properties of CNTs, like high surface area, chemical stability, and thermal and electrical conductivity due to this CNTs could be versatile nanomaterials in the treatment of CRC [77].
In a recent investigation, Gonzalez and colleagues developed functionalized SWCNTs with nanocrystalline cellulose II (II NCC) colloidal system platforms to enhance the activity in colon cancer therapy. The TEM images disclosed that the dispersion of SWCNTs/NCC hybrids resulted in a rod-shaped nanocrystal structure. The SWCNTs/NCC containing combinations resulted in efficient size and width. The nanohybrids do not affect normal cells and considerably decrease the tumor cells. These nanohybrid platforms and Capecitabine potency were analyzed using IC50 value against Caco-2 cells. The outcomes indicated that the IC50 value of 1a-SWCNT (0.201 ± 0.115) and 2a-SWCNT (0.113 ± 0.115) is less than that of Capecitabine (0.279 ± 0.110). The results disclosed that the same anti-cancer effects resulted with a lower dose than the capecitabine drug alone. The authors concluded that NCC stabilized SWCNTs in aqueous dispersion, resulting in nanohybrids showing no toxicity. Aqueous dispersions of fluorescein functionalized SWCNTs/NCC II exhibited enhanced intrinsic activity against Caco 2 cells compared with the non-functionalized chemotherapeutic drug capecitabine [78].
Similarly, 5-Fluorouracil (5-FU) is a well-known chemotherapeutic agent against colorectal tumors, but it is associated with several drawbacks [79], and there was a need to develop a prodrug. Capecitabine is a prodrug of 5-FU, and this has been designed to overcome limitations associated with the 5-FU [80]. To check their efficacy, Randive and teammates developed chitosan (CHI) and folic acid (FA) functionalized CNTs (fCNTs) containing Capecitabine against colon cancer cells. The Raman spectroscopy, FTIR, and X-ray diffraction studies confirmed the conjugation of Capecitabine in CNTs. SEM studies showed the size range of CNTs around 200–500 nm, and drug loading capacity was around 94.63 ± 1.07%. The cytotoxicity study using COLO320DM cells showed % inhibition of 86.45 ± 0.5788% for FA–CHI-fSWCNT- Capecitabine, and 50.52 ± 0.3106% for pure drug. Against HT29, the % inhibition was observed to be 82.76 ± 0.4668% (FA–CHI-F-SWCNT-Capecitabine) and 56.41 ± 0.2316% (pure drug). In vivo studies in rabbit models revealed that the developed novel system targets the drug specifically in the colon region without premature release. Hence, the research team concluded that the developed system facilitates drug delivery to colon, rectal, and intestinal cancers (Fig. 3(i)) [81].
SWCNTs emerged as a novel therapeutic tool to optimize the efficacy of many drugs. Despite its excellent properties, this is limited because its poor solubility leads to reduced bioavailability, absorption, blood transportation, and secretion. To overcome the above limitations, Shejawal and co-workers developed functionalized CNTs (fSWCNTs) of isolated lycopene (LYC) for its targeted release in colon cancer cells. The Phosphatidylcholine (PC) and Polyvinylpyrrolidone (PVP) K30 were used as stabilizing agents. SEM studies showed that LYC-PC-PVP-fSWCNTs exhibited a size range of 10–300 nm. TEM studies confirmed normal size distribution in the developed novel system was 272 nm with a ζ–potential of -40mV. The resulting novel approach exhibited 90 ± 0.256% of the loading capacity of LYC and 93.81 ± 1.2172% of %CDR in 60 min. The cytotoxicity activity against COLO320DM and HT 29 was better than pure lycopene. The results of in vitro and in vivo studies revealed that the drug targeted the site of the colon entirely without any premature release (Fig. 3(ii)) [82]. PDT is a well-known cancer therapy, and chlorine e6 (Ce6) is a naturally derived photosensitizer (PS) that is commonly used in PDT. The solubility of PS is the major challenge in PDT [83]. To overcome this issue, Sundaram and Abrahamse synthesized nano biocomposites of hyaluronic acid (HA) coupled and Ce6-coated SWCNTs for PDT to treat colon cancer. The average particle size of nano biocomposite was increased in SWCNTs-HA-Ce6 (203 ± 6.6 nm) compared to plain SWCNTs (191 ± 4.6 nm). The ζ–potential analysis indicated that developed MWCNTs (− 17.8 ± 1.2 mV) and SWCNTs-HA-Ce6 (− 18.9 ± 1 mV) are stable. The SWCNTs-HA-Ce6 exhibited a significantly higher percentage of cell death (Fig. 3(iii)) in irradiated Caco-2 cells (84.9%). The authors concluded that the novel synthesized nano biocomposite (SWCNTs-HA-Ce6 ) intensified the ability of PDT and induced the death of colon cancer cells [84].
Conventional treatment modes have limited therapeutic efficacy for colon cancer due to the lack of specificity and selectivity against colon cancer cells. Hence, site-specific drug delivery to colon tumor cells is needed, which upturns the concentration at the targeted site and minimizes the dose and side effects [85]. To overcome this, Prajapati and colleagues developed Gemcitabine (GEM) MWCNTs (plain), coupled with HA (GEM/HA/MWCNTs), PEGylated (GEM/PEG-MWCNTs) and HA coupled and PEGylated (GEM-HA-PEG-MWCNTs) (Fig. 3(iv)) as a novel delivery to an intracellular system and evaluation its efficacy in vitro and in vivo models. The effectiveness of the current approach was compared with the free GEM. The FT-IR study confirmed the HA and PEG conjugation on MWCNTs. TEM images confirmed tubular shape open-ended structure with nano size for purified MWCNTs and GEM/HA-MWCNTs. The average particle size and PDI of GEM-MWCNTs (28 ± 2.1 nm & 0.412 ± 0.034), GEM/HA-PEG-MWCNTs (39 ± 1.9 nm & 0.192 ± 0.053), GEM/HA-MWCNTs (32 ± 2.4 nm & 0.298 ± 0.046), and GEM/PEG-MWCNTs (35 ± 1.4 nm & 0.202 ± 0.074) respectively. The % entrapment efficiency was decreased GEM/HA-MWCNTs than GEM-MWCNTs (91.3 ± 1.7%) due to π-π interaction of MWCNTs and GEM after HA conjugation. The enhanced cell growth inhibition was observed in GEM/HA-PEG-MWCNTs (27 ± 1.1%) & GEM/HA-MWCNTs (22 ± 0.9%) was observed in HT29 cells. The hemolytic toxicity was comparatively less in GEM/HA-PEG-MWCNTs (7.73 ± 0.4%) when compared to free GEM (18.71 ± 0.44%). The anti-tumor study revealed that GEM/HA-PEG-MWCNTs significantly reduced tumor volume and increased survival. The improved pharmacokinetic parameter was observed in GEM/HA-MWCNTs and GEM/HA-PEG-MWCNTs treated group than in free GEM. The research team concluded that engineered MWCNTs as a safe and effective treatment for colon cancer therapy [86].
(i) Conceptual illustration of the preparation of FA–CHI-F-SWCNT-Capecitabine and characterization reproduced with permission from Ref. [81], (ii) Graphical representation of lycopene isolation, loading to SWCNTs and characterization reproduced with permission from Ref. [82], (iii) Macroscopic images of Caco 2 cell lines using PDT at a fluence of (a) untreated cells at 0 and 24 h, (b) 5 J/cm2 at 0 and 24 h c 10 J/cm2 at 0 and 24 h, reproduced with permission from Ref. [84] (iv) Schematic representation of purification and drug loading into MWCNTs reproduced with permission from Ref. [86]
Quantum dots
QDs are semiconductor NPs with a diameter of < 10 nm [87]. These are unique kinds of NPs because of their electrochemical, optical, physicochemical, and structural properties. Due to their sensing ability, they are widely used in biosensors, bioimaging, and drug delivery. These are also called fluorescent semiconductor inorganic NPs composed of groups II–VI, III–V, or IV elements [88]. QDs are classified based on their chemical composition, core type, core-shell, and alloyed QDs. The fluorescence imaging ability of QDs is because of their higher fluorescence output and photochemical stability [89]. QDs have taken attention in cancer therapy because of their unique physicochemical properties, and the drug can be selectively targeted into the tumor with less invasive techniques and without affecting normal cells [90]. The studies have reported that they are suitable for targeting colorectal tumors due to the exceptional properties of QDs, i.e., electrical conductivity, mechanical strength, thermal conductivity, optical, fluorescence emission, and excitation properties [91].
CRC is one of the most curable types of cancer if it is detected in an early stage. Chemotherapeutics are limited by their side effects and less responsible in an advanced stage. The currently available imaging techniques are limited due to a lack of diagnostic precision. Targeted NPs will be useful and bind to specific antigens to overcome this. Placenta-specific protein 1 (PLAC-1) is overexpressed in CRC [92]. Hence, Haider and teammates developed QDs of graphene oxide with a peptide named (GILGFVFTL) that has a high affinity towards (PLAC-1). The physicochemical and morphological characterization outcomes confirmed the coupling of QDs and peptides. Functionalized Q (QD-P) enhanced uptake in cells and cell cytotoxicity more than non-functionalized QDs. The QD-P toxicity downregulated the PLAC-I expression in HT-29 (53%) and HCT-116 (33%), respectively (Fig. 4(i)). The authors concluded that developed QD-P is a potential theranostic tool in detecting and treating colon carcinoma cells expressing the antigen PLAC-1protein [93]. In addition, biosensors have a significant role in the biomedical and pharmaceutical fields. Biosensors are sensing devices with unique characteristics like selectivity, sensitivity, rapid response time, etc. However, the limiting factor was a lack of sensitivity to low concentrations of cancer biomarkers. Combining nanotechnology and biosensors can partially overcome this issue [94]. Hence Pourakbari and colleagues attempted to develop a novel biosensing method for tungsten disulfide QDs (WS2-QD with electrochemical deposition of AuNPs for rapid and selective determination of c-Met proteins. Biosensor (M-13 Bacteriophage) is used as proof of concept to detect c-Met protein as a colon cancer biomarker. SEM studies showed size range of WS2-QD was 25 nm, and the average of the electrodeposited AuNPs was 25–45 nm. The outcomes revealed that the developed novel biosensor detected some concentration of c-Met protein in the colon cancer serum sample, which was unable to be seen by the ELISA kit. The authors concluded that the developed novel bioassay system has potential applications in the biomedical area [95].
Recently carbon-based QDs (CQD) emerged as a novel class of nanomaterials. Nowadays, naturally, available phytochemicals and secondary metabolites are generally used in cancer therapy because of their specificity and tissue-protective nature [96]. Based on this concept Mishra and colleagues developed a high fluorescence carbon QDs/Ag heterostructure from orange juice (Fig. 4(ii)) and carried out in vitro anti-cancer activity using HCT-116 and HEK-293 cell line. Energy dispersive X-ray analysis confirmed the uniform distribution of carbon and Ag in their heterostructure. The carbon QDs of orange extract TEM images approved the uniform distribution of spherical-shaped particle particles without aggregation. The average diameter of particles was around 12 nm by Gaussian fitting and obtained around 200 particles. The carbon QDs/Ag heterostructure TEM images confirmed the uniform distribution of the spherical-shaped 60 particle’s average diameter was about 10 nm. In HCT-116 cells, higher concentration, cQDs/Ag induced 50% of cell death within 24 h. The HEK − 293 cell line showed 6 µg/ml induced negligible cell death [91]. Tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) is well known anti-cancer therapeutic agent. The use of TRAIL is limited due to the weak pharmacokinetic profile leading to poor circulation and a short half-life resulting in fast renal excretion. Hence it may lead to insufficient availability of TRAIL to the targeted site and also resistance in cancer cells [97]. To address this issue, Lotfollahzadeh and co-workers developed a novel TRAIL-S-layer (S-TRAIL) fusion protein that was coupled to QDs of graphene(GQDs) via noncovalent interactions on colon cancer cells (Fig. 4(iii)). TEM results of S-TRAIL, GQD, and the S-TRAIL/GQD revealed that GQDs were in narrow size distribution with average diameters of 2.01 ± 0.28 nm, S-TRAIL exhibited as amorphous aggregates and S-TRAIL/GQDs appeared as a spherical shape with an average diameter of 17.77 ± 2.03 nm. The anti-cancer efficacy in HT-29 by MTT and flow cytometry assay revealed that 80% of cell death. The authors concluded that the developed novel S-TRAIL/GQD complex is a promising nanohybrid technique against colon cancer [98]. PDT emerged as the best treatment technique for various cancers. It utilizes laser light and radiation to kill the cancerous cells. However, this procedure is restricted due to the precision of the imaging technique. Hence to enhance the efficacy, the treatment system should facilitate the imaging techniques [99]. To address this, Liu and colleagues produced Au and Ag-doped CQD nanohybrid composite for effective PDT against colon cancer cells. TEM studies showed that developed Au/Ag-CQD-NC exhibited a quasi-spherical shape with a homogenous distribution. Energy dispersive X-Ray spectra confirmed the presence of C, O, N, Ag, Au, and Cu. This demonstrated the successful preparation of Au/Ag-CQD-NC. The cell viability was 88% upon treatment with Au/Ag-CQD-NC (100 µg/ml) and 85% with Au/Ag-CQD-NC (4000 µg/ml). This indicated prepared novel nanocomposite was not cytotoxic. In vivo studies revealed that after irradiation with NIR and injection of Au/Ag-CQD-NC, the tumor size reduced to small and turned black (Fig. 4(iv)). Hence authors concluded that Hybrid nanocomposite has advantages over medical conditions [100].
(i) Graphical representation of QD-P of graphene oxide as CRC theranostics, reproduced with permission from Ref. [93] (ii) Graphical representation of the development of CQD/Ag heterostructure from orange juice extract and characterization, reproduced with permission from Ref. [91]. (iii) Diagramatic illustration of S-TRAIL and S-TRAIL-GQD development and action on HT-29 cells, reproduced with permission from Ref. [98], (iv) Evaluation of tumor size (A) Tumor treated and irradiated with Ag-CQD-NC & Au/Ag-CQD-NC after with NIR laser, (B) Size of tumor treated and irradiated with Ag-CQD-NC, Au/Ag-CQD-NC with NIR laser, (C) Tumor volume (14 days) treated and irradiated with Ag-CQD-NC, Au/Ag-CQD-NC with NIR laser, (D) Total histone estimation in different groups. *p < 0.05, compared to normal, reproduced with permission from Ref. [100]
Mesoporous silica nanoparticles
MSNs are a porous, solid framework that chemically has the honeycomb-like structure of silica (SiO2). They have several advantages, like the tunable size of particles, uniform porous structure, high surface area, and good biocompatibility. There are three types of MSNs (i) Ordered type MSNs, (ii) hollow type MSNs, and (iii) core type MSNs [101, 102]. The therapeutic agent loads into the core or to the surface of MSNs by covalent binding and electrostatic adsorption. The mesoporous form of silica has the potential to load high quantities of the drug in its core; with low toxicity and subsequent drug release, these unique features make them suitable for targeted drug delivery systems. These are stable NPs because of the strong bond between the Si-O [103]. Studies reported that MSNs induced endocytosis mechanisms in different mammalian cancer cells [104]. Conventional treatment for CRC is ineffective because the drug’s desired concentration has not reached the targeted site. Also, several studies have reported drug-related toxicities due to a lack of site-specificity. To overcome this, Narayan and teammates developed Chitosan glucuronic acid conjugated pH-responsive MSNs of capecitabine to target CRC (Fig. 5(i)). The study showed that developed MSNs exhibit average pore diameter and particle sizes of 8.12 ± 0.43 nm and 245.24 ± 5.75 nm with a pore volume of 0 0.73 ± 0.21 cm3/g. The high drug loading (180.51 ± 5.23 mg/g) was reported for larger pore volume MSNs. The drug release was around 85.16 ± 1.45% at acidic pH (5.5) within 72 h. The high uptake of the developed novel system was observed in HCT 116 cells. In vivo, study outcomes revealed that developed glycosylated MSNs reduced the tumors, aberrant crypt foci, inflammation, and toxicity. The authors concluded that the promising outcome from developed NPs can be used as an effective carrier against CRC [105]. The developing novel system should consider the physicochemical properties of NPs and tumor microenvironment features to upgrade the drawbacks associated with conventional therapy and enhance therapeutic efficacy. Studies reported that including traceable imaging systems and therapeutic agents in single nano delivery systems develops a better theranostic action plan, resulting in the most efficient targeted delivery through image-guided delivery [106]. So, Iranpour and teammates developed a novel system of magnetic MSNs as a smart and targeted drug delivery system for CRC therapy. Developed NPs exhibited a mean final size diameter of 58.22 ± 8.54 nm. SEM studies confirmed that developed nanocarriers had a uniform and spherical morphology. TEM studies confirmed the porous structure of nanocarriers. In the EpCAM-positive HT-29 cells, higher cytotoxicity, and cellular uptake of nanocarriers were observed than in EpCAM-negative CHO cells. In vivo studies in a mouse model showed that targeted nanocarriers could effectively doxorubicin accumulation in the tumor area, inhibit its growth, and ultimately reduce the adverse side effects (Fig. 5(ii)) [107]. Epithelial cell adhesion molecule (EpCAM) is a transmembrane glycoprotein, and they have a vital role in cell signaling, migration, proliferation, and differentiation. Several studies reported that this biomarker is associated with CRC metastasis; hence this can be considered an excellent therapeutic agent for CRC treatment. Considering this, Mosawi and colleagues developed non-targeted PEGylated superparamagnetic iron oxide NPs (SPION) conjugated MSNs for the controlled release of 5-FU. The developed nanocarriers exhibited a diameter of 78 nm. The surface area of SPION-MSNs was found to be 636 m2g− 1. The drug release was initially rapid, and sustained release was up to 96 h at pH5.4. In vitro studies using HT-29 cells proved that cellular uptake of developed nanocarrier was at a high rate. The study revealed that the targeted SPION-MSNs significantly reduced tumor growth in C57BL/6 mice bearing HT-29 tumors compared to the injection of free 5-FU. The authors concluded that the developed nanocarrier is a potential theranostic platform for Ep-CAM-positive CRC [108]. CRC is usually diagnosed at the last stage; hence radiotherapy emerged as an enormous treatment approach. Studies have shown that to enhance the therapeutic efficacy of radiotherapy using radionuclides, the process of nanotechnology can be used effectively by using multifunctional nanomaterials [109]. Hence Viana and his team developed 177Lu labeled-Eu doped pyridine 2, 6-dicarboxylic acid (DPA) designed into 177Lu-Eu-DPA/SiO2-NH2 functionalized hybrid NPs (Fig. 5(iii)) SEM studies of 177Lu-Eu-DPA/SiO2-NH2 functionalized hybrid NPs exhibited small blocks of irregular patterns. TEM studies confirmed small particles having a size of less than 20 nm. DLS study showed an average particle size of 21.7 nm for silica NPs and 63.3 nm for 177Lu-Eu-DPA/SiO2-NH2. The loading of 177Lu into Eu-DPA/SiO2-NH2 was efficient, and up to 93% of the radioactivity resulted from the final compound. The SPECT/CT image analysis confirmed the localization of the tumor was maintained up to 48 h after intratumoral administration. The tumor growth was significantly reduced after treatment of 177Lu-Eu-DPA/SiO2-NH2 NPs. The authors confirmed that it is a promising agent for future development in the clinical treatment of CRC [110]. MSNs can achieve controlled delivery of drugs into CRC cells, which can be boosted by switching to the gatekeeper modifications like stimuli sensitive exhibits system more advantages [111]. Hence Fan and co-workers developed CEA/CD44 targeting nanobody (11C12)-conjugated HA-modified doxorubicin MSNs (DOX@MSNs-HA-11C12) with pH and redox sensitivity (Fig. 5(iv)). TEM study results showed that the average particle size of MSNs-NH2 was around 144.9 ± 5.2 nm, which is barely changed with DOX@MSNs-HA-11C12. The in vitro drug release study showed that the release of drug rate accelerated in a weak acidic environment pH [pH 7.4 (GSH) + pH 6.8 (GSH) + pH 5.4 (GSH)] and the presence of glutathione. In vitro, cytotoxicity study showed good biocompatibility, safety, and DOX@MSNs-HA-11C12) led to higher cell apoptosis in LoVo CRC. The authors concluded that developed dual CEA/CD44 formulation can promote more accurate drug delivery to CRC cells [112].
(i) Representation of study protocol (A) Synthesis of MSNPs, (B) Treatment protocol reproduced with permission from Ref. [105]. (ii) Graphical representation of the development of magnetic MSNPs, reproduced with permission from Ref. [107]. (iii) Graphical representation of the development of 177Lu-Eu-DPA/SiO2-NH2 NPs for CRC, reproduced with permission from Ref. [110]. (iv) Diagrammatic illustration of nanobody conjugated HA MSNPs reproduced with permission from Ref. [112]
Iron oxide nanoparticles
On Earth, iron is abundantly available in the form of iron oxides. The synthesis of multifunctional magnetic NPs is widely preferred and has enormous applications as a contrasting agent in MRI [113]. These have wide applications in the biomedical field, which include molecular imaging, hyperthermia treatment techniques, therapeutic agent delivery, etc. This system delivers the drug directly into the cytoplasm through the endocytosis mechanism. It mainly avoids the pump transporter recognition in multi-drug resistance cancer cells [114]. The dual ability of Fe3O4NPs, i.e., its magnetic and photothermal properties, made it suitable for cancer therapy [115].
The studies have shown that the approach of conjugated NPs with ligands exhibited the promised outcomes in targeted drug delivery systems [116]. So, Mirzaghavami and teammates synthesized a triblock copolymer (PEG-PCL-PEG), 5-FU loaded magnetite NPs conjugation within folic acid as a ligand (5-FU-PEG-PCL-PEG-FA) (Fig. 6(i)). The TEM studies exhibited the spherical shape of particles with a hydrodynamic diameter of 85 nm. The release studies showed that drug release is dependent on pH with a %CDR of 23% at 24 h. The uptake studies revealed the uptake of 5-FU-PEG-PCL-PEG-FA-NPs colon carcinoma cells (HT-29) than in normal endothelial cells (HUVEC). The resulting novel system exhibited enhanced anti-tumor efficiency, high mouse model survival rate, and tumor inhibitory volume. The authors concluded that a developed novel system could potentially treat colon cancer [117]. Similarly, Chen and colleagues developed iron oxide nanocrystals with sonosensitizer conjugated as a combination of chemodynamic (CDT) and sonodynamic therapy (SDT) against CRC (Fig. 6(ii)). The Fe3O4 nanoparticles were synthesized using a basic co-precipitation method using bovine serum albumin conjugation with Ce6. The resulting nanocrystals exhibited strong catalytic ability and highly efficient sonodynamic features. The cellular uptake studies I CT 26 tumor cells resulted in a high uptake of nanocrystals via a homologous targeting mechanism. The developed nanocrystals combination with SDT and CDT induced apoptosis of cells in vitro and significantly inhibited growth of tumor in tested mice model. The authors concluded that biomimetic nanocrystals could be a potential approach for CRC tumor targets [118].
Iron-based nanomedicine system that is the principal representative of INPs approved and widely applicable in the biomedical field. So, Chen and teammates designed DOX ferumoxytol Chitosan-based iron oxide nano hydrogel (DOX-FMT-CS-Fe3O4-HYD) as chemotherapy in HT-29 cells (Fig. 6(iii)). The resulting DOX-FMT-CS-Fe3O4-HYD with an angular frequency of 3.5 rad/s loss and storage modulus cross with equal modulus. The release study showed that DOX was released from the HYD, and FMT-embedded hydrogel generated heat. The content of leaked iron concentration was increased over time (In 16 h, 7.6 to 9.2%, and 40 h 89–98%). Cell apoptosis assay showed synergetic efficacy with 32.4% on HT 29 cells. Xenografted mice tumor model exhibited better heating performance from the DOX-FMT-CS-Fe2O3-HYD complex [119]. Recently PDT (PDT) has been widely used in CRC because it causes less toxicity to normal healthy cells. However, this treatment system has limitations like tumor selectivity, shorter ROS lifetime, and less laser penetrability. Studies have shown that this problem can be overcome using a metal NP hybrid system [120]. Hence Alkahtane and co-workers developed oleic acid (OA) CS-coated Fe3O4 NPs (OA-C- Fe3O4−NPs) against CRC carcinoma. TEM studies exhibited that OA-C- Fe3O4−NPs had a network-like appearance with a segment length of 300–500 nm and a width of 350–450 nm. The morphology was found like nano egg-like properties with a size of 15 nm and a width of 5 nm. The study outcomes revealed that the light-induced enhanced ROS activity of the OA-C- Fe3O4NPs led to cell death via activating caspase 9/3 (Fig. 6(iv)). The authors concluded that in vitro MRI experiments in HCT 116 cells showed that the nano-hybrid system is a highly therapeutic MRI contrasting agent for diagnosing CRC [121]. Several studies reported that combinational therapy suppresses tumor growth [119]. So, Dabaghi and co-workers formulated functionalized CS-coated magnetic NPs of 5-FU (5-FU-MNPs) and termed this combination therapy “thermo-chemotherapy.” The developed 5-FU-MNPs exhibited a mean diameter of 176 ± 7 nm, followed by PDI of 0.17 ± 0.01 and the ζ-potential of − 27.9 ± 0.5 mV. The developed thermo-chemotherapy resulted in distinct tumor regression. The authors concluded that thermo-chemotherapy induces thrombogenic collagen fiber leading to an imbalance in nutrient supply to the tumor cells and cell death [122].
(i) Graphical representation of folic acid conjugated 5-FU-PEG-PCL-PEG-FA mechanism of action on colon cancer cells, reproduced with permission from Ref. [117]. (ii) Graphical representation of biomimetic sonosensitizer targeted iron oxide nanocrystals, reproduced with permission from Ref. [118]. (iii) Graphical representation of the synthesis of DOX-ferumoxytol iron oxide nanoparticles, reproduced with permission from Ref. [119]. (iv) Expression of p53, p21, and cH2AX upon light exposure in treated HCT 116, reproduced with permission from Ref. [121]
Gold nanoparticles
AuNPs are wine-red compounds with antioxidant properties, and the size varies from 1 nm to 1000 nm. It has distinct forms like spherical, sub-octahedral, irregular shapes, nanorods, nanotriangles, etc. [123]. Au is a non-reactive chemical element and this widely used due to its occurrence, easy handling, simple fabrication, easy modification, corrosive resistance, chemical stability, biocompatibility, etc. [124]. AuNPs are widely applicable in the biomedical field due to their small size, stability, low toxicity, optical properties, bioimaging, and large surface area [125]. AuNPs absorb incidence photons and convert them into heat; hence generated heat destroys the cancer cells. Different types of AuNPs include Au-nanorods, Au-nanostars, and Au-nanocages [126].
PTT is an effective treatment for CRC condition. This therapy is mainly based on photosensitizers. The irradiation of photosensitizers with NIR 808 nm leads to the activation of photosensitizers. AuNPs efficiently convert high light into heat; hence, they are widely used as photothermal agents in PTT. So, Costantini et al. synthesized highly photostable branched AuNPs (B-AuNPs) to eradicate colon cancer cells. The synthesized B-AuNPs exhibited an average particle size of 180 ± 10 nm. The cell granularity was enhanced 1.74-fold in B-AuNP cells compared to unexposed cells. This confirmed the uptake of B-AuNPs by colon cells. The treated colon cancer cells resulted in a significant decrease in cell viability. The authors concluded that the synthesized photostable B-AuNPs could target colon cancer cells [127].
Similarly, Zhang and colleagues developed laser-triggered AuNPs of doxorubicin modified by AS1411 and DNA rich of intercalation (Fig. 7(i)). TEM images confirmed that the diameter of NPs increased after the modification of AuNPs with DNA and DOX. The average size of 399.5 nm with PDI 0641 and ζ–potential − 18.9 mV. The developed system bound toward SW480 colon cancer cells and enhanced the uptake of NPs in vitro. The AS1411-based AuNPs increased the cytotoxicity by inhibiting cell proliferation in SW480 colon cancer cells under laser exposure. They concluded that the developed study emerged as a novel alternative platform with targeted PTT against colon cancer cells [128]. Studies have shown that survival of CRC is significantly associated with the overexpression of programmed cell death ligand (PDL1). Hence developing a system targeting PDL1 may enhance the survival rate [129]. So, Emami and teammates developed AuNPs of DOX and anti-PD-L1 antibody (PD-L1-AuNPs-DOX) for CRC photochemotherapy (Fig. 7(ii)). The developed PD-L1-AuNPs-DOX exhibited an average particle size of 40.0 ± 3.1 nm. The Intracellular uptake of DOX increased in CT-26 cells. Due to enhanced uptake, the apoptosis of cells was around 66% in CT-26 cells. The association of PD-L1-AuNPs-DOX with irradiation of NIR leads to cell cycle arrest and cell apoptosis in CT-26 cells. The authors concluded that PD-L1-AuNPs-DOX, in association with PTT, acts as a synergistic treatment for localizing the CRC [130]. The studies showed that CRC cells overexpressed EGFRs. Hence targeting this receptor may be targeted therapy to treat CRC [131]. Therefore, Liszbinski and co-workers developed 5-FU-AuNPs coated with anti-EGFR antibodies (Fig. 7(iii)). The developed AuNPs-5FU-anti-EGFR exhibited a particle size of 18.83 ± 1.52 nm, followed by a ζ–potential of -33.1 ± 3.78 mV. TEM results confirmed the presence of spherical-shaped particles. The developed novel system induced cell death in HT-29 and HCT-116 cells. The AuNPs-5FU-anti-EGFR enhanced cell apoptosis and impaired cell proliferation in both cell lines. The authors concluded that functionalized AuNPs with monoclonal antibodies potentially delivered 5-FU to colon cancer cells and could be a novel strategy against CRC [132]. The 5-FU is a well-known chemotherapeutic agent against CRC. However, clinical use of this is hindered by several side effects. Hence to overcome these side effects and enhance the anti-cancer efficacy, it is loaded with the AuNPs using two thiol-containing ligands, namely thioglycolic acid (TGA) and glutathione, by Safwat and colleagues (Fig. 7(iv)). The resulting NPs exhibited a size of ∼9–17 nm. The high loading of 5-FU was observed with a ligand molar ratio of 1:1 for TGA-GNPs and 2:1 for GSH-GNPs, respectively. The drug release from AuNPs was pH-dependent. The developed 5-FU/GSH-AuNPs inhibited cell apoptosis and cycle progression. The study also revealed that the developed novel system exhibited a 2-fold higher anti-cancer effect when compared with free 5-FU. The authors concluded that developed AuNPs potential to enhance 5-FU anti-cancer efficacy in colon cancer cells [133].
(i) Diagrammatic representation of chemophothermal effect of AuNPs on colon cancer cells, reproduced with permission from Ref. [128]. (ii) Graphical representation of PD-L1-AuNPs-DOX targeting colon cancer cells, reproduced with permission from Ref. [130]. (iii) Diagrammatic illustration of the effect of AuNPs- 5FU-EGFR on colon cancer cells, reproduced with permission from Ref. [132]. (iv) Graphical illustration of 5FU-AuNPs inducing cell death in CRC cells, reproduced with permission from Ref. [133]
Silver nanoparticles
AgNPs are widely used in the industry, food, and medical fields due to their distinctive physical and chemical properties. Due to its broad spectrum of anti-microbial properties, it is used in the manufacture of household products, industries, health care products, pharmaceutical industries, etc. [134]. The AgNPs have anti-cancer properties and have a mechanism of action that releases Ag + ions from AgNPs enters into the cell and reach the mitochondria and interact with the thiol groups. After interaction binds to the NADPH dehydrogenase enzymes and releases the ROS, this reacts with respiratory enzymes and blocks ATP synthesis. Also, the formed ROS binds to DNA and RNA, hindering the cell replication and synthesis of proteins resulting in cell death [135]. So, AgNPs are widely used as an anti-cancer drug carrier to the tumor site [136].
Recently green nanotechnology emerged as a new approach that uses various metal NPs for targeted drug delivery systems. Studies have shown that Ag is a metal that has a strong cytotoxic effect against cancer cells; it is widely used in the biomedical field [137]. So, Mashwani and teammates developed AgNPs of Mentha longifolia leaves aqueous extract and evaluated their action against colon cancer cells (Fig. 8(i)). Spectrophotometric analysis exhibited that 120 °C with acidic pH 3mM of AgNO3 when mixed in a 1:9 ratio (plant extract: AgNO3) optimized condition for the synthesis of AgNPs. The studies showed that NPs are spherical and have an average size in the 10–100 nm range. The AgNP concentration of ∼10 µg/ml destructed population of ∼66% of Leishmania and 8.73 µg/ml was selected as IC50. Cell apoptosis assay on HCT 116 cells showed plant extract and AgNPs not active against colon cancer cells. The AgNPs of Mentha longifolia revealed that they can generate free radicals and not result in any photothermal activity [138]. Several studies reported curcumin efficacy against CRC, and clinical studies reported its poor bioavailability and low absorption with rapid metabolism. Hence Freitas and colleagues developed a multimodal platform of incorporating curcumin and AgNPs into polysaccharide-based hydrogels to enhance the photodynamic effect of curcumin via the metal-enhanced singlet oxygen effect (Fig. 8(ii)). The resulting NPs exhibited an average diameter of 4.1 nm. HR-TEM analysis confirmed that the particles are spherical with uniform distribution. The chitosan and chondroitin sulfate hydrogel containing curcumin AgNPs exhibited a homogenous macrostructure with rough surface and excellent miscibility and stability. Cellular study results revealed that developed hydrogel containing curcumin AgNPs is safe for healthy cells. The PDT selective illumination study showed that developed AgNPs inhibited Caco-2 colon cancer cells. The authors concluded that curcumin could be an efficient diagnostic fluorescent probe in the theranostic system [139]. In another investigation, Lu and his team developed reusable composite graphene and AgNPs (GO-AgNPs) for the catalytic reduction of 4-nitrophenol and as an efficient treatment of CRC. FE-SEM images revealed that small quantities of AgNPs were distributed on the surface of GO in the developed system. EDX analysis confirmed that the developed nanocomposite comprises Ag, C, and O elements. TEM images confirmed that GO-AgNPs were uniformly distributed with a narrow size distribution (5 nm). The developed GO-AgNPs showed significant cell death in CRC cell lines (HT-29, HCT 116, HCT-8 [HRT-18], and Ramos.2G6.4C10). The high percentage of cell apoptosis results were exhibited in HT-29 cells. The antioxidant study revealed that GO-AgNPs inhibited 50% of the DPPH molecules in 344 mg/mL concentration. The authors concluded that GO-AgNPs could be a chemotherapeutic supplement for CRC [140]. Several studies reported that TRAIL could induce cell death, but the use of this is limited due to its short half-life and less stability. Due to this issue, TRAIL could not reach the tumor site [141]. So, Birtekocak and co-workers synthesized TRAIL-conjugated PEGylated AgNPs (AgCTP-NPs) against colon cancer cells (Fig. 8(iii)). The synthesized AgCTP-NPs were light brown and had good water solubility. The average particle size was around 128.12 ± 8.02 nm, and the ζ–potential of -28.75 ± 2.02 mV. The AgCTP-NPs showed a significant decrease in HT-29 cell colonies and confirmed the upregulation of apoptotic proteins. The authors concluded that AgNPs could be a good vehicle for TRAIL therapeutic proteins, which can be used to treat colon tumors [142].
Similarly, Rozalen and colleagues synthesized PEGylated AgNPs of methotrexate embedded in GO (AgNPs-MTX) (Fig. 8(iv)). The developed AgNPs-MTX exhibited a spherical shape of NPs with an average size of 13 nm with the polydisperse size of particles with a wide distribution range of 7–21 nm. In vitro drug release profile results revealed that 77–85% of MTX is released from AgNPs. The release profile was fitted to the Higuchi model and followed the diffusion mechanism. The AgNPs-MTX significantly reduced HCT-116 cells, and the reduced IC50 from 186 to 63 µg mL− 1 was observed after 12 and 48 h of exposure. The authors concluded that a synergetic effect was observed when MTX was conjugated with AgNPs [143].
(i) Graphical representation of biogenesis of silver nanoparticles, reproduced with permission from Ref. [138]. (ii) Graphical representation of hydrogel containing curcumin and AgNPs, reproduced with permission from Ref. [139]. (iii) Schematic representation of the synthesis of AgCTP nanoparticles, reproduced with permission from Ref. [142]. (iv) Schematic representation of AgNPs-MTX against colon and lung cancer cells, reproduced with permission from Ref. [143]
Zinc oxide nanoparticles
Zinc is essential for various human body parts, like muscles, bones, skin, etc. Recently, ZnONPs emerged as an outstanding tool in optical, electrical, and biomedical research [144]. Studies have been shown that ZnONPs widely used in cancer therapeutics due to their cancer cell-specific toxicity. ZnONPs lead to cancer cell death via a pH-dependent mechanism. The Zn2+ dissolves in low pH and generates ROS, leading to cancer cell death [145]. ZnONPs have biomedical applications like bioimaging, targeted drug delivery systems, and tissue engineering. These are also anti-oxidant, anti-diabetic, and anti-microbial agents [146].
Recently, Racca and colleagues developed an NP’s (oleic acid-Zn oxide nanocrystals) anti-tumor effect with external high-energy shock waves as a combinational therapy against CRC. The undoped ZnO nanocrystals (ZnONC) and iron (Fe) doped with oleic acid and aminopropyl functionalization ensure the colloidal stability of nanocrystals. The FE-SEM images revealed that both the nanocrystals are in a unique shape with average particle diameters of 4 and 10 nm. HR-TEM analysis revealed that Fe-doped and undoped nanocrystals were exhibited as single crystals in a spherical shape. The cell lines studies showed that both the nanocrystals had good bio-tolerance and rate of internalization in HT-29 and Duke’s type C Colo 320-DM cells. Cell cytotoxicity studies showed that combined therapy boosted anti-tumor activity in Colo 320-DM cells more than in control groups. The continued shock wave treatment thrice per day further resulted in the mortality of CRC cells (Fig. 9(i)). The authors concluded that combination therapy enhanced the anti-tumor efficacy of CRC cells [147]. Studies have reported that the upregulation of hydrogen sulfide (H2S) aids the growth and progression of colorectal tumors. Hence, removing H2S can be a significant step in treating CRC [148]. So, Pan and co-workers developed the H2S-responsive ZnO nanosphere coated with virus-like silica (VZnO) for CRC therapy (Fig. 9(ii)). The virus-like formation MSNs exhibited a mono-distributed average particle size of ≈ 120 nm. The elemental mapping analysis showed the distribution of Si and O elements in ZnONPs. The H2S scavenging study using HCT-116 and CT 26 cells resulted in a reduction in glutathione levels as well as cell viability within 24 h. TEM images revealed that the treatment with VZnO led to an abnormality in mitochondria of malignant epithelial cells with shrinkage and disappearance. In vivo biodistribution study in Balb/c mice model showed IV injections of VZnO@fluroscence isothiocyanate signals were detected within 30 min and reached peak level within 4 h after injection. The authors concluded that H2S scavenging-induced ferroptosis strategy using VZnONPs could be a promising therapy for CRC [149]. Ye and teammates developed ZnONPs in oxaliplatin-resistant CRC cells in another investigation. The developed ZnONPs reduced the viability of HCT116 and HCT8 cells. Also, ZnONPs reduced the viability of oxaliplatin resistance HCT-116 and HCT8 cells. Also, this study revealed intensified cell proliferation after hindrance of miR-1321 or overexpression of HIF-2a compared with the ZnONPs treated cells.
The administration of ZnONPs suppressed oxaliplatin resistance HCT116 cell-induced tumors in the Balb/C mice model. The authors concluded that ZnONPs are the effective treatment technique against chemoresistant CRC [150]. In another investigation, Shahanaz and colleagues synthesized the ZnONPs of the aqueous extract of leaves of Artocarpus heterophyllus (Fig. 9(iii)). The SEM analysis exhibited roughness of developed ZnONPs. TEM images confirmed the well-dispersed spherical shape of particles in the size range of 12–24 nm. The synthesized ZnONPs of the extract resulted in a significant cytotoxic effect on the colon cancer cell line (HCT-116). AO/EB staining revealed that ZnONPs treated HCT-116 cells characterized by nuclear condensation, fragmentation, and cell apoptosis. The authors concluded that ZnONPs of Artocarpus heterophyllus act as an excellent anti-cancer agent in colorectal adenocarcinoma cell lines [151].
Similarly, another team evaluated the anti-proliferation effect of ZnONPs and Aluminium hydroxide [Al(OH)3] NPs on colon cancer cell lines (HT-29). The resulting NPs exhibited an average particle size of 50.89 nm. TEM images confirmed that both Al(OH)3-NPs and ZnONPs revealed high homogenous surfaces of particles. The developed ZnONPs significantly inhibited cell proliferation in a dose-dependent manner (Fig. 9(iv)). The cell viability in HT-29 cells treated with 50 and 100 µg/ml of ZnONPs showed 49% & 33% and Al(OH)3-NPs 65% and 41%, respectively. The authors concluded that ZnONPs are a potential anti-proliferative agent against human colon cancer cell lines [152]. Recent research findings on inorganic NPs for colorectal cancer treatment are listed in Table 1.
(i) Graphical representation of the synergetic effect of ZnO-nanocrystals and shock waves on CRC, reproduced with permission from Ref. [147]. (ii) Schematic illustration of the mechanism of ZnO nanospheres in H2S scavenging and ferroptosis in CRC, reproduced with permission from Ref. [149]. (iii) Schematic representation of the synthesis of ZnO-NPs and its evaluation in HCT-116 cell line, reproduced with permission from Ref. [151]. (iv) Graphical representation of anti-proliferative effects of ZnO-NPs and Al(OH)3-NPs on HT-29 cells, reproduced with permission from Ref. [152]
Toxicity aspects of INPs and potential ways to overcome them
The cytotoxic effects of INPs present both advantages and challenges. On one hand, these NPs can effectively kill cancer cells, making them useful in cancer treatment. On the other hand, they can also damage healthy cells by triggering cell apoptosis, necrosis, or autophagy. To maximize the therapeutic potential of INPs, it is essential to design NPs that are selectively toxic to cancer cells while remaining biocompatible and harmless to normal cells. Recently, there has been growing interest in functionalizing INPs with protein molecules and using green synthesis methods (plants, microbes, biodegradable waste, etc.) [175]. These approaches have shown promise in reducing the toxicities associated with INPs, compared to their non-functionalized and chemically synthesized counterparts [21, 176].
An interesting study by Mukherjee and colleagues explored the toxicity differences between green synthesized silver NPs and their chemically synthesized counterparts. They discovered that green synthesized silver NPs exhibited notable anticancer activity across three cancer cell lines: A549, B16F10, and MCF-7. These green synthesized NPs significantly inhibited the proliferation of the cancer cells in a dose-dependent manner, with concentrations ranging from 3 to 30 µM. In contrast, chemically synthesized silver NPs failed to suppress cancer cell proliferation within the same concentration range. Additionally, biocompatibility assessments on human umbilical vein endothelial cells (HUVEC) and Chinese hamster ovary (CHO) cells showed no toxicity for green synthesized NPs within the 3–30 µM range. However, a slight toxicity was observed in rat cardiomyoblast cells (H9C2) at the highest concentration of 30 µM. Conversely, the chemically synthesized NPs exhibited toxicity to these normal cells even at lower doses [177].
Another interesting investigation by Kummara and teammates revealed that chemically synthesized silver NPs were toxic to human dermal fibroblast cells at concentrations between 120 and 240 ppm. Conversely, green synthesized silver NPs demonstrated no toxicity to normal cells within the same concentration range of 0 to 240 ppm. Yet, green synthesized silver NPs effectively inhibited the growth of NCI-H460 lung cancer cells at concentrations of 160, 200, and 240 ppm. In contrast, similar concentrations of chemically synthesized silver NPs did not result in significant inhibition of lung cancer cell proliferation. Additionally, toxicity tests on brine shrimp showed that green synthesized NPs caused 100% mortality at 240 ppm and 56% mortality at 120 ppm. On the other hand, chemically synthesized NPs led to 100% mortality at both 240 ppm and 120 ppm. These results underscore the excellent biocompatibility of green synthesized INPs compared to their chemically synthesized counterparts [178].
Surface functionalization of INPs with proteins has been shown to enhance their biocompatibility through various mechanisms. These include reducing the release of free metal ions, masking residual toxic capping agents, improving water dispersibility, decreasing immunotoxicity, enhancing renal excretion, and lowering thrombogenic activity. Modifying INPs with biocompatible polymers such as proteins enhances their cytocompatibility by mitigating the release of free metal ions, thus reducing ROS generation and related cytotoxic effects [176]. For example, Fe3O4 NPs coated with silk fibroin exhibited diminished iron ion release and ROS production, facilitating neural cell differentiation instead of apoptosis [179]. Similarly, HSA-coated Gd-DTPA and gelatin-coated CdTe quantum dots showed improved biocompatibility and lower cytotoxicity by minimizing the efflux of toxic ions [180].
An interesting investigation reported that encapsulating CTAB-coated AuNRs with an HSA shell reduced the harmful effects of residual CTAB on cell membranes, resulting in improved biocompatibility with over 91% viability in 4T1 breast cancer cells. Conversely, uncoated AuNRs without albumin exhibited significantly reduced cell viability due to the presence of toxic CTAB residues. A significant obstacle with free CNTs is their propensity to aggregate due to hydrophobic interactions between the sp2 carbon tube shells. Protein coating enhances the water dispersibility of CNTs by breaking these hydrophobic interactions, thus making them safe for mesenchymal stem cells. Likewise, protein functionalization enables the synthesis of photostable QDs, typically created in hydrophobic environments, in aqueous media, thereby facilitating their use in biological systems [181]. In another study, ultra-small BSA-conjugated Gd nanoparticles (less than 3 nm) were identified as valuable MRI contrast agents due to their renal excretion capability. Following intravenous administration, these nanoparticles produced significant negative contrast enhancements in mouse liver MRI images, which returned to baseline after 24 h, primarily due to renal clearance. This excretion process is crucial for reducing potential toxicity in the body [182]. Free and PEGylated CNTs are notably thrombogenic, likely due to their negatively charged surfaces promoting clotting factor activity. Plasma proteins like fibrinogen and von Willebrand factor can bind to CNTs, causing platelet adhesion and activation. Thus, a study showed that modifying the surface of CNTs with HAS inhibits protein adsorption and platelet adhesion, reducing their prothrombotic effects, decreasing the risk of platelet aggregation and thrombus formation, thereby improving their biocompatibility [183].
Conclusion and future perspectives
CRC stands as a formidable adversary in the realm of oncology. However, amid this challenge, INPs emerge as promising platforms in the fight against this malignancy. They represent a potential paradigm shift in CRC therapy, offering the prospect of addressing and surmounting the limitations entrenched in conventional chemotherapy. INPs surpass organic NPs due to their unique attributes such as heightened photosensitivity, remarkable conductivity, magnetic allure, and thermal proficiency, thus serving dual roles as drug carriers and therapeutic agents. Derived primarily from metals, metal oxides, and non-metallic materials, INPs exhibit superior drug-loading capacity and facilitate advanced photothermal and PDT. As we mentioned earlier in the introduction, to date, only two INP-based products, NanoTherm® and Hensify®, have garnered approval by EMA for the treatment of cancer. Additionally, other INP-based formulations like Arimune, Auroshell, etc., are in clinical trials, highlighting the ongoing exploration of INPs in cancer therapy. Yet, no INPs have been approved for colorectal cancer therapy.
Although INP-based approaches to CRC therapy offer substantial benefits, significant hurdles remain before their seamless integration into clinical practice. Understanding the pathways through which INPs are eliminated from the body is crucial for their successful clinical application. While renal elimination via urine is not viable for most INPs due to their non-biodegradable nature and larger particle sizes, hepatobiliary elimination through feces emerges as a potential alternative route. However, this elimination process is influenced by INPs’ interactions with liver cells, impacting their fate. The complex journey of INPs through liver sinusoids, hepatocytes, bile ducts, intestines, and eventual excretion via feces underscores the intricate dynamics involved. Extensive investigation into biosafety, biodegradation, biodistribution, excretion, and in vivo behavior of inorganic nanomaterials is imperative due to the limited data available regarding their safety profiles. While concerns remain over the toxicity associated with inorganic NPs, particularly at higher concentrations, strategic measures such as utilizing biologically sourced materials in NP synthesis and encasing them within degradable polymers/lipids hold promise in mitigating these risks. Moreover, surface functionalization of INPs with biological materials introduces further safety considerations, amplifying their potential interactions with tissues and biomolecules upon in vivo administration. Nevertheless, ensuring homogeneity in INPs is crucial; yet, scaling up production while preserving reproducibility and uniformity presents a significant challenge, especially for drug-loaded complex targeted INPs. Despite both advantages and limitations, ample opportunities exist for further research on INPs for efficient CRC therapy, while also considering toxicity and clinical translation. Consequently, this review is poised to offer significant assistance to scientists and researchers engaged in CRC research.
Data availability
No datasets were generated or analysed during the current study.
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin [Internet]. 2021 [cited 2023 Apr 14];71:209–49. https://pubmed.ncbi.nlm.nih.gov/33538338/.
Ashique S, Kumar S, Hussain A, Mishra N, Garg A, Gowda BHJ et al. A narrative review on the role of magnesium in immune regulation, inflammation, infectious diseases, and cancer. J Health Popul Nutr [Internet]. 2023 [cited 2023 Aug 8];42:74. https://jhpn.biomedcentral.com/articles/https://doi.org/10.1186/s41043-023-00423-0.
Dekker E, Tanis PJ, Vleugels JLA, Kasi PM, Wallace MB. Colorectal cancer. Lancet [Internet]. 2019 [cited 2024 Mar 27];394:1467–80. http://www.thelancet.com/article/S0140673619323190/fulltext.
Sharma R, Abbasi-Kangevari M, Abd-Rabu R, Abidi H, Abu-Gharbieh E, Acuna JM et al. Global, regional, and national burden of colorectal cancer and its risk factors, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Gastroenterol Hepatol [Internet]. 2022 [cited 2024 Mar 27];7:627–47. http://www.thelancet.com/article/S2468125322000449/fulltext.
Lin JS, Perdue LA, Henrikson NB, Bean SI, Blasi PR. Screening for Colorectal Cancer: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA [Internet]. 2021 [cited 2024 Mar 27];325:1978–97. https://pubmed.ncbi.nlm.nih.gov/34003220/.
Biller LH, Schrag D. Diagnosis and Treatment of Metastatic Colorectal Cancer: A Review. JAMA [Internet]. 2021 [cited 2024 Mar 27];325:669–85. https://jamanetwork.com/journals/jama/fullarticle/2776334.
Colon cancer - Diagnosis. and treatment - Mayo Clinic [Internet]. [cited 2024 Jun 17]. https://www.mayoclinic.org/diseases-conditions/colon-cancer/diagnosis-treatment/drc-20353674.
Bien J, Lin A. A Review of the Diagnosis and Treatment of Metastatic Colorectal Cancer. JAMA [Internet]. 2021 [cited 2024 Mar 27];325:2404–5. https://jamanetwork.com/journals/jama/fullarticle/2781060.
Radiation Therapy for Colorectal Cancer. | Radiation for Colorectal Cancer | American Cancer Society [Internet]. [cited 2024 Jun 17]. https://www.cancer.org/cancer/types/colon-rectal-cancer/treating/radiation-therapy.html.
Strickler JH, Yoshino T, Graham RP, Siena S, Bekaii-Saab T. Diagnosis and Treatment of ERBB2-Positive Metastatic Colorectal Cancer: A Review. JAMA Oncol [Internet]. 2022 [cited 2024 Mar 27];8:760–9. https://jamanetwork.com/journals/jamaoncology/fullarticle/2789766.
Xie YH, Chen YX, Fang JY. Comprehensive review of targeted therapy for colorectal cancer. Signal Transduct Target Ther 2020 51 [Internet]. 2020 [cited 2024 Mar 26];5:1–30. https://www.nature.com/articles/s41392-020-0116-z.
Gowda BHJ, Ahmed MG, Almoyad MAA, Wahab S, Almalki WH, Kesharwani P. Nanosponges as an Emerging Platform for Cancer Treatment and Diagnosis. Adv Funct Mater [Internet]. 2023 [cited 2023 Dec 18];2307074. https://onlinelibrary.wiley.com/doi/full/10.1002/adfm.202307074.
Zeng L, Gowda BHJ, Ahmed MG, Abourehab MAS, Chen ZS, Zhang C et al. Advancements in nanoparticle-based treatment approaches for skin cancer therapy. Mol Cancer 2023 221 [Internet]. 2023 [cited 2023 Mar 24];22:1–50. https://link.springer.com/articles/https://doi.org/10.1186/s12943-022-01708-4.
Hani U, Gowda BHJ, Haider N, Ramesh K, Paul K, Ashique S et al. Nanoparticle-Based Approaches for Treatment of Hematological Malignancies: a Comprehensive Review. AAPS PharmSciTech [Internet]. 2023 [cited 2023 Dec 18];24:1–24. https://link.springer.com/article/https://doi.org/10.1208/s12249-023-02670-0.
Khan MS, Jaswanth Gowda BH, Almalki WH, Singh T, Sahebkar A, Kesharwani P. Unravelling the potential of mitochondria-targeted liposomes for enhanced cancer treatment. Drug Discov Today. 2024;29:103819.
Damiri F, Gowda BHJ, Andra S, Balu S, Rojekar S, Berrada M. Chitosan Nanocomposites as Scaffolds for Bone Tissue Regeneration. 2023 [cited 2023 Jul 3];377–94. https://link.springer.com/chapter/10.1007/978-981-19-9646-7_16.
Barani M, Bilal M, Rahdar A, Arshad R, Kumar A, Hamishekar H et al. Nanodiagnosis and nanotreatment of colorectal cancer: an overview. J Nanoparticle Res 2021 231 [Internet]. 2021 [cited 2024 Jun 17];23:1–25. https://link.springer.com/article/https://doi.org/10.1007/s11051-020-05129-6.
Banazadeh M, Behnam B, Ganjooei NA, Gowda BHJ, Kesharwani P, Sahebkar A. Curcumin-based nanomedicines: a promising avenue for brain neoplasm therapy. J Drug Deliv Sci Technol. 2023;89:105040.
Mohanto S, Narayana S, Merai KP, Kumar JA, Bhunia A, Hani U, et al. Advancements in gelatin-based hydrogel systems for biomedical applications: a state-of-the-art review. Int J Biol Macromol. 2023;253:127143.
Gowda BHJ, Ahmed MG, Alshehri SA, Wahab S, Vora LK, Singh Thakur RR, et al. The cubosome-based nanoplatforms in cancer therapy: seeking new paradigms for cancer theranostics. Environ Res. 2023;237:116894.
Gowda BHJ, Ahmed MG, Chinnam S, Paul K, Ashrafuzzaman M, Chavali M, et al. Current trends in bio-waste mediated metal/metal oxide nanoparticles for drug delivery. J Drug Deliv Sci Technol. 2022;71:103305.
He X, Jiang Z, Akakuru OU, Li J, Wu A. Nanoscale covalent organic frameworks: from controlled synthesis to cancer therapy. Chem Commun [Internet]. 2021 [cited 2024 Jun 17];57:12417–35. https://pubs.rsc.org/en/content/articlehtml/2021/cc/d1cc04846e.
Jiang Z, Han X, Zhao C, Wang S, Tang X. Recent Advance in Biological Responsive Nanomaterials for Biosensing and Molecular Imaging Application. Int J Mol Sci. 2022, Vol 23, Page 1923 [Internet]. 2022 [cited 2024 Jun 17];23:1923. https://www.mdpi.com/1422-0067/23/3/1923/htm.
Hani U, Jaswanth Gowda BH, Siddiqua A, Wahab S, Begum Y, Sathishbabu P, et al. Herbal approach for treatment of cancer using curcumin as an anticancer agent: a review on novel drug delivery systems. J Mol Liq. 2023;390:123037.
Khan MS, Gowda BHJ, Nasir N, Wahab S, Pichika MR, Sahebkar A, et al. Advancements in dextran-based nanocarriers for treatment and imaging of breast cancer. Int J Pharm. 2023;643:123276.
Paul K, Jaswanth Gowda BH, Damiri F, Hemalatha YR, Chandan RS, Berrada M. Nanophytomedicine and Their Applications: A Brief Overview 1. Polym Nanocomposites [Internet]. 2023 [cited 2023 Jul 23];83–94. https://www.taylorfrancis.com/chapters/edit/10.1201/9781003343912-6/nanophytomedicine-applications-karthika-paul-jaswanth-gowda-fouad-damiri-hemalatha-chandan-mohammed-berrada.
Ahamed J, Jaswanth Gowda BH, Almalki WH, Gupta N, Sahebkar A, Kesharwani P. Recent advances in nanoparticle-based approaches for the treatment of brain tumors: Opportunities and challenges. Eur Polym J [Internet]. 2023 [cited 2023 May 15];193:112111. https://linkinghub.elsevier.com/retrieve/pii/S001430572300294X.
Dubey SK, Parab S, Achalla VPK, Narwaria A, Sharma S, Jaswanth Gowda BH et al. Microparticulate and nanotechnology mediated drug delivery system for the delivery of herbal extracts. https://doi.org/101080/0920506320222065408 [Internet]. 2022 [cited 2023 Mar 31];33:1531–54. https://www.tandfonline.com/doi/abs/https://doi.org/10.1080/09205063.2022.2065408.
Hani U, Osmani RAM, Yasmin S, Gowda BHJ, Ather H, Ansari MY et al. Novel Drug Delivery Systems as an Emerging Platform for Stomach Cancer Therapy. Pharm 2022, Vol 14, Page 1576 [Internet]. 2022 [cited 2022 Oct 10];14:1576. https://www.mdpi.com/1999-4923/14/8/1576/htm.
Sameer Khan M, Jaswanth Gowda BH, Hasan N, Gupta G, Singh T, Md S, et al. Carbon nanotube-mediated platinum-based drug delivery for the treatment of cancer: advancements and future perspectives. Eur Polym J. 2024;206:112800.
Nag S, Mitra O, Bhattacharjee PS, Mohanto A, Gowda S. Exploring the emerging trends in the synthesis and theranostic paradigms of cerium oxide nanoparticles (CeONPs): a comprehensive review. Mater Today Chem. 2024;35:101894.
Amaldoss MJN, Yang JL, Koshy P, Unnikrishnan A, Sorrell CC. Inorganic nanoparticle-based advanced cancer therapies: promising combination strategies. Drug Discov Today. 2022;27:103386.
Nanomaterials-assisted Photothermal Therapy for Breast Cancer. State-of-the advances and future perspectives. Photodiagnosis Photodyn Ther. 2024;103959.
Cancer Nano-Therapies in the Clinic and Clinical Trials. - NCI [Internet]. [cited 2024 Mar 27]. https://www.cancer.gov/nano/cancer-nanotechnology/current-treatments.
Hossain MS, Karuniawati H, Jairoun AA, Urbi Z, Ooi DJ, John A et al. Colorectal Cancer: A Review of Carcinogenesis, Global Epidemiology, Current Challenges, Risk Factors, Preventive and Treatment Strategies. Cancers (Basel) [Internet]. 2022 [cited 2023 Jul 23];14:1732. http://www.pmc/articles/PMC8996939/.
Al-Joufi FA, Setia A, Salem-Bekhit MM, Sahu RK, Alqahtani FY, Widyowati R et al. Molecular Pathogenesis of Colorectal Cancer with an Emphasis on Recent Advances in Biomarkers, as Well as Nanotechnology-Based Diagnostic and Therapeutic Approaches. Nanomater 2022, Vol 12, Page 169 [Internet]. 2022 [cited 2024 Mar 27];12:169. https://www.mdpi.com/2079-4991/12/1/169/htm.
Aghagolzadeh P, Radpour R. New trends in molecular and cellular biomarker discovery for colorectal cancer. World J Gastroenterol [Internet]. 2016 [cited 2024 Mar 27];22:5678. http://www.pmc/articles/PMC4932205/.
Knowlton CA, Mackay MK, Speer TW, Vera RB, Arthur DW, Wazer DE et al. Colon Cancer. Encycl Radiat Oncol [Internet]. 2023 [cited 2024 Mar 27];77–77. https://www.ncbi.nlm.nih.gov/books/NBK470380/.
Bien SA, Su YR, Conti DV, Harrison TA, Qu C, Guo X et al. Genetic variant predictors of gene expression provide new insight into risk of colorectal cancer. Hum Genet [Internet]. 2019 [cited 2024 Mar 27];138:307–26. https://pubmed.ncbi.nlm.nih.gov/30820706/.
Li X, Lovell JF, Yoon J, Chen X. Clinical development and potential of photothermal and photodynamic therapies for cancer. Nat Rev Clin Oncol 2020 1711 [Internet]. 2020 [cited 2023 Mar 25];17:657–74. https://www.nature.com/articles/s41571-020-0410-2.
Zhang Y, Zhang S, Zhang Z, Ji L, Zhang J, Wang Q, et al. Recent progress on NIR-II Photothermal Therapy. Front Chem. 2021;9:728066.
Sun J, Zhao H, Xu W, Jiang GQ. Recent advances in photothermal therapy-based multifunctional nanoplatforms for breast cancer. Front Chem [Internet]. 2022 [cited 2024 Mar 27];10. Available from: /pmc/articles/PMC9528973/.
Xu N, Zhang X, Qi T, Wu Y, Xie X, Chen F et al. Biomedical applications and prospects of temperature-orchestrated photothermal therapy. MedComm – Biomater Appl [Internet]. 2022 [cited 2024 Mar 27];1:e25. https://onlinelibrary.wiley.com/doi/full/https://doi.org/10.1002/mba2.25.
Overchuk M, Weersink RA, Wilson BC, Zheng G, Photodynamic, Therapies P. Synergy Opportunities for Nanomedicine. ACS Nano [Internet]. 2023 [cited 2024 Mar 27];17:7979–8003. https://pubs.acs.org/doi/full/https://doi.org/10.1021/acsnano.3c00891.
Xiong Y, Rao Y, Hu J, Luo Z, Chen C. Nanoparticle-Based Photothermal Therapy for Breast Cancer Noninvasive Treatment. Adv Mater [Internet]. 2023 [cited 2024 Mar 27];2305140. https://onlinelibrary.wiley.com/doi/full/https://doi.org/10.1002/adma.202305140.
Alamdari SG, Amini M, Jalilzadeh N, Baradaran B, Mohammadzadeh R, Mokhtarzadeh A, et al. Recent advances in nanoparticle-based photothermal therapy for breast cancer. J Control Release. 2022;349:269–303.
Gallo J, Villasante A. Recent Advances in Biomimetic Nanocarrier-Based Photothermal Therapy for Cancer Treatment. Int J Mol Sci. 2023, Vol 24, Page 15484 [Internet]. 2023 [cited 2024 Mar 27];24:15484. https://www.mdpi.com/1422-0067/24/20/15484/htm.
Zhao L, Zhang X, Wang X, Guan X, Zhang W, Ma J. Recent advances in selective photothermal therapy of tumor. J Nanobiotechnology [Internet]. 2021 [cited 2024 Mar 27];19:1–15. https://jnanobiotechnology.biomedcentral.com/articles/https://doi.org/10.1186/s12951-021-01080-3.
Mater J, Chem B, Thirumurugan S, Ramanathan S, Muthiah KS, Lin Y-C et al. Inorganic nanoparticles for photothermal treatment of cancer. J Mater Chem B [Internet]. 2024 [cited 2024 Mar 27]; https://pubs.rsc.org/en/content/articlehtml/2024/tb/d3tb02797j.
Han X, Zhao C, Wang S, Pan ZY, Jiang Z, Tang X. Multifunctional TiO2/C nanosheets derived from 3D metal–organic frameworks for mild-temperature-photothermal-sonodynamic-chemodynamic therapy under photoacoustic image guidance. J Colloid Interface Sci. 2022;621:360–73.
Fernandes N, Rodrigues CF, Moreira AF, Correia IJ. Overview of the application of inorganic nanomaterials in cancer photothermal therapy. Biomater Sci [Internet]. 2020 [cited 2024 Mar 27];8:2990–3020. https://pubs.rsc.org/en/content/articlehtml/2020/bm/d0bm00222d.
Dolmans DEJGJ, Fukumura D, Jain RK. Photodynamic therapy for cancer. Nat Rev Cancer 2003 35 [Internet]. 2003 [cited 2023 Mar 25];3:380–7. https://www.nature.com/articles/nrc1071.
Lobo CS, Mendes MIP, Pereira DA, Gomes-da-Silva LC, Arnaut LG. Photodynamic therapy changes tumour immunogenicity and promotes immune-checkpoint blockade response, particularly when combined with micromechanical priming. Sci Reports 2023 131 [Internet]. 2023 [cited 2024 Mar 27];13:1–14. https://www.nature.com/articles/s41598-023-38862-8.
Kim WS, Khot MI, Woo HM, Hong S, Baek DH, Maisey T et al. AI-enabled, implantable, multichannel wireless telemetry for photodynamic therapy. Nat Commun 2022 131 [Internet]. 2022 [cited 2024 Mar 27];13:1–11. https://www.nature.com/articles/s41467-022-29878-1.
Aziz B, Aziz I, Khurshid A, Raoufi E, Esfahani FN, Jalilian Z et al. An Overview of Potential Natural Photosensitizers in Cancer Photodynamic Therapy. Biomedicines [Internet]. 2023 [cited 2024 Mar 27];11. http://www.pmc/articles/PMC9855789/.
Montaseri H, Kruger CA, Abrahamse H. Inorganic Nanoparticles Applied for Active Targeted Photodynamic Therapy of Breast Cancer. Pharmaceutics [Internet]. 2021 [cited 2024 Mar 27];13:1–33. Available from: /pmc/articles/PMC7996317/.
Shang L, Zhou X, Zhang J, Shi Y, Zhong L. Metal Nanoparticles for Photodynamic Therapy: A Potential Treatment for Breast Cancer. Mol 2021, Vol 26, Page 6532 [Internet]. 2021 [cited 2024 Mar 27];26:6532. https://www.mdpi.com/1420-3049/26/21/6532/htm.
Younis MR, He G, Qu J, Lin J, Huang P, Xia XH. Inorganic Nanomaterials with Intrinsic Singlet Oxygen Generation for Photodynamic Therapy. Adv Sci [Internet]. 2021 [cited 2024 Mar 27];8:2102587. https://onlinelibrary.wiley.com/doi/full/10.1002/advs.202102587.
Cheng S-H, Lo L-W. Inorganic nanoparticles for enhanced photodynamic Cancer therapy. Curr Drug Discov Technol. 2011;8:269–76.
Montaseri H, Kruger CA, Abrahamse H. Recent Advances in Porphyrin-Based Inorganic Nanoparticles for Cancer Treatment. Int J Mol Sci. 2020, Vol 21, Page 3358 [Internet]. 2020 [cited 2024 Mar 27];21:3358. https://www.mdpi.com/1422-0067/21/9/3358/htm.
Mitchell MJ, Billingsley MM, Haley RM, Wechsler ME, Peppas NA, Langer R. Engineering precision nanoparticles for drug delivery. Nat Rev Drug Discov 2020 202 [Internet]. 2020 [cited 2023 Mar 31];20:101–24. https://www.nature.com/articles/s41573-020-0090-8.
Ghosn Y, Kamareddine MH, Tawk A, Elia C, Mahmoud A, El, Terro K et al. Inorganic nanoparticles as Drug Delivery systems and their potential role in the treatment of chronic myelogenous leukaemia. 2019;18:1–12.
Unnikrishnan G, Joy A, Megha M, Kolanthai E, Senthilkumar M. Exploration of inorganic nanoparticles for revolutionary drug delivery applications: a critical review. Discov Nano 2023 181 [Internet]. 2023 [cited 2024 Mar 27];18:1–44. https://link.springer.com/article/https://doi.org/10.1186/s11671-023-03943-0.
Liang R, Wei M, Evans DG, Duan X. Inorganic nanomaterials for bioimaging, targeted drug delivery and therapeutics. Chem Commun [Internet]. 2014 [cited 2024 Mar 27];50:14071–81. https://pubs.rsc.org/en/content/articlehtml/2014/cc/c4cc03118k.
Bayda S, Hadla M, Palazzolo S, Riello P, Corona G, Toffoli G et al. Inorganic nanoparticles for Cancer Therapy: a transition from lab to Clinic. 2018;4269–303.
Pugazhendhi A, Edison TNJI, Karuppusamy I, Kathirvel B. Inorganic nanoparticles: a potential cancer therapy for human welfare. Int J Pharm. 2018;539:104–11.
Yuan Z, Cho-Chun Hu H-TC. and CL. Gold nanoparticles as sensitive optical probes. 2016.
Gao X, Cui Y, Levenson RM, Chung LWK, Nie S. In vivo cancer targeting and imaging with semiconductor quantum dots. 2004;22:969–76.
Wang S, Wang Z, Zha Z, Wang S, Wang Z. Metal nanoparticles or metal oxide nanoparticles, an efficient and promising family of novel heterogeneous catalysts in organic synthesis. 2009;9363–73.
Wang F, Li C, Cheng J, Yuan Z. Recent Advances on Inorganic Nanoparticle-Based Cancer Therapeutic Agents. 1957.
Liu Q, Kim Y, Im G, Zhu J, Wu Y, Liu Y, et al. Inorg Nanopart Appl as Funct Ther. 2020;2008171:1–24.
Tang L, Xiao Q, Mei Y, He S, Zhang Z, Wang R et al. Insights on functionalized carbon nanotubes for cancer theranostics. J Nanobiotechnology 2021 191 [Internet]. 2021 [cited 2022 Nov 10];19:1–28. https://jnanobiotechnology.biomedcentral.com/articles/https://doi.org/10.1186/s12951-021-01174-y.
Chen D, Dougherty CA, Zhu K, Hong H. Theranostic applications of Carbon nanomaterials in Cancer: Focus on Imaging and Cargo Delivery. J Control Release. 2015.
Costa PM, Bourgognon M, Wang JT, Al-jamal KT. Functionalized carbon nanotubes: from intracellular uptake and cell-related toxicity to systemic brain delivery. J Control Release. 2016;241:200–19.
Ma J, Wang G, Ding X, Wang F, Zhu C, Rong Y. Carbon-based nanomaterials as Drug Delivery agents for Colorectal Cancer: clinical preface to Colorectal Cancer citing their markers and existing theranostic approaches. 2023.
Lherminier J, Gharbi T, Micheau O, Herlem G. Nanovectorization of TRAIL with single Wall Carbon Nanotubes enhances. Tumor Cell Killing. 2014;2–6.
Brar B, Ranjan K, Palria A, Kumar R, Ghosh M. Nanatechnol Colorectal Cancer Precision Diagnosis Therapy. 2021;3:1–21.
Criado A, Ans A. Intrinsic and selective activity of functionalized carbon nanotube / nanocellulose platforms against colon cancer cells. 2022;212.
Vazospazm K, Sunumu V. Coronary vasospasm secondary to 5-Fluorouracil and its management. Case Rep. 2011;2010–2.
Cassidy J, Roos B, Banken L. Preferential activation of capecitabine in tumor following oral administration to colorectal cancer patients. 2000;291–7.
Randive DS, Gavade AS, Shejawal KP, Mangesh A, Bhinge SD, Jadhav NR, et al. Colon targeted dosage form of capecitabine using folic acid anchored modified carbon nanotube: in vitro cytotoxicity, apoptosis and in vivo roentgenographic study. Drug Dev Ind Pharm. 2021;47:1401–12.
Kiran P, Shejawal DS, Randive SD, Bhinge, Mangesh A, Bhutkar, Ganesh H. Wadkar SST& SKM. Functionalized carbon nanotube for colon-targeted delivery of isolated lycopene in colorectal cancer: in vitro cytotoxicity and in vivo roentgenographic study. J Mater Res. 2021;36:4894–907.
Anyanee Kamkaew1,‡, Liang Cheng1, 2,‡, Shreya Goel3,‡, Hector F. Valdovinos4, Todd E. Barnhart4, Zhuang Liu2, and Weibo Cai1, 3, 4 5. Cerenkov Radiation Induced Photodynamic Therapy Using Chlorin e6-Loaded Hollow Mesoporous Silica Nanoparticles Anyanee. 2017;8:26630–7.
Abrahamse PS. and H. E ff ective photodynamic therapy for Colon cancer cells using Chlorin e6 coated Hyaluronic Acid-based. Carbon Nanotubes. 2020;6.
Gulbake A, Jain A, Jain A, Jain A, Jain SK. Insight to drug delivery aspects for colorectal cancer. http://www.wjgnet.com/. 2016;22:582–99.
Prajapati SK, Jain A, Shrivastava C, Jain AK. Hyaluronic acid conjugated multi-walled carbon nanotubes for colon cancer targeting. Int J Biol Macromol. 2018;#pagerange#.
Iga AM, Robertson JHP, Winslet MC, Seifalian AM, Free R, Nhs H et al. Clinical Potential of Quantum Dots. 2007;2007.
Farzin MA, Abdoos H. A critical review on quantum dots: from synthesis toward applications in electrochemical biosensors for determination of disease-related biomolecules. Talanta. 2020;121828.
Devi S, Kumar M, Tiwari A, Tiwari V, Kaushik D. Quantum dots: an Emerging Approach for Cancer Therapy. 2022;8:1–18.
Naik K, Chaudhary S, Ye L, Parmar AS. A Strategic Review on Carbon Quantum dots for Cancer-Diagnostics and Treatment. 2022;10:1–21.
Mishra S, Chatterjee S, Sahoo P, Kundu S, Pal M, Bhaumik A et al. Facile and Green Synthesis of Novel Fluorescent Carbon Quantum Dots and Their Silver Heterostructure: An In Vitro Anticancer Activity and Imaging on Colorectal Carcinoma. 2023.
Ren Y, Lv Y, Li T, Jiang Q. High expression of PLAC1 in colon cancer as a predictor of poor prognosis: a study based on TCGA data. Gene. 2020;763:145072.
Mohamed Haider a b 1, Roberta Cagliani b 1, Jayalakshmi Jagal b, Manju N. Jayakumar b, Bahgat Fayed b c, Sarra B. Shakartalla b d e, Renu Pasricha f, Khaled Greish g RE-A b h. Peptide-functionalized graphene oxide quantum dots as colorectal cancer theranostics. J Colloid Interface Sci. 2023;630:698–713.
Wang Z, Zhang J, Guo Y, Wu X, Yang W, Xu L, et al. Biosensors and Bioelectronics A novel electrically magnetic-controllable electrochemical biosensor for the ultra sensitive and specific detection of attomolar level oral cancer-related microRNA. Biosens Bioelectron. 2013;45:108–13.
Pourakbari R, Yousefi M, Khalilzadeh B, Haddad M, Khataee A. Early stage evaluation of colon cancer using tungsten disulfide quantum dots and bacteriophage nano – biocomposite as an efficient electrochemical platform. Cancer Nanotechnol. 2022;1–17.
Duc Dung Nguyen a J-YL. Synthesis, bioactive properties, and biomedical applications of intrinsically therapeutic nanoparticles for disease treatment. Chem Eng J. 2022;435:134970.
Shepard BD, Badley AD. The Biology of TRAIL and the role of TRAIL-Based therapeutics in Infectious diseases. 2011;8:87–101.
Lotfollahzadeh S, Hosseini ES, Aznaveh HM. OPEN TRAIL / S – layer / graphene quantum dot nanohybrid enhanced stability and anticancer activity of TRAIL on colon cancer cells. Sci Rep. 2022;1–12.
Goodrich GP, Gill-sharp K, Sang KL. Photothermal therapy in a murine colon cancer model using near-infrared absorbing gold nanorods. 2023;15:1–8.
Liu F, Wang X, Du S. Production of gold / silver doped carbon nanocomposites for effective photothermal therapy of colon cancer. Sci Rep. 2020;1–9.
Rajani C, Borisa P, Karanwad T, Borade Y, Patel V, Rajpoot K et al. Cancer-targeted chemotherapy: Emerging role of the folate anchored dendrimer as drug delivery nanocarrier. Pharm. Appl. Dendrimers. Elsevier Inc.; 2019.
Hossen S, Hossain MK, Basher MK, Mia MNH, Rahman MT, Uddin MJ. Smart nanocarrier-based drug delivery systems for cancer therapy and toxicity studies: a review. J Adv Res. 2019;15:1–18.
Bharti C, Nagaich U, Pal AK, Gulati N. Mesoporous silica nanoparticles in target drug delivery system. Rev. 2015;5.
Ahmadi F, Sodagar-taleghani A, Ebrahimnejad P. A review on the latest developments of mesoporous silica nanoparticles as a promising platform for diagnosis and treatment of cancer. Int J Pharm. 2022;625:122099.
Narayan R, Gadag S, Pragnya S, Raichur AM, Minhthu C, Garg S, et al. Chitosan-glucuronic acid conjugate coated mesoporous silica nanoparticles: a smart pH-responsive and receptor-targeted system for colorectal cancer therapy. Carbohydr Polym. 2021;261:117893.
Janib SM, Moses AS, Mackay JA. Imaging and drug delivery using theranostic nanoparticles ☆. Adv Drug Deliv Rev. 2010;62:1052–63.
Iranpour S, Bahrami AR, Nekooei S, Saljooghi AS, Matin MM. Improving anti – cancer drug delivery performance of magnetic mesoporous silica nanocarriers for more efficient colorectal cancer therapy. J Nanobiotechnol. 2021;1–22.
Al-mosawi AKM, Bahrami AR. Using magnetic mesoporous silica nanoparticles armed with EpCAM aptamer as an ef fi cient platform for speci fi c delivery of 5- fl uorouracil to colorectal cancer cells. 2023;1–18.
Ramos AP. Biomedical applications of nanotechnology. Biophys Rev. 2017;79–89.
Viana S, Amaral L, Costa DM, Harmon A, Adriao M, Adrião G, et al. Lu-Labeled Eu-Doped Mesoporous SiO nanoparticles as a Theranostic Radiopharmaceutical for Colorectal Cancer Therapy Eu-Doped Mesoporous SiO 2 nanoparticles. as a Theranostic Radiopharmaceutical for Colorectal Cancer; 2020.
Cheng W, Nie J, Xu L, Liang C, Peng Y, Liu G et al. Article a pH-sensitive delivery vehicle based on folic acid-conjugated polydopamine- modified mesoporous silica nanoparticles for targeted cancer therapy. 2017.
Xiying F, Wang aT, Han aM. b Yi Gu, ac Guochuan Sun, a Xinying Peng, ac Qinghui Shou, a Haipeng Song d WL and RN. Dual CEA/CD44 targeting to colorectal cancer cells using nanobody-conjugated hyaluronic acid- modified mesoporous silica nanoparticles with pH- and redox-sensitivity. 2022;4707–17.
Ramasami P, Baabu S, Kumar HK, Gumpu MB, Kulandaisamy KJB et al. AJ,. Iron Oxide Nanoparticles: A Review on the Province of Its Compounds, Properties and Biological Applications. 2023.
Islam A. Hassanin 1 2, Ahmed N. Shama 1 AOE. Overcoming cancer drug resistance via nanomedicine-based combined drug delivery. 2022.
Espinosa A, Corato R, Di, Kolosnjaj-tabi J, Flaud P, Pellegrino T, Wilhelm C. The duality of Iron Oxide nanoparticles in Cancer Therapy. Amplification of Heating Efficiency by Magnetic Hyperthermia and Photothermal Bimodal Treatment; 2016.
Kumari S, Kondapi AK. Lactoferrin nanoparticle mediated targeted delivery of 5-Fluorouracil for enhanced therapeutic efficacy. Int J Biol Macromol. 2016.
Mirzaghavami PS, Khoei S, Khoee S, Shirvalilou S. Folic acid – conjugated magnetic triblock copolymer nanoparticles for dual targeted delivery of 5 – fluorouracil to colon cancer cells. Cancer Nanotechnol. 2022;3:1–18.
Chen X. ‡ab Danling Cheng,‡c Mengbin Ding, c Ningyue Yu, c Jiansheng Liu c JLO logo *c and LL. Tumor-targeting biomimetic sonosensitizer-conjugated iron oxide nanocatalysts for combinational chemodynamic–sonodynamic therapy of colorectal cancer. J Mater Chem B. 2022;10:4595–604.
Chen B, Xing J, Li M, Liu Y, Ji M. DOX@Ferumoxytol-Medical Chitosan as magnetic hydrogel therapeutic system for effective magnetic hyperthermia and chemotherapy in vitro. Colloids Surf B Biointerfaces. 2020;110896.
Li L, Jeong Y, Lee Y. A photosensitizer-conjugated magnetic iron oxide / gold hybrid nanoparticle as an activatable platform for photodynamic cancer therapy †. 2014;2929–37.
Alkahtane AA, Alghamdi HA, Aljasham AT, Alkahtani S. Saudi Journal of Biological Sciences Original article A possible theranostic approach of chitosan-coated iron oxide nanoparticles against human colorectal carcinoma (HCT-116) cell line. Saudi J Biol Sci. 2022;29:154–60.
Dabaghi M, Mohammad S, Rasa M, Cirri E, Ori A, Neri F et al. Iron Oxide nanoparticles carrying 5-Fluorouracil in combination with magnetic hyperthermia induce thrombogenic collagen fibers, Cellular stress, and Immune responses in Heterotopic human Colon cancer in mice. 2021.
Nafisi S, Maibach HI. Nanotechnology in Cosmetics. 2017.
Sztandera K, Gorzkiewicz M, Klajnert-Maculewicz B. Gold Nanoparticles in Cancer Treatment. Mol Pharm [Internet]. 2019 [cited 2022 Sep 5];16:1–23. https://pubs.acs.org/doi/abs/https://doi.org/10.1021/acs.molpharmaceut.8b00810.
Cabuzu D, Cirja A, Puiu R. AMG Biomedical Appl gold Nanopart. 2015;15:1605–13.
Lu SW. and G. Applications of Gold Nanoparticles in Cancer Imaging and Treatment.
Costantini PE, Giosia M, Di, Ulfo L, Petrosino A, Saporetti R, Fimognari C et al. Spiky Gold Nanoparticles for the Photothermal Eradication of Colon Cancer Cells. 2021.
Zhang Y, Zhou L, Tan J, Liu J, Shan X, Ma Y. Laser-triggered collaborative chemophotothermal e ff ect of gold nanoparticles for targeted colon cancer therapy. Biomed Pharmacother. 2020;130:110492.
Lote H, Cafferkey C, Chau I. PD-1 and PD-L1 blockade in gastrointestinal malignancies. Cancer Treat Rev. 2015;41:893–903.
Fakhrossadat Emami JOK. Jee-Heon Jeong,* and SY. Doxorubicin and Anti-PD-L1 Antibody Conjugated Gold Nanoparticles for Colorectal Cancer Photochemotherapy. Mol Pharm. 2019;16:1184–99.
Pabla B, Bissonnette M, Konda VJ. Colon cancer and the epidermal growth factor receptor: current treatment paradigms, the importance of diet, and the role of chemoprevention. 2015;6:133–42.
Liszbinski RB, Romagnoli GG, Gorgulho CM, Basso CR, Pedrosa VA, Kaneno R. Anti-EGFR-Coated Gold nanoparticles in Vitro carry 5-Fluorouracil to Colorectal Cancer cells. 2020.
Safwat MA, Soliman GM, Sayed D, Attia MA. Gold nanoparticles enhance 5-fluorouracil anticancer efficacy against colorectal cancer cells. Int J Pharm 2016.
Zhang X, Liu Z, Shen W, Gurunathan S. Silver Nanoparticles: Synthesis, Characterization, Properties, Applications, and Therapeutic Approaches. 2016.
Sunil T, Galatage AS, Hebalkar SV, Dhobale OR, Mali, Pranav S, Kumbhar SVN. and SGK. Silver Nanoparticles: Properties, Synthesis, Characterization, Applications and Future Trends.
Gomes HIO, Martins CSM, Prior JAV. Silver Nanoparticles as Carriers of Anticancer Drugs for Efficient Target Treatment of Cancer Cells. Nanomaterials [Internet]. 2021 [cited 2022 Sep 5];11. http://www.pmc/articles/PMC8069134/.
Gund M, Roopan SM, Khan FN, Jin JS, Kumar R, Kumar AS. Regioselective O-alkylation: synthesis of 1-{2-[(2- chloroquinolin-3-yl)methoxy]-6-chloro-4- phenylquinolin-3-yl}ethanones. 2012;1111–8.
Bilal, Javed. Zia-Ur-Rehman Mashwani, Abdullah Sarwer NIR& AN. Synergistic response of physicochemical reaction parameters on biogenesis of silver nanoparticles and their action against colon cancer and leishmanial cells. Artif Cells Nanomed Biotechnol. 2020;48:1340–53.
Fabiano C, Freitas D, Kimura E, Forti A, Curti E. Curcumin and silver nanoparticles carried out from polysaccharide-based hydrogels improved the photodynamic properties of curcumin through metal-enhanced singlet oxygen e ff ect. Mater Sci Eng C. 2020;112:110853.
Lu Y, Wan X, Li L, Sun P, Liu G. Synthesis of a reusable composite of graphene and silver nanoparticles for catalytic reduction of 4- nitrophenol and performance as anti-colorectal carcinoma. J Mater Res Technol 12:1832–43.
Perlstein B, Finniss SA, Miller C, Okhrimenko H, Kazimirsky G, Cazacu S et al. TRAIL conjugated to nanoparticles exhibits increased anti-tumor activities in glioma cells and glioma stem cells in vitro and in vivo Benny. 2013;15:29–40.
Birtekocak F, Melike G, Cevik O. Conjugated silver nanoparticle synthesis, characterization and therapeutic effects on HT-29. Colon Cancer Cells. 2021;20:45–56.
Rozalen M, *a M, S´anchez-Polo aM. Fern´andez-Perales a TJW and JR-U. Synthesis of controlled-size silver nanoparticles for the administration of methotrexate drug and its activity in colon and lung cancer cells. 2020;10646–60.
Pushpalatha C, Suresh J, Gayathri VS, Sowmya SV, Augustine D, Alamoudi A, et al. Zinc Oxide Nanoparticles: Rev Its Appl Dentistry. 2022;10:1–9.
Singh TA, Das J, Sil PC. Zinc oxide nanoparticles: a comprehensive review on its synthesis, anticancer and drug delivery applications as well as health risks. Adv Colloid Interface Sci. 2020;102317.
Mishra PK, Mishra H, Ekielski A. Zinc oxide nanoparticles: a promising nanomaterial for biomedical applications. Drug Discov Today. 2017;6446.
Racca L, Rosso G, Carofiglio M, Fagoonee S, Mesiano G, Altruda F et al. Effective combination of biocompatible zinc oxide nanocrystals and high – energy shock waves for the treatment of colorectal cancer. Cancer Nanotechnol. 2023;1–21.
Szabo C, Ransy C, Módis K, Andriamihaja M, Murghes B, Coletta C et al. Regulation of mitochondrial bioenergetic function by hydrogen sulfide. Part I. Biochemical and physiological mechanisms. 2014.
Pan X, Qi Y, Du Z, He J, Yao S, Lu W et al. Zinc oxide nanosphere for hydrogen sulfide scavenging and ferroptosis of colorectal cancer. J Nanobiotechnol. 2021;1–17.
Ye H, Wu K, Liu Y, Zhu Y, Luo H. Zinc oxide nanoparticle attenuates chemotherapy resistance by inducing cell stemness progression of colorectal cancer via miR-1321/HIF-2α axis. Arab J Chem. 2022;15:103938.
Shahnaz M, Danish M, Hisyamuddin M, Ismail B, Tahir M, Nasir M et al. Anticancer and apoptotic activity of biologically synthesized zinc oxide nanoparticles against human colon cancer HCT-116 cell line- in vitro study. 2019;14.
Subramaniam VD, Ramachndran M, Marotta F. Comparative study on anti-proliferative potentials of zinc oxide and aluminium oxide nanoparticles in colon cancer cells. 2019;90:241–7.
Chen Z, Zhuang J, Pang J, Liu Z, Zhang P, Deng H et al. Application of a cationic amylose derivative loaded with single-walled carbon nanotubes for gene delivery therapy and photothermal therapy of colorectal cancer. 2022;1052–61.
Jin H, Gao S, Song D, Liu Y, Chen X. Intratumorally CpG immunotherapy with carbon nanotubes inhibits local tumor growth and liver metastasis by suppressing the epithelial – mesenchymal transition of colon cancer cells. 2020;278–85.
Boncel SB, Pluta A, Skonieczna M, Gondela A, Maciejewska B, Herman AP et al. Hybrids of Iron-Filled Multiwall Carbon Nanotubes and Anticancer Agents as Potential Magnetic Drug Delivery Systems: In Vitro Studies against Human Melanoma, Colon Carcinoma, and Colon Adenocarcinoma. 2017;2017.
Lee P, Chiou Y, Wong J, Peng C, Shieh M. Biomaterials Targeting colorectal cancer cells with single-walled carbon nanotubes conjugated to anticancer agent SN-38 and EGFR antibody. Biomaterials. 2013;34:8756–65.
Awad NS, Haider M, Paul V, Alsawaftah NM, Jagal J, Pasricha R et al. Ultrasound-Triggered Liposomes Encapsulating Quantum Dots as Safe Fluorescent Markers for Colorectal Cancer. 2021.
Khan FA, Lammari N, Shezad A, Siar M. Quantum dots encapsulated with curcumin inhibit the growth of colon cancer. Breast cancer Bacterial Cells. 2020.
Fakhroueian Z, Vahabpour R, Assmar M, Massiha A, Zahedi A, Esmaeilzadeh P et al. ZnO Q-dots as a potent therapeutic nanomedicine for in vitro cytotoxicity evaluation of mouth KB44, breast MCF7, colon HT29 and HeLa cancer cell lines, mouse ear swelling tests in vivo and its side effects using the animal model. Artif Cells, Nanomedicine, Biotechnol. 2018;46:S96–111.
Pilch J, Kowalik P, Bujak P, Nowicka AM. Quantum Dots as a Good Carriers of Unsymmetrical Bisacridines for Modulating Cellular Uptake and the Biological Response in Lung and Colon Cancer Cells. 2021.
Pang J, Galactosylated PW, Liu W, Zhu Y, Wang F, Li X et al. Galactosylated chitosan- functionalized mesoporous silica nanoparticles for efficient colon cancer cell-targeted drug delivery. 2018.
Chen N, Souris JS, Cheng S, Chu C, Konda V, Dougherty U et al. Lectin-functionalized mesoporous silica nanoparticles for endoscopic detection of Premalignant Colonic lesions Nai-Tzu. 2017;13:1941–52.
Eikanger MM, Dakota S, States U, Antony RM. Targeting Colon cancer cells with enzyme-triggered Casein-gated Release of Cargo from Mesoporous silica-based nanoparticles. 2022;32:2353–65.
Hirad, Sinafar. Hassan Noorbazargan FT& VN. Evaluation of apoptotic and anti-metastatic effects by modified mesoporous silica nanoparticles (MSNs) with zn and NH2 containing cisplatin on HT-29 cell line. J Nanoparticle Res. 2023;83.
Nobahari M. Curcumin-Loaded Iron Oxide nanoparticles coated with Sodium Alginate and Hydroxyapatite and their cytotoxic effects against the HT-29 and MCF-7. Cancer Cell Lines. 2022;17:1–11.
Esmaelbeygi E, Khoei S, Khoee S, Eynali S, Esmaelbeygi E, Khoei S et al. Role of iron oxide core of polymeric nanoparticles in the thermosensitivity of colon cancer cell line role of iron oxide core of polymeric nanoparticles in the thermosensitivity of colon cancer cell line HT-29. 2015;6736.
Jamuna Bai Aswathanarayan RRV&, UM, To. Anticancer activity of metal nanoparticles and their peptide conjugates against human colon adenorectal carcinoma cells. Artif Cells Nanomed Biotechnol. 2018;46:1444–51.
Akbari A. Eshkiki, Sara Mayahi SMA. In-vitro investigation of curcumin coated gold nanoparticles effect on human colorectal adenocarcinoma cell line. Nanomed Res the. 2022;7:66–72.
Al-ani LA, Kadir FA, Hashim NM, Julkapli NM, Seyfoddin A, Lu J, et al. The impact of curcumin-graphene based nanoformulation on cellular interaction and redox-activated apoptosis: an in vitro colon cancer study. Heliyon. 2020;6:e05360.
Akhtar S, Asiri SM, Alam F, Gunday ST, Iqbal A, Alrushaid N, et al. Formulation of gold nanoparticles with hibiscus and curcumin extracts induced anti-cancer activity. Arab J Chem. 2022;15:103594.
Shathviha PC, Ezhilarasan D, Rajeshkumar S. β -sitosterol mediated silver nanoparticles induce cytotoxicity in human Colon cancer HT-29 cells. 2021;13:42–6.
Aboulthana WM, Ibrahim NE, Mohamed N, Seif MM, Hassan AK, Mahmoud A et al. Evaluation of the Biological efficiency of silver nanoparticles biosynthesized using Croton tiglium L. Seeds Extract against Azoxymethane Induced Colon cancer in rats. 2020;21:1369–89.
Berehu HM, Anupriya S, Khan I, Chakraborty R. Cytotoxic potential of Biogenic Zinc Oxide nanoparticles synthesized from Swertia Chirayita Leaf Extract on Colorectal Cancer cells. 2021;9:1–13.
Boskabadi SH, Balanezhad SZ, Neamati A. The green-synthesized zinc oxide nanoparticle as a novel natural apoptosis inducer in human breast (MCF7 and MDA-MB231) and colon (HT-29) cancer cells. Inorg Nano-Metal Chem. 2020;0:1–11.
Paul K, Gowda BHJ, Hani U, Chandan RS, Mohanto S, Ahmed MG et al. Traditional Uses, Phytochemistry, and Pharmacological Activities of Coleus amboinicus: A Comprehensive Review. Curr Pharm Des [Internet]. 2024 [cited 2024 May 15];30:519–35. https://pubmed.ncbi.nlm.nih.gov/38321896/.
Elzoghby AO, Hemasa AL, Freag MS. Hybrid protein-inorganic nanoparticles: from tumor-targeted drug delivery to cancer imaging. J Control Release. 2016;243:303–22.
Mukherjee S, Chowdhury D, Kotcherlakota R, Patra S, Vinothkumar B, Bhadra MP et al. Potential Theranostics Application of Bio-Synthesized Silver Nanoparticles (4-in-1 System). Theranostics [Internet]. 2014 [cited 2024 Jun 17];4:316–35. http://www.thno.org.
Kummara S, Patil MB, Uriah T. Synthesis, characterization, biocompatible and anticancer activity of green and chemically synthesized silver nanoparticles – A comparative study. Biomed Pharmacother. 2016;84:10–21.
Deng M, Huang Z, Zou Y, Yin G, Liu J, Gu J. Fabrication and neuron cytocompatibility of iron oxide nanoparticles coated with silk-fibroin peptides. Colloids Surf B Biointerfaces. 2014;116:465–71.
Byrne SJ, Williams Y, Davies A, Corr SA, Rakovich A, Gunko YK et al. Jelly Dots: Synthesis and Cytotoxicity Studies of CdTe Quantum Dot–Gelatin Nanocomposites. Small [Internet]. 2007 [cited 2024 Jun 17];3:1152–6. https://onlinelibrary.wiley.com/doi/full/https://doi.org/10.1002/smll.200700090.
Peralta DV, Heidari Z, Dash S, Tarr MA. Hybrid paclitaxel and gold nanorod-loaded human serum albumin nanoparticles for simultaneous chemotherapeutic and photothermal therapy on 4T1 breast cancer cells. ACS Appl Mater Interfaces [Internet]. 2015 [cited 2024 Jun 17];7:7101–11. https://doi.org/10.1021/acsami.5b00858.
Ahmad MW, Kim CR, Baeck JS, Chang Y, Kim TJ, Bae JE, et al. Bovine serum albumin (BSA) and cleaved-BSA conjugated ultrasmall Gd2O3 nanoparticles: synthesis, characterization, and application to MRI contrast agents. Colloids Surf Physicochem Eng Asp. 2014;450:67–75.
Vakhrusheva TV, Gusev AA, Gusev SA, Vlasova II. Albumin reduces thrombogenic potential of single-walled carbon nanotubes. Toxicol Lett. 2013;221:137–45.
Acknowledgements
The authors extend their appreciation to the Deanship of Scientific Research at King Khalid University for funding this work through a Large Group Research Project under grant number RGP.2/21/45.
Funding
The authors extend their appreciation to the Deanship of Scientific Research at King Khalid University for funding this work through a Large Group Research Project under grant number RGP.2/21/45.
Author information
Authors and Affiliations
Contributions
Soumya Narayana: Writing – Original draft, Artwork. B.H. Jaswanth Gowda: Supervision, Conceptualization, Visualization, Writing – Original draft, Review & Editing, Artwork. . Umme Hani: Writing – Original draft, Review & Editing. Sharmin Sultana Shimu: Writing – Review & Editing. Karthika Paul: Supervision, Writing – Review & Editing, Artwork, Project administration. Avinaba Das: Writing – Review & Editing. Sumel Ashique: Writing – Review & Editing. Mohammed Gulzar Ahmed: Writing – Review & Editing. Maryam Abbasi Tarighat: Writing – Review & Editing. Gholamreza Abdi: Writing – Review & Editing, Visualization, Project administration.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Narayana, S., Gowda, B., Hani, U. et al. Inorganic nanoparticle-based treatment approaches for colorectal cancer: recent advancements and challenges. J Nanobiotechnol 22, 427 (2024). https://doi.org/10.1186/s12951-024-02701-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12951-024-02701-3