Abstract
Research into mRNA vaccines is advancing rapidly, with proven efficacy against coronavirus disease 2019 and promising therapeutic potential against a variety of solid tumors. Adjuvants, critical components of mRNA vaccines, significantly enhance vaccine effectiveness and are integral to numerous mRNA vaccine formulations. However, the development and selection of adjuvant platforms are still in their nascent stages, and the mechanisms of many adjuvants remain poorly understood. Additionally, the immunostimulatory capabilities of certain novel drug delivery systems (DDS) challenge the traditional definition of adjuvants, suggesting that a revision of this concept is necessary. This review offers a comprehensive exploration of the mechanisms and applications of adjuvants and self-adjuvant DDS. It thoroughly addresses existing issues mentioned above and details three main challenges of immune-related adverse event, unclear mechanisms, and unsatisfactory outcomes in old age group in the design and practical application of cancer mRNA vaccine adjuvants. Ultimately, this review proposes three optimization strategies which consists of exploring the mechanisms of adjuvant, optimizing DDS, and improving route of administration to improve effectiveness and application of adjuvants and self-adjuvant DDS.
Similar content being viewed by others
Introduction
Since the outbreak of coronavirus disease 2019 (COVID-19) in early 2020, severe acute respiratory syndrome coronavirus 2 has spread globally, resulting in over 250 million confirmed cases [1]. In the fight against COVID-19, mRNA vaccines have emerged as a prominent solution. These vaccines offer significant advantages over traditional vaccine technologies, including higher production efficiency and enhanced safety. Moderna, a leading entity among mRNA vaccine developers, rapidly identified the antigenic sequence of the virus and produced the first mRNA-1273 vaccine within just 45 days. This vaccine later demonstrated a 94.1% efficacy rate in a phase III clinical trial, underscoring the promising potential of mRNA vaccine technology for future infectious disease responses [2]. The mRNA vaccine has emerged as a vital tool in humanity’s arsenal against the novel coronavirus. Beyond their application in viral infections, mRNA vaccines are also being explored for their potential in cancer treatment. Several clinical trials involving mRNA-based cancer vaccines have yielded promising outcomes, highlighting the potential of mRNA vaccines in oncology. This development points to a broader scope of application for mRNA technology, potentially revolutionizing the approach to cancer treatment [3, 4].
The remarkable success of mRNA vaccines can be attributed to several key advantages, we summarized them by the acronym “WESP” (Fig. 1): (1) Wide applicability. mRNA vaccines can encode almost any protein and facilitate post-translational modifications within cells. This capability reduces immunogenicity while ensuring the functionality of protein products, leading to significant breakthroughs in treating various diseases [5,6,7]. (2) Efficiency. Appropriate modification and optimization of sequence can significantly improve mRNA stability and translation efficiency. Currently, there is already an efficient drug delivery systems (DDS) that can achieve rapid uptake and cytoplasmic expression of mRNAs [8,9,10]. (3) Safety. Unlike DNA vaccines, the mRNA platform is non-infectious and non-integrating, eliminating the risk of infection or gene insertion [11]. (4) Productiveness. Once the genome sequence of a pathogen is known, mRNA encoding the antigenic protein can be swiftly designed and produced. This was exemplified by the rapid development of mRNA vaccines for COVID-19. Furthermore, the high yield from in vitro transcription not only ensures rapid production but also makes the process cost-effective and scalable [2].
Aforementioned attributes collectively underscore the effectiveness and potential of mRNA vaccines as a pivotal tool in modern medicine, capable of addressing both infectious diseases and complex conditions like cancer. The mRNA vaccines offer a promising approach to cancer treatment by their ability to encode tumor-related antigens and elicit an immune response. The core principle of mRNA cancer vaccines involves transporting transcripts that encode for tumor-associated antigens or tumor-specific antigens into the cytoplasm of host cells, particularly antigen-presenting cells (APCs). This capability allows the immune system to recognize and target cancer cells effectively, potentially transforming cancer therapy by providing a highly specific and adaptive treatment option [12,13,14]. Currently, mRNA cancer vaccines made significant achievements in the treatment of prostate cancer. The prostate cancer vaccines CV9103, developed by Curevacs (Germany), has already undergone phase I/II clinical trials, during which it was demonstrated to be well tolerated and to elicit a favorable immune-activation [15]. In addition, in the phase I trial of a novel personalized mRNA neoantigen vaccine, it stimulated high-magnitude neoantigen-specific and long-lived polyfunctional CD8 + T cells in pancreatic cancer, resulting in a longer recurrence-free survival [16]. To date, mRNA vaccines have made notable achievements in the field of cancer treatment.
However, mRNA vaccines still face several challenges. The primary concern is their instability and inability to penetrate the physiological barriers in human body, which prevents them from reaching target cells [17]. In the human body, mRNAs are susceptible to degradation by RNases or recognition and phagocytosis by macrophages or dendritic cells (DCs) in the liver [1]. While the naked mRNA can still be taken up by the cell, the process is too inefficient. To increase effectiveness, repeated administrations are required [17]. Nevertheless, excessive amounts of drug can lead to immune-related adverse reactions [18]. In addition, the mRNA vaccine (BNT162b2) administered by intramuscularly injection was mainly distributed in the site of injection and the liver, resulting in reversible liver damage in animals [19]. Therefore, it is challenging to achieve specific organ targeting for mRNA cancer vaccines.
To cope with these challenges, the design and selection of adjuvants is crucial. Adjuvants have the ability to enhance body’s immune response, optimize drug delivery routes, reduce drug toxicity, and enhance drug efficacy by precisely targeting and reducing the total drug volume. However, the definition of adjuvant remains controversial. According to the traditional view, an mRNA vaccine comprises three components: mRNA sequence containing antigen, DDS or vector, and adjuvant. Adjuvant is an immunostimulant that is added in addition to a vector or DDS to non-specifically enhance the body’s specific immune response to the antigen [7]. Conversely, some scholars argue that adjuvants should include DDS in addition to traditional adjuvants [20]. This is because some self-adjuvant delivery materials, such as mesoporous silicon rods, have the ability to enhance the strength, breadth and durability of the immune response itself [21]. In this review, we will discuss immunostimulants or DDS that have an immunopotentiation effect on mRNA vaccines, all of which will be considered as adjuvants. The lack of a systematic overview and summary of the mechanism of action, combined with the complexity of the mechanism and the broad definition of adjuvant, has caused inconvenience and confusion for researchers in designing appropriate vaccine adjuvants [20]. To aid researchers in comprehending adjuvants of mRNA vaccines, this review will describe the design strategies for adjuvants, introduce their mechanisms, and summarize the limitations and side effects of existing adjuvants, and provide prospects for future improvement.
Design strategies for immunostimulants
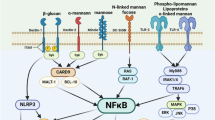
Immunostimulants, often recognized as danger signal molecules, function as pathogen-associated molecular patterns (PAMPs), damage-associated molecular patterns, or their mimics. These substances are pivotal in triggering the innate immune response. They achieve this by targeting pattern recognition receptors (PRRs) on APCs. Upon activation, APCs undergo a maturation process during which their antigen phagocytic activity ceases, and their capability to present antigens is enhanced. Concurrently, these matured APCs express higher levels of co-stimulatory signals and cytokines, which are crucial for initiating and amplifying adaptive immune responses [22]. Currently, the design strategies for immunostimulants are targeting different PRRs to lead different cytokine secretion [23]. Based on the different targeting pathway, there are four dominant design strategies for immunostimulants. Besides, there is a special design strategy for immunostimulants, which is using cytokines as immunostimulants (Fig. 2).
The design strategies for immunostimulants. Various types of immunostimulants activate different PRRs, leading to the secretion of various cytokines and inducing diverse adaptive immune responses. Immunostimulants activate TLRs, cGAS-STING, CLRs, other PRRs, or directly release cytokines to induce and modulate adaptive immune responses. Binding to TLRs heterodimers initiates MyD88 pathway and activated NF-κB and ERK1/2 to enhance pro-inflammatory cytokines. The mtDNA and dsDNA initiates the conversion of cGAS into cGAMP and consequently activates STING to release TBK to activates IRF 3 and IKKi to activates NF-κB. Finally, IRF 3 induces type 1 interferons, cross presentation and CTL and NF-κB induces pro-inflammatory cytokines and activate Th1 cells. Targeting to most CLRs activated NF-κB to enhance pro-inflammatory cytokines. Notably, targeting to CD205 and CD206 can enhance endocytosis and antigen presentation. Common immunostimulants targeting to NLRs (NOD 1 and NOD 2) activated NF-κB ultimately produces a predominantly Th2-type of immune response. Alternatively, immunostimulants targeting to MDA5 and RIG-I activate IRF 3 and IRF 7 respectively. At length, IRF 3 and IRF 7 induce type 1 interferons, cross presentation, CTL and activate Th1 cells
Targeting TLRs pathway
Immunostimulants can enhance antigen presentation and upregulate costimulatory signals and cytokine expression by targeting Toll-like receptors (TLRs) on APCs, ultimately enhancing the adaptive immune response [24,25,26,27,28]. One classical mechanism of action for immunostimulants involves their binding to TLRs heterodimers, specifically TLR2/1 or TLR2/6. This interaction initiates signaling through the myeloid differentiation primary response 88 (MyD88) pathway. Subsequent to this signaling event, the transcription factor nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) is activated. NF-κB activation leads to the production of pro-inflammatory cytokines, which play a critical role in the differentiation of naive T cells into T helper 1 cells [29, 30]. Simultaneously, the activation of the extracellular signal-regulated kinase 1/2 (ERK1/2) pathway enhances the signaling cascade, leading to increased expression of the c-Fos protein. This increase in c-Fos levels plays a pivotal role in modulating cytokine expression; specifically, it enhances the production of interleukin-10 (IL-10) and suppresses the expression of interleukin-12 (IL-12). This drives the conversion of naive T cells into Th2-type cells [31]. Consequently, immunostimulants targeting TLR2 primarily induce Th2-type adaptive immune response. Here are some common immunostimulants that target TLRs: lipopolysaccharide (LPS), monophosphoryl lipid (MPL), cytosine phospho-guanosine oligonucleotides [CpG (TLR9a)] and R848. LPS, a potent TLR4 agonist, is a natural immune adjuvant from the outer membrane of Gram-negative bacteria [32]. MPL contains the adjuvant active principle of LPS (lipid A) [33]. CpG (TLR9a), an agonist of TLR9, has been widely used as an adjuvant in mRNA cancer vaccine. R848 is recognized by TLR7 and TLR8. The immune cells like monocytes and macrophages are activated by TLR7 and TLR8 and then secrete cytokines to mediate innate and adaptive immune responses [34].
Targeting cGAS-STING pathway
The cyclic guanosine monophosphate (GMP)-adenosine monophosphate (AMP) synthase (cGAS) functions as a cytoplasmic DNA sensor that is activated by the presence of double-stranded DNA (dsDNA) and mitochondrial DNA (mtDNA). Upon activation by such DNA, cGAS catalyzes the conversion of cytoplasmic AMP and GMP into cyclic guanosine monophosphate-adenosine monophosphate (cGAMP) [35]. Subsequently, cGAMP aggregates and activates STING through conformational change, which then activates NF-κB and interferon regulatory factor 3 (IRF 3), promoting the production of type I interferons and pro-inflammatory cytokines. Finally, the APCs will have a terrific ability to present or cross-present antigens [36, 37]. Immunostimulants that target the cGAS-STING pathway include nucleotide and non-nucleotide small molecule agonists. The former are mainly natural ligand molecules which based on cyclic dinucleotides. For instance, the cyclic dimeric adenosine monophosphate, cyclic dimeric guanosine monophosphate, 3’,3’-cGAMP, and 2’,3’-cGAMP [38]. Examples of common non-nucleotide small molecule agonists include CF 501 and DMXAA [39, 40].
Targeting CLRs pathway
The C-type lectin receptors (CLRs) superfamily comprises various receptors, such as MINCLE, DC-SIGN, Dectin-1, Dectin-2, CD205, CD206, and others. CLRs are primarily located on cell membranes and act as antigen receptors for capturing and presenting antigens [41, 42]. In most instances, immunostimulants that have a carbohydrate structure can activate the CLRs and stimulate the APCs to initiate the internalization, presentation and processing of antigens, thereby enhancing the adaptive immune response [43, 44]. Comparatively, there has been less research on the potential of immunostimulants targeting CLRs pathway. However, it has been found that fungal mannans can act as immune adjuvants, which can elicit a potent antigen-specific neutralizing antibodies to increase the immune response and can be harnessed for vaccine [45]. Immunostimulants that targeted CLRs pathway has a great deal of untapped potential.
Targeting other PRRs
In addition to the three major pathways mentioned above, the nucleotide-binding oligomerization domain-like receptors (NLRs) family which includes nucleotide-binding oligomerization domain 1 (NOD1), nucleotide-binding oligomerization structural domain 2 (NOD2), and NOD-like receptor thermal protein domain associated protein 3 (NLRP 3), can also be targeted [46]. Common immunostimulants that target NLRs are muramyl dipeptide and complete Freund’s adjuvant, which ultimately produces a predominantly Th2-type of immune response [28]. Furthermore, retinoic acid-inducible gene I-like receptors family can also be targeted as they primarily recognize RNA. The main members of this family are melanoma differentiation-associated gene 5 (MDA5) and retinoic acid-induced gene I (RIG-I) [47].
Cytokine immunostimulant
In addition to the immunostimulants mentioned above, there is a distinct category of immunostimulants called cytokines. Cytokines are small soluble polypeptide proteins secreted by immune and non-immune cells under certain conditions. They play a regulatory role intercellularly and intracellularly. Their effectiveness as adjuvants highly depends on the dose, form, route of administration, and the type of co-administered vaccine [48]. Interleukin-2 (IL-2) and granulocyte-macrophage colony stimulating factor (GM-CSF) are two mature cytokine immunostimulant. GM-CSF promotes the maturation and activation of APCs and IL-2 enhances immune response of T cells [49]. Interferon-alpha (IFN-alpha) and tumor necrosis factor (TNF) are also being investigated for their potential adjuvant effects. They enhance the immunoregulatory function of natural killer cells, promote the differentiation of T lymphocytes and play a broad up-regulatory role in the body’s immune response [50].
Application of immunostimulant in mRNA cancer vaccines.
The current applications of immunostimulant in mRNA cancer vaccines are as follows: (1) Protamine. Arginine-rich protamine peptides have been demonstrated to form a complex with mRNA, subsequently activating TLR7/8 pathways to elicit T-cell and B-cell-dependent immune responses against non-small-cell lung cancer, prostate cancer, and melanoma [51,52,53,54]. (2) DP7. The cationic peptide DP7 with cholesterol-modified (VQWRIR-VAVIRK) activates the TLR2-MyD88-IKK-NF-κB pathway and enhance the immune responses stimulated by the mRNA cancer vaccine. Notably, DP7 has been identified as an effective immunostimulant for personalized mRNA cancer vaccines [55]. (3) R848. The TLR7/8 agonist R848 modified with palmitic acid (C16-R848) has been demonstrated to effectively activate the adaptive immune response and to enhance the delivery efficiency of the mRNA cancer vaccine in prostate and lymphoma tumor model mice [56]. (4) α-galactosylceramide (α-GC). The α-GC is a glycolipid antigen that can be presented in the CD1d, the MHC-I-like molecule on APCs, to stimulate invariant natural killer T cells and evoke pluripotent innate and adaptive antitumor immune response in lymphoma animal models [57].
Design strategies for self-adjuvant drug delivery systems for mRNA vaccines
The definition of DDS is carrier materials that load antigen and increase the ability of APCs to uptake and present antigen [20]. The main function of DDS for mRNA vaccines is to aid in antigen presentation by assisting mRNAs in crossing the three barriers of the body: the extracellular barrier, lysosomal escape, and intracellular immunity. This results in an increase in the antigenic signals on the surface of APCs [1]. Notably, the precise DDS for mRNA vaccines can reduce the toxicity of the vaccine, reduce the total amount of drug, and increase the efficacy of the vaccine. Currently, there are two main types of delivery vectors: viral vectors and non-viral vectors. Although viral vectors have the advantage of high transfection efficiency, the safety concerns remain a significant issue. The enthusiasm for viral vector research has largely waned after two clinical trials in which the use of viral vectors resulted in the deaths of volunteers [58, 59]. Attention has shifted to non-viral vectors due to the stagnation of viral vector research. Non-viral vectors, with their low toxicity and low immunogenicity, have become one of the hottest research directions at present. Nevertheless, drugs delivered by non-viral vectors also have the disadvantage of low escape efficiency in nuclear endosomes or lysosomes and weak ability to target to cells, tissues, and organs [60, 61]. There is an urgent need for novel non-viral DDS for mRNA vaccines to overcome these difficulties. There are four common strategies (Fig. 3):
The design strategies for self-adjuvant drug delivery systems. Self-adjuvant delivery systems increased antigen presentation to enhance adaptive immune responses by enhancing the bioavailability of antigens; targeting immune organs or cells; promoting antigen cross-presentation; and activating inflammasome. Enhancing the bioavailability of antigens can be achieved by sustained releasing antigens, formatting immune niches, and protecting antigens from breakdown. Targeting APCs can be achieved by using nanoscale materials, constructing highly ordered and repetitive spatial structures to mimic pathogens and targeting specific receptors on APCs. Targeting lymph node can achieved through the design of suitable dimensions (20 to 200 nm), surface properties (negative charge and hydrophobicity) and albumin-hitchhiking. Promoting antigen cross-presentation can be enabled in three main ways, proton sponge effect, destabilization of membranes and photochemical internalization
Enhancing the bioavailability of antigens
(1) Sustained Release: By prolonging the presence of antigens within the immune system through their sustained release, there is a prolonged opportunity for immune system interaction. This method ensures that antigens are continuously available to stimulate an immune response [62]. (2) Formation of Immune Niches: Creating immune niches at the site of injection can recruit additional immune cells. This influx of immune cells enhances antigen uptake and activates the adaptive immune response, thereby stimulating the release of cytokines and chemokines. This process intensifies the immune system’s engagement with the antigen [63]. (3) Protection Against Breakdown: Protecting antigen from breakdown by mRNA enzymes and slowing down the antigen digestion process are crucial, which is one of the benefits of lipid nano-particle (LNP) [4, 64, 65].
Targeting immune organs or cells
Targeting APCs in the immune microenvironment can enhance immune presentation and phagocytosis. Several methods can be employed to achieve this: (1) Using microscale or nanoscale materials to adjust the dimension of antigen to mimic pathogens [66]. (2) Constructing highly ordered and repetitive spatial structures similar to those inherent in pathogens allows the immune system to recognize and respond to these structural features with greater sensitivity [67]. Furthermore, these structures can facilitate the co-aggregation of B cell receptor (BCR) and the eventually produce high-affinity antibodies and memory B cells [68, 69]. (3) Targeting the specific receptors on APCs, such as Fc receptors [70,71,72,73].
Lymph node metastasis is a significant prognostic factor that signals a worse prognosis and reflects the necessity of systemic therapy in the majority of cancer patients [74]. For patients with oral squamous cell carcinomas, the five-year survival rate can decline to below 20% when lymph node metastasis occur [75]. Consequently, targeting the mRNA vaccines to lymph nodes is an ideal design strategy for DDS. The precise delivery of mRNA vaccine to lymph nodes can change the pharmacokinetics, activate a long-lasting and potent immune response, and reduce undesired systemic toxicity and side effects [76, 77]. In addition, targeting lymph nodes can significantly augment the innate immune response, particularly by activating macrophages within the lymph nodes, which in turn enhances the anti-tumor efficacy of mRNA vaccines [78]. The high anti-tumor efficacy of the lymph node-targeting DDS demonstrates considerable potential as a design strategy for mRNA vaccines [77].
Effective strategies to achieve this include: (1) Designing a DDS for mRNA vaccines of suitable dimensions (20 to 200 nm) and surface properties (net negative charge and hydrophobicity) that relies on passive diffusion to enter the afferent lymphatics and subsequently enter lymph nodes [79,80,81]. (2) Albumin-hitchhiking, which exploits the ability of endogenous albumin to circulate in the lymphatic system. Binding the antigen to endogenous albumin, thereby antigen is transported to the lymph nodes via the albumin train [82, 83]. Notably, this method is found to be highly effective in inhibiting the growth of primary or metastatic tumors in mice [84].
Alternatively, in LNP, there is a special target needs to be considered cautiously, the non-liver tissues target [85]. LNP is a mature technique for the delivery of genetic medicines. However, its therapeutic application is limited due to the liver accumulation. The apolipoprotein E in serum binds to LNP and causes mRNA to preferentially enter the liver, which produces enzymes that interfere with the effectiveness of the mRNA vaccine, preventing the LNP@mRNA from achieving its full potential [86]. In order to address liver accumulation, the addition of the selective organ targeting (SORT) lipids can achieve specific targeting of organs such as the liver, lungs, spleen, etc., thus enabling non-liver tissues target [85].
Promoting antigen cross-presentation
In most cases, exogenous antigens are just internalized by APCs and only presented to CD4+ T cells by major histocompatibility complex II (MHC II) molecules, without any cross-presentation which is the process by which exogenous antigens are presented to CD8 + T cells by major histocompatibility complex I (MHC I) molecules [87, 88]. This type of presentation elicits a weak immune response. To increase the strength of the immune response, some DDS for mRNA vaccines have enabled antigen cross-presentation by facilitating the escape of antigens from lysosomes or endosomes [20]. Cross-presentation can be enabled in three main ways: (1) Proton sponge effect. When some DDS for mRNA vaccines containing protonable amine groups are internalized by the APCs, substantial protons are absorbed by the APCs. To neutralize the acidic environment of the lysosome or endosomal of APCs, chloride ions and water will flow from the cytoplasm into endosomes or lysosomes in large amounts, which causes swelling and rupture of the endosomes. Subsequently, the antigens are released into the cytoplasm, which facilitates the cross-presentation of the antigen by MHC I molecules [89]. (2) Binding or fusing to the membranes of endosomal or lysosomal. This process destabilizes the endosomal/lysosomal membrane, releasing the antigen into the cytoplasm, which facilitates the cross-presentation of the antigen by MHC I molecules [90]. (3) Photochemical internalization release technology. This is an emerging technology that uses photosensitizers to release antigens into the cytoplasm through light-induced disruption of endosomal membranes [91,92,93].
Activating inflammasome
Inflammasome, multi-protein complexes assembled with the participation of PRRs, is an important component of the innate immune system [94]. Gold nanoparticles, one of the most mature inorganic nano-drug delivery systems, can promote the production of antigen-specific antibodies and Th2 cytokines through the activation of NLRP3 inflammasome, in addition to the protection of antigens from hydrolysis by mRNA enzymes and targeting to the lymph nodes mentioned above [95].
Application of self-adjuvant DDS in mRNA cancer vaccines
Currently, the applications of self-adjuvant DDS in mRNA cancer vaccines are listed as follows: (1) LNP. The use of endogenously LN-targeting LNP can improve the effectiveness of mRNA vaccine by stimulating robust humoral responses and T follicular helper cell [96]. The 113-O12B is an effective LN-targeting DDS for mRNA cancer vaccines and can improve the effectiveness of anti-tumour treatment [77]. The BNT-113, an mRNA vaccine encapsulated within LNP, has demonstrated encouraging efficacy against head and neck cancer. It is currently undergoing phase II clinical trials (NCT04534205) [97]. In addition, the mRNA-4157 is a personalized mRNA vaccine encapsulated in LNP too. It can encode multiple neoantigens, thereby inducing neoantigen-specific T cells and eliciting anti-tumor immune responses in patients with head and neck cancer [98]. Furthermore, loading comb-structured mRNA, which consists of antigen-producing single-stranded mRNA, and adjuvant short double-stranded RNA, onto LNP enables immunostimulation in different formulations of mRNA cancer vaccines [99]. The mRNA vaccine combining all-trans-retinoic acid with LNP has shown significant tumor inhibition effects in animal model for the treatment of orthotopic colorectal tumors [100]. (2) Polyguanidine (PolyGu). Branched PolyGu nanovaccines are used to integrate immunostimulant functions into the DDS, resulting in self-adjuvating PolyGu nanovaccines. It can effectively stimulate and promoted the maturation of DCs through TLR4 and NLRP3 pathways, and exhibited strong immune activity in vivo. In addition, PolyGu can improve the delivery efficiency of mRNA as a DDS and effectively suppress tumour growth, thereby prolonging the survival of mice [101]. . (3) Self-assembled RNA origami (RNA-OG). The RNA-OG nanostructure functions as a TLR 3 agonist and is a suitable DDS for mRNA cancer vaccines due to its versatility in modification and robust synthesis. Studies have shown that the assembled RNA-OG-peptide nanovaccines induce DCs maturation, mobilize tumor-specific CD8 + T cell responses, and reduce tumor-mediated immunosuppression [102]. In the field of colorectal cancer, the lantern-shaped flexible origami can compress mRNA to nanoscale, thereby promoting its endocytosis by cells and improving translation efficiency. The mRNA nano-lantern facilitates the overexpression of Smad4, a tumor suppressor gene, in orthotopic colorectal tumor models, effectively inhibiting their growth [103]. This origami strategy offers a competitive DDS for mRNA-based therapies in the treatment of colorectal cancer. (4) Outer membrane vesicles (OMVs). OMVs contain numerous PAMPs that can effectively stimulate the innate immune system, facilitating T cell activation and antigen presentation. OMVs with surface decoration of lysosomal escape protein listeriolysin O and RNA binding protein, L7Ae, (OMV-LL) can be cross-presentation and OMV-LL mRNA significantly inhibits the progression of melanoma [104]. (5) Porous silica nanoparticles. This mRNA DDS is based on polyethylenimine-modified porous silica nanoparticles. It promotes effective antitumor immunity without evidence of systemic toxicity and off-target translation of mRNA [105]. It also inhibited distant metastatic tumors and improved anti-tumor responses in murine cancer models. (6) Iron oxide. This is a magnetically multi-functional RNA-loaded liposome that is capable of generating a robust anti-cancer immune response. Comparing to electroporation, this mRNA DDS activates DCs more effectively, resulting in superior tumor growth inhibition in animal models [106].
Deficiencies of the adjuvants of mRNA cancer vaccines
Current clinical trials of mRNA vaccines have shown that adverse reactions such as fatigue, pain at the injection site, myalgia, narcolepsy and neurological side effects can be triggered by the mRNA vaccines [107,108,109]. Notably, there are also a number of adverse reactions and limitations associated with adjuvants that need to be considered. Immune-related adverse event is one of the most notable side effects. Reports of anaphylactic reactions induced by mRNA vaccines are still being received [110]. At the injection site, adjuvanted vaccines are more reactogenic than non-adjuvanted vaccines, which can cause immune-related adverse events such as anaphylaxis. However, the symptoms are typically mild to moderate and of short duration [18]. Notably, immune activation and cytotoxicity may be triggered when injected above a certain dose of mRNA or ionizable lipids. Ultimately, allergic reactions and even cytokine storms may be triggered [111]. In preclinical mRNA vaccine studies, LNP were found to be highly inflammatory in mice, triggering a severe inflammatory response [112]. The ionizes lipids SM-102, which is ionizable and used in vaccines, may cause vaccinator to experience adverse effects such as nausea [113]. Besides, in PEGylated lipids, PEG may cause allergic reactions [114].
In addition, the unsatisfactory outcomes and unclear mechanisms of most approved adjuvants cannot be ignored. Comparatively, the mRNA vaccines have low immunogenicity and produce weak and short-lived immunity in the body [115]. The vaccine’s protective effect is low until two vaccinations were completed. Additionally, the effectiveness of vaccine declined with age, and is ineffective in the old age group [116]. Alternatively, the development of mRNA adjuvants is still in its early stages, and the construction of DDS for mRNA vaccines is immature. The mechanisms of many adjuvants remain unclear. Some basic problem such as the endosomal escape process remain unclear too [117]. Unclear mechanisms of adjuvants can result in improper use of adjuvants and the emergence of side effects. The deficiencies of the adjuvants of mRNA vaccines are summarized in Fig. 4.
The deficiencies of mRNA adjuvants and the prospects for improvement. The immune-related adverse event, unsatisfactory outcomes in old age group and unclear mechanism of adjuvants are three main deficiencies of the mRNA adjuvants. Alternatively, optimizing drug delivery systems, improving route of administration and further explored the mechanisms of adjuvant are three main prospects for the improvement of mRNA adjuvants
Prospects for improvement
Further explored the mechanisms of adjuvant
The formation of an immune niche (antigen depot), as mentioned above, is traditionally thought to be one of the important mechanisms of adjuvants. Nevertheless, with the deepening of researches, some researchers found that the removal of the “immune niche” after aluminum adjuvant administration didn’t substantial reduce the generation of B cell responses and antigen-specific T [118]. This suggests that the formation of an immune niche may not be the key mechanism of action of aluminum adjuvants. From this, we can further hypothesize whether other adjuvant systems are also like the aluminum adjuvant system. For more rational use of adjuvants and the development of new adjuvants, the mechanism of adjuvants should be better explored. Alternatively, the source of the adjuvant effect requires further researched. Immunostimulants and self-adjuvanted DDS for mRNA vaccines are not the only methods for achieving adjuvant effects. Editing the RNA itself can also produce adjuvant effect. However, the mechanism behind it requires further investigation [99].
Optimizing drug delivery systems
Using LNP as an example, DDS optimization can be achieved by optimizing lipid structure and targeted molecular. It has been shown that by modifying the lipid structure of lipid nanoparticles, including tail length, linkages and amine heads, and by optimizing the proportion of different lipid components of lipid nanoparticle formulations, lymph node-targeted delivery can be achieved to enhance vaccine immunity effects [77, 119]. Notably, the modification of targeting molecules on the surface of LNP or alteration of the properties of the LNP can enhance the efficacy of vaccine by targeting LNP delivery to specific cells or organs. For example, mannosylation of lipid nanoparticles can enhance the uptake of APCs [120]. Modulation of the surface charge of RNA-lipid complexes enables precise and efficient targeting of DCs [121]. In addition, for non-liver tissues target, permanent cationic SORT lipids (EPC, DDAB, and DOTAP) can be applied to shift tissue tropism from the liver to the lung [13]. Alternating the alkyl length of a lipid or changing the intermediate connecting group of LNP from an ester bond to an amide bond in the tail can also change the organ targeting of LNP to liver or lung [122, 123].
In addition to the existing DDS for mRNA vaccines, there are many novel DDS besides liposomes waiting to be discovered. For instance, engineered extracellular vesicles (EVs) with pathogen proteins is a promising alternative to LNP-mRNA vaccines. With their ability to naturally target and transport bioactive molecules, these engineered EVs are expected to overcome the problems of complex and non-continuous manufacturing processes and expensive materials involved in mRNA vaccine production [124].
Improving route of administration
Route of administration can greatly influence expression, kinetics organ distribution, and therapeutic outcome of LNP-mRNA vaccine [125, 126]. Intravenous administration, as mentioned above, has the potential to enhance the immune response to mRNA vaccines, although issues of targeting non-liver tissues remain to be addressed [121]. Nevertheless, topical administration also has its own unique local therapeutic effect, allowing for the supplementation of therapeutic proteins in specific tissues such as brain, heart, eyes [127,128,129]. In addition, there are other routes of administration such as the intranasal route [130]. In order to better exploit the advantages of different routes of administration, a comprehensive decision on which route to use needs to be made after careful consideration of factors such as the nature of the nanoparticles and the therapeutic indications. The prospects for improving of mRNA vaccines are summarized in Fig. 4.
Conclusion
Although there is still a long way to go in optimizing mRNA vaccines, their excellent biocompatibility, high tissue penetration, high nucleic acid encapsulation efficiency, low occurrence of off-target effects, cytotoxicity, and immunogenicity have made mRNA cancer vaccine one of the hottest research areas in vaccines today. Further researches are required to elucidate the mechanism of adjuvants and to optimize the design strategy and DDS for mRNA vaccines. Additionally, the development of new DDS for mRNA cancer vaccines and personalized mRNA cancer vaccines are promising avenues for future investigations. With the continued refinement of next-generation adjuvants, this new technology will help solve problems that traditional small-molecule and antibody therapies cannot, providing more effective and longer-lasting therapeutic outcome in the treatment of a wide range of diseases, including tumors, and improving healthcare in the near future.
Data availability
No datasets were generated or analysed during the current study.
Abbreviations
- DDS:
-
drug delivery systems
- COVID-19:
-
coronavirus disease 2019
- APCs:
-
antigen-presenting cells
- DCs:
-
dendritic cells
- PAMPs:
-
pathogen-associated molecular patterns
- PRRs:
-
pattern recognition receptors
- TLRs:
-
Toll-like receptors
- MyD88:
-
myeloid differentiation primary response 88
- NF-κB:
-
nuclear factor kappa-light-chain-enhancer of activated B cells
- ERK1/2:
-
extracellular signal-regulated kinase 1/2
- IL-10:
-
interleukin-10
- IL-12:
-
interleukin-12
- LPS:
-
lipopolysaccharide
- MPL:
-
monophosphoryl lipid
- CpG (TLR9a):
-
cytosine phospho-guanosine oligonucleotides
- dsDNA:
-
double-stranded DNA
- mtDNA:
-
mitochondrial DNA
- GMP:
-
guanosine monophosphate
- AMP:
-
adenosine monophosphate
- cGAS:
-
cyclic GMP-AMP synthase
- cGAMP:
-
cyclic guanosine monophosphate-adenosine monophosphate
- IRF 3:
-
interferon regulatory factor 3
- TBK:
-
TANK-binding kinase
- IKKi:
-
inhibitor of NF-kappaB kinase
- CTL:
-
cytotoxic T lymphocyte
- CLRs:
-
C-type lectin receptors
- NLRs:
-
nucleotide-binding oligomerization domain-like receptors
- NOD1:
-
nucleotide-binding oligomerization domain 1
- NOD2:
-
nucleotide-binding oligomerization structural domain 2
- NLRP 3:
-
NOD-like receptor thermal protein domain associated protein 3
- MDA5:
-
melanoma differentiation-associated gene 5
- RIG-I:
-
retinoic acid-induced gene I
- IRF 7:
-
interferon regulatory factor 7
- IL-2:
-
interleukin-2
- GM-CSF:
-
granulocyte-macrophage colony stimulating factor
- IFN-alpha:
-
interferon-alpha
- TNF:
-
tumor necrosis factor
- α-GC:
-
α-galactosylceramide
- PLGA:
-
poly(lactic-co-glycolic acid)
- LNP:
-
lipid nano-particle
- BCR:
-
B cell receptor
- SORT:
-
selective organ targeting
- MHC II:
-
major histocompatibility complex II
- MHC I:
-
major histocompatibility complex I
- PolyGu:
-
polyguanidine
- RNA-OG:
-
RNA origami
- OMVs:
-
outer membrane vesicles
- OMV-LL:
-
OMVs with surface decoration of listeriolysin O and L7Ae
- EVs:
-
extracellular vesicles
References
Yang L, Gong L, Wang P, Zhao X, Zhao F, Zhang Z, Li Y, Huang W. Recent Advances in Lipid Nanoparticles for Delivery of mRNA. Pharmaceutics 2022, 14.
Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, Diemert D, Spector SA, Rouphael N, Creech CB, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–16.
Whitley J, Zwolinski C, Denis C, Maughan M, Hayles L, Clarke D, Snare M, Liao H, Chiou S, Marmura T, et al. Development of mRNA manufacturing for vaccines and therapeutics: mRNA platform requirements and development of a scalable production process to support early phase clinical trials. Transl Res. 2022;242:38–55.
Lorentzen CL, Haanen JB, Met Ö, Svane IM. Clinical advances and ongoing trials on mRNA vaccines for cancer treatment. Lancet Oncol. 2022;23:e450–8.
Schlake T, Thess A, Thran M, Jordan I. mRNA as novel technology for passive immunotherapy. Cell Mol Life Sci. 2019;76:301–28.
Barbier AJ, Jiang AY, Zhang P, Wooster R, Anderson DG. The clinical progress of mRNA vaccines and immunotherapies. Nat Biotechnol. 2022;40:840–54.
Liu X, Huang P, Yang R, Deng H. mRNA Cancer vaccines: construction and boosting strategies. ACS Nano. 2023;17:19550–80.
Dunbar CE, High KA, Joung JK, Kohn DB, Ozawa K, Sadelain M. Gene therapy comes of age. Science 2018, 359.
Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science. 2012;337:816–21.
Posey AD Jr., Schwab RD, Boesteanu AC, Steentoft C, Mandel U, Engels B, Stone JD, Madsen TD, Schreiber K, Haines KM, et al. Engineered CAR T cells targeting the Cancer-Associated Tn-Glycoform of the membrane mucin MUC1 control Adenocarcinoma. Immunity. 2016;44:1444–54.
Maruggi G, Zhang C, Li J, Ulmer JB, Yu D. mRNA as a transformative technology for Vaccine Development to Control Infectious diseases. Mol Ther. 2019;27:757–72.
Cobb M. Who discovered messenger RNA? Curr Biol. 2015;25:R526–532.
Palucka K, Banchereau J. Cancer immunotherapy via dendritic cells. Nat Rev Cancer. 2012;12:265–77.
Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature. 2011;480:480–9.
Rausch S, Schwentner C, Stenzl A, Bedke J. mRNA vaccine CV9103 and CV9104 for the treatment of prostate cancer. Hum Vaccin Immunother. 2014;10:3146–52.
Rojas LA, Sethna Z, Soares KC, Olcese C, Pang N, Patterson E, Lihm J, Ceglia N, Guasp P, Chu A, et al. Personalized RNA neoantigen vaccines stimulate T cells in pancreatic cancer. Nature. 2023;618:144–50.
Mobasher M, Ansari R, Castejon AM, Barar J, Omidi Y. Advanced nanoscale delivery systems for mRNA-based vaccines. Biochim Biophys Acta Gen Subj. 2024;1868:130558.
Tavares Da Silva F, Di Pasquale A, Yarzabal JP, Garçon N. Safety assessment of adjuvanted vaccines: methodological considerations. Hum Vaccin Immunother. 2015;11:1814–24.
Aldén M, Olofsson Falla F, Yang D, Barghouth M, Luan C, Rasmussen M, De Marinis Y. Intracellular reverse transcription of Pfizer BioNTech COVID-19 mRNA vaccine BNT162b2 in Vitro in Human Liver Cell line. Curr Issues Mol Biol. 2022;44:1115–26.
Zhao T, Cai Y, Jiang Y, He X, Wei Y, Yu Y, Tian X. Vaccine adjuvants: mechanisms and platforms. Signal Transduct Target Ther. 2023;8:283.
Li WA, Lu BY, Gu L, Choi Y, Kim J, Mooney DJ. The effect of surface modification of mesoporous silica micro-rod scaffold on immune cell activation and infiltration. Biomaterials. 2016;83:249–56.
Hafner AM, Corthésy B, Merkle HP. Particulate formulations for the delivery of poly(I:C) as vaccine adjuvant. Adv Drug Deliv Rev. 2013;65:1386–99.
Turley JL, Lavelle EC. Resolving adjuvant mode of action to enhance vaccine efficacy. Curr Opin Immunol. 2022;77:102229.
Luchner M, Reinke S, Milicic A. TLR Agonists as Vaccine Adjuvants Targeting Cancer and Infectious Diseases. Pharmaceutics 2021, 13.
Duthie MS, Windish HP, Fox CB, Reed SG. Use of defined TLR ligands as adjuvants within human vaccines. Immunol Rev. 2011;239:178–96.
Wang Y, Zhang S, Li H, Wang H, Zhang T, Hutchinson MR, Yin H, Wang X. Small-molecule modulators of toll-like receptors. Acc Chem Res. 2020;53:1046–55.
Ong GH, Lian BSX, Kawasaki T, Kawai T. Exploration of Pattern Recognition receptor agonists as candidate adjuvants. Front Cell Infect Microbiol. 2021;11:745016.
Maisonneuve C, Bertholet S, Philpott DJ, De Gregorio E. Unleashing the potential of NOD- and toll-like agonists as vaccine adjuvants. Proc Natl Acad Sci U S A. 2014;111:12294–9.
Watford WT, Moriguchi M, Morinobu A, O’Shea JJ. The biology of IL-12: coordinating innate and adaptive immune responses. Cytokine Growth Factor Rev. 2003;14:361–8.
Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3:133–46.
Pulendran B. Modulating vaccine responses with dendritic cells and toll-like receptors. Immunol Rev. 2004;199:227–50.
Oberli MA, Reichmuth AM, Dorkin JR, Mitchell MJ, Fenton OS, Jaklenec A, Anderson DG, Langer R, Blankschtein D. Lipid nanoparticle assisted mRNA delivery for Potent Cancer Immunotherapy. Nano Lett. 2017;17:1326–35.
Mitchell TC, Casella CR. No pain no gain? Adjuvant effects of alum and monophosphoryl lipid A in pertussis and HPV vaccines. Curr Opin Immunol. 2017;47:17–25.
Zhuang X, Chen L, Yang S, Xia S, Xu Z, Zhang T, Zeng B, Yu T, Yu N, Wang W, et al. R848 Adjuvant Laden with Self-assembled nanoparticle-based mRNA vaccine elicits protective immunity against H5N1 in mice. Front Immunol. 2022;13:836274.
Barnett KC, Coronas-Serna JM, Zhou W, Ernandes MJ, Cao A, Kranzusch PJ, Kagan JC. Phosphoinositide interactions position cGAS at the plasma membrane to ensure efficient distinction between self- and viral DNA. Cell. 2019;176:1432–e14461411.
Wang Y, Luo J, Alu A, Han X, Wei Y, Wei X. cGAS-STING pathway in cancer biotherapy. Mol Cancer. 2020;19:136.
Diamond MS, Kinder M, Matsushita H, Mashayekhi M, Dunn GP, Archambault JM, Lee H, Arthur CD, White JM, Kalinke U, et al. Type I interferon is selectively required by dendritic cells for immune rejection of tumors. J Exp Med. 2011;208:1989–2003.
Van Herck S, Feng B, Tang L. Delivery of STING agonists for adjuvanting subunit vaccines. Adv Drug Deliv Rev. 2021;179:114020.
Lu T, Hu F, Yue H, Yang T, Ma G. The incorporation of cationic property and immunopotentiator in poly (lactic acid) microparticles promoted the immune response against chronic hepatitis B. J Control Release. 2020;321:576–88.
Liu Z, Zhou J, Xu W, Deng W, Wang Y, Wang M, Wang Q, Hsieh M, Dong J, Wang X, et al. A novel STING agonist-adjuvanted pan-sarbecovirus vaccine elicits potent and durable neutralizing antibody and T cell responses in mice, rabbits and NHPs. Cell Res. 2022;32:269–87.
Geijtenbeek TB, Gringhuis SI. Signalling through C-type lectin receptors: shaping immune responses. Nat Rev Immunol. 2009;9:465–79.
Lepenies B, Lee J, Sonkaria S. Targeting C-type lectin receptors with multivalent carbohydrate ligands. Adv Drug Deliv Rev. 2013;65:1271–81.
Engering A, Geijtenbeek TB, van Vliet SJ, Wijers M, van Liempt E, Demaurex N, Lanzavecchia A, Fransen J, Figdor CG, Piguet V, van Kooyk Y. The dendritic cell-specific adhesion receptor DC-SIGN internalizes antigen for presentation to T cells. J Immunol. 2002;168:2118–26.
Jiang W, Swiggard WJ, Heufler C, Peng M, Mirza A, Steinman RM, Nussenzweig MC. The receptor DEC-205 expressed by dendritic cells and thymic epithelial cells is involved in antigen processing. Nature. 1995;375:151–5.
Borriello F, Poli V, Shrock E, Spreafico R, Liu X, Pishesha N, Carpenet C, Chou J, Di Gioia M, McGrath ME, et al. An adjuvant strategy enabled by modulation of the physical properties of microbial ligands expands antigen immunogenicity. Cell. 2022;185:614–e629621.
Kvarnhammar AM, Petterson T, Cardell LO. NOD-like receptors and RIG-I-like receptors in human eosinophils: activation by NOD1 and NOD2 agonists. Immunology. 2011;134:314–25.
Rehwinkel J, Gack MU. RIG-I-like receptors: their regulation and roles in RNA sensing. Nat Rev Immunol. 2020;20:537–51.
Charerntantanakul W. Adjuvants for swine vaccines: mechanisms of actions and adjuvant effects. Vaccine. 2020;38:6659–81.
Ma X, Kadir Z, Li J, Zhang F. The effects of GM-CSF and IL-5 as molecular adjuvants on immune responses and contraception induced by mZP3 DNA vaccination. Am J Reprod Immunol. 2012;68:476–85.
Zeng Z, Wang H, Zhang Z, Yi Y. [Research progress of new vaccine adjuvants]. Sheng Wu Gong Cheng Xue Bao. 2021;37:78–87.
Kallen KJ, Heidenreich R, Schnee M, Petsch B, Schlake T, Thess A, Baumhof P, Scheel B, Koch SD, Fotin-Mleczek M. A novel, disruptive vaccination technology: self-adjuvanted RNActive(®) vaccines. Hum Vaccin Immunother. 2013;9:2263–76.
Weide B, Pascolo S, Scheel B, Derhovanessian E, Pflugfelder A, Eigentler TK, Pawelec G, Hoerr I, Rammensee HG, Garbe C. Direct injection of protamine-protected mRNA: results of a phase 1/2 vaccination trial in metastatic melanoma patients. J Immunother. 2009;32:498–507.
Kübler H, Scheel B, Gnad-Vogt U, Miller K, Schultze-Seemann W, Vom Dorp F, Parmiani G, Hampel C, Wedel S, Trojan L, et al. Self-adjuvanted mRNA vaccination in advanced prostate cancer patients: a first-in-man phase I/IIa study. J Immunother Cancer. 2015;3:26.
Papachristofilou A, Hipp MM, Klinkhardt U, Früh M, Sebastian M, Weiss C, Pless M, Cathomas R, Hilbe W, Pall G, et al. Phase ib evaluation of a self-adjuvanted protamine formulated mRNA-based active cancer immunotherapy, BI1361849 (CV9202), combined with local radiation treatment in patients with stage IV non-small cell lung cancer. J Immunother Cancer. 2019;7:38.
Zhang R, Tang L, Tian Y, Ji X, Hu Q, Zhou B, Zhenyu D, Heng X, Yang L. Cholesterol-modified DP7 enhances the effect of individualized cancer immunotherapy based on neoantigens. Biomaterials. 2020;241:119852.
Islam MA, Rice J, Reesor E, Zope H, Tao W, Lim M, Ding J, Chen Y, Aduluso D, Zetter BR, et al. Adjuvant-pulsed mRNA vaccine nanoparticle for immunoprophylactic and therapeutic tumor suppression in mice. Biomaterials. 2021;266:120431.
Verbeke R, Lentacker I, Breckpot K, Janssens J, Van Calenbergh S, De Smedt SC, Dewitte H. Broadening the message: a Nanovaccine co-loaded with Messenger RNA and α-GalCer induces Antitumor immunity through conventional and natural killer T cells. ACS Nano. 2019;13:1655–69.
Lehrman S. Virus treatment questioned after gene therapy death. Nature. 1999;401:517–8.
Hacein-Bey-Abina S, Von Kalle C, Schmidt M, McCormack MP, Wulffraat N, Leboulch P, Lim A, Osborne CS, Pawliuk R, Morillon E, et al. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science. 2003;302:415–9.
Dirisala A, Uchida S, Li J, Van Guyse JFR, Hayashi K, Vummaleti SVC, Kaur S, Mochida Y, Fukushima S, Kataoka K. Effective mRNA protection by poly(l-ornithine) synergizes with endosomal escape functionality of a Charge-Conversion Polymer toward maximizing mRNA introduction efficiency. Macromol Rapid Commun. 2022;43:e2100754.
Patel S, Ashwanikumar N, Robinson E, DuRoss A, Sun C, Murphy-Benenato KE, Mihai C, Almarsson Ö, Sahay G. Boosting intracellular delivery of lipid nanoparticle-encapsulated mRNA. Nano Lett. 2017;17:5711–8.
Roth GA, Picece V, Ou BS, Luo W, Pulendran B, Appel EA. Designing spatial and temporal control of vaccine responses. Nat Rev Mater. 2022;7:174–95.
Adu-Berchie K, Mooney DJ. Biomaterials as local niches for Immunomodulation. Acc Chem Res. 2020;53:1749–60.
Jackson LA, Anderson EJ, Rouphael NG, Roberts PC, Makhene M, Coler RN, McCullough MP, Chappell JD, Denison MR, Stevens LJ, et al. An mRNA vaccine against SARS-CoV-2 - preliminary Report. N Engl J Med. 2020;383:1920–31.
Mulligan MJ, Lyke KE, Kitchin N, Absalon J, Gurtman A, Lockhart S, Neuzil K, Raabe V, Bailey R, Swanson KA, et al. Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature. 2020;586:589–93.
Wang YQ, Wu J, Fan QZ, Zhou M, Yue ZG, Ma GH, Su ZG. Novel vaccine delivery system induces robust humoral and cellular immune responses based on multiple mechanisms. Adv Healthc Mater. 2014;3:670–81.
Bachmann MF, Zinkernagel RM. Neutralizing antiviral B cell responses. Annu Rev Immunol. 1997;15:235–70.
Marcandalli J, Fiala B, Ols S, Perotti M, de van der Schueren W, Snijder J, Hodge E, Benhaim M, Ravichandran R, Carter L, et al. Induction of potent neutralizing antibody responses by a designed protein nanoparticle vaccine for respiratory Syncytial Virus. Cell. 2019;176:1420–e14311417.
Ingale J, Stano A, Guenaga J, Sharma SK, Nemazee D, Zwick MB, Wyatt RT. High-density array of Well-ordered HIV-1 spikes on synthetic liposomal nanoparticles efficiently activate B cells. Cell Rep. 2016;15:1986–99.
Soleimanpour S, Farsiani H, Mosavat A, Ghazvini K, Eydgahi MR, Sankian M, Sadeghian H, Meshkat Z, Rezaee SA. APC targeting enhances immunogenicity of a novel multistage Fc-fusion tuberculosis vaccine in mice. Appl Microbiol Biotechnol. 2015;99:10467–80.
Lu L, Palaniyandi S, Zeng R, Bai Y, Liu X, Wang Y, Pauza CD, Roopenian DC, Zhu X. A neonatal fc receptor-targeted mucosal vaccine strategy effectively induces HIV-1 antigen-specific immunity to genital infection. J Virol. 2011;85:10542–53.
Levin D, Golding B, Strome SE, Sauna ZE. Fc fusion as a platform technology: potential for modulating immunogenicity. Trends Biotechnol. 2015;33:27–34.
Shafifar M, Mozhgani SH, Razavi Pashabayg K, Mosavat A, Karbalaei M, Norouzi M, Rezaee SA. Selective APC-targeting of a novel Fc-fusion multi-immunodominant recombinant protein ((t)Tax-(t)env:mFcγ2a) for HTLV-1 vaccine development. Life Sci. 2022;308:120920.
Padera TP, Meijer EF, Munn LL. The lymphatic system in disease processes and Cancer Progression. Annu Rev Biomed Eng. 2016;18:125–58.
Kligerman J, Lima RA, Soares JR, Prado L, Dias FL, Freitas EQ, Olivatto LO. Supraomohyoid neck dissection in the treatment of T1/T2 squamous cell carcinoma of oral cavity. Am J Surg. 1994;168:391–4.
Bhagchandani S, Johnson JA, Irvine DJ. Evolution of toll-like receptor 7/8 agonist therapeutics and their delivery approaches: from antiviral formulations to vaccine adjuvants. Adv Drug Deliv Rev. 2021;175:113803.
Chen J, Ye Z, Huang C, Qiu M, Song D, Li Y, Xu Q. Lipid nanoparticle-mediated lymph node-targeting delivery of mRNA cancer vaccine elicits robust CD8(+) T cell response. Proc Natl Acad Sci U S A. 2022;119:e2207841119.
Kubara K, Yamazaki K, Miyazaki T, Kondo K, Kurotaki D, Tamura T, Suzuki Y. Lymph node macrophages drive innate immune responses to enhance the anti-tumor efficacy of mRNA vaccines. Mol Ther 2024.
Moyer TJ, Zmolek AC, Irvine DJ. Beyond antigens and adjuvants: formulating future vaccines. J Clin Invest. 2016;126:799–808.
Swartz MA. The physiology of the lymphatic system. Adv Drug Deliv Rev. 2001;50:3–20.
Jiang H, Wang Q, Sun X. Lymph node targeting strategies to improve vaccination efficacy. J Control Release. 2017;267:47–56.
Famta P, Shah S, Jain N, Srinivasarao DA, Murthy A, Ahmed T, Vambhurkar G, Shahrukh S, Singh SB, Srivastava S. Albumin-hitchhiking: fostering the pharmacokinetics and anticancer therapeutics. J Control Release. 2023;353:166–85.
Abdallah M, Müllertz OO, Styles IK, Mörsdorf A, Quinn JF, Whittaker MR, Trevaskis NL. Lymphatic targeting by albumin-hitchhiking: applications and optimisation. J Control Release. 2020;327:117–28.
Zhu G, Lynn GM, Jacobson O, Chen K, Liu Y, Zhang H, Ma Y, Zhang F, Tian R, Ni Q et al. Albumin/vaccine nanocomplexes that assemble in vivo for combination cancer immunotherapy. Nat Commun : 2017, 8:1954.
Dilliard SA, Cheng Q, Siegwart DJ. On the mechanism of tissue-specific mRNA delivery by selective organ targeting nanoparticles. Proc Natl Acad Sci U S A 2021, 118.
Hou X, Zaks T, Langer R, Dong Y. Lipid nanoparticles for mRNA delivery. Nat Rev Mater. 2021;6:1078–94.
Cruz FM, Colbert JD, Merino E, Kriegsman BA, Rock KL. The Biology and underlying mechanisms of Cross-presentation of Exogenous antigens on MHC-I molecules. Annu Rev Immunol. 2017;35:149–76.
Embgenbroich M, Burgdorf S. Current concepts of Antigen Cross-presentation. Front Immunol. 2018;9:1643.
Benjaminsen RV, Mattebjerg MA, Henriksen JR, Moghimi SM, Andresen TL. The possible proton sponge effect of polyethylenimine (PEI) does not include change in lysosomal pH. Mol Ther. 2013;21:149–57.
Du G, Sun X. Engineering nanoparticulate vaccines for enhancing antigen cross-presentation. Curr Opin Biotechnol. 2020;66:113–22.
Jerjes W, Theodossiou TA, Hirschberg H, Høgset A, Weyergang A, Selbo PK, Hamdoon Z, Hopper C, Berg K. Photochemical internalization for Intracellular Drug Delivery. From Basic mechanisms to Clinical Research. J Clin Med 2020, 9.
Otterhaug T, Janetzki S, Welters MJP, Håkerud M, Nedberg AG, Edwards VT, Boekestijn S, Loof NM, Selbo PK, Olivecrona H, et al. Photochemical internalization enhanced vaccination is safe, and gives Promising Cellular Immune responses to an HPV peptide-based vaccine in a phase I clinical study in healthy volunteers. Front Immunol. 2020;11:576756.
Haug M, Brede G, Håkerud M, Nedberg AG, Gederaas OA, Flo TH, Edwards VT, Selbo PK, Høgset A, Halaas Ø. Photochemical internalization of peptide antigens provides a Novel Strategy to realize therapeutic Cancer vaccination. Front Immunol. 2018;9:650.
Broz P, Dixit VM. Inflammasomes: mechanism of assembly, regulation and signalling. Nat Rev Immunol. 2016;16:407–20.
Zhu M, Du L, Zhao R, Wang HY, Zhao Y, Nie G, Wang RF. Cell-penetrating nanoparticles activate the Inflammasome to enhance antibody production by Targeting Microtubule-Associated protein 1-Light chain 3 for degradation. ACS Nano. 2020;14:3703–17.
Alameh MG, Tombácz I, Bettini E, Lederer K, Sittplangkoon C, Wilmore JR, Gaudette BT, Soliman OY, Pine M, Hicks P, et al. Lipid nanoparticles enhance the efficacy of mRNA and protein subunit vaccines by inducing robust T follicular helper cell and humoral responses. Immunity. 2021;54:2877–e28922877.
Qian J. mRNA vaccines: a powerful tool in cancer treatment. In International Conference on Biological Engineering and Medical Science. 2024.
Burris HA, Patel MR, Cho DC, Clarke JM, Gutierrez M, Zaks TZ, Frederick J, Hopson K, Mody K, Binanti-Berube A. A phase I multicenter study to assess the safety, tolerability, and immunogenicity of mRNA-4157 alone in patients with resected solid tumors and in combination with pembrolizumab in patients with unresectable solid tumors. American Society of Clinical Oncology; 2019.
Tockary TA, Abbasi S, Matsui-Masai M, Hayashi A, Yoshinaga N, Boonstra E, Wang Z, Fukushima S, Kataoka K, Uchida S. Comb-structured mRNA vaccine tethered with short double-stranded RNA adjuvants maximizes cellular immunity for cancer treatment. Proc Natl Acad Sci U S A. 2023;120:e2214320120.
Li W, Li Y, Li J, Meng J, Jiang Z, Yang C, Wen Y, Liu S, Cheng X, Mi S et al. All-trans-retinoic acid-adjuvanted mRNA vaccine induces mucosal Anti-tumor Immune responses for treating Colorectal Cancer. Adv Sci (Weinh) 2024:e2309770.
Zhang X, Wang K, Zhao Z, Shan X, Wang Y, Feng Z, Li B, Luo C, Chen X, Sun J. Self-Adjuvanting Polyguanidine Nanovaccines for Cancer Immunotherapy. ACS Nano. 2024;18:7136–47.
Yip T, Qi X, Yan H, Chang Y. RNA origami functions as a self-adjuvanted nanovaccine platform for Cancer Immunotherapy. ACS Nano. 2024;18:4056–67.
Hu M, Feng C, Yuan Q, Liu C, Ge B, Sun F, Zhu X. Lantern-shaped flexible RNA origami for Smad4 mRNA delivery and growth suppression of colorectal cancer. Nat Commun. 2023;14:1307.
Li Y, Ma X, Yue Y, Zhang K, Cheng K, Feng Q, Ma N, Liang J, Zhang T, Zhang L, et al. Rapid Surface Display of mRNA antigens by Bacteria-derived outer membrane vesicles for a personalized Tumor Vaccine. Adv Mater. 2022;34:e2109984.
Shin H, Kang S, Won C, Min DH. Enhanced local delivery of Engineered IL-2 mRNA by porous silica nanoparticles to promote effective Antitumor immunity. ACS Nano. 2023;17:17554–67.
Grippin AJ, Wummer B, Wildes T, Dyson K, Trivedi V, Yang C, Sebastian M, Mendez-Gomez HR, Padala S, Grubb M, et al. Dendritic cell-activating magnetic nanoparticles enable early prediction of Antitumor Response with magnetic resonance imaging. ACS Nano. 2019;13:13884–98.
Im JH, Kim E, Lee E, Seo Y, Lee Y, Jang Y, Yu S, Maeng Y, Park S, Park S, et al. Adverse events with the Pfizer-BioNTech COVID-19 vaccine among Korean Healthcare Workers. Yonsei Med J. 2021;62:1162–8.
Riad A, Pokorná A, Attia S, Klugarová J, Koščík M, Klugar M. Prevalence of COVID-19 Vaccine Side effects among Healthcare Workers in the Czech Republic. J Clin Med 2021, 10.
García-Grimshaw M, Ceballos-Liceaga SE, Hernández-Vanegas LE, Núñez I, Hernández-Valdivia N, Carrillo-García DA, Michel-Chávez A, Galnares-Olalde JA, Carbajal-Sandoval G, Del Mar Saniger-Alba M, et al. Neurologic adverse events among 704,003 first-dose recipients of the BNT162b2 mRNA COVID-19 vaccine in Mexico: a nationwide descriptive study. Clin Immunol. 2021;229:108786.
Banerji A, Wickner PG, Saff R, Stone CA Jr., Robinson LB, Long AA, Wolfson AR, Williams P, Khan DA, Phillips E, Blumenthal KG. mRNA vaccines to Prevent COVID-19 Disease and reported allergic reactions: current evidence and suggested Approach. J Allergy Clin Immunol Pract. 2021;9:1423–37.
Halamoda-Kenzaoui B, Bremer-Hoffmann S. Main trends of immune effects triggered by nanomedicines in preclinical studies. Int J Nanomed. 2018;13:5419–31.
Ndeupen S, Qin Z, Jacobsen S, Bouteau A, Estanbouli H, Igyártó BZ. The mRNA-LNP platform’s lipid nanoparticle component used in preclinical vaccine studies is highly inflammatory. iScience. 2021;24:103479.
Tahtinen S, Tong AJ, Himmels P, Oh J, Paler-Martinez A, Kim L, Wichner S, Oei Y, McCarron MJ, Freund EC, et al. IL-1 and IL-1ra are key regulators of the inflammatory response to RNA vaccines. Nat Immunol. 2022;23:532–42.
Kostoff RN, Calina D, Kanduc D, Briggs MB, Vlachoyiannopoulos P, Svistunov AA, Tsatsakis A. Retraction notice to Why are we vaccinating children against COVID-19? [Toxicol. Rep. 8 (2021) 1665–1684]. Toxicol Rep 2022, 9:1065.
Olotu A, Fegan G, Wambua J, Nyangweso G, Leach A, Lievens M, Kaslow DC, Njuguna P, Marsh K, Bejon P. Seven-year efficacy of RTS,S/AS01 malaria vaccine among young African children. N Engl J Med. 2016;374:2519–29.
Ranzani OT, Hitchings MDT, Dorion M, D’Agostini TL, de Paula RC, de Paula OFP, Villela EFM, Torres MSS, de Oliveira SB, Schulz W et al. Effectiveness of the CoronaVac vaccine in older adults during a gamma variant associated epidemic of covid-19 in Brazil: test negative case-control study. BMJ 2021, 374n2015.
Patel S, Kim J, Herrera M, Mukherjee A, Kabanov AV, Sahay G. Brief update on endocytosis of nanomedicines. Adv Drug Deliv Rev. 2019;144:90–111.
Hutchison S, Benson RA, Gibson VB, Pollock AH, Garside P, Brewer JM. Antigen Depot is not required for alum adjuvanticity. Faseb j. 2012;26:1272–9.
Zhang NN, Li XF, Deng YQ, Zhao H, Huang YJ, Yang G, Huang WJ, Gao P, Zhou C, Zhang RR, et al. A thermostable mRNA vaccine against COVID-19. Cell. 2020;182:1271–e12831216.
Zhuang X, Qi Y, Wang M, Yu N, Nan F, Zhang H, Tian M, Li C, Lu H, Jin N. mRNA vaccines encoding the HA protein of Influenza A H1N1 Virus delivered by Cationic lipid nanoparticles induce Protective Immune responses in mice. Vaccines (Basel) 2020, 8.
Kranz LM, Diken M, Haas H, Kreiter S, Loquai C, Reuter KC, Meng M, Fritz D, Vascotto F, Hefesha H, et al. Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature. 2016;534:396–401.
Qiu M, Tang Y, Chen J, Muriph R, Ye Z, Huang C, Evans J, Henske EP, Xu Q. Lung-selective mRNA delivery of synthetic lipid nanoparticles for the treatment of pulmonary lymphangioleiomyomatosis. Proc Natl Acad Sci U S A 2022, 119.
Liu S, Cheng Q, Wei T, Yu X, Johnson LT, Farbiak L, Siegwart DJ. Membrane-destabilizing ionizable phospholipids for organ-selective mRNA delivery and CRISPR-Cas gene editing. Nat Mater. 2021;20:701–10.
Zhang B, Sim WK, Shen TL, Lim SK. Engineered EVs with pathogen proteins: promising vaccine alternatives to LNP-mRNA vaccines. J Biomed Sci. 2024;31:9.
Pardi N, Tuyishime S, Muramatsu H, Kariko K, Mui BL, Tam YK, Madden TD, Hope MJ, Weissman D. Expression kinetics of nucleoside-modified mRNA delivered in lipid nanoparticles to mice by various routes. J Control Release. 2015;217:345–51.
Melo M, Porter E, Zhang Y, Silva M, Li N, Dobosh B, Liguori A, Skog P, Landais E, Menis S, et al. Immunogenicity of RNA replicons encoding HIV Env Immunogens designed for self-assembly into nanoparticles. Mol Ther. 2019;27:2080–90.
Zangi L, Lui KO, von Gise A, Ma Q, Ebina W, Ptaszek LM, Später D, Xu H, Tabebordbar M, Gorbatov R, et al. Modified mRNA directs the fate of heart progenitor cells and induces vascular regeneration after myocardial infarction. Nat Biotechnol. 2013;31:898–907.
Nabhan JF, Wood KM, Rao VP, Morin J, Bhamidipaty S, LaBranche TP, Gooch RL, Bozal F, Bulawa CE, Guild BC. Intrathecal delivery of frataxin mRNA encapsulated in lipid nanoparticles to dorsal root ganglia as a potential therapeutic for Friedreich’s ataxia. Sci Rep. 2016;6:20019.
Patel S, Ryals RC, Weller KK, Pennesi ME, Sahay G. Lipid nanoparticles for delivery of messenger RNA to the back of the eye. J Control Release. 2019;303:91–100.
Kim J, Eygeris Y, Gupta M, Sahay G. Self-assembled mRNA vaccines. Adv Drug Deliv Rev. 2021;170:83–112.
Acknowledgements
Figures were created on BioRender.com.
Funding
This study was supported by the Fundamental Research Funds for the Central Universities (Wuhan University, Clinical Medicine + X, 2042024YXB017), Hubei Province Chinese Medicine Research Project (ZY2023Q015), Natural Science Foundation of Hubei Province (2023AFB665), Medical Young Talents Program of Hubei Province, Wuhan Young Medical Talents Training Project to L.-L. Bu., Youth Interdisciplinary Special Fund of Zhongnan Hospital of Wuhan University (ZNQNJC2022003), Health Commission of Hubei Province Scientific Research Project (WJ2023M068), Knowledge Innovation Program of Wuhan-Shugung Project (2023020201020510), Youth Fund of the National Natural Science Foundation of China (82001644), Medical Young Talents Program of Hubei Province (7020206), and “Dawn of Scientific and Technological Innovation” Program of Wuhan (703030804).
Author information
Authors and Affiliations
Contributions
C.L.: Methodology, Conceptualization, Writing - Original Draft, Writing - Review & Editing. Y.Y.: Investigation, Methodology, Conceptualization, Writing - Original Draft, Visualization, Writing - Review & Editing. L.Z.: Investigation, Methodology, Conceptualization, Writing - Original Draft, Writing – Review & Editing. Z.N.: Investigation, Methodology, Conceptualization, Writing - Original Draft, Writing – Review & Editing. W.G.: Investigation, Methodology, Conceptualization, Writing – Review & Editing. X.Y.: Writing - Review & Editing, Conceptualization. L.B.: Supervision, Writing - Review & Editing, Project Administration. W.Q.: Conceptualization, Methodology, Writing - Review & Editing, Supervision, Funding acquisition. F.C.: Supervision, Review & Editing, Funding acquisition. B.L.: Conceptualization, Supervision, Project Administration, Funding Acquisition, Writing - Review & Editing.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Cao, LM., Yu, YF., Li, ZZ. et al. Adjuvants for cancer mRNA vaccines in the era of nanotechnology: strategies, applications, and future directions. J Nanobiotechnol 22, 308 (2024). https://doi.org/10.1186/s12951-024-02590-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12951-024-02590-6