Abstract
Synthetic nanoparticles with surface bioconjugation are promising platforms for targeted therapy, but their simple biological functionalization is still a challenging task against the complex intercellular environment. Once synthetic nanoparticles enter the body, they are phagocytosed by immune cells by the immune system. Recently, the cell membrane camouflage strategy has emerged as a novel therapeutic tactic to overcome these issues by utilizing the fundamental properties of natural cells. Macrophage, a type of immune system cells, plays critical roles in various diseases, including cancer, atherosclerosis, rheumatoid arthritis, infection and inflammation, due to the recognition and engulfment function of removing substances and pathogens. Macrophage membranes inherit the surface protein profiles and biointerfacing properties of source cells. Therefore, the macrophage membrane cloaking can protect synthetic nanoparticles from phagocytosis by the immune cells. Meanwhile, the macrophage membrane can make use of the natural correspondence to accurately recognize antigens and target inflamed tissue or tumor sites. In this review, we have summarized the advances in the fabrication, characterization and homing capacity of macrophage membrane cloaking nanoparticles in various diseases, including cancers, immune diseases, cardiovascular diseases, central nervous system diseases, and microbial infections. Although macrophage membrane-camouflaged nanoparticles are currently in the fetal stage of development, there is huge potential and challenge to explore the conversion mode in the clinic.
Similar content being viewed by others
Introduction
Most therapeutics, including small molecules and biological macromolecules used in the clinic of various diseases, have to effectively work to act on targets to cells. However, the major problem in the treatment of many diseases is to deliver therapeutics into the target site. These therapeutics are characterized by limited effectiveness, poor biodistribution, and lack of selectivity [1]. Studies have shown that nanoparticle drug delivery systems can overcome these limitations and drawbacks by controlling drug delivery to the inflamed site or tumor so that the influence on normal cells or tissues can be minimized. Currently, an increasing number of nanocarriers have been permitted by the Food and Drug Administration (FDA) on the market, such as poly(D,L-lactic-co-glycolic acid) (PLGA), liposomes, polylactic acid (PLA), polycaprolactone (PCL), iron oxide nanoparticles, nanocrystals and albumin nanoparticles [2, 3].
The transportation of foreign substance nanoparticles to lesions is still a challenge due to the reticuloendothelial system (RES) and the mononuclear phagocytic system (MPS) [4]. When nanoparticles enter the body, they are first bound by proteins that make nanoparticles more recognizable by phagocytic cells. Subsequently, they are rapidly cleared by RES and MPS, which limits their delivery and distribution [5]. For nanoparticles to efficiently enter lesion sites, they need to evade clearance by the immune system. The most common method is to modify the surface of nanoparticles with polyethylene glycol (PEG) [6]. The stability of PEG in vivo, the accelerated blood clearance (ABC) effect and the difficulty of phagocytosis by target cells limit the application [7]. But simple surface functionalization cannot accurately simulate the complex interface in the body and cannot avoid foreign body recognition and subsequent immune responses [8]. To solve this problem, methods based on active targeting have been developed. The surface of the nanocarrier is connected with affinity ligands such as antibodies, peptides, aptamers or other small molecules to bind to target cells through ligand-receptor interactions and release drugs in the target cells [9]. For example, nucleic acid strands as known as aptamers, show relatively strong binding affinity and target specificity, but easily degraded and may cause immune response in body [10]. Protein-based nanomedicine is used in tumor chemotherapy due to their merits in bioavailability, biocompatibility, biodegradability, and low toxicity. Ding et al. have prepared human serum albumin nanoparticles to deliver paclitaxel (NPs-PTX) [11]. It shows that NPs-PTX enhance the cytotoxicity in MCF-7 and A549 cells. Nevertheless, antibodies conjugated nanoparticle encompass high production costs and complex preparation process.
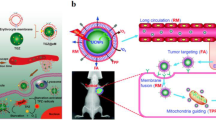
Recently, the cell membrane cloaking strategy has emerged as an epidemic means in various diseases. Natural cell membranes have a variety of source cell-relevant functions, such as ‘self’ markers, biological targeting, and correspondence with the immune system [12]. Cell membrane-coated nanoparticles endow them with certain biological functions, including long circulation, targeted recognition, enhanced accumulation at disease sites and deep tumor penetration. To date, this biomimetic strategy has involved various kinds of cells, such as red blood cell membranes [13], macrophage membranes [14], platelet membranes [15], mesenchymal stem cell membranes [16], and cancer cell membranes [17] and so on. Red blood cells were the first cell types used in cell therapy. CD47, a membrane protein widely expressed on the red blood cells, prevents the clearance from the immune system by delivering a “don’t eat me” signal [13]. This approach significantly extends the circulation time of the core nanoparticles, but do not specifically target inflamed site or tumor. Platelet expressing P-selectin can bind specifically to CD44 on the surface of cancer cells, thus platelet membranes coated nanoparticles can target to tumor tissues. Currently, numerous studies focus on the application of platelet membrane biomimetic nanoparticles in various diseases, including cancer, atherosclerosis, and immune diseases [18]. However, it is the difficulty to isolate and culture of platelet that limited its clinical transformation. Similarly, the harsh culture conditions of mesenchymal stem cells increase the cost of fabricating drug delivery systems. Cancer cell membranes can only be applied to tumors [19]. Macrophages are immune cells responsible for protecting the body against infections, clearing foreign invaders, repairing injured tissue, and resisting pathogens. The membranes from macrophages have cellular self-recognition mechanisms to avoid phagocytosis by immune cells, and the ligands inherited from the membrane can bind to receptors at disease sites. Meanwhile, macrophage membrane-coated nanoparticles can recognize and respond to pathogens and immune signals, including bacterial toxins, viruses and inflammatory cytokines. Furthermore, the strategy can activate anticancer immunity. Taken together, macrophage membrane cloaking is a biomimetic platform with promising therapeutic potential. Macrophage membrane cloaking nanoparticles have been applied in tumors, immune diseases, anti-infection, neurological diseases and cardiovascular diseases (Fig. 1).
Here, we summarize the synthesis and characterization of macrophage membrane-coated nanoparticles and their applications in different diseases. Meanwhile, we conclude how the macrophage membrane functions in various diseases. Finally, we put forward some challenges faced at present in their application in the clinic or factory.
Macrophages and macrophage membranes
The initial response to an infection is mediated by the innate immune system. The process recruits immune cells, including macrophages, dendritic cells, neutrophils, eosinophils, basophils, mast cells, and natural killer cells. Immune cells can use their pattern recognition receptors (PRRs), including Toll-like receptors (TLRs), RIG-I-like receptors (RLRs) and NOD-like receptors (NLRs), to recognize microorganisms through structural patterns, which are expressed only on pathogens called pathogen-associated molecular patterns (PAMPs) [20]. Among the cellular components of the innate immune system, macrophages and neutrophils are the predominant cells responding to pathogens in the body. Regrettably, neutrophils live just 48 h, but macrophages can live several months or more [21]. Macrophages possess the capacity for active targeting, high immune compatibility and long circulation.
Macrophages are phagocytic cells with broad sources, and they are effectors in the process of inflammation and tissue repair. Tissue-resident macrophages can differentiate from circulating monocytes, which develop from hematopoietic stem cells in the bone marrow or during embryonic development in the fetal liver, yolk sac, or dorsal aorta [22]. They are highly specialized cells that mediate tissue homeostasis in all organs, such as splenic macrophages of the spleen, intraocular macrophages of the eye, intestinal macrophages of the gut, osteoclast macrophages of the bone, Kupffer macrophages of the liver, alveolar macrophages of the lungs, Langerhans macrophages of the skin, and microglial cells of the brain [23]. Macrophages are involved in innate immune responses to protect the body by phagocytosing microorganisms and apoptotic cells and presenting antigens [24, 25]. In the early stages of inflammation, macrophages play an important role by releasing cytokines and chemokines that in turn recruit other immune cells to sites of inflammation to start the adaptive response of the immune system.
Macrophages have two phenotypes, named the M1 and M2 phenotypes. M2 is further classified into M2a, M2b, M2c and M2d. Previous studies have shown that lipopolysaccharide (LPS), interferon-γ (IFN-γ) and transforming factor-α (TNF-α) can induce monocytes to produce the M1 phenotype, while interleukin-4 (IL-4), interleukin-10 (IL-10), interleukin-13 (IL-13) transforming factor-β (TNF-β), bacterial infection, colony-stimulating factor 1 (CSF-1) and interleukin-21 (IL-21) are used to produce the M2 phenotype. M1 and M2 macrophages have different functions. M1 macrophages mainly play a role through innate and adaptive immune responses in Th1-cell recruitment, pathogen resistance, and tumor control [26]. They produce proinflammatory factors, including IL-1, IL-6, TNF-α, NO and reactive oxygen species (ROS), to promote T cells to produce Th1 cytokines [27]. Therefore, under certain conditions, M1 macrophages exacerbate inflammatory processes, which are detrimental to healthy tissue. In contrast to M1 macrophages, M2 macrophages express opposite functions, responding to parasites, tissue remodeling, angiogenesis, and allergic diseases by downregulating IL-12 and IL-23 but upregulating IL-10 and IL-1RA [28]. The M2 type promotes the release of anti-inflammatory cytokines, wound healing, and the growth and metastasis of tumor cells in the tumor microenvironment [29, 30]. In summary, M1 macrophages kill pathogens by recruiting inflammatory cells in the process of tissue inflammation. M2 macrophages are used to inhibit the recruitment of inflammatory cells to protect against excess proinflammatory cytokines and promote angiogenesis and tissue repair. However, how did this process develop?
Studies have shown that there is a special recognition mechanism between macrophages and pathogens. This process is primarily initiated by PAMPs and damage-associated molecular patterns (DAMPs) in response to infection and injury [31, 32]. The migration of macrophages depends on the expression of adhesive molecules by chemical mediators on venous endothelial surfaces. Cell adhesion molecules are cell surface proteins that mediate cell-cell and/or cell-extracellular matrix (ECM) interactions. These adhesion molecules contain integrins, selectins, cadherins, immunoglobulin superfamily and others. Macrophage membranes express P-selectin glycoprotein ligand-1 (PSGL-1), L-selectin, lymphocyte function-associated antigen 1 (LFA-1), integrin, and very late antigen-4 (VLA-4), which result in their cell adhesion with inflammatory cells and cancer cells. It has been suggested that macrophage membrane-cloaking nanoparticles can actively target tumors by binding with integrin to vascular cell adhesion protein 1 (VCAM-1) or LFA-1, which is highly expressed in active cell membranes [33,34,35,36].
Therefore, using these properties of macrophages, macrophage membrane cloaking nanoparticles can avoid clearance by the immune system and achieve tumor as well as inflammatory tissue targeting capacity. This strategy has shown great potential to overcome the biocompatibility, short cycle time, and immunogenicity of traditional materials.
Fabrication of macrophage membrane biomimetic nanoparticles
Multistep synthesis of cell membrane cloaking nanoparticles
Cell membrane-camouflaged nanoparticles are usually composed of a thin layer of cell membrane encapsulating therapeutic nanoparticles, thus forming a “shell-core” structure with nanoparticles as the core and the cell membrane as the outer shell [37]. The synthesis of macrophage membrane-coated nanoparticles usually involves the following three steps [38,39,40]: (1) macrophage cell membrane extraction, (2) fabrication of the core, and (3) the formation of membrane-wrapped nanoparticles (Fig. 2).
Source and isolation of macrophage membrane
Previous studies have shown two major classes of macrophage membranes, primary macrophages isolated from animals and macrophages cultured in plates. Cells are composed of membranes, intracellular biomacromolecules, intracellular vesicles, and nuclei. Membrane extraction needs to remove these intracellular components while leaving the whole functional surface protein of membranes. These proteins can transport molecules in or out of the cells, correspond between the cell and cell and are related to other processes [41, 42]. Proteins on the cell membrane are important for biomimetic therapy because they can endow bioinspired nanoparticles with targeting ability and immune escape capacity. Therefore, the isolation of the cell membrane needs to be carried out moderately to reduce the denaturation of membrane proteins [43]. Usually, this process requires a mass of cells to be harvested from culture dishes such as RAW264.7, J774A.1, THP-1 and blood or tissues [44, 45]. The harvested cells can be subjected to hypotonic treatment, freeze-thaw cycling and ultrasonic waves. After discontinuous sucrose gradient centrifugation, the pellets were collected. Then, the cell suspension was disrupted by ultrasound with appropriate power and extrusion through a porous polycarbonate membrane to obtain the membrane vesicles required for the experiment. Extracted macrophage membranes are stored together with protease inhibitors at 4 °C to maintain the stability of biological activity [46].
Inner core
The selection of the inner core depends on the intended application, as they are the effective loads delivered to targeted tissues [47]. They are particulate dispersions or solid particles with a particle size in the range of 10–1000 nm [48]. In recent years, various types of materials for cell membrane cloaking have been widely explored and applied, including PLGA, liposomes, SiO2, mesoporous silica, nanocapsules, gold, iron oxide, upconversion nanoparticles (UCNPs) and metal-organic frameworks (MOFs) (Table 1). The inner cores can be classified as organic and inorganic nanoparticles in terms of the properties of materials [49]. Organic particles are formed by organic molecules through a self-assembly process, including polymeric nanoparticles and lipid nanoparticles [50]. They have simple design principles, high biocompatibility and high drug-loading capacity. However, the high cost of organic materials and manufacturing equipment, poor stability in vivo, difficulty in controlling size, large differences between different batches and residual organic solvents limit the application of organic cores [50]. Inorganic cores include silica nanoparticles [14], gold nanocage (AuNC) nanoparticles [51], iron oxide nanoparticles [52], upconversion nanoparticles (UCNPs) [53], metal-organic frameworks (MOFs) [54]. They have various advantages in terms of controllable size, good stability, large specific surface area for surface functionalization. Meanwhile, their unique photothermal or electromagnetic properties give them potential therapeutic and imaging functions. However, the low biodegradability and high toxicity in vivo of inorganic vectors hinder their clinical transformation [55].
Preparation of macrophage membrane biomimetic nanoparticles
The last and most important step is to coat the prepared macrophage membrane on the synthetic nanoparticles. Currently, there are four methods to prepare macrophage membrane biomimetic nanoparticles: incubation, membrane extrusion, sonication or electroporation. An incubation approach has been described in which nanoparticles with an indicated concentration are incubated with cells for several hours [56]. This method is good for maintaining the stability of membrane proteins. However, few macrophage-coated nanoparticles are collected. For membrane extrusion, cell membrane vesicles and nanoparticles are coextruded several times through polycarbonate porous membranes of different pore sizes, such as 200 nm, to develop the biomimetic nanoparticles. In physical extrusion, the mechanical force prompts them to form “shell-core” structures by disrupting the membranes and reforming them [15, 57,58,59,60]. Although this strategy does not require sophisticated equipment, large-scale production and integrity of membrane proteins have become major obstacles to clinical application. In sonication-based methods, the cores are coincubated with cell membrane vesicles and ultrasound at an appropriate parameter; this process is easy to achieve in the clinic or factory and prepares good shape and size cell membrane-coated nanoparticles [61, 62]. Recently, electroporation has become a new approach to prepare macrophage-coated nanoparticles. Cores and cell membrane vesicles are infused into a microfluidic chip, and then, electric pulses produce transient pores in the membranes to promote NPs into membrane vesicles. This strategy shows excellent advantages in maintaining the integrity of the nanovesicles and a higher success of the CM-NPs [63].
Characterization of macrophage membrane coated nanoparticles
The macrophage membrane-coated nanoparticles were further investigated to confirm that the macrophage membrane successfully coated the surface of the NPs. Physicochemical and biological properties, such as size, zeta potential, and membrane protein composition, need to be characterized, as does the function of macrophage-coated nanoparticles (Fig. 3).
Characterization of cell-membrane coated nanoparticles. A Hydrodynamic diameter and zeta potential of PLGA NPs, M-vesicles and PLGA@M after formulation in water. Adapted with permission from [102], copyright © 2022 BioMed Central Ltd unless otherwise stated. Typical SEM (B) and TEM (C, D) image of core nanoparticles before and after cell-membrane coating. B Adapted with permission from [68], Copyright © 2022 Elsevier B.V. C Adapted with permission from [94], Copyright © 2022 National Academy of Science. D Adapted with permission from [33], copyright © 2020 American Chemical Society. E Immunogold TEM images of membrane and membrane coated NPs samples probed for α4 (red arrows) and vascular cell adhesion molecule-1 (yellow arrows). Adapted with permission from [72], copyright © 2022 BioMed Central Ltd unless otherwise stated. F Co-localization of membranes (red) and core (green) by CLSM. Adapted with permission from [131], copyright © 2022 Elsevier B.V. G Representative SDS-PAGE result showing the membrane proteins analysis of PLGA cores (lane 1), AM membranes (lane 2), AM vesicles (lane 3), TN@AM NPs (lane 4), and AM cell lysate (lane 5). Lane M: marker. Adapted with permission from [36], © 2021 The Authors. Advanced Science. H Western blotting analysis demonstrating the retention of characteristic membrane proteins. Adapted with permission from [33], copyright © 1999–2022 John Wiley & Sons, Inc. I The α4 and β1 integrins in RAW264.7 cells measured by FACS analysis. Adapted with permission from [35], copyright © 2016 American Chemical Society
Physicochemical properties
Successful wrapping can be validated by observing a 10–20 nm increase in particle size, which is equal to the thickness of the membrane layer, and the charge of membrane-coated nanoparticles should be close to the potential of the macrophage membranes [64]. To prepare membrane-coated nanoparticles, Wang et al. designed the optimal ratio of microvesicles at different mass ratios to screen uniform and stable sizes and zeta potentials by dynamic light scattering (DLS) [65]. DLS is one of the most accurate and economical methods to determine the size distribution and zeta potential of nanoparticles. Wei et al. reported that the mean size of membrane-coated nanoparticles increased by 10–15 nm compared with that of bare nanoparticles, and the zeta potential of membrane-coated nanoparticles was close to that of macrophage membranes (Fig. 3A) [66]. The naked nanoparticles can form a distinct “shell-core” structure after coating by macrophage membranes. Scanning electron microscopy (SEM) and transmission electron microscopy (TEM) are mainly used to examine the structure and morphology of the sample, such as size, shape, aggregation and surface morphology. SEM images revealed that the bare core and macrophage-coated core exhibited a spherical shape with a smooth surface (Fig. 3B). TEM images showed a typical ‘core–shell’ structure and a single dimer macrophage membrane layer (~ 10 nm) on the core nanoparticles (Fig. 3C, D). Recently, immunogold staining fluorescence and colocalization analysis have been used to verify the success of the membrane coating through a method of visual evidence. Immunogold staining showed that the specific markers were simultaneously present on the surface of the core (Fig. 3E). Colocalization assays showed the overlap of fluorescence of macrophage membranes and core, indicating the success of membrane coating (Fig. 3F). Infrared spectroscopy, nuclear magnetic resonance spectroscopy and mass spectrometry are also required to determine the successful preparation of drugs or nanoparticles. Finally, in vitro release experiments are also required to measure the release rate of the drug under different acidic environments.
Biological function properties
Particle size, zeta potential, electron microscopy and others confirmed the success of the membrane coating. However, the integrity of the membrane components and the retention of membrane protein activity directly determine whether the encapsulated nanoparticles can perform their specific functions. Therefore, to detect the retention of membrane activity, we can examine its protein profile as well as the concentration of characteristic proteins. Western blotting and sodium dodecyl polyacrylamide gel electrophoresis (SDS-PAGE) were used to analyze the protein environment of the biomimetic nanoparticles. SDS-PAGE was used to verify the successful migration of overall proteins on the cell membrane and compare the difference in membrane proteins present on the cell membrane of source cells, extracted membrane and cell membrane-camouflaged nanoparticles (Fig. 3G). Furthermore, western blotting was validated against key membrane proteins with specific protein markers (Fig. 3H). As shown in Table 2, integrin α4 and integrin β1 are usually detected in macrophage membranes because they can actively bind to vascular cell adhesion molecule-1 of cancer cells or inflamed endothelium. CD63 and TSG101 are usually detected in M-exo and macrophage-derived microvesicles. In addition, flow cytometry and qPCR can also verify membrane proteins and mRNAs. For example, Rao et al. made use of flow cytometry and qPCR to verify the success of genetically modified cell membranes (Fig. 3I).
Application of macrophage membrane biomimetic nanoparticles
Macrophage membranes inherit the function of the natural membranes so that endow the nanomaterials with immune evasion, enhanced compatibility, ability to target inflammation and tumors. Recently, this biomimetic strategy has been employed successfully in the treatment of various of diseases including cancers, infection diseases, cardiovascular diseases, CNS diseases, immune disease, and other inflammatory. The macrophage membranes coated nanoparticles have a capacity to target tumors and inflamed tissues by the surface receptors such as integrins, MAC-1 and CSF1R. Table 3 has summarized the biomedical applications of macrophage membranes coated nanosystems.
Target delivery in cancer therapy
Considering the role of macrophages in the tumor microenvironment, macrophages can target tumors and phagocytose cancer cells. Macrophage membranes bioinspired nanocarriers have exhibited many advantages such as prolonged circulation and specific target tumor tissue. Currently, macrophage membrane-coated nanoparticles have shown unique essential therapeutic effects in cancers, including breast, skin, lung, colon and others.
Breast cancer
Breast cancer has already become a worldwide public health problem for women. Macrophages are the main cellular components of the breast tumor microenvironment and play an important role in the development and metastasis of tumors. Zhang et al. have developed natural macrophage membrane-coated paclitaxel-loaded nanoparticles (cskc-PPiP/PTX@Ma) to exhibit an enhanced therapeutic effect for the treatment of primary breast cancer [67]. Cskc-PPiP/PTX@Ma showed higher accumulation at the tumor site because the macrophage membrane with its associated membrane protein served as a concealing cloak against RES clearance and a tumor-homing navigator. For the treatment of breast cancer metastasis, Sun et al. created a polymer of PLGA that was used to encapsulate saikosaponin D to formulate biomimetic polymer nanoparticles coated by macrophage membranes hybridized with T7 peptide (SCMNPs) [68]. The T7 peptide is a targeting ligand for transferrin receptors that are overexpressed in tumor cells. The in vitro cellular uptake and in vivo biodistribution studies showed that SCMNPs could specifically target tumor cells and exhibited an immune escape effect on RES. In vivo antitumor performance demonstrated that SCMNPs significantly inhibited tumor growth compared with the control groups. Pretreated macrophage membranes are also used for the encapsulation strategy. For example, Cao’s group synthesized macrophage membrane-coated emtansine liposomes (MEL) to target metastatic foci in the lung [35]. The macrophage membranes were isolated from RAW264.7 cells with high expression of α4 and β1 integrins, which can specifically bind to VCAM-1 on cancer cells. An in vitro cell uptake study showed a 2.0-fold higher internalization into 4T1 cells by FACS than into emtansine liposomes but a lower internalization into RAW264.7 cells. The in vivo pharmacokinetic study displayed the intensity of fluorescence signals of MEL was much higher than emtansine liposome at 1.0 and 4.0 h after administration. Meanwhile, the in vivo distribution of MEL showed a higher fluorescence signal in the lung than in other organs owing to the high expression of α4 and β1 integrins on the macrophage membranes.
A previous study confirmed that M1 macrophages had the natural ability to migrate into tumor tissues and secrete proinflammatory factors [69]. Furthermore, Zheng et al. found that exosomes secreted from the macrophage RAW264.7 by LPS can induce neuroprotective function after ischemic stroke by enhancing the anti-inflammatory M2 phenotype polarization of microglia [70]. Wang et al. used M1 exosomes loaded with paclitaxel (PTX-M1-Exos) to enhance the antitumor activity of breast cancer [71]. The results show that M1 exosomes produced proinflammatory cytokines and enhanced the antitumor efficiency. After loading the chemotherapeutic agent paclitaxel, PTX-M1-Exos exhibited a higher antitumor effect than M1-Exos alone.
Currently, some investigators transfer their attention to macrophage hybrid membrane biomimetic nanoparticles. For example, Gong et al. developed macrophage-4T1 hybrid membrane-camouflaged PLGA loaded with the FGL1/LAG3 blockade molecule siFGL1 and the immune-metabolic adjuvant metformin [72]. The hybrid membrane made use of the homologous targeting capacity of cancer cells and the ability of immune escape of macrophages to realize precise targeting. All studies showed that macrophage membranes and macrophage-derived membrane-coated nanoparticles have great potential in the treatment of primary and metastatic tumors.
Phototherapy is emerging as a new therapeutic strategy for the treatment of cancer because of its noninvasiveness, high selectivity and low systemic toxicity, such as the well-known photothermal therapy (PTT) and photodynamic therapy (PDT) [73]. For PTT, Xuan et al. designed macrophage cell membrane-coated gold nanoshells (MPCM-AuNSs) that exhibited good colloidal stability and maintained the original NIR adsorption of AuNSs [74]. The formulation showed higher accumulation at the tumor site. By incubating FITC-dextran-loaded bare AuNSs and MPCM-AuNSs with 4T1 mouse breast cancer cells, 83.18% of the MPCM-AuNSs were internalized into 4T1 cells, whereas only 42.15% of the AuNSs were internalized. The pharmacokinetic studies showed that MPCM-AuNSs had significantly enhanced blood retention time compared to the bare AuNSs. Upon NIR laser irradiation, local heat generated by the MPCM-AuNSs achieves high efficiency in suppressing tumor growth and selectively ablating cancerous cells within the illuminated zone. For PDT, Chen et al. reported tumor-associated macrophage membranes (TAMMs) coated with UCNPs (NPR@TAMM) for the treatment of cancer and improving immunotherapy [75]. TAMMs were derived from purified primary TAMs, which highly expressed specific protein markers, including CSF1R, CD206, F4/80, and CD11b. The membranes had the ability to deplete the CSF1 secreted by the tumor cells in the TME, resulting in blockade of the interaction between TAMs and cancer cells. After irradiation with a 980 nm laser for 5 min, NPR@TAMMs inhibited cell proliferation in 4T1 cells with an IC50 value of 59.7 μg/mL, which was significantly lower than that for NPR (301.5 μg/mL) or NPR@MMs (129.2 μg/mL). The TAMM coating strategy allowed the core nanoparticle to be a passive and active target to the tumor while avoiding oxidation and invasion by the immune system. Based on the great effects of chemotherapy, PTT and PDT, researchers have combined all three. Poudel et al. established a nanoplatform (PTX@CuS@MMNPs) containing three therapies to kill tumor cells [33]. CuS was used as a nanocarrier for chemotherapeutic drugs, PTT and PDT. Cellular internalization was improved by membrane encapsulation. In vivo tumor accumulation, tumor inhibition rate, and apoptotic marker expression were significantly improved.
The above examples demonstrate that macrophage membrane macrophages and macrophage-derived membrane-coated phototherapeutic agents have the ability to target tumors, escape immune responses and treat breast cancer. Combined with phototherapy, these synergistic systems achieve a high-precision treatment of tumors and an excellent treatment efficiency.
Skin cancer
Skin cancer is a malignant tumor due to subcutaneous tissue lesions. Macrophages play a crucial role in the development and migration of skin cancer. Studies have shown that macrophages in squamous cell carcinoma (SCC) can indirectly inhibit T-cell activity by expressing arginase-1, which breaks down L-arginine [76, 77]. Chen et al. reported macrophage membrane-coated nanoparticles to increase the circulation time and targeting ability in a model of SSC-7 tumor-bearing mice [54]. Additionally, macrophages can secrete periostin and thus promote the metastasis of melanoma [78]. Melanoma is a kind of aggressive skin cancer that originates from melanocytes. The current limited available treatment options for melanoma are due to its high metastasis rate. Parodi et al. have shown that leukocyte membrane biomimetic nanoparticles enhance circulation time and tumoritropic accumulation in mice with murine B16 melanoma [79]. Furthermore, Cao et al. developed albumin nanoparticles coated with macrophage membranes loaded with paclitaxel to treat melanoma [80]. Albumin nanoparticles can accumulate in the tumor site, and macrophage membranes can reduce the clearance of albumin by RES and enhance the target capacity to tumor. Melanoma is an aggressive tumor. Even though it works well on primary tumors, there is no better treatment for cancer cells in the circulation. Cancer cells form circulating tumor cells (CTCs) by binding to immune cells, including macrophages and platelets, thus avoiding the clearance of the immune system and low efficiency of metastasis. Rao et al. developed hybrid nanovesicles (hNVs), including M1 macrophage membranes, platelet membranes and cancer cell membranes, that overexpressed high-affinity SIRPα variants by loading a stimulator of interferon genes (STING) agonist for the treatment of B16F10 tumors and 4T1 tumors (Fig. 4A, B) [81]. The hybrid nanovesicles increased the affinity for CD47 because they overexpressed SIRPα and promoted the M2-to-M1 repolarization of macrophages. Notably, researchers have used six nanovesicles, including liposomes, red blood cells, platelets, M1 macrophages, engineered cancer cells and hNVs, for comparison. The in vivo pharmacokinetic study and fluorescence imaging showed that M1-NVs exhibited a longer blood retention time and more significant accumulation at the tumor site in the B16F10 mouse melanoma model. The mRNA levels of M2 macrophages after treatment with M1-NVs demonstrated a lower level of M2 markers and a higher level of M1 markers. As a result, M1-NV treatment induced a significant increase in tumor-infiltrating T cells, especially CD8+ T cells, effectively improving the antitumor effects. According to B16F10 mouse melanoma models, hNVs significantly suppressed local recurrence and distant metastasis of tumors (Fig. 4C, D). Due to the suppression of tumor recurrence, the survival rate of the mouse groups treated with hNVs increased to 66% in 60 days. Additionally, hNVs demonstrated a higher inhibition of 4T1 tumors in a poorly immunogenic triple-negative breast cancer model (Fig. 4E, F). Remarkably, the platelet-derived NVs suggested the damaged tissue targeting capability and effective binding of pNVs with CTCs.
A Schematic showing the hNVs consist of engineered SαV-C-NVs, M1-NVs, and P-NVs. B Schematic showing the hNVs efficiently interact with CTCs in the blood, accumulate in the post-surgical tumor bed, repolarize TAMs towards M1 phenotype, and block the CD47-SIRPα ‘don’t eat me’ pathway, thus promoting macrophage phagocytosis of cancer cells, as well as boosting antitumor T cell immunity. Average C tumor growth kinetics in 4T1 tumor groups. D Metastasis rates after indicated treatments. Average E tumor growth kinetics in B16F10 tumor groups. F Numbers of lung metastatic foci after different treatments. Adapted with permission from [81], copyright © 2022 Springer Nature Limited
The most critical aspect of the capture for CTCs is that the researchers have combined the advantages of the three cells. Platelet and macrophage membranes evade recognition by the immune system, thus allowing high-affinity cancer cell membrane variants to target tumors more precisely.
Lung cancer
Lung cancer is characterized by the uncontrolled proliferation of cancer cells in the lung. Meanwhile, the lung is the most common site of metastasis and tumor recurrence, which is the leading cause of cancer-related death. Non-small cell lung cancer accounts for > 80% of lung cancer cases and currently has an overall 5 year survival rate of only 15%. Evangelopoulos et al. have made use of electroporation to load doxorubicin into J774.1 macrophages; thereby, the system can deliver the drug into the pulmonary environment or lungs by its tropism for neoplastic and release drug through efflux bump [82]. In the LL/2 mouse model, this system has demonstrated favorable suppression of tumor growth in the lungs. Notably, the cell viability of macrophages is inactive, and previous work showed that electroporated cells maintain the expression of adhesion-based transmembrane proteins. With the application of electroporation, they have developed a low-cost and high-efficiency solution to generate effective pulmonary drug delivery vehicles. The aforementioned macrophage membranes are not focused on the phenotype of macrophages. According to a report, non-small cell lung cancer overexpresses the sigma receptor, which is a membrane-bound protein [83]. Based on this finding, Kim et al. prepared PTX-loaded exosomes modified with aminoethylanisamide-polyethylene glycol (AA-PEG) to target pulmonary metastases [84]. Exosomes are harvested from the supernatants of RAW264.7 cells and primary bone marrow-derived macrophages to enhance the blood circulation time and tumor accumulation rate. In vivo therapeutic efficacy was greater than that of nonvectorized exosome-PTX and Taxol. Choi et al. used mouse peritoneal macrophages induced by 3% Brewer thioglycollate medium as a biocarrier [85]. They provided liposome-loaded doxorubicin and the imaging agent iron oxide, which can reach the tumor site in vivo, maintain continuous and durable action in tumor death and exhibit imaging capacity.
Others
Apart from the above types of cancer, macrophage membrane-mimetic nanoparticles are also being applied to other cancers. For example, Fang et al. prepared artificially assembled macrophages with drug-loaded liposomes for colorectal cancer [86]. Integrin α4, integrin β1 and C-C chemokine receptor 2 (CCR2) help them target tumors and evade the mononuclear phagocyte system. He et al. designed polyelectrolyte multilayer (PEM) capsules coated with gold nanoparticles and THP-1-cell membranes on the two sides to be used as photoactive cancer cell detectors to kill HeLa cells by PTT [62]. Upon illumination with NIR light, the gold shell could be heated to evaporate water violently and melt the capsule and cellular walls. In vitro experiments of biofunctionalized Janus capsules indicated that they can successfully kill cancer cells with a laser power density of 23.6 mW/μm2. Qiang et al. designed reduced graphene oxide-loaded doxorubicin, followed by internalization of macrophages to work on RM-1 mouse prostate cancer [87]. Ji et al. created hybrid membranes, including H22 cells, HepG2 cells and RAW264.7 cells, to target hepatocellular carcinoma [88].
Overall, the aforementioned studies indicate the potency of macrophage membrane-cloaking nanoparticles in the treatment of cancers. Macrophage membrane coating does not influence the function of the core but endows the core with targeting and immune evasion. Additionally, macrophage membrane camouflage nanoparticles can steadily improve the solubility and target capability of drugs. This strategy can also utilize the membrane protein to overcome the challenge faced by nanoparticles during systemic circulation. Macrophage membrane- or membrane-derived exosomes can be edited and modified. Notably, macrophages play important roles in tumor metastasis. A previous study showed that tumor macrophages promote epithelial-mesenchymal plasticity to fit the shear stress in blood vessels [89]. Based on this mechanism, macrophages have a potent ability to kill CTCs, such as leukemia, multiple myeloma and malignant lymphoma. Taking into account the role of macrophages, macrophages have natural superiority in cancer treatment.
Anti-microbe
Pathogenic microorganisms refer to microorganisms, or pathogens, that can invade the human body and cause infections or even infectious diseases. Among the pathogens, bacteria, viruses and parasites are harmful. The membranes from immune cells, including macrophages, have a mass of bacterial-specific cellular receptors that allow the nanoparticles to attract and neutralize the membrane active toxins. This phenomenon is called the ‘nanosponge effect’ due to its high affinity for toxins and cytokines [90].
Antibacterial
With the discovery of penicillin, antibacterial agents have been emerging as a new therapeutic strategy. However, the emergence of bacterial resistance to antibacterial agents has increased the use of antibiotics in clinical treatments for infection, mainly because of three biological properties: cell envelope blockages, biofilm protection, and macrophage shelter [91, 92]. Indeed, macrophages can specifically recognize and neutralize bacteria through the connection between PRRs on the macrophage membrane and PAMPs of bacteria. For example, in sepsis, endotoxin, referred to as lipopolysaccharide (LPS), is released from the bacteria and is recognized as a PAMP by macrophages [93]. Subsequently, macrophages can bind and neutralize endotoxins and cytokines. Soracha et al. prepared a PLGA core coated with a macrophage membrane (MΦ-NPs) for the treatment of sepsis [94]. MΦ-NPs could bind to endotoxins and cytokines, inhibiting their ability to enhance downstream inflammatory cascades. In a mouse Escherichia coli bacteremia model, treatment with MΦ-NPs significantly reduced proinflammatory cytokine levels and inhibited bacterial dissemination. In another study, scholars found that macrophage-derived biomimetic nanoparticles (leukosomes) could be used for the treatment of an LPS-induced murine model of sepsis [95]. In vitro studies elucidated that leukosomes induce an anti-inflammatory response in endothelial cells through the interaction of leukosomes with macrophages. The interaction could trigger a decrease in proinflammatory genes (IL-6, IL-1b, TNF-α) and an increase in anti-inflammatory genes (IL-10, TGF-β).
Interestingly, when macrophages were cultured with specific bacteria, the expression of recognition receptors, such as TLR2, TLR4 and TLR6, increased. Toll-like receptors function as PRRs to produce an immune response. Wei et al. reported a nanotoxoid formulation for use as a vaccine against gram-negative bacterial infections by a multiantigenic formulation [96]. This formulation was first performed on PLGA core-coated J774 macrophage cell membranes with a weight ratio of 1:1, followed by sonication. Then, they collected P. aeruginosa secretions (PaS) from the bacterial culture supernatant and incubated them with macrophage membrane-coated nanoparticles for 15 min to obtain the final macrophage nanotoxoids (MΦ-toxoids). MΦ-toxoids endowed the nanoparticles with multiantigenic characteristics to clear bacteria. Likewise, on account of this phenomenon, this group used a pretreated macrophage membrane to coat a gold-silver nanocage (Sa-M-GSNCs) to more precisely target bacterial cell surfaces [97]. GSNC has been approved as an effective antibacterial agent because of its photothermal effect. Both in vitro and in vivo live bacterial infection of cells and mice showed that Sa-M-GCNCs had a better therapeutic effect. Li et al. regarded the use of bacterially pretreated macrophage membranes as highly impractical for intracellular bacterial infections [98]. They designed a self-assembled micelle composed of antimicrobial triclosan and ciprofloxacin coated with macrophage membranes (Me-ANPs) to target infected macrophage cells where bacteria hide. Both in vitro and in vivo showed efficiency in killing S. aureus. Me-ANPs eradicated the infection in a mouse peritoneal infection model and mouse organ infection model.
Antiviral
A virus is a much smaller microbe than fungi and bacteria that relies on the metabolic system of host cells for reproduction. Infected cells often die because the virus blocks their normal physiological functions, subsequently resulting in the release of new viruses to infect other cells. The mechanism of action of antiviral infection drugs is mostly to interfere with the replication of the virus [99]. However, when antiviral drugs enter the body, in addition to being able to attack viruses and infected cells, they can also produce certain toxicity to normal cells in the human body [100]. The antiretroviral delivery system is an effective measure among antiviral drugs currently on the market. However, there are still three problems: weak antiviral effects, serious side effects, and rapid drug resistance.
Dou et al. developed a nanoparticle-loaded indinavir (NP-IDV) formulation packaged into carrier bone marrow-derived macrophages (BMDMs) for human immunodeficiency virus type 1 (HIV-1) [56]. Macrophage membrane protein endowed NP-IDV with the ability to target sites, promoting sustained “local” drug release for periods of approximately 2 weeks, avoiding the destruction of the immune system in the HIV-1 mouse model and increasing the therapeutic effects. In addition, they used IDV-NP-BMM to target HIV-1 encephalitis (HIVE) rodent model [101]. The results showed continuous IDV release for 14 days and a reduction in HIV-1 replication in HIVE brain regions. Similarly, severe COVID-19 was highly associated with cytokine storm syndrome (CSS). Tan et al. designed macrophage membrane-coated PLGA NPs loaded with lopinavir (PLGA-LPV@M) [102]. The receptors of IL-1β and IL-6 on the macrophage membrane neutralize proinflammatory cytokines to suppress macrophages and neutrophils. Moreover, macrophages expressed angiotensin-converting enzyme 2 (ACE II), which could help PLGA-LPV@M target SARS-CoV-2 by the affinity between ACE II and the spike protein on SARS-CoV-2. Owing to the synergistic effects, PLGA-LPV@M exhibited a significant effect in the mouse model of coronavirus infection. Li’s group reported that alveolar macrophages (AMs) were the first line of defense for the host immune system against SARS-CoV-2 infection [36]. Therefore, an AM membrane was used to coat PLGA nanoparticles embedding photothermal material (TN@AM NPs) to enhance antiviral efficiency (Fig. 5A). In a surrogate mouse model of COVID-19, TN@AM NP treatment decreased the lung virus burden compared to the untreated group (Fig. 5B). Anti-cytokine ability analysis demonstrated that the TN@AM NP group significantly decreased the expression of various proinflammatory cytokines (Fig. 5C). Furthermore, lung histopathological alteration analysis showed that TN@AM NPs significantly reduced lung damage compared to the other NPs.
A Schematic illustration of multifunctional alveolar macrophage-like nanoparticles for coronavirus cellular entry blockage, virus photothermal disruption, and inflammatory cytokines absorption. Physicochemical and membrane protein characterizations of TN@AM NPs B Detection of MHV-A59 burden in the lung tissues through a standard plaque assay at 5 days post different treatments. C Detection of the mRNA expression of various proinflammatory cytokines in the lung tissues through a standard RT-qPCR assay at 5 days post different treatments. Adapted with permission from [36], copyright © 1999–2022 John Wiley & Sons, Inc.
Anti-parasite
Macrophages also play an important role in parasite infection, participating in parasite-host immune interactions [103]. A study confirmed that parasites can induce M1-type polarization of macrophages. M1 macrophages clear parasites by producing TNF-α, IL-12 and iNOS. M2 macrophages can metabolize L-arginine into proline and polyamine to promote parasite survival through Arg1, which is related to the inflammatory process and regression of tissue repair [104]. Leishmania donovani, an intracellular parasite, adheres to macrophages and enters into macrophage cells along with phagocytosis activity to induce transformation to the M2. Amphotericin B has been encapsulated into macrophage nanogghosts to Leishmania-infected macrophages [105]. It has been shown that an increased concentration at the infected site and the production of protective cytokines and ROS-RNS with lower collateral toxicity to healthy cells compared to the free amphotericin B group.
Taken together, these results showed the ability of macrophage membrane-coated nanoparticles to regulate the inflammatory response in target cells, acting as a bioactive nanotherapeutic.
Cardiovascular diseases
Atherosclerosis (AS) is a chronic inflammatory vascular disease caused by the accumulation of many lipids around the arterial wall. As an oxidized form of low-density lipoprotein (Ox-LDL) in the intimal layer, the local endothelial wall produces excessive ROS and subsequently secretes a series of adhesion molecules to recruit immune cells [106]. Subsequently, local inflammation leads to fatal cardiovascular events and stroke by stenosis of the arterial lumen and rupture of unstable plaques [107]. Integrin α4 and β1 are major leukocyte receptors on the macrophage membrane that recruit macrophages to atherosclerotic plaques by recognizing VCAM-1 on the surface of vascular endothelial cells [108, 109].
Fe3O4 has been used as a typical magnetic resonance imaging (MRI) agent. Huang et al. confirmed that Fe3O4 nanoparticles coated with a RAW264.7 membrane (Fe3O4@M) could effectively target the early process of AS [110]. Compared with the conventional MR agent, Fe3O4@Ms had more safety for research objects in vivo or in vitro and were more effective in imaging than Fe3O4@PEG. The molecular mechanism study showed that integrin α4 and β1 overexpression on macrophages specifically recognized VCAM-1 on endothelial cells. Wang et al. fabricated a macrophage membrane coating on the surface of rapamycin-loaded PLGA (MM/RAPNPs) to target atherosclerotic lesions [111]. The release kinetics showed that 38.51% and 35.62% of rapamycin were released from RAPNPs and MM/RAPNPs after 72 h of incubation, respectively. MM/RAPNPs approved sustained drug release. Cell phagocytosis assays were performed to evaluate immune evasion. Both CLSM images and FACS analysis showed that macrophage membrane-coated DID-labeled NPs (MM/DIDNPs) could significantly inhibit internalization by macrophages. Similarly, an overexpression VCAM-1 model of HUVECs activated by TNF-α demonstrated a higher internalization compared with DIDNPs. Rapamycin is practically insoluble in water, resulting in low bioavailability. The in vivo therapeutic efficacy of MM/RAPNPs showed the lowest atherosclerotic lesions in 6.59% compared with 18.3% of free RAP and 14.43% of RAPNPs. ORO and toluidine blue further verified the therapeutic efficacy of MM/RAPNPs. Overall, MM/RAPNPs can effectively attenuate the progression of AS.
Based on the excessive ROS in the local inflammatory site, researchers have developed a delivery system including ROS-responsive NPs coated with macrophage membranes for AS [112]. For example, amphiphilic oxidation-sensitive chitosan oligosaccharide (Oxi-COS) was chosen as a ROS-responsive material to load atorvastatin (AT-NPs). AT-NPs possessed a drug encapsulation efficiency of 48.3% and a drug loading content of 5.1%. After coating the macrophage membrane by coextrusion and bath sonication, a spherical core–shell structure was observed. From the TEM image and DLS, a single layer of cell membrane wrapped the Oxi-COSNPs, and the diameter of the nanoparticles increased from ~ 204 to 227 nm. Considering the key role of foam cells in AS, researchers selected LPS-induced inflammation in macrophages and oxLDL-treated macrophages as in vitro models to evaluate the therapeutic efficacy of MM-AT-NPs. The results revealed the ROS responsiveness of AT-NPs and MM-AT-NPs in vitro, as well as their attenuation. After incubation with macrophages, MM-AT-NPs provided a potential targeted capacity to inflammatory macrophage cells and foam cells, except for the remaining macrophage cells. For the in vivo targeting capability of MM-NPs and NPs/Mas to atheromatous plaques, cyanine 7.5 NHS ester was employed to prepare Cy7.5-labeled formulations. The aortic tissues from MM-Cy7.5-NPs had stronger fluorescence due to their inherent immune propensity for inflammation. Next, they examined the therapeutic effect through a model of female ApoE−/− mice. There was an obvious improvement in plaque area with the treatment of MM-AT-NPs in comparison with AT-NPs, which decreased to ~ 8% of the total aorta tissue area. Interestingly, Ahn et al. designed a formulation by encapsulating Ce6 within the triple-helix structure of Glu in aqueous solution [113]. Then, macrophages derived from foam cells internalized the Glu/Ce6 nanocomplexes and delivered nanocomplexes to treat atherogenesis. With laser irradiation, the Glu/Ce6 nanocomplexes significantly damaged the foam cell membranes and generated ROS to reduce ICD. In another article, boronic ester-modified dextran was used to load rapamycin (MM/RNPs) [114]. The average hydrodynamic diameter of MM/RNPs was 164.7 nm. The cumulative release of rapamycin with or without H2O2 was lower than that of RNPs due to the coating membrane. However, MM/RNPs still have ROS responsiveness to H2O2. An in vitro uptake assay demonstrated that MM/RNPs could effectively escape from macrophages and inflamed endothelial cells. Zebrafish were used as an animal model to confirm the in vivo biocompatibility of MM/PCD NPs. Various concentrations of MM/PCD NPs and time points were applied to embryos from zebrafish, and the live zebrafish suggested good biocompatibility. These results showed a potential candidate for anti-AS applications.
Although the natural macrophage membrane has many specific membrane recognition proteins, researchers can still modify the expression of membrane proteins to suit their own needs for treatment. Li’s team reported a bionic nanoparticle with overexpressed CD47 and integrin α4 and β1 [115]. Firstly, they used endothelin-1 to enhance the expression of integrin α4/β1. Second, the functionalized CD47 plasmid was transferred into macrophages to upregulate CD47. The modified macrophage membrane vesicles were mixed with PLGA NPs loaded with colchicine (MMM/COL NPs), sonicated and extruded. DLS analysis showed that the diameter of MMM/COL NPs increased from ~ 180.76 to 202.02 nm, which is the thickness of the macrophage membrane shell. The fluorescence of macrophage cells treated with MMM/COL NPs was lower than that of cells treated with DID NPs or MM/COL NPs, suggesting that the overexpressed CD47 and integrin α4 and β1 were functional. Additionally, the MMM/COL NP group had significantly enhanced fluorescence of inflamed HUVECs. Furthermore, an in vivo targeting study showed the best accumulation of MMM/Dil NPs in atherosclerotic lesions and the best targetability to atherosclerotic plaques.
As recently reported, exosomes isolated from macrophages have been applied in AS. Wu et al. confirmed and clarified that M2 exosomes had a lower level of proinflammatory factors but anti-inflammatory factors, including IL-10, IL-1Ra, and TGF-β [116]. Hexyl 5-aminolevulinate hydrochloride (HAL) enhanced the anti-inflammatory effect by driving the intrinsic biosynthesis and metabolism of heme, which induced the production of anti-inflammatory carbon monoxide and bilirubin. Next, HAL was taken into M2 Exos via electroporation (HAL@M2 Exos) to treat AS (Fig. 6A, B). DLS analysis revealed HAL@M2 Exos with a diameter of 180 nm and excellent stability after 1 week in PBS. A series of experiments were conducted to determine the mechanism. The expression of adhesion molecules, including E-selectin, VCAM1, ICAM1, CD44, VLA4 and LFA1, on inflammatory endothelial cells was measured. The recognition mechanism led to the excellent transmigration and accumulation of M2 Exos at the inflammation site. The in vivo therapeutic effect of HAL@M2 Exo was evaluated in ApoE−/− mice fed a cholesterol-rich diet. From the fluorescence imaging originating from endogenously biosynthesized protoporphyrin IX (PpIX) in the aortas of mice, the HAL@M2 EXO group exhibited the highest PpIX fluorescence signal (Fig. 6C). Oil Red O (ORO) staining of entire aortas indicated that HAL@M2 Exo observably reduced aortic lesions and aortic valve lesions by 75.2% and 73.9%, respectively, compared with PBS alone (Fig. 6D, E). The H&E staining results showed that the HAL@M2 EXO group had diminished aortic values. Finally, the expression of ABCA-1 and SR-B1 receptors in aortas showed a greatly upregulated tendency, which further confirmed that HAL@M2 EXOs could relieve chronic inflammation-induced AS in vivo.
A The preparation of HAL@M2 Exo. B The inflammation-tropism and anti-inflammation effects of HAL@M2 Exo. Treatment of early atherosclerotic plaques with HAL@M2 Exo. C Fluorescence imaging of the aortas excised from mice. D Photographs of the excised aortas stained by ORO and the corresponding quantitative analyses of plaque areas. E Cryosection photographs of the aortic valves stained by ORO and the corresponding quantitative analyses of plaque areas. Adapted with permission from [116], copyright © 2020 The Authors. Publishing services by Elsevier B.V
Neurological disease
Neuroinflammation is an inflammatory response within the central nervous system and the main hallmark of common neurodegenerative diseases, including Alzheimer’s disease (AD), Parkinson’s disease (PD), and amyotrophic lateral sclerosis (ALS). The main cause of pathogenesis is that hyperactivated microglia release proinflammatory cytokines, which induce neuronal death and accelerate neurodegeneration [117]. However, it is extremely difficult to deliver drugs to microglia. The main obstacle is the blood-brain barrier (BBB), which is difficult for drugs to cross due to its unique and compact physiological structure. It refers to the barrier between blood plasma and brain cells formed by the walls of brain capillaries and glial cells and the barrier between plasma and cerebrospinal fluid formed by the choroid plexus, which can prevent certain substances from the blood from entering the brain tissue [118,119,120]. Macrophages are naturally capable of crossing the BBB, which can traverse the endothelial wall due to increased margination and extravasation [121].
PD is a neurological disorder. This disease is attributed to the selective loss of neurons in the substantia nigra and is associated with brain inflammation, microglial activation and secretory neurotoxin activities such as ROS [122]. Batrakova et al. have developed a BMM system to deliver catalase to an animal model of PD. Catalase was packaged into a cationic block copolymer, polyethyleneimine-poly(ethylene glycol) (PEI-PEG). It has been shown that the nanoparticle could retain catalytic activity and be released in its active form after 24 h. In vitro experiments have indicated potent antioxidant effects for ROS produced by microglia activated with either N-α-syn or TNF-α. Additionally, an in vivo assay showed that BMM could increase the delivery of labeled enzymes into tissues, including a twofold increase in the number of enzymes in the brains of MPTP-treated mice. Furthermore, this team has explored the pharmacokinetics and biodistribution of BMM-incorporated nanozymes. After coating with a macrophage cell membrane, the BMM-nanozyme processed longer blood circulation than bare nanozyme and more precisely targeted diseased sites in models of PD [123, 124]. A previous study proved that exosomes function as source membranes and cross biological barriers, including the BBB. Haney et al. developed an exosome-based delivery system to treat PD by loading catalase [125, 126]. These findings indicated that exosomes loaded with catalase efficiently accumulated in neurons and microglial cells in the brain and produced a potent neuroprotective effect. They internalized nanozymes into BMMs to attract neurons and endothelial cells by endocytosis-independent mechanisms. The results showed that nanozyme trafficking in and between cells could facilitate a neuroprotective response and provide insights.
New therapeutic targets have been developed for AD, including neuronal mitochondrial dysfunction and aggregation of beta-amyloids. Han et al. designed a system in which macrophage membrane-coated solid lipid nanoparticles attached rabies virus glycoprotein (RVG29) and triphenylphosphine cation (TPP) molecules to deliver genistein (GS) (RVG/TPP-MASLNs-GS) to neuronal mitochondria (Fig. 7A) [127]. RVG29 helped the system cross the BBB and target neurons, and TPP further delivered the system to mitochondria via its positive charge. Physical parameters displayed a diameter of 100 nm and a “shell-core” structure. In vivo imaging verified the high brain accumulation of DIR-labeled RVG-MASLNs (Fig. 7B). An in vitro antioxidative stress assay was performed to confirm the protective effect of RVG-MASLN. H&E staining of the hippocampal region of AD mice was further verified. Finally, the swimming path tracing of AD mice showed a more significant learning ability Fig. 7C. In conclusion, RVG-MASLN-GS exhibited excellent effects on relieving AD symptoms in vitro and in vivo.
A Preparation and characterization of RVG/TPP-MASLNs-GS. B In vivo brain-targeting ability. Biodistribution of DIR contained in various formulations determined by IVIS Lumina II. C Representative swimming path tracings of different groups. Adapted with permission from [127], copyright © 1999–2022 John Wiley & Sons, Inc.
Spinal cord injury (SCI) is also a common neurological disease, including primary injury and secondary injury [128]. Primary injury refers to injury caused by an external force acting directly or indirectly on the spinal cord. Secondary injury refers to spinal cord edema caused by an external force, hematoma formed by hemorrhage of small blood vessels in the spinal canal, compression fracture, and broken intervertebral disc tissue [129]. Secondary injury is the main obstacle to motor function recovery after spinal cord injury. Macrophages are characterized by chemotaxis to inflammatory sites, penetrating blood vessels, and targeting the damaged central nervous system through the interaction of adhesion factors and integrins [130]. Xia’s group encapsulated nerve growth factor (NGF) into the macrophage membrane (NGF-NVs) to treat SCI [131]. Cell viability assays showed that NGF-NVs were effectively taken up by PC12 cells and inhibited neuronal apoptosis. Furthermore, they found that NGF-NVs sharply increased the survival of neurons by activating the PI3K/AKT signaling pathway and had a good effect on SCI. In another article, researchers developed the use of membranes from different macrophage subtypes to coat liposomes loaded with minocycline [132]. All experiments showed that the formulation decreased cellular uptake by macrophages, prolonged drug circulation time and actively targeted the trauma site of SCI.
Regarding neuronal survival in ischemic stroke, reperfusion injury is still a major obstacle. Compared with glial cells and vascular cells, neurons are the most vulnerable cells because of the rapid loss of normal function under ischemic conditions. Li et al. developed a macrophage-disguised honeycomb manganese dioxide (MnO2) nanosphere loaded with fingolimod (Ma@(MnO2 + FTY) [133]. On the one hand, Ma@(MnO2 + FTY) crosses the BBB, and MnO2 nanospheres can reduce oxidative stress and promote the transition of M1 microglia to M2 by consuming excess hydrogen peroxide (H2O2). On the other hand, consuming ROS led to the inhibition of the NF-κB signaling pathway in microglia to mitigate the proinflammatory response. Fingolimod can activate the signal transducer and activator of the transcription-3 (STAT3) pathway. The results indicated that Ma@(MnO2 + FTY) successfully protected neurons and provided new possibilities for the treatment of brain disease.
Pang et al. used primary M1 macrophages as a carrier combined with PLGA nanoparticles to deliver doxorubicin (DOX@M1-NPs) to glioma tumors [134]. The results of the in vivo endothelial barrier model revealed that DOX@M1-NPs significantly boosted transcytosis across the endothelial barrier and uptake by U87 cells. In vivo imaging experiments verified that M1-NPs exhibited significant superiority in brain U87 tumors. The in vivo antiglioma effect showed that DOX@M1-NPs markedly prolonged mouse survival by 38.5 days.
Immune diseases
Macrophage membrane-camouflaged nanoparticles are used for the treatment of immune diseases. Rheumatoid arthritis (RA) is an autoimmune disease that leads to joint inflammation, resulting in functional disabilities. The pathology of RA mainly involves the proliferation of synovial lining cells, the infiltration of a large number of inflammatory cells in the interstitium, the neovascularization of microvessels, the formation of pannus, and the destruction of cartilage and bone tissue. Evidence has shown that macrophages adhere to the synovium or the pannus of inflamed vascular tissue via specific ligands. In this case, macrophage membranes or macrophage exosome membranes can take the burden of biomimetic nanoparticles to decrease inflammatory cytokines [65, 135,136,137].
The current treatment of autoimmune disease presents systemic side effects. Flavia et al. developed composite platforms made of porous silicon (Psi) coated with KG-1 macrophage cell membranes (TCPSi@KG-1) for RA [138]. They analyzed the size, surface morphology, and stability in different biological buffers of TCPSi@KG-1, followed by the biological evaluation of cytocompatibility and immunological profile. As a result, TCPSi@KG-1 greatly enhanced the stability of the hydrophobic particles in plasma and simulated synovial fluid. The cytocompatibility of TCPSi@KG-1 in different cell lines, including target organs, blood vessels, kidneys and livers, reached 48 h at concentrations ranging from 0.5 to 50 μg/mL. In addition, the immunological profile investigated in KG-1 macrophages showed that the nanoplatforms decreased the immunostimulatory potential and avoided the activation of the immune system. Li et al. made use of macrophage-derived microvesicles by stimulating cytochalasin B to coat PLGA nanoparticles loaded with tacrolimus (T-MNPs) (Fig. 8A) [139]. The in vitro binding of T-MNPs to inflamed HUVECs was significantly stronger than that of erythrocyte membrane-coated nanoparticles (RNPs). The results showed that macrophage membrane-coated nanoparticles significantly enhanced in vivo targeting in mice with collagen-induced arthritis compared with DIR, bare NPs and RNP. The in vivo tissue distribution further clarified the better targeting capacity of the MNPs to the paws than the others. Proteomic analysis has revealed the targeting mechanism, suggesting that MAC-1 and CD44 contribute to the prominent targeting of MNPs. Therefore, in vivo immunofluorescence revealed higher levels of P-selectin and ICAM-1 expression in the arthritic paw (Fig. 8B). The inflamed endothelium in RA highly expresses P-selectin and ICAM-1, which can be recognized by CD44 and MAC-1. The in vivo drug release of T-MNPs demonstrated sustained release and long retention. The expression of proinflammatory cytokines provided that T-MNPs could remarkably reduce their production. The therapeutic effect of RA showed that T-MNPs significantly suppressed RA and maintained a stable period (Fig. 8C). H&E staining of joint tissues further confirmed this effect.
A Schematic illustration of MMV-coated nanoparticle (MNP) targeting sites of RA. MNP could target sites of RA through ICAM-1 or P-selectin adhesion. B Representative images of different nanoparticles accumulation in arthritic paws or nonarthritic (NA) paws; n = 3. C Arthritis index in different groups over 14 days of treatment. Adapted with permission from [139], copyright © 2022 American Chemical Society
More recently, Li’s team reported that macrophage-derived microvesicle membranes coated nanoparticles to treat RA [65, 137]. One used M2-type exosomes to load plasmids encoding IL-10 cytokine and betamethasone sodium phosphate, and another used exosome membranes to coat zeolitic imidazolate framework-8 (ZIF-8)-loaded dexamethasone sodium phosphate. All studies have indicated that the macrophage biomimetic platform could be applied to RA and is a promising potent for other immune diseases.
Other diseases
Macrophages are the cells of the immune system. They can phagocytose foreign materials from bacteria and debris to dead cells. A large number of leukocytes migrate to the inflamed lesion to regulate inflammatory processes. Macrophage membranes and macrophage-derived microvesicles are used to deliver the drug to the lesions.
Tang et al. generated dexamethasone-loaded macrophage-derived microvesicles (MV-DXs) for the treatment of renal inflammation and renal fibrosis [140]. The high expression of integrin αLβ2 (LFA-1) and α4β1 (VAL-4) caused them to adhere to the inflamed kidney. Furthermore, MV-DEX significantly decreased renal injury in murine models of LPS- or ADR-induced nephropathy compared with free DEX. Meanwhile, MV-DEX sharply reduced the side effect of DEX due to the precise targetability. Ulcerative colitis (UC) is characterized by relapsing inflammation in the colon. Sun et al. developed a macrophage membrane-coated ROS-sensitive β-cyclodextrin loaded with rosiglitazone (RMN NPs) to treat UC [39]. Owing to the inflammatory homing effects, the macrophage membrane assisted RMN NPs in inflammatory colonic tissues to suppress inflammation. Based on the overproduction of ROS and upregulation of transmembrane glycoprotein CD98 in UC, Ma et al. developed a macrophage membrane to coat a pH-responsive MOF carrier loaded with carbon nanodots and CD98 CRISPR/Cas9 plasmid (CCZM) [141]. The results of cellular uptake in RAW264.7 cells showed that macrophage membrane-coated nanoparticles had a 2.5-fold higher fluorescence intensity than nanoparticles without membrane decoration. Regarding the in vivo inflammation targeting ability of the macrophage membrane, CCZM demonstrated significantly stronger fluorescence in the inflammatory colon. The combined effect of nanozymes and the CRISPR/Cas9 system significantly alleviated UC inflammation and provided a promising approach for the personalized treatment of inflammation. Zhang et al. reported a biomimetic nanoparticle with a ‘lure and kill’ mechanism that was made by embedding with a macrophage membrane doped with a PLGA core loaded with melittin and MJ-33 for pancreatitis (MФ-NP) [142]. In pancreatitis models, MФ-NP effectively inhibited PLA2 activity and PLA2-induced pancreatic injury. In allergic asthma (AA), the adoptive transfer of M2 macrophages could aggravate pathologies of allergic airway inflammation. Pei et al. explored the exosome membrane of M2 macrophages coated PLGA@Dnmt3aossmart silencer to treat AA [143]. Dnmt3aossmart silencer is a small interfering RNA (siRNA) that is easily degraded. Therefore, they established PLGA NPs to obtain a nanocomplex with siRNA and exosome decoration to improve the biodistribution, stability, efficacy and biocompatibility of NPs. The encapsulation efficiency of Dnmt3aossmart silencer into PLGA was determined to be 70.70 ± 1.82%. The release kinetics of the Dnmt3aossmart silencer showed a sustained release profile of more than 50% of the cumulative release rate at 24 h. Teo et al. designed macrophage membrane-coated NPs originating from M0, M1 and M2 macrophage membranes for the treatment of osteoarthritis [144]. Interestingly, M2 macrophage membrane-coated NPs showed superior efficacy in sponging proinflammatory cytokines and relieving osteoarthritis.
In conclusion, macrophage membrane-mimetic nanoparticles can effectively target inflamed tissue and mitigate the inflammatory response by utilizing the natural inflammatory targeting effect of macrophage membranes. Meanwhile, the anti-inflammatory type of M2 macrophages exhibited a superior anti-inflammatory effect. This lays the foundation for the clinical application of cell membrane-mimetic nanoparticles loaded with clinical applications.
Relevance to clinical studies
Over the past years, the development and application of macrophage-coated nanoparticles have been an increasing number of relevant patents published. As shown in Table 4, these patents of drug delivery were initially performed by using macrophages to directly carry cargoes. Subsequently, macrophage membranes were used to wrap various nanomaterials with more diversified therapeutic actions. Then, modified macrophage membranes were used as the envelope material. These patents show that macrophage membrane bionic nanoparticles can combine with other therapy, offering a potential novel platform for disease treatment. And the mimetic platform was progressing in a more efficient and intelligent direction in the past years.
Summary and future challenges
Cell membrane cloaking is a potential platform for drug delivery and therapy strategies. As a type of immune monocyte cell, macrophages are key factors in the physiological functions and pathology of various diseases. Macrophage membrane-coated nanoparticles enhance the retention of the loaded drug. Moreover, macrophage membrane-coated nanoparticles can target inflamed sites or tumor sites and neutralize endotoxins, thus achieving safe and efficient therapeutic effects. This paper reviews the preparation, characterization, application and clinical challenges of macrophage membrane-coated core nanoparticles. The mimetic strategy has been studied in the treatment of cancer, immune disease, atherosclerosis, infection, and inflammatory disease. The above discussion also proves that macrophages are potential cell membrane donors. It is expected that macrophage membrane cloaking will be translated into clinical trials and act as an important part in the field of biomedicine.
However, there are many challenges faced in clinical translation. Firstly, how to obtain macrophage membranes on a large scale under the premise of ensuring the biological function of membrane proteins. Secondly, among the currently known coating methods, including ultrasound, coextrusion and electroporation, the membrane can be successfully coated on the surface of nanoparticles, but it is difficult to ensure the coating efficiency in large-scale processing. To ensure consistent physical and chemical parameters, the preparation and processing methods of different nanoparticles are also different. Therefore, it is necessary to further optimize membrane-coated nanoparticle technology and develop a simple, efficient and large-scale preparation of new technology. Moreover, ultralong-term stability studies should be conducted under different storage conditions to ensure the efficacy and stability of the product. To our knowledge, macrophage membrane-encapsulated nanoparticles can be used in a variety of diseases to enhance therapeutic efficacy in vivo and in vitro. However, the biosafety of cell membranes should be carefully studied before applying in clinical trials. Firstly, immunogenicity between immune cell membrane donors and acceptors may lead to serious safety concerns. Secondly, certain specific modifications of cell membranes may also lead to some unexpected side effects. Thirdly, issues about pharmacokinetics, safety, and interaction with materials in the body should be addressed to improve the clinical translation of macrophage membrane cloaking. More importantly, there is no formal regulatory standard for bionic products, so more attention should be given to the safety and effectiveness of bionic nanoparticles in humans. Therefore, in future studies, these limitations must be overcome before such methods can be used as standards for clinical treatment.
Availability of data and materials
Not applicable.
Abbreviations
- FDA:
-
Food and drug administration
- PLGA:
-
Poly [D, L-lactic-co-glycolic acid]
- PLA:
-
Polylactic acid
- PCL:
-
Polycaprolactone
- EPR:
-
Enhanced permeability and retention
- PEG:
-
Polyethylene glycol
- ABC:
-
Accelerated blood clearance
- RES:
-
Reticuloendothelial system
- PRRs:
-
Pattern recognition receptors
- DAMPs:
-
Damage associated molecular patterns
- PAMPS:
-
Pathogen-associated molecular patterns
- LPS:
-
Lipopolysaccharide
- IFN-γ:
-
Interferon-γ
- TNF-α:
-
Transforming factor-α
- IL-4:
-
Interleukin-4
- IL-10:
-
Interleukin-10
- IL-13:
-
Interleukin-13
- IL-21:
-
Interleukin-21
- ECM:
-
Cell-extracellular matrix
- PSGL-1:
-
P-selectin glycoprotein ligand-1
- LFA-1:
-
Lymphocyte function-associated antigen 1
- VLA-4:
-
Very late antigen-4
- VCAM-1:
-
Vascular cell adhesion protein 1
- UCNPs:
-
Upconversion nanoparticles
- MOFs:
-
Metal-organic frameworks
- DLS:
-
Dynamic light scattering
- TEM:
-
Transmission electron microscopy
- SEM:
-
Scanning electron microscopy
- SDS-PAGE:
-
Sodium dodecyl polyacrylamide gel electrophoresis
- PTX:
-
Paclitaxel
- PTT:
-
Photothermal therapy
- PDT:
-
Photodynamic therapy
- BMMs:
-
Bone marrow derived macrophages
- DDS:
-
Drug delivery system
- TAMMs:
-
Tumor associated macrophage membranes
- CSS:
-
Cytokine storm syndrome
- ACE II:
-
Angiotensin converting enzyme 2
- RA:
-
Rheumatoid arthritis
- AD:
-
Alzheimer’s disease
- PD:
-
Parkinson’s disease
- ALS:
-
Amyotrophic lateral sclerosis
- BBB:
-
Blood-brain barrier
- SCI:
-
Spinal cord injury
- NGF:
-
Nerve growth factor
- AS:
-
Atherosclerosis
References
Mitragotri S, Burke PA, Langer R. Overcoming the challenges in administering biopharmaceuticals: formulation and delivery strategies. Nat Rev Drug Discov. 2014;13(9):655–72.
Pombo García K, Zarschler K, Barbaro L, Barreto JA, O’Malley W, Spiccia L, et al. Zwitterionic-coated “stealth” nanoparticles for biomedical applications: recent advances in countering biomolecular corona formation and uptake by the mononuclear phagocyte system. Small. 2014;10(13):2516–29.
Batty CJ, Bachelder EM, Ainslie KM. Historical perspective of clinical nano and microparticle formulations for delivery of therapeutics. Trends Mol Med. 2021;27(6):516–9.
Gunatillake PA, Adhikari R. Biodegradable synthetic polymers for tissue engineering. Eur Cell Mater. 2003;5:1–16.
Song G, Petschauer JS, Madden AJ, Zamboni WC. Nanoparticles and the mononuclear phagocyte system: pharmacokinetics and applications for inflammatory diseases. Curr Rheumatol Rev. 2014;10(1):22–34.
Kolate A, Baradia D, Patil S, Vhora I, Kore G, Misra A. PEG—a versatile conjugating ligand for drugs and drug delivery systems. J Control Release. 2014;192:67–81.
Wang Y, Wang C, Fu S, Liu Q, Dou D, Lv H, et al. Preparation of Tacrolimus loaded micelles based on poly(Ɛ-caprolactone)-poly(ethylene glycol)-poly(ɛ-caprolactone). Int J Pharm. 2011;407(1–2):184–9.
Hu CM, Fang RH, Wang KC, Luk BT, Thamphiwatana S, Dehaini D, et al. Nanoparticle biointerfacing by platelet membrane cloaking. Nature. 2015;526(7571):118–21.
Bahrami B, Hojjat-Farsangi M, Mohammadi H, Anvari E, Ghalamfarsa G, Yousefi M, et al. Nanoparticles and targeted drug delivery in cancer therapy. Immunol Lett. 2017;190:64–83.
Vandghanooni S, Eskandani M, Barar J, Omidi Y. As1411 aptamer-decorated cisplatin-loaded poly(lactic-co-glycolic acid) nanoparticles for targeted therapy of miR-21-inhibited ovarian cancer cells. Nanomedicine. 2018;13(21):2729–58.
Ding D, Tang X, Cao X, Wu J, Yuan A, Qiao Q, et al. Novel self-assembly endows human serum albumin nanoparticles with an enhanced antitumor efficacy. AAPS PharmSciTech. 2014;15(1):213–22.
Wang H, Wu J, Williams GR, Fan Q, Niu S, Wu J, et al. Platelet-membrane-biomimetic nanoparticles for targeted antitumor drug delivery. J Nanobiotechnology. 2019;17(1):60.
Xia Q, Zhang Y, Li Z, Hou X, Feng N. Red blood cell membrane-camouflaged nanoparticles: a novel drug delivery system for antitumor application. Acta Pharm Sin B. 2019;9(4):675–89.
Xuan M, Shao J, Dai L, He Q, Li J. Macrophage cell membrane camouflaged mesoporous silica nanocapsules for in vivo cancer therapy. Adv Healthcare Mater. 2015;4(11):1645–52.
Liu G, Zhao X, Zhang Y, Xu J, Xu J, Li Y, et al. Engineering biomimetic platesomes for pH-responsive drug delivery and enhanced antitumor activity. Adv Mater. 2019;31(32):e1900795.
Toledano Furman NE, Lupu-Haber Y, Bronshtein T, Kaneti L, Letko N, Weinstein E, et al. Reconstructed stem cell nanoghosts: a natural tumor targeting platform. Nano Lett. 2013;13(7):3248–55.
Li J, Zhen X, Lyu Y, Jiang Y, Huang J, Pu K. Cell membrane coated semiconducting polymer nanoparticles for enhanced multimodal cancer phototheranostics. ACS Nano. 2018;12(8):8520–30.
Kunde SS, Wairkar S. Platelet membrane camouflaged nanoparticles: biomimetic architecture for targeted therapy. Int J Pharm. 2021;598:120395.
Harris JC, Scully MA, Day ES. Cancer cell membrane-coated nanoparticles for cancer management. Cancers. 2019;11:12.
Newton K, Dixit VM. Signaling in innate immunity and inflammation. Cold Spring Harbor Perspect Biol. 2012;4(3):19.
Gomez Perdiguero ESC, Geissmann F. Development and homeostasis of resident myeloid cells the case of the microglia. Glia. 2013 Jan; 112-120.
Wynn TA, Chawla A, Pollard JW. Macrophage biology in development homeostasis and disease. Nature. 2013;496(7446):445–55.
Epelman S, Lavine Kory J, Randolph GJ. Origin and functions of tissue macrophages. Immunity. 2014;41(1):21–35.
Lemke G. How macrophages deal with death. Nat Immunol. 2019;19(9):539–49.
Tom Cotter AMd, Henson Peter M. The final step in programmed cell death phagocytes carry apoptotic cells to the grave. Biochem. 2003;39:105–17.
Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32(5):593–604.
Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol. 2010;11(10):889–96.
Chen Y, Zhang X. Pivotal regulators of tissue homeostasis and cancer: macrophages. Exp Hematol Oncol. 2017;6:23.
Andón FT, Digifico E, Maeda A, Erreni M, Mantovani A, Alonso MJ, et al. Targeting tumor associated macrophages: the new challenge for nanomedicine. Semin Immunol. 2017;34:103–13.
Hagemann T, Lawrence T, McNeish I, Charles KA, Kulbe H, Thompson RG, et al. “Re-educating” tumor-associated macrophages by targeting NF-kappaB. J Exp Med. 2008;205(6):1261–8.
Tang D, Kang R, Coyne CB, Zeh HJ, Lotze MT. PAMPs and DAMPs: signal 0s that spur autophagy and immunity. Immunol Rev. 2012;249(1):158–75.
Zindel J, Kubes P. DAMPs, PAMPs, and LAMPs in immunity and sterile inflammation. Annu Rev Pathol. 2020;15:493–518.
Poudel K, Banstola A, Gautam M, Soe Z, Phung CD, Pham LM, et al. Macrophage-membrane-camouflaged disintegrable and excretable nanoconstruct for deep tumor penetration. ACS Appl Mater Interfaces. 2020;12(51):56767–81.
Si J, Shao S, Shen Y, Wang K. Macrophages as active nanocarriers for targeted early and adjuvant cancer chemotherapy. Small. 2016;12(37):5108–19.
Cao H, Dan Z, He X, Zhang Z, Yu H, Yin Q, et al. Liposomes coated with isolated macrophage membrane can target lung metastasis of breast cancer. ACS Nano. 2016;10(8):7738–48.
Li B, Wang W, Song W, Zhao Z, Tan Q, Zhao Z, et al. Antiviral and anti-inflammatory treatment with multifunctional alveolar macrophage-like nanoparticles in a surrogate mouse model of COVID-19. Adv Sci. 2021;8(13):2003556.
Vijayan V, Uthaman S, Park IK. Cell membrane-camouflaged nanoparticles: a promising biomimetic strategy for cancer theragnostics. Polymers. 2018;10(9):983.
Rao L, Zhao SK, Wen C, Tian R, Lin L, Cai B, Sun Y, Kang F, Yang Z, He L, Mu J, Meng QF, Yao G, Xie N, Chen X. Activating macrophage-mediated cancer immunotherapy by genetically edited nanoparticles. Adv Mater. 2020;32:47.
Sun T, Kwong CHT, Gao C, Wei J, Yue L, Zhang J, et al. Amelioration of ulcerative colitis via inflammatory regulation by macrophage-biomimetic nanomedicine. Theranostics. 2020;10(22):10106–19.
Gong C, Yu X, You B, Wu Y, Wang R, Han L, et al. Macrophage-cancer hybrid membrane-coated nanoparticles for targeting lung metastasis in breast cancer therapy. J Nanobiotechnol. 2020;18(1):92.
Fang RH, Jiang Y, Fang JC, Zhang L. Cell membrane-derived nanomaterials for biomedical applications. Biomaterials. 2017;128:69–83.
Simons K, Vaz WL. Model systems, lipid rafts, and cell membranes. Annu Rev Biophys Biomol Struct. 2004;33:269–95.
Choi B, Park W, Park S-B, Rhim W-K, Han DK. Recent trends in cell membrane-cloaked nanoparticles for therapeutic applications. Methods. 2020;177:2–14.
Zhang Q, Dehaini D, Zhang Y, Zhou J, Chen X, Zhang L, et al. Neutrophil membrane-coated nanoparticles inhibit synovial inflammation and alleviate joint damage in inflammatory arthritis. Nat Nanotechnol. 2018;13(12):1182–90.
Kang T, Zhu Q, Wei D, Feng J, Yao J, Jiang T, et al. Nanoparticles coated with neutrophil membranes can effectively treat cancer metastasis. ACS Nano. 2017;11(2):1397–411.
Zhai Y, Su J, Ran W, Zhang P, Yin Q, Zhang Z, et al. Preparation and application of cell membrane-camouflaged nanoparticles for cancer therapy. Theranostics. 2017;7(10):2575–92.
Vijayan V, Uthaman S, Park IK. Cell membrane coated nanoparticles: an emerging biomimetic nanoplatform for targeted bioimaging and therapy. Adv Exp Med Biol. 2018;1064:45–59.
Mohanraj VJ, Chen Y. Nanoparticles-a review. Trop J Pharm. 2007;5:561–73.
Wang C, Wu S. Research update on cell membrane camouflaged nanoparticles for cancer therapy. Front Bioeng Biotechnol. 2022;10:944518.
Aghebati-Maleki A, Dolati S, Ahmadi M, Baghbanzhadeh A, Asadi M, Fotouhi A, et al. Nanoparticles and cancer therapy: perspectives for application of nanoparticles in the treatment of cancers. J Cell Physiol. 2020;235(3):1962–72.
Nowacek AS, Balkundi S, McMillan J, Roy U, Martinez-Skinner A, Mosley RL, et al. Analyses of nanoformulated antiretroviral drug charge, size, shape and content for uptake, drug release and antiviral activities in human monocyte-derived macrophages. J Control Release. 2011;150(2):204–11.
Rao L, Zhao S-K, Wen C, Tian R, Lin L, Cai B, et al. Activating macrophage-mediated cancer immunotherapy by genetically edited nanoparticles. Adv Mater. 2020;32(47):e2004853.
Liang B, Deng T, Li J, Ouyang X, Na W, Deng D. Biomimetic theranostic strategy for anti-metastasis therapy of breast cancer via the macrophage membrane camouflaged superparticles. Mater Sci Eng C Mater Biol Appl. 2020;115:111097.
Chen LJ, Zhao X, Liu YY, Yan XP. Macrophage membrane coated persistent luminescence nanoparticle@MOF-derived mesoporous carbon core-shell nanocomposites for autofluorescence-free imaging-guided chemotherapy. J Mater Chem B. 2020;8(35):8071–83.
Kumari P, Ghosh B, Biswas S. Nanocarriers for cancer-targeted drug delivery. J Drug Target. 2016;24(3):179–91.
Dou H, Destache CJ, Morehead JR, Mosley RL, Boska MD, Kingsley J, et al. Development of a macrophage-based nanoparticle platform for antiretroviral drug delivery. Blood. 2006;108(8):2827–35.
Zuo H, Tao J, Shi H, He J, Zhou Z, Zhang C. Platelet-mimicking nanoparticles co-loaded with W18O49 and metformin alleviate tumor hypoxia for enhanced photodynamic therapy and photothermal therapy. Acta Biomater. 2018;80:296–307.
Rao L, Xu JH, Cai B, Liu H, Li M, Jia Y, et al. Synthetic nanoparticles camouflaged with biomimetic erythrocyte membranes for reduced reticuloendothelial system uptake. Nanotechnology. 2016;27(8):085106.
Rao L, Bu LL, Cai B, Xu JH, Li A, Zhang WF, et al. Cancer cell membrane-coated upconversion nanoprobes for highly specific tumor imaging. Adv Mater. 2016;28(18):3460–6.
Liu T, Shi C, Duan L, Zhang Z, Luo L, Goel S, et al. A highly hemocompatible erythrocyte membrane-coated ultrasmall selenium nanosystem for simultaneous cancer radiosensitization and precise antiangiogenesis. J Mater Chem B. 2018;6(29):4756–64.
Liang X, Ye X, Wang C, Xing C, Miao Q, Xie Z, et al. Photothermal cancer immunotherapy by erythrocyte membrane-coated black phosphorus formulation. J Control Release. 2019;296:150–61.
He W, Frueh J, Wu Z, He Q. Leucocyte membrane-coated janus microcapsules for enhanced photothermal cancer treatment. Langmuir. 2016;32(15):3637–44.
Chou LY, Ming K, Chan WC. Strategies for the intracellular delivery of nanoparticles. Chem Soc Rev. 2011;40(1):233–45.
Liu L, Wang Y, Guo X, Zhao J, Zhou S. A biomimetic polymer magnetic nanocarrier polarizing tumor-associated macrophages for potentiating immunotherapy. Small. 2020;16(38):e2003543.
Wang Y, Zhang D, Jia M, Zheng X, Liu Y, Wang C, et al. ZIF-8 nanoparticles coated with macrophage-derived microvesicles for sustained, targeted delivery of dexamethasone to arthritic joints. J Drug Target. 2022.
Wei Y, Zhu M, Li S, Hong T, Guo X, Li Y, et al. Engineered biomimetic nanoplatform protects the myocardium against ischemia/reperfusion injury by inhibiting pyroptosis. ACS Appl Mater Interfaces. 2021;13(29):33756–66.
Zhang Y, Cai K, Li C, Guo Q, Chen Q, He X, et al. Macrophage-membrane-coated nanoparticles for tumor-targeted chemotherapy. Nano Lett. 2018;18(3):1908–15.
Sun K, Yu W, Ji B, Chen C, Yang H, Du Y, et al. Saikosaponin D loaded macrophage membrane-biomimetic nanoparticles target angiogenic signaling for breast cancer therapy. Appl Mater Today. 2020;18:100505.
Papa S, Ferrari R, De Paola M, Rossi F, Mariani A, Caron I, et al. Polymeric nanoparticle system to target activated microglia/macrophages in spinal cord injury. J Control Release. 2014;174:15–26.
Zheng Y, He R, Wang P, Shi Y, Zhao L, Liang J. Exosomes from lps-stimulated macrophages induce neuroprotection and functional improvement after ischemic stroke by modulating microglial polarization. Biomater Sci. 2019;7(5):2037–49.
Wang P, Wang H, Huang Q, Peng C, Yao L, Chen H, et al. Exosomes from M1-polarized macrophages enhance paclitaxel antitumor activity by activating macrophages-mediated inflammation. Theranostics. 2019;9(6):1714–27.
Gong C, Yu X, Zhang W, Han L, Wang R, Wang Y, et al. Regulating the immunosuppressive tumor microenvironment to enhance breast cancer immunotherapy using pH-responsive hybrid membrane-coated nanoparticles. J Nanobiotechnol. 2021;19(1):58.
Dougherty TJ, Gomer CJ, Henderson BW, Jori G, Kessel D, Korbelik M, et al. Photodynamic therapy. J Natl Cancer Inst. 1998;90(12):889–905.
Xuan M, Shao J, Dai L, Li J, He Q. Macrophage cell membrane camouflaged au nanoshells for in vivo prolonged circulation life and enhanced cancer photothermal therapy. ACS Appl Mater Interfaces. 2016;8(15):9610–8.
Chen C, Song M, Du Y, Yu Y, Li C, Han Y, et al. Tumor-associated-macrophage-membrane-coated nanoparticles for improved photodynamic immunotherapy. Nano Lett. 2021;21(13):5522–31.
Cyrus N, Mai-Anh Bui C, Yao X, Kohn LL, Galan A, Rhebergen AM, et al. Density and polarization states of tumor-associated macrophages in human cutaneous squamous cell carcinomas arising in solid organ transplant recipients. Dermatol Surg. 2016;42(Suppl 1):S18-23.
Rodriguez PC, Quiceno DG, Ochoa AC. L-arginine availability regulates T-lymphocyte cell-cycle progression. Blood. 2007;109(4):1568–73.
Fukuda K, Sugihara E, Ohta S, Izuhara K, Funakoshi T, Amagai M, et al. Periostin is a key niche component for wound metastasis of melanoma. PLoS ONE. 2015;10(6): e0129704.
Parodi A, Quattrocchi N, van de Ven AL, Chiappini C, Evangelopoulos M, Martinez JO, et al. Synthetic nanoparticles functionalized with biomimetic leukocyte membranes possess cell-like functions. Nat Nanotechnol. 2013;8(1):61–8.
Cao X, Tan T, Zhu D, Yu H, Liu Y, Zhou H, et al. Paclitaxel-loaded macrophage membrane camouflaged albumin nanoparticles for targeted cancer therapy. Int J Nanomed. 2020;15:1915–28.
Rao L, Wu L, Liu Z, Tian R, Yu G, Zhou Z, et al. Hybrid cellular membrane nanovesicles amplify macrophage immune responses against cancer recurrence and metastasis. Nat Commun. 2020;11(1):4909.
Evangelopoulos M, Yazdi IK, Acciardo S, Palomba R, Giordano F, Pasto A, et al. Biomimetic cellular vectors for enhancing drug delivery to the lungs. Sci Rep. 2020;10(1):172.
Vilner BJ, John CS, Bowen WD. Sigma-1 and sigma-2 receptors are expressed in a wide variety of human and rodent tumor cell lines. Cancer Res. 1995;55(2):408–13.
Kim MS, Haney MJ, Zhao Y, Yuan D, Deygen I, Klyachko NL, et al. Engineering macrophage-derived exosomes for targeted paclitaxel delivery to pulmonary metastases: in vitro and in vivo evaluations. Nanomedicine. 2018;14(1):195–204.
Choi J, Kim HY, Ju EJ, Jung J, Park J, Chung HK, et al. Use of macrophages to deliver therapeutic and imaging contrast agents to tumors. Biomaterials. 2012;33(16):4195–203.
Fang Y, Zhang Z, Liu Y, Gao T, Liang S, Chu Q, et al. Artificial assembled macrophage co-deliver black phosphorus quantum dot and CDK4/6 inhibitor for colorectal cancer triple-therapy. ACS Appl Mater Interfaces. 2022;14(18):20628–40.
Qiang L, Cai Z, Jiang W, Liu J, Tai Z, Li G, et al. A novel macrophage-mediated biomimetic delivery system with NIR-triggered release for prostate cancer therapy. J Nanobiotechnol. 2019;17(1):83.
Ji B, Cai H, Yang Y, Peng F, Song M, Sun K, et al. Hybrid membrane camouflaged copper sulfide nanoparticles for photothermal-chemotherapy of hepatocellular carcinoma. Acta Biomater. 2020;111:363–72.
Osmulski PA, Cunsolo A, Chen M, Qian Y, Lin CL, Hung CN, et al. Contacts with macrophages promote an aggressive nanomechanical phenotype of circulating tumor cells in prostate cancer. Cancer Res. 2021;81(15):4110–23.
Zhang Q, Honko A, Zhou J, Gong H, Downs SN, Vasquez JH, et al. Cellular nanosponges inhibit SARS-COV-2 infectivity. Nano Lett. 2020;20(7):5570–4.
Aslam B, Wang W, Arshad MI, Khurshid M, Muzammil S, Rasool MH, et al. Antibiotic resistance: a rundown of a global crisis. Infect Drug Resist. 2018;11:1645–58.
Halstead SB, Mahalingam S, Marovich MA, Ubol S, Mosser DM. Intrinsic antibody-dependent enhancement of microbial infection in macrophages: disease regulation by immune complexes. Lancet Infect Dis. 2010;10(10):712–22.
Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. 2001;2(8):675–80.
Thamphiwatana S, Angsantikul P, Escajadillo T, Zhang Q, Olson J, Luk BT, et al. Macrophage-like nanoparticles concurrently absorbing endotoxins and proinflammatory cytokines for sepsis management. Proc Natl Acad Sci USA. 2017;114(43):11488–93.
Molinaro R, Pastò A, Corbo C, Taraballi F, Giordano F, Martinez JO, et al. Macrophage-derived nanovesicles exert intrinsic anti-inflammatory properties and prolong survival in sepsis through a direct interaction with macrophages. Nanoscale. 2019;11(28):13576–86.
Wei X, Ran D, Campeau A, Xiao C, Zhou J, Dehaini D, et al. Multiantigenic nanotoxoids for antivirulence vaccination against antibiotic-resistant gram-negative bacteria. Nano Lett. 2019;19(7):4760–9.
Wang C, Wang Y, Zhang L, Miron RJ, Liang J, Shi M, et al. Pretreated macrophage-membrane-coated gold nanocages for precise drug delivery for treatment of bacterial infections. Adv Mater. 2018;30(46): e1804023.
Li Y, Liu Y, Ren Y, Su L, Li A, An Y, et al. Coating of a novel antimicrobial nanoparticle with a macrophage membrane for the selective entry into infected macrophages and killing of intracellular staphylococci. Adv Funct Mater. 2020;30:48.
Law GL, Korth MJ, Benecke AG, Katze MG. Systems virology: host-directed approaches to viral pathogenesis and drug targeting. Nat Rev Microbiol. 2013;11(7):455–66.
Ison MG. Antiviral treatments. Clin Chest Med. 2017;38(1):139–53.
Dou H, Grotepas CB, McMillan JM, Destache CJ, Chaubal M, Werling J, et al. Macrophage delivery of nanoformulated antiretroviral drug to the brain in a murine model of neuroaids. J Immunol. 2009;183(1):661–9.
Tan Q, He L, Meng X, Wang W, Pan H, Yin W, et al. Macrophage biomimetic nanocarriers for anti-inflammation and targeted antiviral treatment in COVID-19. J Nanobiotechnol. 2021;19(1):173.
Ivashkiv LB. Epigenetic regulation of macrophage polarization and function. Trends Immunol. 2013;34(5):216–23.
Muraille E, Leo O, Moser M. Th1/Th2 paradigm extended: macrophage polarization as an unappreciated pathogen-driven escape mechanism? Front Immunol. 2014;5:603.
Kumar P, Bose PP. Macrophage ghost entrapped amphotericin b: a novel delivery strategy towards experimental visceral leishmaniasis. Drug Deliv Transl Res. 2019;9(1):249–59.
Libby P, Buring JE, Badimon L, Hansson GK, Deanfield J, Bittencourt MS, et al. Atherosclerosis. Nat Rev Dis Primers. 2019;5(1):56.
Moore KJ, Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell. 2011;145(3):341–55.
Foster CA. VCAM-1/alpha 4-integrin adhesion pathway: therapeutic target for allergic inflammatory disorders. J Allergy Clin Immunol. 1996;98(6 Pt 2):S270–7.
Wolf D, Ley K. Immunity and inflammation in atherosclerosis. Circ Res. 2019;124(2):315–27.
Huang X, Lin C, Luo C, Guo Y, Li J, Wang Y, et al. Fe3O4@M nanoparticles for MRI-targeted detection in the early lesions of atherosclerosis. Nanomedicine. 2021;33: 102348.
Wang Y, Zhang K, Li T, Maruf A, Qin X, Luo L, et al. Macrophage membrane functionalized biomimetic nanoparticles for targeted anti-atherosclerosis applications. Theranostics. 2021;11(1):164–80.
Gao C, Huang Q, Liu C, Kwong CHT, Yue L, Wan JB, et al. Treatment of atherosclerosis by macrophage-biomimetic nanoparticles via targeted pharmacotherapy and sequestration of proinflammatory cytokines. Nat Commun. 2020;11(1):2622.
Ahn JW, Kim JH, Park K. In vitro photodynamic effects of the inclusion nanocomplexes of glucan and chlorin e6 on atherogenic foam cells. Int J Mol Sci. 2020;22:1.
Tang D, Wang Y, Wijaya A, Liu B, Maruf A, Wang J, et al. ROS-responsive biomimetic nanoparticles for potential application in targeted anti-atherosclerosis. Regen Biomater. 2021;8:4.
Li Y, Che J, Chang L, Guo M, Bao X, Mu D, et al. CD47- and integrin α4/β1-comodified-macrophage-membrane-coated nanoparticles enable delivery of colchicine to atherosclerotic plaque. Adv Healthc Mater. 2022;11(4):e2101788.
Wu G, Zhang J, Zhao Q, Zhuang W, Ding J, Zhang C, et al. Molecularly engineered macrophage-derived exosomes with inflammation tropism and intrinsic heme biosynthesis for atherosclerosis treatment. Angew Chem. 2020;59(10):4068–74.
Zhu FD, Hu YJ, Yu L, Zhou XG, Wu JM, Tang Y, et al. Nanoparticles: a hope for the treatment of inflammation in CNS. Front Pharmacol. 2021;12:683935.
Langen UH, Ayloo S, Gu C. Development and cell biology of the blood-brain barrier. Annu Rev Cell Dev Biol. 2019;35:591–613.
Liebner S, Dijkhuizen RM, Reiss Y, Plate KH, Agalliu D, Constantin G. Functional morphology of the blood-brain barrier in health and disease. Acta Neuropathol. 2018;135(3):311–36.
Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis. 2010;37(1):13–25.
Daneman R. The blood-brain barrier in health and disease. Ann Neurol. 2012;72(5):648–72.
Samii A, Nutt JG, Ransom BR. Parkinson’s disease. Lancet. 2004;363(9423):1783–93.
Zhao Y, Haney MJ, Mahajan V, Reiner BC, Dunaevsky A, Mosley RL, et al. Active targeted macrophage-mediated delivery of catalase to affected brain regions in models of Parkinson’s disease. J Nanomed Nanotechnol. 2011;S4:003.
Batrakova EV, Li S, Reynolds AD, Mosley RL, Bronich TK, Kabanov AV, et al. A macrophage-nanozyme delivery system for Parkinson’s disease. Bioconjug Chem. 2007;18(5):1498–506.
Haney MJ, Klyachko NL, Zhao Y, Gupta R, Plotnikova EG, He Z, et al. Exosomes as drug delivery vehicles for Parkinson’s disease therapy. J Control Release. 2015;207:18–30.
Haney MJ, Suresh P, Zhao Y, Kanmogne GD, Kadiu I, Sokolsky-Papkov M, et al. Blood-borne macrophage-neural cell interactions hitchhike on endosome networks for cell-based nanozyme brain delivery. Nanomedicine. 2012;7(6):815–33.
Han Y, Gao C, Wang H, Sun J, Liang M, Feng Y, et al. Macrophage membrane-coated nanocarriers co-modified by RVG29 and TPP improve brain neuronal mitochondria-targeting and therapeutic efficacy in Alzheimer’s disease mice. Bioact Mater. 2021;6(2):529–42.
Caron I, Papa S, Rossi F, Forloni G, Veglianese P. Nanovector-mediated drug delivery for spinal cord injury treatment. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2014;6(5):506–15.
Yanagisawa S, Katoh H, Imai T, Nomura S, Watanabe M. The relationship between inflammasomes and the endoplasmic reticulum stress response in the injured spinal cord. Neurosci Lett. 2019;705:54–9.
Teng YD, Choi H, Onario RC, Zhu S, Desilets FC, Lan S, et al. Minocycline inhibits contusion-triggered mitochondrial cytochrome C release and mitigates functional deficits after spinal cord injury. Proc Natl Acad Sci U S A. 2004;101(9):3071–6.
Xia N, Gao Z, Hu H, Li D, Zhang C, Mei X, et al. Nerve growth factor loaded macrophage-derived nanovesicles for inhibiting neuronal apoptosis after spinal cord injury. J Biomater Appl. 2021;36(2):276–88.
Tang W, Yang Y, Yang L, Tang M, Chen Y, Li C. Macrophage membrane-mediated targeted drug delivery for treatment of spinal cord injury regardless of the macrophage polarization states. Asian J Pharm Sci. 2021;16(4):459–70.
Li C, Zhao Z, Luo Y, Ning T, Liu P, Chen Q, et al. Macrophage-disguised manganese dioxide nanoparticles for neuroprotection by reducing oxidative stress and modulating inflammatory microenvironment in acute ischemic stroke. Adv Sci. 2021;8(20):e2101526.
Pang L, Zhu Y, Qin J, Zhao W, Wang J. Primary M1 macrophages as multifunctional carrier combined with PLGA nanoparticle delivering anticancer drug for efficient glioma therapy. Drug Deliv. 2018;25(1):1922–31.
Chuang SY, Lin CH, Huang TH, Fang JY. Lipid-based nanoparticles as a potential delivery approach in the treatment of rheumatoid arthritis. Nanomaterials. 2018;8(1):42.
Chen M, Li MH, Zhang N, Sun WW, Wang H, Wang YA, et al. Pro-angiogenic effect of exosomal microRNA-103a in mice with rheumatoid arthritis via the downregulation of hepatocyte nuclear factor 4 alpha and activation of the JAK/STAT3 signaling pathway. J Biol Regul Homeost Agents. 2021;35(2):629–40.
Li H, Feng Y, Zheng X, Jia M, Mei Z, Wang Y, et al. M2-type exosomes nanoparticles for rheumatoid arthritis therapy via macrophage re-polarization. J Control Release. 2022;341:16–30.
Fontana F, Albertini S, Correia A, Kemell M, Lindgren R, Makila E, et al. Bioengineered porous silicon nanoparticles@macrophages cell membrane as composite platforms for rheumatoid arthritis. Adv Fun Mater. 2018;28(22):1801355.
Li R, He Y, Zhu Y, Jiang L, Zhang S, Qin J, et al. Route to rheumatoid arthritis by macrophage-derived microvesicle-coated nanoparticles. Nano Lett. 2019;19(1):124–34.
Tang TT, Lv LL, Wang B, Cao JY, Feng Y, Li ZL, et al. Employing macrophage-derived microvesicle for kidney-targeted delivery of dexamethasone: an efficient therapeutic strategy against renal inflammation and fibrosis. Theranostics. 2019;9(16):4740–55.
Ma Y, Gao W, Zhang Y, Yang M, Yan X, Zhang Y, et al. Biomimetic MOF nanoparticles delivery of C-Dot nanozyme and CRISPR/Cas9 system for site-specific treatment of ulcerative colitis. ACS Appl Mater Interfaces. 2022;14(5):6358–69.
Zhang Q, Zhou J, Zhou J, Fang RH, Gao W, Zhang L. Lure-and-kill macrophage nanoparticles alleviate the severity of experimental acute pancreatitis. Nat Commun. 2021;12(1):4136.
Pei W, Li X, Bi R, Zhang X, Zhong M, Yang H, et al. Exosome membrane-modified M2 macrophages targeted nanomedicine: treatment for allergic asthma. J Control Release. 2021;338:253–67.
Teo KYW, Sevencan C, Cheow YA, Zhang S, Leong DT, Toh WS. Macrophage polarization as a facile strategy to enhance efficacy of macrophage membrane-coated nanoparticles in osteoarthritis. Small Sci. 2022;2(4):2100116.
Hu C, Lei T, Wang Y, Cao J, Yang X, Qin L, et al. Phagocyte-membrane-coated and laser-responsive nanoparticles control primary and metastatic cancer by inducing anti-tumor immunity. Biomaterials. 2020;255: 120159.
Zhao H, Li L, Zhang J, Zheng C, Ding K, Xiao H, et al. C-C chemokine ligand 2 (CCL2) recruits macrophage-membrane-camouflaged hollow bismuth selenide nanoparticles to facilitate photothermal sensitivity and inhibit lung metastasis of breast cancer. ACS Appl Mater Interfaces. 2018;10(37):31124–35.
Rao L, He Z, Meng Q-F, Zhou Z, Bu L-L, Guo S-S, et al. Effective cancer targeting and imaging using macrophage membrane-camouflaged upconversion nanoparticles. J Biomed Mater Res Part A. 2017;105(2):521–30.
Bhattacharyya S, Ghosh SS. Transmembrane TNFα-expressed macrophage membrane-coated chitosan nanoparticles as cancer therapeutics. ACS Omega. 2020;5(3):1572–80.
Li Y, Yan T, Chang W, Cao C, Deng D. Fabricating an intelligent cell-like nano-prodrug via hierarchical self-assembly based on the DNA skeleton for suppressing lung metastasis of breast cancer. Biomater Sci. 2019;7(9):3652–61.
Rayamajhi S, Nguyen TDT, Marasini R, Aryal S. Macrophage-derived exosome-mimetic hybrid vesicles for tumor targeted drug delivery. Acta Biomater. 2019;94:482–94.
Fu J, Wang D, Mei D, Zhang H, Wang Z, He B, et al. Macrophage mediated biomimetic delivery system for the treatment of lung metastasis of breast cancer. J Controlled Release. 2015;204:11–9.
Haney MJ, Zhao Y, Jin YS, Li SM, Bago JR, Klyachko NL, et al. Macrophage-derived extracellular vesicles as drug delivery systems for triple negative breast cancer (TNBC) therapy. J Neuroimmune Pharmacol. 2020;15(3):487–500.
Wayne EC, Long C, Haney MJ, Batrakova EV, Leisner TM, Parise LV, et al. Targeted delivery of sirna lipoplexes to cancer cells using macrophage transient horizontal gene transfer. Adv Sci. 2019;6(21):1900582.
Nguyen VD, Min H-K, Kim D-H, Kim C-S, Han J, Park J-O, et al. Macrophage-mediated delivery of multifunctional nanotherapeutics for synergistic chemo-photothermal therapy of solid tumors. Appl Mater Interfaces. 2020;12(9):10130–41.
Zuo W, Chen W, Liu J, Huang S, Chen L, Liu Q, et al. Macrophage-mimic hollow mesoporous fe-based nanocatalysts for self-amplified chemodynamic therapy and metastasis inhibition via tumor microenvironment remodeling. ACS Appl Mater Interfaces. 2022;14(4):5053–65.
Hou L, Gong X, Yang J, Zhang H, Yang W, Chen X. Hybrid-membrane-decorated prussian blue for effective cancer immunotherapy via tumor-associated macrophages polarization and hypoxia relief. Adv Mater. 2022;34(14): e2200389.
Evans MA, Huang PJ, Iwamoto Y, Ibsen KN, Chan EM, Hitomi Y, et al. Macrophage-mediated delivery of light activated nitric oxide prodrugs with spatial temporal and concentration control. Chem Sci. 2018;9(15):3729–41.
Liu R, An Y, Jia W, Wang Y, Wu Y, Zhen Y, et al. Macrophage-mimic shape changeable nanomedicine retained in tumor for multimodal therapy of breast cancer. J Control Release. 2020;321:589–601.
Cai JX, Liu JH, Wu JY, Li YJ, Qiu XH, Xu WJ, et al. Hybrid cell membrane-functionalized biomimetic nanoparticles for targeted therapy of osteosarcoma. Int J Nanomed. 2022;17:837–54.
Shi M, Shen K, Yang B, Zhang P, Lv K, Qi H, et al. An electroporation strategy to synthesize the membrane-coated nanoparticles for enhanced anti-inflammation therapy in bone infection. Theranostics. 2021;11(5):2349–63.
Cai C, Koch B, Morikawa K, Suda G, Sakamoto N, Rueschenbaum S, et al. Macrophage-derived extracellular vesicles induce long-lasting immunity against hepatitis C virus which is blunted by polyunsaturated fatty acids. Front Immunol. 2018;9:723.
Yin C, Zhao Q, Li W, Zhao Z, Wang J, Deng T, et al. Biomimetic anti-inflammatory nano-capsule serves as a cytokine blocker and M2 polarization inducer for bone tissue repair. Acta Biomater. 2020;102:416–26.
Wu B, Lin L, Zhou F, Wang X. Precise engineering of neutrophil membrane coated with polymeric nanoparticles concurrently absorbing of proinflammatory cytokines and endotoxins for management of sepsis. Bioprocess Biosyst Eng. 2020;43(11):2065–74.
Lee S. Monocytes: a novel drug delivery system targeting atherosclerosis. J Drug Target. 2014;22(2):138–45.
Liu B, Yan W, Luo L, Wu S, Wang Y, Zhong Y, et al. Macrophage membrane camouflaged reactive oxygen species responsive nanomedicine for efficiently inhibiting the vascular intimal hyperplasia. J Nanobiotechnol. 2021;19(1):374.
Ou Z, Zhong H, Zhang L, Deng M, Zhang W, Wang J, et al. Macrophage membrane-coated nanoparticles alleviate hepatic ischemia-reperfusion injury caused by orthotopic liver transplantation by neutralizing endotoxin. Int J Nanomed. 2020;15:4125–38.
Klyachko NL, Polak R, Haney MJ, Zhao Y, Gomes Neto RJ, Hill MC, et al. Macrophages with cellular backpacks for targeted drug delivery to the brain. Biomaterials. 2017;140:79–87.
Lai J, Deng G, Sun Z, Peng X, Li J, Gong P, et al. Scaffolds biomimicking macrophages for a glioblastoma NIR-Ib imaging guided photothermal therapeutic strategy by crossing blood-brain barrier. Biomaterials. 2019;211:48–56.
Xiao T, He M, Xu F, Fan Y, Jia B, Shen M, et al. Macrophage membrane-camouflaged responsive polymer nanogels enable magnetic resonance imaging-guided chemotherapy/chemodynamic therapy of orthotopic glioma. ACS Nano. 2021;15(12):20377–90.
Madsen SJ, Christie C, Hong SJ, Trinidad A, Peng Q, Uzal FA, et al. Nanoparticle-loaded macrophage-mediated photothermal therapy: potential for glioma treatment. Lasers Med Sci. 2015;30(4):1357–65.
Zhang W, Wang M, Tang W, Wen R, Zhou S, Lee C, et al. Nanoparticle-laden macrophages for tumor-tropic drug delivery. Adv Mater. 2018;30(50): e1805557.
Zhang Z, Li D, Li X, Guo Z, Liu Y, Ma X, et al. PEI-modified macrophage cell membrane-coated PLGA nanoparticles encapsulating dendrobium polysaccharides as a vaccine delivery system for ovalbumin to improve immune responses. Int J Biol Macromol. 2020;165(Pt A):239–48.
Wu W, Wu D, Yan W, Wang Y, You J, Wan X, et al. Interferon-induced macrophage-derived exosomes mediate antiviral activity against hepatitis B virus through miR-574-5p. J Infect Dis. 2021;223(4):686–98.
Acknowledgements
Not applicable.
Funding
This research was supported by The Science and Technology Planning Project of Sichuan Province of China (Nos. 2022NSFSC1429, 23NSFSC5467 and 2022JDJQ0061), The National Natural Science Foundation of China (No. 22208269 and 82074129), The Joint Project between Luzhou Municipal People's Government and Southwest Medical University (Nos. 2020LZXNYDZ03, 2021LZXNYD-J17 and 2020LZXNYDP01), Foundation of Southwest Medical university (No. 2021ZKZD012).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. YW and JW conceived the idea, designed the study and revised the manuscript. The first draft of the manuscript was written by YW, SW and SY. HH, CZ and JL reviewed and made significant revisions to the manuscript. JZ, WC, XT, JL, XZ, LY and LW revised the manuscript. JW, QF and AW provided direction and guidance throughout the preparation of this manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors are consent for publication.
Competing interests
The authors declare that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wu, Y., Wan, S., Yang, S. et al. Macrophage cell membrane-based nanoparticles: a new promising biomimetic platform for targeted delivery and treatment. J Nanobiotechnol 20, 542 (2022). https://doi.org/10.1186/s12951-022-01746-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12951-022-01746-6