Abstract
Aims
About 20–40% patients with type 2 diabetes mellitus (T2DM) had an increased risk of developing diabetic nephropathy (DN). Dipeptidyl peptidase-4 inhibitors (DPP-4i) were recommended for treatment of T2DM, while the impact of DPP-4i on renal function remained unclear. This study aimed to explore the effect of DPP-4i on renal parameter of estimated glomerular filtration rate (eGFR) and albumin-to-creatinine ratio (ACR) in T2DM.
Methods
A systematic search was performed across PubMed, Embase and Cochrane Library. A fixed or random-effects model was used for quantitative synthesis according to the heterogeneity, which was assessed with I2 index. Sensitivity analysis and publication bias were performed with standard methods, respectively.
Results
A total of 17 randomized controlled trials were identified. Administration of DPP-4i produced no significant effect on eGFR (WMD, -0.92 mL/min/1.73m2, 95% CI, -2.04 to 0.19) in diabetic condition. DPP-4i produced a favorable effect on attenuating ACR (WMD, -2.76 mg/g, 95% CI, -5.23 to -0.29) in patients with T2DM. The pooled estimate was stable based on the sensitivity test. No publication bias was observed according to Begg’s and Egger’s tests.
Conclusions
Treatment with DPP-4i preserved the renal parameter of eGFR in diabetic condition. Available evidences suggested that administration of DPP-4i produced a favorable effect on attenuating ACR in patients with T2DM.
International Prospective Register for Systematic Review (PROSPERO) number
CRD.42020144642.
Similar content being viewed by others
Introduction
The number of patients with type 2 diabetes mellitus (T2DM) was increasing annually across the world. An increased morbidity or mortality partially stem from macrovascular and/or microvascular complications occurred during T2DM progression. Diabetic nephropathy (DN), one common microvascular complication, was characterized as a marked decrease of estimated glomerular filtration rate (eGFR) and/or a persistent increase of albuminuria [1]. Evidence suggested that 20–40% of patients developed microvascular complications of DN in diabetic condition [2]. A chronic exposure to hyperglycaemia led to progressive impairment of the renal microvasculature [3]. Therapeutic strategies should not modulate glycaemic balance alone, while other measures including an attenuation of blood pressure and/or preserving renal function should also be performed in diabetic context [4].
Traditional antidiabetic agents mainly focused on glucose control in treatment of T2DM. Dipeptidyl peptidase-4 inhibitors (DPP-4i) were developed as noninsulin hypoglycaemic agents since 2006, and these agents were orally administered in clinical practice. Preclinical study demonstrated that DPP-4 was expressed in the kidney, and increased DPP-4 activity was positively correlated with levels of creatinine and proteinuria [5]. Inhibition of DPP-4 effectively improved renal outcomes by decreasing tubular and glomerular proteinuria in diabetic setting [6]. Evidence indicated that DPP-4i potentially attenuated renal biomarkers for tubular injury in patients with diabetic kidney disease (DKD) [7]. In contrast, some studies yielded different estimates on renal parameters during treatment with DPP-4i. A long-term treatment with linagliptin produced no significant effect on eGFR compared to placebo (-0.8 vs. -2.2 mL/min/1.73 m2) in diabetic participants with renal impairment [8]. Similarly, administration of linagliptin did not significantly modulate albuminuria in diabetic individuals with renal dysfunction [9]. However, a pooled analysis demonstrated that treatment with DPP-4i significantly reduced eGFR (-1.11 mL/min/1.73 m2; 95% CI, -1.78 to -0.44; P = 0.001) in patients with T2DM [10]. It was an important issue to explore the extent to which DPP-4i modulated renal parameters in patients with T2DM. Therefore, this study was performed to evaluate an impact of DPP-4i on eGFR and albumin-to-creatinine ratio (ACR) in patients with T2DM.
Methods
Search strategy
This study was designed based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [11]. PubMed, Embase and Cochrane Library were searched for trials published before April 30, 2024. Relevant items included (“dipeptidyl peptidase-4 inhibitors” OR “sitagliptin” OR “vildagliptin” OR “teneligliptin” OR “saxagliptin” OR “linagliptin” OR “alogliptin”) AND (“type 2 diabetes” OR “type 2 diabetes mellitus” OR “T2DM”) AND (randomized controlled trials).
Study selection
Two reviewers screened databases independently and searched the reference lists for eligible articles manually. Randomized controlled trials (RCTs) evaluating the impact of DPP-4i on eGFR and/or ACR were selected. Inclusion criteria were established as follows: (i) an effect of DPP-4i on eGFR or ACR was studied; (ii) relative information on renal parameter was recorded at baseline and follow-up, or a change was indicated directly; and (iii) patients were diagnosed with T2DM. The exclusion criteria were listed as follows: (i) non-human studies; (ii) lack of records on eGFR or ACR; and (iii) meetings, abstracts or reviews.
Data extraction
Detailed records were extracted into the table, including (i) first author; (ii) publication year; (iii) trial location; (iv) number of participants in DPP-4i and control groups; (v) age and body mass index (BMI); (vi) follow-up and diabetes duration; (vii) HbA1c% at baseline; and (viii) eGFR and ACR at baseline. Studies with multiple follow-ups were extracted as the longest duration.
Quality assessment
Quality of RCTs was evaluated based on the Cochrane criteria [14]. Related items included random sequence generation, allocation concealment, blinding of participants, personnel, outcome assessment, incomplete outcome data, selective outcome reporting, and other potential sources of bias. A judgement of ‘yes’ indicated a low risk of bias, while a judgement of ‘no’ indicated a high risk of bias. A judgement of ‘unclear’ indicated an unknown or unclear risk of bias.
Quantitative data synthesis
A pooled calculation was performed on the renal parameter of eGFR or ACR. Weighted mean difference (WMD) and 95% confidence interval (CI) were calculated for changes of eGFR and ACR. A fixed- or random-effects model was used according to the heterogeneity, which was quantified by the index of I2. Sensitivity test was used to examine the influence of individual study on an overall estimate. In case of possible important heterogeneity, subgroup analysis was accordingly performed on related parameters. Publication bias was also examined by Begg’s and Egger’s tests if there were at least five studies reporting changes of eGFR or ACR. All these analysis were performed by using Review Manager (5.3) and STATA (12.0) software.
Results
Characteristics of the included studies
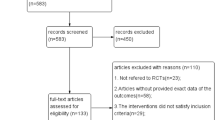
The literature search produced 8,738 records, and 17 publications (19 studies) met an inclusion criteria (Fig. 1). In addition, 17 studies reported the change of eGFR, while 11 studies reported the change of ACR during DPP-4i treatment. Fourteen studies lasted less than 1 year (ranging from 1 to 6 months), and three studies lasted longer than 1 year (ranging from 13 to 26 months). Two studies had a sample size of larger than 100, respectively. Characteristics of eligible were detailed illustrated (Table 1).
Quality evaluation
Study quality was objectively evaluated by two reviewers with Cochrane criteria(Fig. 2). All the studies were randomly designed, and three studies provided sufficient data about allocation concealment. Ten studies had detection bias on the basis of blinding of outcome assessment. Additionally, thirteen trials had performance bias as blinding methods were not implemented.
Effect of DPP-4i on eGFR in T2DM
A pooled estimate suggested that administration of DPP-4i preserved eGFR (WMD, -0.92 mL/min/1.73 m2, 95% CI, -2.04 to 0.19, I2 = 0%, P = 0.10) in patients with T2DM (Fig. 3). Subgroup analysis indicated that HbA1c at baseline, lengths of follow-up, BMI, comparator type and dosage did not influence the effect of DPP4i on the eGFR. In addition, no significant differences were observed in subgroups of DPP-4i alone, combined with other antidiabetic agents or inhibitors of renin-angiotensin-aldosterone system (RAASi) (Table 2).
Effect of DPP-4i on ACR in T2DM
Administration of DPP-4i produced a significant effect on reducing ACR (WMD, -2.76 mg/g, 95% CI, -5.23 to -0.29, I2 = 0%, P = 0.03) in T2DM (Fig. 4). In addition, DPP-4i significantly reduced ACR in subgroups of HbA1c ≤ 7.5, BMI ≥ 30 kg/m2 and coadministration of RAASi. However, no significant effects were indicated in subgroups of BMI, comparator type or coadministration with other antidiabetic agents during DPP4i treatment (Table 2).
Evaluation of publication bias
The pooled estimates on eGFR and ACR were stable according to the leave-one-out sensitivity test (Supplementary Figs. 1–2). This result proved that a significant difference was an overall effect of all the identified studies. No publication bias was observed on the association of DDP4i with eGFR or ACR according to Begg’s test (eGFR, P = 0.48, ACR, P = 1.00) or Egger’s test (eGFR, P = 0.478, ACR, P = 0.217) (Supplementary Figs. 3–4). In addition, no significant interactions were detected on the pooled estimates of eGFR or ACR across subgroup analysis (Table 2).
Discussion
DPP-4i were commonly recommended for treatment of patients with or without DN. Pooled analysis demonstrated that DPP-4i preserved renal function of eGFR in patients with T2DM. This finding was consistent with that of previous study in which DPP-4i were safely administered in diabetic patients with or without chronic kidney disease (CKD). Administration of sitagliptin resulted in no significant change of eGFR as that of glipizide in diabetic patients with CKD [12]. Additionally, outcomes from the Trial Evaluating Cardiovascular Outcomes with Sitagliptin (TECOS) demonstrated that sitagliptin did not significantly modulate eGFR after a long-term treatment [13]. A retrospective analysis also uncovered that teneligliptin could be safely used at an early stage in diabetic patients with DKD [14]. In addition, sitagliptin did not significantly modulate eGFR (-6 mL/min/1.73 m2, 95% CI, -14 to 3) in overweight patients with T2DM [15]. Similarly, a nonsignificant change of eGFR was observed in subgroup analysis on BMI. Pooled estimates might stem from a lack of significant renal haemodynamic changes during DPP-4i treatment. Different hyperfiltration ranges might also participate in attenuating eGFR in T2DM [16].
The preserved effect of DPP-4i on eGFR was consistent with outcomes of SAVOR-TIMI 53 trial. Saxagliptin did not significantly modulate eGFR while showing a beneficial effect on ACR in T2DM [17]. The pooled analysis also revealed that DPP-4i favorably reduced ACR in patients with T2DM. A preclinical study showed that DPP-4i reduced ACR and slowed the progression of renal impairment independent of blood pressure [18]. Evidence showed that saxagliptin and vildagliptin significantly reduced albuminuria, respectively, in diabetic patients with hypertension. Saxagliptin might present a stronger effect on reducing albuminuria compared to vildagliptin, an action independent of glycaemic control [19]. In fact, administration of saxagliptin ameliorated microalbuminuria in patients with or without renal impairment [20]. These results indicated that DPP-4i might produce an effect on ACR in a direct pathway. Most DPP-4i were predominantly excreted by the kidneys, except for linagliptin. A pooled analysis demonstrated that linagliptin significantly reduced ACR in patients receiving treatment of RAASi [21]. Subgroup analysis revealed that DPP-4i significantly reduced ACR in subgroup of HbA1c < 7.5. A previous study revealed that no significant correlation of DPP-4i with albuminuria was found in patients with different levels of HbA1c [20]. This might come from multiple parameters applied by different teams, namely, ACR and albuminuria alone. Correlation analysis also indicated that changes of ACR were associated with eGFR and systolic blood pressure in sitagliptin-treated participants [22]. This analysis suggested that an impact of DPP-4i on ACR partially dependent on eGFR at baseline. A significant effect of DPP-4i on ACR was also observed in patients with BMI > 30 kg/m2, while the underlying mechanism remained unclear in patients with T2DM.
The potential mechanism by which DPP-4i attenuated renal function might involve multiple pathways. First, DPP-4i increased the levels of glucagon-like peptide-1 (GLP-1), thereby inhibiting glomerular hyperfiltration [23]. Second, inflammation played a key role in the progression of CKD, and DPP-4i produced an anti-inflammatory effect by targeting toll-like receptor 4 (TLR4) in diabetic model [24]. Third, oxidative stress participated in the occurrence of renal impairment. Vildagliptin alleviated the process of renal sclerosis by inhibiting p22phox in diabetic condition [25]. DPP4i also significantly reduced an accumulation of reactive oxygen species (ROS) and promoted the activation of superoxide dismutase (SOD). DPP-4i reduced oxidative stress through modulating haem oxygenase-1 (HO-1) and NF-E2-related factor 2 (Nrf2) [26]. Fourth, kidney fibrosis was recognized as a final step in progression of CKD, which was ameliorated by an inhibition of endothelial-to mesenchymal transition (EndMT) during DPP-4i treatment [27]. In addition, DPP-4i produced a vasodilating effect on vessels by inducing a release of endothelial nitric oxide synthase (eNOS) [28]. Finally, DPP-4i improved pancreatic β-cell function in both fasting and postprandial states in patients with T2DM, which potentially presented vasodilatory effects on renal system [29].
In addition to DPP-4i, other antidiabetic agents had been reported to exert multiple effects on renal function in T2DM. Incretin-based GLP-1 receptor agonists (GLP-1RA) could improve renal function by presenting an antioxidant and/or anti-atherosclerotic effect in diabetic condition. Evidence demonstrated that weight reduction also contributed to a decrease of albuminuria during semaglutide treatment [30]. Administration of sodium–glucose cotransporter 2 inhibitors (SGLT2i) was reported to show a transient reduction of eGFR and proteinuria in diabetic patients [31]. The reduction of glomerular filtration might result from an effect of renal adenosine under hyperglycaemic conditions [32]. Metformin was proved to improve renal function by slowing the progression of kidney fibrosis. Preclinical evidence suggested that metformin targeted the AMPK signalling pathway, thus contributing to the normalization of kidney structure [33]. Pioglitazone, a peroxisome proliferator-activated receptor γ (PPAR-γ) agonist, was also found to modulate the progression of renal fibrosis and ameliorate DN in diabetic model [34]. Pioglitazone showed a reno-protective effect by attenuating mitochondrial function and stabilizing membrane potential [35]. Similarly, glibenclamide stabilized kidney structure by downregulating an expression of inflammatory markers. This action was accompanied with an alleviation of inflammatory cell infiltration in the kidney [36]. In the present study, DPP-4i did not demonstrate a stronger effect on renal parameters compared to other antidiabetic agents. The composite impact of multiple agents might ultimately surpass the effects of DPP-4i on renal parameters in diabetic participants. Head-to-head studies comparing DPP-4i with other antidiabetic agents should be designed to evaluate the effect on eGFR.
Finally, given the protective effect of RAASi on DN, it was important to determine whether DPP-4i showed a synergistic effect on renal function with RAASi. Angiotensin II (Ang II) downregulated the expression of megalin by activating DPP-4 in the proximal tubules, thereby resulting in an impairment of renal function. Inhibition of DPP-4 upregulated the expression of megalin in an Ang II-mediated way, thus decreasing the phosphorylation of extracellular regulated kinase (ERK) [37]. Linagliptin marked decreased glycosylated haemoglobin levels and preserved renal function when added to a conventional dose of RAASi in DN [38]. A pooled estimate demonstrated that coadministration of DPP-4i with RAASi produced a favorable effect on reducing ACR in T2DM. Evidence uncovered that an addition of DPP-4i to a maximal dose of RAASi markedly reduced ACR in patients with renal dysfunction [39]. This suggested that coadministration of DPP-4i with RAASi produced a synergistic effect on improving renal function in diabetic patients with renal impairment. In addition, a previous study showed that sitagliptin potentially targeted the sympathetic nervous system, thus weakening the hypotensive effect of angiotensin-converting enzyme inhibitors (ACEI) in patients with metabolic syndrome [40]. Therefore, essential measures should be performed to monitor blood pressure when patients received a maximal dose of RAASi during treatment with DPP-4i.
Strengths
This meta-analysis had some strengths to be stated. This meta-analysis firstly combined evidence on changes of eGFR and ACR during DPP-4i treatment. Pooled results suggested that DPP-4i potentially produced a favorable effect in patients with DN. In addition, subgroup analysis was performed to explore the influence of related parameters on renal function.
Limitations
It should be noted that this study had some limitations. Firstly, included studies had relatively small sample sizes, and a few number of trials were identified. Secondly, the identified trials showed differences in characteristics of participants, eGFR or ACR at baseline, and dosage of DPP-4i. Variations of these parameters might present an impact on an overall estimate. Thirdly, only publications in related databases were included, which also produce an inevitable publication bias.
Conclusions
Administration of DPP-4i potentially reduced ACR and prevented the decline of eGFR in T2DM. These results suggested that diabetic participants with or without albuminuria potentially benefit more from DPP-4i treatment in clinical practice.
Data availability
No datasets were generated or analysed during the current study.
Abbreviations
- T2DM:
-
Type 2 diabetes mellitus
- DN:
-
Diabetic nephropathy
- DPP-4i:
-
Dipeptidyl peptidase-4 inhibitors
- eGFR:
-
Estimated glomerular filtration rate
- ACR:
-
Albumin-to-creatinine ratio
- DKD:
-
Diabetic kidney disease
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- BMI:
-
Body mass index
- WMD:
-
Weighted mean difference
- CI:
-
Confidence interval
- CKD:
-
Chronic kidney disease
- TECOS:
-
Trial Evaluating Cardiovascular Outcomes with Sitagliptin
- RAASi:
-
Inhibitors of renin-angiotensin-aldosterone system
- TLR4:
-
Toll-like receptor 4
- ROS:
-
Reactive oxygen species
- SOD:
-
Superoxide dismutase
- HO-1:
-
Heme oxygenase-1
- Nrf2:
-
NF-E2-related factor 2
- EndMT:
-
Endothelial-to mesenchymal transition
- eNOS:
-
Endothelial nitric oxide synthase
- GLP-1:
-
Glucagon-like peptide-1
- GLP-1RA:
-
GLP-1 receptor agonists
- SGLT2i:
-
Sodium–glucose cotransporter 2 inhibitors
- AMPK:
-
AMP-activated protein kinase
- PPAR-γ:
-
Proliferator-activated receptorγ
- And II:
-
Angiotensin II
- ERK:
-
Extracellular regulated kinase
- ACEI:
-
Angiotensin-converting enzyme inhibitors
References
Faselis C, Katsimardou A, Imprialos K, Deligkaris P, Kallistratos M, Dimitriadis K. Microvascular complications of type 2 diabetes Mellitus. Curr Vasc Pharmacol. 2020;18:117–24.
ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, Collins BS, Hilliard ME, Isaacs D, Johnson EL, et al. 11. Chronic kidney Disease and Risk Management: standards of Care in Diabetes-2023. Diabetes Care. 2023;46:S191–202.
Li Y, Liu Y, Liu S, Gao M, Wang W, Chen K, Huang L, Liu Y. Diabetic vascular diseases: molecular mechanisms and therapeutic strategies. Signal Transduct Target Ther. 2023;8:152.
KDOQI Clinical Practice Guideline for Diabetes and CKD. 2012 Update. Am J Kidney Dis. 2012;60:850–86.
Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368:1696–705.
Benetti A, Martins FL, Sene LB, Shimizu MHM, Seguro AC, Luchi WM, Girardi ACC. Urinary DPP4 correlates with renal dysfunction, and DPP4 inhibition protects against the reduction in megalin and podocin expression in experimental CKD. Am J Physiol Ren Physiol. 2021;320:F285–96.
Trakarnvanich T, Satirapoj B, Suraamornkul S, Chirananthavat T, Sanpatchayapong A, Claimon T. Effect of Dipeptidyl Peptidase-4 (DPP-4) Inhibition on Biomarkers of Kidney Injury and Vascular Calcification in Diabetic Kidney Disease: A Randomized Controlled Trial. J Diabetes Res 2021, 2021:7382620.
McGill JB, Sloan L, Newman J, Patel S, Sauce C, von Eynatten M, Woerle HJ. Long-term efficacy and safety of linagliptin in patients with type 2 diabetes and severe renal impairment: a 1-year, randomized, double-blind, placebo-controlled study. Diabetes Care. 2013;36:237–44.
Groop PH, Cooper ME, Perkovic V, Hocher B, Kanasaki K, Haneda M, Schernthaner G, Sharma K, Stanton RC, Toto R, et al. Linagliptin and its effects on hyperglycaemia and albuminuria in patients with type 2 diabetes and renal dysfunction: the randomized MARLINA-T2D trial. Diabetes Obes Metab. 2017;19:1610–9.
Bae JH, Kim S, Park EG, Kim SG, Hahn S, Kim NH. Effects of Dipeptidyl Peptidase-4 inhibitors on renal outcomes in patients with type 2 diabetes: a systematic review and Meta-analysis. Endocrinol Metab (Seoul). 2019;34:80–92.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. J Clin Epidemiol. 2021;134:178–89.
Arjona Ferreira JC, Marre M, Barzilai N, Guo H, Golm GT, Sisk CM, Kaufman KD, Goldstein BJ. Efficacy and safety of sitagliptin versus glipizide in patients with type 2 diabetes and moderate-to-severe chronic renal insufficiency. Diabetes Care. 2013;36:1067–73.
Cornel JH, Bakris GL, Stevens SR, Alvarsson M, Bax WA, Chuang LM, Engel SS, Lopes RD, McGuire DK, Riefflin A, et al. Effect of Sitagliptin on kidney function and Respective Cardiovascular outcomes in type 2 diabetes: outcomes from TECOS. Diabetes Care. 2016;39:2304–10.
Shah K. Teneligliptin in Early Diabetic kidney disease: an Observation in Asian Indian patients with type 2 diabetes Mellitus in Real-Life scenario. J Clin Diagn Res. 2017;11:Oc22–5.
Tonneijck L, Smits MM, Muskiet MH, Hoekstra T, Kramer MH, Danser AH, Ter Wee PM, Diamant M, Joles JA, van Raalte DH. Renal effects of DPP-4 inhibitor sitagliptin or GLP-1 receptor agonist liraglutide in overweight patients with type 2 diabetes: a 12-Week, Randomized, Double-Blind, placebo-controlled trial. Diabetes Care. 2016;39:2042–50.
Bjornstad P, Cherney DZ, Snell-Bergeon JK, Pyle L, Rewers M, Johnson RJ, Maahs DM. Rapid GFR decline is associated with renal hyperfiltration and impaired GFR in adults with type 1 diabetes. Nephrol Dial Transpl. 2015;30:1706–11.
Mosenzon O, Leibowitz G, Bhatt DL, Cahn A, Hirshberg B, Wei C, Im K, Rozenberg A, Yanuv I, Stahre C, et al. Effect of Saxagliptin on renal outcomes in the SAVOR-TIMI 53 Trial. Diabetes Care. 2017;40:69–76.
Sharkovska Y, Reichetzeder C, Alter M, Tsuprykov O, Bachmann S, Secher T, Klein T, Hocher B. Blood pressure and glucose independent renoprotective effects of dipeptidyl peptidase-4 inhibition in a mouse model of type-2 diabetic nephropathy. J Hypertens. 2014;32:2211–23. discussion 2223.
Mohsen M, Elberry AA, Mohamed Rabea A, Abdelrahim MEA, Hussein RRS. Saxagliptin and vildagliptin lowered albuminuria in patients with diabetes and hypertension independent on glycaemic control. Int J Clin Pract. 2021;75:e13769.
Bloomgarden Z. The kidney and cardiovascular outcome trials. J Diabetes. 2018;10:88–9.
Kanasaki K. The role of renal dipeptidyl peptidase-4 in kidney disease: renal effects of dipeptidyl peptidase-4 inhibitors with a focus on linagliptin. Clin Sci (Lond). 2018;132:489–507.
Kawasaki I, Hiura Y, Tamai A, Yoshida Y, Yakusiji Y, Ikuno Y, Okada M, Ueno H, Tanaka N, Yamagami K, et al. Sitagliptin reduces the urine albumin-to-creatinine ratio in type 2 diabetes through decreasing both blood pressure and estimated glomerular filtration rate. J Diabetes. 2015;7:41–6.
Vallon V, Docherty NG. Intestinal regulation of urinary sodium excretion and the pathophysiology of diabetic kidney disease: a focus on glucagon-like peptide 1 and dipeptidyl peptidase 4. Exp Physiol. 2014;99:1140–5.
Ibrahim SSA, Salama MA, Selima E, Shehata RR. Sitagliptin and tofacitinib ameliorate adjuvant induced arthritis via modulating the cross talk between JAK/STAT and TLR-4/NF-κB signaling pathways. Life Sci. 2020;260:118261.
Vavrinec P, Henning RH, Landheer SW, Wang Y, Deelman LE, Dokkum RP, Buikema H. Vildagliptin restores renal myogenic function and attenuates renal sclerosis independently of effects on blood glucose or proteinuria in zucker diabetic fatty rat. Curr Vasc Pharmacol. 2014;12:836–44.
Si J, Meng R, Gao P, Hui F, Li Y, Liu X, Yang B. Linagliptin protects rat carotid artery from balloon injury and activates the NRF2 antioxidant pathway. Exp Anim. 2019;68:81–90.
Kanasaki K, Shi S, Kanasaki M, He J, Nagai T, Nakamura Y, Ishigaki Y, Kitada M, Srivastava SP, Koya D. Linagliptin-mediated DPP-4 inhibition ameliorates kidney fibrosis in streptozotocin-induced diabetic mice by inhibiting endothelial-to-mesenchymal transition in a therapeutic regimen. Diabetes. 2014;63:2120–31.
Vellecco V, Mitidieri E, Gargiulo A, Brancaleone V, Matassa D, Klein T, Esposito F, Cirino G, Bucci M. Vascular effects of linagliptin in non-obese diabetic mice are glucose-independent and involve positive modulation of the endothelial nitric oxide synthase (eNOS)/caveolin-1 (CAV-1) pathway. Diabetes Obes Metab. 2016;18:1236–43.
Luksch A, Polak K, Matulla B, Dallinger S, Kapiotis S, Rainer G, Wolzt M, Schmetterer L. Glucose and insulin exert additive ocular and renal vasodilator effects on healthy humans. Diabetologia. 2001;44:95–103.
Greco EV, Russo G, Giandalia A, Viazzi F, Pontremoli R, De Cosmo S. GLP-1 receptor agonists and kidney protection. Med (Kaunas) 2019, 55.
Heerspink HJ, Perkins BA, Fitchett DH, Husain M, Cherney DZ. Sodium glucose cotransporter 2 inhibitors in the treatment of diabetes Mellitus: Cardiovascular and kidney effects, potential mechanisms, and clinical applications. Circulation. 2016;134:752–72.
Vallon V, Schroth J, Satriano J, Blantz RC, Thomson SC, Rieg T. Adenosine A(1) receptors determine glomerular hyperfiltration and the salt paradox in early streptozotocin diabetes mellitus. Nephron Physiol. 2009;111:p30–38.
Lv Z, Guo Y. Metformin and its benefits for various diseases. Front Endocrinol (Lausanne). 2020;11:191.
Wang Z, Liu Q, Dai W, Hua B, Li H, Li W. Pioglitazone downregulates Twist-1 expression in the kidney and protects renal function of Zucker diabetic fatty rats. Biomed Pharmacother. 2019;118:109346.
Sun L, Yuan Q, Xu T, Yao L, Feng J, Ma J, Wang L, Lu C, Wang D. Pioglitazone improves mitochondrial function in the remnant kidney and protects against Renal Fibrosis in 5/6 nephrectomized rats. Front Pharmacol. 2017;8:545.
Diwan V, Gobe G, Brown L. Glibenclamide improves kidney and heart structure and function in the adenine-diet model of chronic kidney disease. Pharmacol Res. 2014;79:104–10.
Aroor A, Zuberek M, Duta C, Meuth A, Sowers JR, Whaley-Connell A, Nistala R. Angiotensin II stimulation of DPP4 activity regulates megalin in the proximal tubules. Int J Mol Sci 2016, 17.
Ueda Y, Ishii H, Kitano T, Shindo M, Miyazawa H, Ito K, Hirai K, Kaku Y, Mori H, Hoshino T, et al. Effects and Safety of Linagliptin as an add-on therapy in Advanced-Stage Diabetic Nephropathy patients taking renin-angiotensin-aldosterone system blockers. Drug Target Insights. 2016;10:13–8.
Groop PH, Cooper ME, Perkovic V, Emser A, Woerle HJ, von Eynatten M. Linagliptin lowers albuminuria on top of recommended standard treatment in patients with type 2 diabetes and renal dysfunction. Diabetes Care. 2013;36:3460–8.
Marney A, Kunchakarra S, Byrne L, Brown NJ. Interactive hemodynamic effects of dipeptidyl peptidase-IV inhibition and angiotensin-converting enzyme inhibition in humans. Hypertension. 2010;56:728–33.
Narimani R, Kachuei A, Rezvanian H, Feizi A, Poorpoone M. Effect of sitagliptin on proteinuria in patients with type 2 diabetes - A renoprotective effect of sitagliptin. J Res Med Sci. 2021;26:35.
Cosenso-Martin LN, Giollo-Júnior LT, Fernandes LAB, Cesarino CB, Nakazone MA, Machado MN, Yugar-Toledo JC, Vilela-Martin JF. Effect of vildagliptin versus glibenclamide on endothelial function and arterial stiffness in patients with type 2 diabetes and hypertension: a randomized controlled trial. Acta Diabetol. 2018;55:1237–45.
Ott C, Kistner I, Keller M, Friedrich S, Willam C, Bramlage P, Schmieder RE. Effects of linagliptin on renal endothelial function in patients with type 2 diabetes: a randomised clinical trial. Diabetologia. 2016;59:2579–87.
Suzuki KTS, AC, Kato K, Jojima T, Aso Y. Greater efficacy and improved endothelial dysfunction in untreated type 2 diabetes with liraglutide versus sitagliptin. Dokkyo J Med Sci. 2014;41:211–20.
Mori H, Okada Y, Arao T, Tanaka Y. Sitagliptin improves albuminuria in patients with type 2 diabetes mellitus. J Diabetes Investig. 2014;5:313–9.
Dei Cas A, Spigoni V, Cito M, Aldigeri R, Ridolfi V, Marchesi E, Marina M, Derlindati E, Aloe R, Bonadonna RC, Zavaroni I. Vildagliptin, but not glibenclamide, increases circulating endothelial progenitor cell number: a 12-month randomized controlled trial in patients with type 2 diabetes. Cardiovasc Diabetol. 2017;16:27.
Takihata M, Nakamura A, Tajima K, Inazumi T, Komatsu Y, Tamura H, Yamazaki S, Kondo Y, Yamada M, Kimura M, Terauchi Y. Comparative study of sitagliptin with pioglitazone in Japanese type 2 diabetic patients: the COMPASS randomized controlled trial. Diabetes Obes Metab. 2013;15:455–62.
Lovshin JA, Rajasekeran H, Lytvyn Y, Lovblom LE, Khan S, Alemu R, Locke A, Lai V, He H, Hittle L, et al. Dipeptidyl Peptidase 4 Inhibition stimulates distal tubular natriuresis and increases in circulating SDF-1α(1–67) in patients with type 2 diabetes. Diabetes Care. 2017;40:1073–81.
Zografou I, Sampanis C, Gkaliagkousi E, Iliadis F, Papageorgiou A, Doukelis P, Vogiatzis K, Douma S. Effect of vildagliptin on hsCRP and arterial stiffness in patients with type 2 diabetes mellitus. Horm (Athens). 2015;14:118–25.
Hayashi T, Fukui T, Nakanishi N, Yamamoto S, Tomoyasu M, Osamura A, Ohara M, Yamamoto T, Ito Y, Hirano T. Dapagliflozin decreases small dense low-density lipoprotein-cholesterol and increases high-density lipoprotein 2-cholesterol in patients with type 2 diabetes: comparison with sitagliptin. Cardiovasc Diabetol. 2017;16:8.
Mita T, Hiyoshi T, Yoshii H, Chimori H, Ikeda K, Shimizu M, Kojima Y, Yamamto H, Yasuda D, Sato J, Watada H. The Effect of Linagliptin versus Metformin Treatment-Related Quality of Life in patients with type 2 diabetes Mellitus. Diabetes Ther. 2019;10:119–34.
Nakamura K, Oe H, Kihara H, Shimada K, Fukuda S, Watanabe K, Takagi T, Yunoki K, Miyoshi T, Hirata K, et al. DPP-4 inhibitor and alpha-glucosidase inhibitor equally improve endothelial function in patients with type 2 diabetes: EDGE study. Cardiovasc Diabetol. 2014;13:110.
Oe H, Nakamura K, Kihara H, Shimada K, Fukuda S, Takagi T, Miyoshi T, Hirata K, Yoshikawa J, Ito H. Comparison of effects of sitagliptin and voglibose on left ventricular diastolic dysfunction in patients with type 2 diabetes: results of the 3D trial. Cardiovasc Diabetol. 2015;14:83.
Mita T, Katakami N, Yoshii H, Onuma T, Kaneto H, Osonoi T, Shiraiwa T, Kosugi K, Umayahara Y, Yamamoto T, et al. Alogliptin, a Dipeptidyl Peptidase 4 inhibitor, prevents the progression of carotid atherosclerosis in patients with type 2 diabetes: the study of Preventive effects of Alogliptin on Diabetic atherosclerosis (SPEAD-A). Diabetes Care. 2016;39:139–48.
Yamada H, Tanaka A, Kusunose K, Amano R, Matsuhisa M, Daida H, Ito M, Tsutsui H, Nanasato M, Kamiya H, et al. Effect of sitagliptin on the echocardiographic parameters of left ventricular diastolic function in patients with type 2 diabetes: a subgroup analysis of the PROLOGUE study. Cardiovasc Diabetol. 2017;16:63.
Roden M, Merker L, Christiansen AV, Roux F, Salsali A, Kim G, Stella P, Woerle HJ, Broedl UC. Safety, tolerability and effects on cardiometabolic risk factors of empagliflozin monotherapy in drug-naïve patients with type 2 diabetes: a double-blind extension of a phase III randomized controlled trial. Cardiovasc Diabetol. 2015;14:154.
Acknowledgements
Relevant advice was provided by Professor Yanxue Xue and Yanping Baoof the National Institute on Drug Dependence and Beijing Key Laboratory of Drug Dependence, Peking University.
Funding
This work was supported by grants from the Natural Science Foundation of China (81700115).
Author information
Authors and Affiliations
Contributions
Y.G. and X.L. wrote the main manuscript text. D.Z. and X.Y.searched the multiple databases and extracted data on renal function. Q.Z and Y.Y evaluated qualities of randomized controlled trials. D.Z and X.Y. prepared Figs. 1 and 2. Y.G. and X.L. prepared Figs. 3 and 4. Q.Z. and Y.Y prepared supplementary Figs. 1-4. Y.Z., J.M. and X.L. had revised the manuscript and had full responsibility for the integrity of the data analysis. All the authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Competing interests
The authors declare no competing interests.
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.




Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Gong, Y., Bai, X., Zhang, D. et al. Effect of DPP-4i inhibitors on renal function in patients with type 2 diabetes mellitus: a systematic review and meta-analysis of randomized controlled trials. Lipids Health Dis 23, 157 (2024). https://doi.org/10.1186/s12944-024-02132-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12944-024-02132-x