Abstract
Objectives
Insulin resistance is a well-established contributor to inflammation; however, the specific association between the triglyceride glucose (TyG) index, a biomarker reflecting insulin resistance, and arthritis remains unexplored. As a result, the main aim of this study was to examine the correlation between the TyG index and arthritis.
Methods
This observational study used data from the National Health and Nutrition Examination Survey (NHANES), which was conducted between 2007 and 2018. To investigate the relationship between the TyG index and arthritis, various statistical analyses were employed, including weighted multivariable logistic regression analysis, subgroup analysis, curve fit analysis, and threshold effect analysis.
Results
In total, 14,817 patients were enrolled in the trial, with 4,191 individuals (28.29%) diagnosed with arthritis. An increased risk of arthritis was found to be significantly correlated with higher TyG index values (odds ratio OR = 1.15, 95% confidence interval CI: 1.07–1.23), according to the results of multivariable logistic regression analysis after full adjustment. Subgroup analysis and interaction tests further indicated that the TyG index exhibited an additive effect when combined with other established risk factors, including age (OR = 1.29; 95% CI: 1.17–1.41), body mass index (BMI) (OR = 1.43; 95% CI: 1.24–1.69), and diabetes (OR = 1.20; 95% CI: 1.11–1.31). Additionally, curve fit analysis and threshold effect analysis demonstrated a nonlinear relationship with a breakpoint identified at 8.08 µmol/L.
Conclusion
The TyG index was positively correlated with arthritis in adults under 60 years of age in the United States who had normal weight and no diabetes. Further large-scale prospective studies are warranted for a comprehensive analysis of the role of the TyG index in arthritis.
Similar content being viewed by others
Introduction
Arthritis is a complex and widespread medical condition characterized by inflammation and stiffness in one or more joints of the body [1]. By 2040, 78.4 million adults in the United States will be diagnosed with arthritis, approximately 25.9% of all adults [2]. It is characterized by inflammation and stiffness in one or more joints of the body, with the most prevalent forms being osteoarthritis, rheumatoid arthritis, and psoriatic arthritis [3; 4]. Although there are differences in the pathological process of different types of arthritis, arthritis is related to inflammation [5]. All of them have the potential to significantly lower patients’ quality of life by causing joint discomfort, functional limitations, and diminished mobility [6; 7]. According to the World Health Organization (WHO), arthritis has emerged as a prevalent cause of disability in the United States. Obviously, the impact of arthritis extends beyond individual suffering, as it imposes substantial economic and healthcare burdens on societies [8; 9]. Given the rising incidence of arthritis and its associated medical costs, early screening of high-risk groups based on risk factors and the development of effective approaches to management are vital for both individuals and society.
The TyG index is an emerging marker that has gained recognition for its utility in assessing insulin resistance and metabolic dysfunction [10]. In the past five years, the TyG index has garnered significant attention as a simple and cost-effective method for assessing insulin resistance, especially when compared to traditional complex techniques such as the euglycemic clamp or the Homeostatic Model Assessment of Insulin Resistance (HOMA-IR) [11; 12]. The appeal of the TyG index lies in its utilization of readily available clinical measurements, including fasting triglyceride and glucose levels, which are routinely obtained in clinical practice. The TyG index has been demonstrated to be a surrogate marker for identifying individuals at risk for metabolic conditions such as type 2 diabetes, nonalcoholic fatty liver disease, and coronary artery disease in several investigations [13; 14; 15]. Its simplicity and potential clinical relevance make the TyG index a potentially promising tool for assessing insulin resistance and its implications in various metabolism-related diseases.
Insulin resistance is linked to chronic low-level inflammation, and inflammation is closely linked to joint disease [5; 16]. As a result, studies related to insulin and arthritis have gained importance in recent years. According to the study conducted by Hamada et al. [17], insulin has been found to play a protective and anti-inflammatory role within the synovium. However, the presence of insulin resistance can hinder this beneficial effect, thereby potentially contributing to joint damage. Araújo et al. [18] found that insulin resistance can affect bone mass and reduce bone density. Furthermore, insulin has been shown to have direct effects on cartilage metabolism, promoting the synthesis of inflammatory mediators and degrading enzymes that can contribute to cartilage degradation and joint inflammation [19]. However, previous studies focused on cellular and animal studies, and there is a scarcity of large-scale population-based investigations focusing on insulin resistance and arthritis risk.
Therefore, the goal of this study was to determine the association between the TyG index and arthritis by conducting a comprehensive cross-sectional analysis utilizing the extensive dataset of the NHANES.
Methods
Survey description
NHANES is a large-scale cross-sectional survey conducted in the United States aimed at collecting data on the health and nutritional status of the general population. NHANES utilizes a stratified multistage random sampling approach to ensure representative coverage [20; 21]. The National Center for Health Statistics study ethical review board approved NHANES, and each participant signed written agreement forms to give their informed consent [22]. The NHANES datasets, along with the accompanying documentation and protocols, can be freely obtained from the website.
Study population
For this analysis, data from six cycles of two years, encompassing the years 2007 to 2018, were collected. All measurements and tests were conducted at mobile testing facilities set up on-site, ensuring standardized procedures and data collection protocols.
Our study applied specific exclusion criteria to ensure the validity and reliability of our findings. The following criteria were used for study exclusions: (1) individuals below 20 years of age, as our focus was on adult populations; (2) pregnant women, as their metabolic profiles can be altered during pregnancy; (3) individuals with missing arthritis data, as their inclusion could introduce bias in our analysis; and (4) individuals with missing data on triglyceride and glucose levels, as these variables were essential for calculating the TyG index. Participants with missing covariate data were excluded from the study.
Assessment of the TyG index
The formula for calculating the TyG index is as follows: TyG = Ln [fasting triglycerides (mg/dL) x fasting glucose (mg/dL)/2] [23]. This equation represents a logarithmic transformation of the product obtained by multiplying the fasting triglyceride and glucose levels, divided by 2. In our analysis, we treated the TyG index as a continuous variable and subsequently categorized participants into tertiles based on their TyG index value for further investigation. It is important to note that the TyG index was considered an exposure variable within the framework of our study design.
Assessment of the diagnosis of arthritis
Arthritis diagnosis was ascertained through a self-report questionnaire (MCQ160a), which included the following question: “Has a healthcare professional ever informed you that you have arthritis?“ Participants were asked to choose between two response options: “Yes” or “No.” [24].
Assessment of covariables of interest
The study incorporated various covariates to account for potential confounding factors. These covariates included age, sex, race, body mass index (BMI), hypertension, diabetes, poverty-to-income ratio (PIR), marital status, education level, moderate activity, and vigorous activity. Age was categorized into two groups: “Below 60” and “Over 60,“ enabling a comparison of the effects between different age ranges. Two categories of marital status were identified: “Married or with partner” and “Single,“ allowing for an examination of potential differences based on relationship status. BMI was divided into three groups using cutoffs of 25 and 30: less than 25 is normal weight, greater than or equal to 30 is obese, and between the cutoffs is overweight. The PIR, which was divided into three levels, “1,“ “1–5,“ and “5,“ was used as a proxy indicator of socioeconomic status (SES).
Statistical analyses
All statistical analyses were performed using R software (version 4.1.3) and EmpowerStats (version: 2.0), and the significance level was set at P < 0.05. Participants with missing covariate data were excluded from the study. The triglyceride glucose index was divided into tertiles, with the lowest tertile (T1) serving as the reference group. Continuous variables are presented as medians ± interquartile ranges according to their distribution status, whereas categorical variables are expressed as frequencies and percentages. The chi-squared test or Kruskal‒Wallis H test was employed for different TyG index tertile groups. Multivariate logistic regression analysis was conducted to investigate the relationship between the triglyceride glucose index and arthritis. Model 1 was unadjusted, while Model 2 was adjusted for sex age, and race. Finally, Model 3, which was our core model, was adjusted for the variables in Model 2 as well as history of education level, poverty-to-income ratio, marital status, body mass index, hypertension, diabetes, moderate activity, and vigorous activity. The same statistical methods presented above were also applied to the age, BMI, and diabetes subgroups, allowing for subgroup analyses to examine potential variations within these specific populations. To better evaluate the nonlinear relationship between the TyG index and arthritis, our study incorporated smooth curve fitting and threshold effect analysis. These statistical techniques enabled a more comprehensive exploration of the potential nonlinearity between the TyG index and the risk of arthritis.
Results
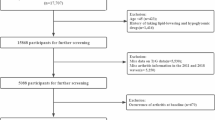
After applying exclusion criteria, our final study cohort comprised a total of 14,817 participants. For a more comprehensive understanding of our study design, sampling methods, and specific exclusion criteria, we refer readers to Fig. 1, which provides additional details on these aspects.
Baseline characteristics of participants
Table 1 presents the baseline characteristics of the participants in the study. A total of 14,817 participants met the selection criteria and were included, with an overall incidence of arthritis in this cohort of 28.29%. Among the participants, 65.33% were below 60 years of age, while 34.67% were aged 60 years or older. In terms of sex distribution, 48.82% were males, and 51.18% were females. Regarding ethnicity, 15.26% were of Mexican American descent, 10.95% were from other Hispanic backgrounds, 41.49% were non-Hispanic whites, 20.02% were non-Hispanic blacks, and 12.28% belonged to other racial groups. The median ± interquartile range concentrations of triglycerides, glucose, and the TyG index were 101.00 ± 79, 101.00 ± 18, and 8.57 ± 0.87, respectively. The participants were classified into tertiles based on their triglyceride glucose index values, with T1 representing values ranging from 5.64 to 8.30, T2 ranging from 8.30 to 8.85, and T3 ranging from 8.85 to 12.84. A significant progressive gain in the prevalence of arthritis was observed as the participants’ triglyceride index values increased (T1: 21.67%, T2: 29.14%, T3: 34.04%, P < 0.001).
The characteristics of participants with arthritis and no arthritis are illustrated in Table 2. Compared with the individuals without arthritis, participants with arthritis were older than 60 years of age. A higher percentage of women had arthritis than men. There was a higher percentage of non-Hispanic white participants with arthritis than people without arthritis. Participants with arthritis had a higher prevalence of education level, obesity and hypertension. The TyG index was higher among arthritis participants than participants without arthritis.
Associations between the TyG index and arthritis
Table 3 displays the results of the multivariate logistic regression study looking at the association between the TyG index and arthritis. In the unadjusted model, a highly significant positive correlation was observed between the TyG index and arthritis (OR = 1.47; 95% CI: 1.39–1.54; P < 0.0001). Upon adjusting for sex, age, and race variables in model 2, this significant positive association remained evident (OR = 1.46; 95% CI: 1.38–1.55; P < 0.0001). Even after accounting for all covariates in model 3, the relationship between the TyG index and arthritis remained significant and positive (OR = 1.15; 95% CI: 1.07–1.23; P = 0.0002). Additionally, when fully adjusting for potential interfering factors, the odds ratios (ORs) with corresponding confidence intervals (CIs) indicated a significant positive association in T2 and T3 compared to T1. Specifically, the ORs were 1.15 (95% CI: 1.03–1.28, P for trend < 0.0001) and 1.19 (95% CI: 1.05–1.34, P for trend = 0.0061) for T2 and T3, respectively, when compared to T1.
Subgroup analyses
Subgroup analyses were performed to estimate the associations between the TyG index and arthritis, and the results are presented in Table 4. Interestingly, some associations remained consistent across different subgroups, including sex, hypertension, vigorous activity, and moderate activity. However, notable interactions were observed between age, BMI, diabetes, and the associations with the TyG index. Subgroup analyses stratified by age, BMI, and diabetes revealed intriguing findings. The positive association between the TyG index and arthritis was more pronounced in participants below 60 years of age (OR = 1.29; 95% CI: 1.17–1.41), those without diabetes (OR = 1.20; 95% CI: 1.11–1.31), and individuals with normal weight (OR = 1.43; 95% CI: 1.24–1.69).
Nonlinear association between the TyG index and arthritis
The adjusted smoothing curve depicted in Fig. 2 demonstrates a saturation effect value relationship between the TyG index and arthritis. Utilizing a two-segment linear regression model, we calculated the turning points to be 8.08 µmol/L for the TyG index. When the TyG index was below 8.08 µmol/L, we observed a significantly positive association with arthritis, with an OR value of 2.01 (95% CI: 1.46–2.75; P < 0.0001). However, once the TyG index reached 8.08 µmol/L, the OR value displayed a slow increase, eventually reaching a saturation point of 1.05 (95% CI: 0.97–1.15; P = 0.2235). Furthermore, we identified distinct inflection points and saturation effect values in the TyG index among individuals aged below 60 years old, without diabetes and with normal weight, with respective inflection points of 8.09, 8.07, and 9.06, as presented in Table 5.
Discussion
In our analysis, we observed a robust correlation between the TyG index and arthritis, which persisted even after adjusting for relevant confounding variables in the fully adjusted model. Subgroup analysis and interaction tests showed that the TyG index may be particularly relevant in identifying individuals with arthritis who are below 60 years of age, have a BMI below 25.0, and do not have diabetes or hypertension. These findings suggest that the impact of the TyG index on arthritis may be influenced by age, BMI, and diabetes status, highlighting the importance of considering these factors when assessing the relationship between the TyG index and arthritis. Curve fit analysis and threshold effect analysis showed a nonlinear relationship with a breakpoint of 8.08 between the TyG index and arthritis. Focusing on the TyG index of patients is a simple but effective tool for the epidemiological study of arthritis.
Biomarkers focusing on arthritis have been progressively reported by scholars over the years as the NHANES database has been extensively studied. In a study conducted by Liu et al. [25], it was shown that the systemic immune-inflammation index serves as a novel and convenient marker of inflammation, suggesting its potential importance in assessing arthritis. Similarly, Xiang et al. [26] found that the dietary inflammation index has an additive effect alongside other risk factors, thereby increasing the likelihood of developing arthritis. Moreover, Chen et al. [27] revealed a significant association between sensitivity to thyroid hormone indices and the prevalence of osteoarthritis, highlighting the intricate correlation between the thyroid system and chondrogenic differentiation. These findings collectively underscore the significance of incorporating various markers in the assessment of arthritis. However, no studies have investigated the correlation between the TyG index, a novel and easy measure of insulin resistance and systemic inflammation, and arthritis.
To the best of our knowledge, this is the first cross-sectional study to investigate the relationship between the TyG index and arthritis. Prior investigations have examined the association between the TyG index and diverse diseases, employing a range of epidemiological methods and focusing on various target populations. According to Shi et al. [28], their study indicates a significant association between TyG index values and the presence of depressive symptoms among adults in the United States. Zheng et al. [29] reported a significant association between a higher TyG index and an increased risk of nephrolithiasis and its recurrence. Their findings suggest that early intervention and management of insulin resistance may potentially improve or alleviate the occurrence and recurrence of kidney stones. Ahn et al. [30] discovered a significant positive association between the TyG index and a low skeletal muscle mass index in Korean participants. According to the findings of Guo et al. [31], an elevated TyG index was associated with a higher risk of long-term complications following percutaneous coronary intervention, such as repeat revascularization and in-stent restenosis. The results presented by Guo et al. highlight the clinical relevance of the TyG index in identifying individuals at higher risk for adverse outcomes after percutaneous coronary intervention and emphasize its potential as a valuable tool in risk assessment and patient management within the context of chronic coronary syndrome. Ren et al. [32] suggested a potential association between the TyG index and the severity and morbidity of COVID-19, indicating that the TyG index could serve as a valuable marker for identifying poor outcomes in patients with COVID-19. Yilmaz et al. [33] conducted a cross-sectional study that revealed a statistically significant elevation of the TyG index in patients with erectile dysfunction (ED) compared to the non-ED group. These findings suggest that the TyG index may hold potential as a diagnostic and monitoring tool for ED. Obviously, there is increasing evidence that the TyG index is not only applicable to diabetics but may also be relevant to other diseases.
The underlying mechanism driving the association between the TyG index and arthritis remains unclear. The observed association suggests potential underlying mechanisms that link insulin resistance, metabolic dysfunction, and chronic inflammation to the pathogenesis of arthritis. The interaction between insulin resistance and inflammation is likely to be facilitated by several mechanisms. On the one hand, arthritis is known to cause the upregulation of proinflammatory cytokines, such as tumor necrosis factor (TNF) and interleukin (IL), which are closely associated with insulin resistance. Elevated systemic levels of TNF-α have been associated with the activation of key signaling proteins, including IKK, p38 MAPK, JNK, and PKC. When activated, these proteins directly target specific serine residues on the insulin receptor substrate (IRS) protein, disrupting its normal functioning. This disruption impedes the process of tyrosine phosphorylation, resulting in impaired insulin signaling and subsequent insulin resistance [34]. TNF-α also upregulates the expression of protein tyrosine phosphatase 1B (PTP1B), which acts by dephosphorylating phosphoserine residues present in insulin receptor and IRS proteins [35]. Through this mechanism, TNF-α-mediated induction of PTP1B disrupts the normal phosphorylation events required for optimal insulin receptor and IRS signaling, ultimately impairing insulin sensitivity and downstream metabolic responses. The upregulation of IL-6 can stimulate the JAK-STAT signaling pathways, resulting in an increase in the expression of suppressor of cytokine signaling 1 (SOCS1) and SOCS3 proteins. These proteins play a role in downregulating the function of the insulin receptor through mechanisms such as sterically blocking its interaction with IRS proteins or modifying kinase activity [36; 37; 38; 39]. Additionally, IL-6 can induce the expression of the TLR-4 gene by activating STAT3. When combined with IL-1β, IL-6 can enhance the activation of STAT3 and NF-κB in hepatocytes, leading to inflammation [40]. Furthermore, IL-1β activates p38 MAPK via the IL-1β receptor, which in turn hinders insulin signaling by inducing serine phosphorylation on IRS1/2 [41].
On the other hand, insulin resistance contributes to oxidative stress and the generation of reactive oxygen species, which are important in the progression of arthritis [42]. Elevated levels of reactive oxygen species (ROS) can have detrimental effects, encompassing not only oxidative damage but also disturbances in redox-regulated cell signaling pathways, such as Akt and MAP kinase signaling. Mitochondrial dysfunction, often observed with aging, plays a role in this process, as it leads to increased generation of ROS within the mitochondria. Age-related changes, including reduced activity of mitochondrial superoxide dismutase, contribute to the augmented production of ROS in chondrocytes, which exacerbates the oxidative stress associated with aging [43]. Consequently, the disruption of redox balance and the accumulation of ROS can have significant implications for cellular function and contribute to the development or progression of age-related conditions, including arthritis [44; 45; 46].
Study strengths and limitations
Our study adds to the existing body of knowledge by providing further evidence supporting the positive correlation between the TyG index and arthritis. By utilizing the NHANES database, we were able to analyze a large representative sample, enhancing the generalizability of our findings. Moreover, our study contributes to the understanding of the TyG index as a potential marker for identifying individuals at risk of developing arthritis, emphasizing the relevance of metabolic health and insulin resistance in the pathogenesis of arthritis.
Nevertheless, this study has some limitations that should be acknowledged. First, the NHANES dataset used in this study is cross-sectional in nature, lacking longitudinal follow-up data. Second, although we adjusted for several relevant confounding factors, the presence of unmeasured or residual confounding cannot be entirely ruled out. Third, data on medications that could influence the TyG index, such as statins, fibrates, and antidiabetic medication, were not included. Furthermore, we did not include data on markers of inflammation that could potentially impact arthritis, such as C-reactive protein and erythrocyte sedimentation rate. In addition, the source of the diagnosis of arthritis in this study was patient self-report, and due to the existence of missing data, we did not further analyze the subtype of arthritis. Therefore, it is essential to interpret our findings with caution and consider them as preliminary evidence that warrants further investigation. Further studies are warranted to clarify the underlying mechanisms driving the observed correlation between the TyG index and arthritis. Prospective studies with longitudinal follow-up can help establish temporal relationships and better understand whether the TyG index is an independent risk factor for arthritis or merely a marker of metabolic dysfunction. Additionally, investigations into specific arthritis subtypes, such as rheumatoid arthritis or osteoarthritis, can shed light on potential variations. As a whole, our research offers insightful information about the association between the TyG index and arthritis, contributing to the cumulative knowledge in this field.
Conclusion
The results of this research point to a relationship between elevated levels of the TyG index and a higher prevalence of arthritis. Our findings contribute to the expanding body of evidence supporting the clinical applicability of the TyG index as a predictive tool for arthritis, providing valuable insights for risk stratification and early intervention strategies in susceptible populations.
Data Availability
Data are available upon request to the corresponding author.
Change history
27 February 2024
A Correction to this paper has been published: https://doi.org/10.1186/s12944-024-02052-w
Abbreviations
- BMI:
-
Body mass index
- CI:
-
Confidence interval
- ED:
-
Erectile dysfunction
- HOMA-IR:
-
Homeostatic Model Assessment of Insulin Resistance
- IL:
-
Interleukin
- IRS:
-
Insulin Receptor Substrate
- NHANES:
-
National Health and Nutrition Examination Survey
- OR:
-
Odds Ratio
- PIR:
-
Poverty Income Ratio
- PTP1B:
-
Protein tyrosine phosphatase 1B
- ROS:
-
Reactive oxygen species
- SES:
-
Socioeconomic Status
- SOCS:
-
Suppressor Of Cytokine Signaling
- TNF:
-
Tumor necrosis factor
- TyG:
-
Triglyceride glucose
- WHO:
-
World Health Organization
References
Harris MF, Frith J. Arthritis in general practice. Med J Aust. 1992;156:854–8.
Hootman JM, Helmick CG, Barbour KE, Theis KA, Boring MA. Updated projected prevalence of self-reported doctor-diagnosed arthritis and arthritis-attributable activity limitation among US adults, 2015–2040. Arthritis & rheumatology (Hoboken, N.J.) 68 (2016) 1582–7.
Park J, Mendy A, Vieira ER. Various types of arthritis in the United States: prevalence and age-related Trends from 1999 to 2014. Am J Public Health. 2018;108:256–8.
Ritchlin CT, Colbert RA, Gladman DD. Psoriatic Arthritis N Engl J Med. 2017;376:957–70.
Spel L, Martinon F. Inflammasomes contributing to inflammation in arthritis. Immunol Rev. 2020;294:48–62.
March LM, Brooks PM. Arthritis: the hidden disability. Med J Aust. 1993;158:369–71.
Schofield D, Cunich M, Shrestha RN, Tanton R, Veerman L, Kelly S, Passey ME. The long-term economic impacts of arthritis through lost productive life years: results from an australian microsimulation model. BMC Public Health. 2018;18:654.
Hunter DJ, Schofield D, Callander E. The individual and socioeconomic impact of osteoarthritis. Nat Rev Rheumatol. 2014;10:437–41.
Safiri S, Kolahi AA, Hoy D, Smith E, Bettampadi D, Mansournia MA, Almasi-Hashiani A, Ashrafi-Asgarabad A, Moradi-Lakeh M, Qorbani M, Collins G, Woolf AD, March L, Cross M. Global, regional and national burden of rheumatoid arthritis 1990–2017: a systematic analysis of the Global Burden of Disease study 2017. Ann Rheum Dis. 2019;78:1463–71.
Ramdas Nayak VK, Satheesh P, Shenoy MT, Kalra S. Triglyceride glucose (TyG) index: a surrogate biomarker of insulin resistance. J Pak Med Assoc. 2022;72:986–8.
Minh HV, Tien HA, Sinh CT, Thang DC, Chen CH, Tay JC, Siddique S, Wang TD, Sogunuru GP, Chia YC, Kario K. Assessment of preferred methods to measure insulin resistance in asian patients with hypertension. J Clin Hypertens (Greenwich). 2021;23:529–37.
Son DH, Lee HS, Lee YJ, Lee JH, Han JH. Comparison of triglyceride-glucose index and HOMA-IR for predicting prevalence and incidence of metabolic syndrome. Nutr Metab Cardiovasc Dis. 2022;32:596–604.
Park B, Lee HS, Lee YJ. Triglyceride glucose (TyG) index as a predictor of incident type 2 diabetes among nonobese adults: a 12-year longitudinal study of the korean genome and epidemiology study cohort. Transl Res. 2021;228:42–51.
Xu L, Wu M, Chen S, Yang Y, Wang Y, Wu S, Tian Y. Triglyceride-glucose index associates with incident heart failure: a cohort study. Diabetes Metab. 2022;48:101365.
Huanan C, Sangsang L, Amoah AN, Yacong B, Xuejiao C, Zhan S, Guodong W, Jian H, Songhe S, Quanjun L. Relationship between triglyceride glucose index and the incidence of non-alcoholic fatty liver disease in the elderly: a retrospective cohort study in China. BMJ open. 2020;10:e039804.
Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116:1793–801.
Hamada D, Maynard R, Schott E, Drinkwater CJ, Ketz JP, Kates SL, Jonason JH, Hilton MJ, Zuscik MJ, Mooney RA. Suppressive Effects of insulin on Tumor Necrosis factor-dependent early osteoarthritic changes Associated with obesity and type 2 diabetes Mellitus. Arthritis & rheumatology (Hoboken N J). 2016;68:1392–402.
de Araújo IM, Parreiras ESLT, Carvalho AL, Elias J Jr, Salmon CEG, de Paula FJA. Insulin resistance negatively affects bone quality not quantity: the relationship between bone and adipose tissue. Osteoporos Int. 2020;31:1125–33.
Ribeiro M, López de Figueroa P, Blanco FJ, Mendes AF, Caramés B. Insulin decreases autophagy and leads to cartilage degradation. Osteoarthritis Cartilage. 2016;24:731–9.
Curtin LR, Mohadjer LK, Dohrmann SM, Kruszon-Moran D, Mirel LB, Carroll MD, Hirsch R, Burt VL, Johnson CL. National Health and Nutrition Examination Survey: sample design, 2007–2010. Vital Health Stat. 2013;2:1–23.
Johnson CL, Dohrmann SM, Burt VL, Mohadjer LK. National health and nutrition examination survey: sample design, 2011–2014. Vital Health Stat. 2014;2:1–33.
Skrivankova VW, Richmond RC, Woolf BAR, Yarmolinsky J, Davies NM, Swanson SA, VanderWeele TJ, Higgins JPT, Timpson NJ, Dimou N, Langenberg C, Golub RM, Loder EW, Gallo V, Tybjaerg-Hansen A, Davey Smith G, Egger M, Richards JB. Strengthening the reporting of Observational Studies in Epidemiology using mendelian randomization: the STROBE-MR Statement. JAMA. 2021;326:1614–21.
Liu X, Tan Z, Huang Y, Zhao H, Liu M, Yu P, Ma J, Zhao Y, Zhu W, Wang J. Relationship between the triglyceride-glucose index and risk of cardiovascular diseases and mortality in the general population: a systematic review and meta-analysis. Cardiovasc Diabetol. 2022;21:124.
Liang X, Chou OHI, Cheung CL, Cheung BMY. Is hypertension associated with arthritis? The United States national health and nutrition examination survey 1999–2018. Ann Med. 2022;54:1767–75.
Liu B, Wang J, Li YY, Li KP, Zhang Q. The association between systemic immune-inflammation index and rheumatoid arthritis: evidence from NHANES 1999–2018. Arthritis Res Ther. 2023;25:34.
Xiang S, Wang Y, Qian S, Li J, Jin Y, Ding X, Xu T. The association between dietary inflammation index and the risk of rheumatoid arthritis in Americans. Clin Rheumatol. 2022;41:2647–58.
Chen S, Sun X, Zhou G, Jin J, Li Z. Association between sensitivity to thyroid hormone indices and the risk of osteoarthritis: an NHANES study. Eur J Med Res. 2022;27:114.
Shi YY, Zheng R, Cai JJ, Qian SZ. The association between triglyceride glucose index and depression: data from NHANES 2005–2018. BMC Psychiatry. 2021;21:267.
Qin Z, Zhao J, Geng J, Chang K, Liao R, Su B. Higher triglyceride-glucose index is Associated with increased likelihood of kidney Stones. Front Endocrinol (Lausanne). 2021;12:774567.
Ahn SH, Lee JH, Lee JW. Inverse association between triglyceride glucose index and muscle mass in korean adults: 2008–2011 KNHANES. Lipids Health Dis. 2020;19:243.
Guo X, Shen R, Yan S, Su Y, Ma L. Triglyceride-glucose index for predicting repeat revascularization and in-stent restenosis in patients with chronic coronary syndrome undergoing percutaneous coronary intervention. Cardiovasc Diabetol. 2023;22:43.
Ren H, Yang Y, Wang F, Yan Y, Shi X, Dong K, Yu X, Zhang S. Association of the insulin resistance marker TyG index with the severity and mortality of COVID-19. Cardiovasc Diabetol. 2020;19:58.
Yilmaz M, Karaaslan M, Tonyali S, Celik M, Toprak T, Odabas O. Triglyceride-glucose index (TyG) is associated with erectile dysfunction: a cross-sectional study. Andrology. 2021;9:238–44.
Qing Z, Xiao-Hui W, Xi-Mei W, Chao-Chun Z. Vitamin C deficiency aggravates tumor necrosis factor α-induced insulin resistance. Eur J Pharmacol. 2018;829:1–11.
Nieto-Vazquez I, Fernández-Veledo S, Krämer DK, Vila-Bedmar R, Garcia-Guerra L, Lorenzo M. Insulin resistance associated to obesity: the link TNF-alpha. Arch Physiol Biochem. 2008;114:183–94.
Senn JJ, Klover PJ, Nowak IA, Zimmers TA, Koniaris LG, Furlanetto RW, Mooney RA. Suppressor of cytokine signaling-3 (SOCS-3), a potential mediator of interleukin-6-dependent insulin resistance in hepatocytes. J Biol Chem. 2003;278:13740–6.
Taniguchi CM, Emanuelli B, Kahn CR. Critical nodes in signalling pathways: insights into insulin action. Nat Rev Mol Cell Biol. 2006;7:85–96.
Ueki K, Kondo T, Kahn CR. Suppressor of cytokine signaling 1 (SOCS-1) and SOCS-3 cause insulin resistance through inhibition of tyrosine phosphorylation of insulin receptor substrate proteins by discrete mechanisms. Mol Cell Biol. 2004;24:5434–46.
Emanuelli B, Peraldi P, Filloux C, Chavey C, Freidinger K, Hilton DJ, Hotamisligil GS, Van Obberghen E. SOCS-3 inhibits insulin signaling and is up-regulated in response to tumor necrosis factor-alpha in the adipose tissue of obese mice. J Biol Chem. 2001;276:47944–9.
Wu H, Ballantyne CM. Metabolic inflammation and insulin resistance in obesity. Circ Res. 2020;126:1549–64.
Watanabe Y, Nagai Y, Takatsu K. Activation and regulation of the pattern recognition receptors in obesity-induced adipose tissue inflammation and insulin resistance. Nutrients. 2013;5:3757–78.
Hu M, Zhang Y, Guo X, Jia W, Liu G, Zhang J, Li J, Cui P, Sferruzzi-Perri AN, Han Y, Wu X, Ma H, Brännström M, Shao LR, Billig H. Hyperandrogenism and insulin resistance induce gravid uterine defects in association with mitochondrial dysfunction and aberrant reactive oxygen species production. Am J Physiol Endocrinol Metab. 2019;316:E794–e809.
Bolduc JA, Collins JA, Loeser RF. Reactive oxygen species, aging and articular cartilage homeostasis. Free Radic Biol Med. 2019;132:73–82.
Khmaladze I, Nandakumar KS, Holmdahl R. Reactive oxygen species in psoriasis and psoriasis arthritis: relevance to human disease. Int Arch Allergy Immunol. 2015;166:135–49.
Cuzzocrea S. Role of nitric oxide and reactive oxygen species in arthritis. Curr Pharm Design. 2006;12:3551–70.
Mirshafiey A, Mohsenzadegan M. The role of reactive oxygen species in immunopathogenesis of rheumatoid arthritis. Iran J Allergy Asthma Immunol. 2008;7:195–202.
Acknowledgements
The authors thank all the staff for their help.
Funding
This research was funded by the National Natural Science Foundation of China (No. 8160922), Jiangsu Provincial Traditional Chinese Medicine Science and Technology Development Project (No. ZD202232), Suzhou Key Clinical Diseases Diagnosis and Treatment Fund Project (No. LCZX201901), and Extracurricular Academical Research Fund of Soochow University (No. KY20201019B).
Author information
Authors and Affiliations
Contributions
Yuxin Yan and Liyu Zhou contributed equally to this paper. Yuxin Yan: Conceptualization, Methodology, Data curation, Investigation, Software, Writing – original draft. Liyu Zhou: Conceptualization, Methodology, Data curation, Investigation, Writing – original draft. Rui La: Conceptualization, Methodology, Data curation, Validation. Ming Jiang: Conceptualization, Methodology, Data curation. Dinghua Jiang: Conceptualization, Methodology, Data curation. Lixin Huang: Conceptualization, Methodology, Data curation. Wu Xu: Conceptualization, methodology, data curation, validation, writing—review & editing, funding acquisition. Qian Wu: Conceptualization, methodology, data curation, validation, writing—review & editing, funding acquisition. All authors approved the manuscript and agreed to publish.
Corresponding authors
Ethics declarations
Conflict of interest
All authors declare that there are no conflicts of interest.
Ethics approval and consent to participate
This study used publicly available summary data, and ethics approval was not necessary.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: the authors have identified errors in Introduction section and table 5.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yan, Y., Zhou, L., La, R. et al. The association between triglyceride glucose index and arthritis: a population-based study. Lipids Health Dis 22, 132 (2023). https://doi.org/10.1186/s12944-023-01899-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12944-023-01899-9