Abstract
Objectives
Thyroid hormones play an instrumental role in chondrogenic differentiation and matrix maturation. However, studies investigating the relationship between thyroid function and the risk of osteoarthritis (OA) remain scarce. This study was designed to investigate the correlation between thyroid status and OA from a novel perspective of sensitivity to thyroid hormones.
Methods
The study included 8478 people from the National Health and Nutrition Examination Survey (NHANES) 2007–2010. The sensitivity to thyroid hormone indices included Thyrotroph Thyroxine Resistance Index (TT4RI), Thyroid-stimulating hormone (TSHI), Thyroid Feedback Quantile-based Index (TFQI), and Free Triiodothyronine /Free thyroxine (FT3/FT4), which were calculated based on serum free triiodothyronine (FT3), free thyroxine (FT4), and thyroid stimulating hormone (TSH). Considering the complex survey design and sample weights, we employed multivariate linear regression models and stratified analysis to evaluate the correlation between sensitivity to thyroid hormone indices and OA.
Results
Study results indicated that participants with OA had elevated TT4RI, TSHI, and TFQI levels, and lower FT3/FT4 levels compared to those with non-arthritis. After adjusting for other covariates, FT3/FT4 was negatively associated with the risk of OA (OR = 1.162, 95%CI 1.048–1.478, P = 0.021); (OR = 1.261, 95%CI 1.078–1.623, P = 0.042). In subgroup analyses stratified by gender and BMI, participants with OA had higher TFQI levels compared to those without OA in both genders. (OR = 1.491, 95%CI 1.070–2.077, P = 0.018); (OR = 2.548, 95%CI 1.929–3.365, P < 0.001). The higher TFQI levels were consistently associated with the increased prevalence of OA in the BMI (< 18.5 kg/m2) group after adjusting for different covariates, but not in other BMI groups. In, addition, TFQI performed better than FT3/FT4, TSHI, and TT4RI on ROC analyses for OA prediction.
Conclusions
The levels of FT3/FT4, TSHI, TT4RI, and TFQI are strongly associated with the prevalence of OA, which illustrates the complex correlation between the thyroid system and chondrogenic differentiation. TFQI may be used as a helpful indicator to predict OA and provide novel ideas for the evaluation and treatment of OA.
Similar content being viewed by others
Introduction
Osteoarthritis (OA) is a common chronic joint disease characterized by articular cartilage degradations and alterations in subchondral bone and synovium [1,2,3]. According to an epidemiological study published in 2020, approximately 3.8% of the global population (250 million people) are affected by OA, and 18% of females and 9.6% of males over 60 years old suffer from symptomatic OA [4, 5]. In addition to the huge physical burden on patients, OA is also a leading cause of rising medical costs in the United States [6]. Thus, early identification and intervention of OA are essential to reduce its prevalence.
Although the etiology and pathogenesis of OA have not yet been fully clarified, aging, obesity, infection, trauma, and metabolic disorders are considered risk factors for OA [7, 8]. Recent studies have demonstrated the important role of thyroid hormone metabolism in articular cartilage maintenance and the pathogenesis of OA [9,10,11]. Thyroid hormone is essential for endochondral ossification and the expression of genes that control chondrocyte maturation and matrix synthesis [12]. The lack of thyroid hormone results in the decrease of calcitonin secretion in the body, which affects calcium balance and bone metabolism, and eventually leads to the occurrence of OA [13]. Furthermore, clinical studies have shown that TSH levels are significantly positively correlated with the prevalence and progression of OA in patients with autoimmune thyroid disease (AITD) [14].
Thyroid function is evaluated clinically by measuring serum FT3, FT4, and TSH levels. However, FT3 or TSH alone may not be sufficient to reflect the regulation of thyroid hormone homeostasis [15]. Therefore, we proposed sensitivity to thyroid hormone indices such as TT4RI, TSHI, TFQI, and FT3/FT4 to comprehensively explain thyroid status [16, 17]. TT4RI, TSHI, and TFQI are introduced as quantitative markers for pituitary thyrotropic function [18, 19]. FT3 to FT4 ratio (FT3/FT4) can estimate the conversion efficiency of FT4 to FT3, which indirectly reflects the peripheral sensitivity of thyroid hormones. Increasing attention has been paid to the relationship between thyroid hormone sensitivity and metabolic disorders in recent years, and sensitivity to thyroid hormone indices have been proved to be reliable predictors of insulin resistance, type 2 diabetes (T2D), cardiometabolic risk, and disorders of glucose and lipid metabolism [20,21,22]. However, studies investigating the relationship between sensitivity to thyroid hormone indices and OA have not been reported. Therefore, we analyzed NHANES (2007–2010) data to explore the correlation between central and peripheral sensitivity to thyroid hormone and OA.
Materials and methods
Study population
The NAHNES is a research project of the National Center for Health Statistics (NCHS) that collects the health and nutrition statistics of the United States population. For the purpose of ensuring that the study participants are representative, the organization implemented a stratified, multistage, and clustered probability sampling design. In addition, all participants have written informed consent for data collection before the start of the investigation.
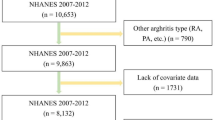
In this research, we used demographic information, laboratory data, examination data, and questionnaire data from NHANES 2007–2010. We included participants who had completed nutrition investigations, medical examinations, and medical conditions questionnaires (n = 20,686). The exclusion criteria were as follows: (1) missing thyroid profile data (n = 12,064); (2) missing arthritis and BMI data (n = 123); (3) patients with a history of cancer diseases (n = 24). Thus, 8478 subjects were finally included in this analysis (Fig. 1).
Definitions of osteoarthritis
Arthritis diagnosis data is part of self-reported personal interview data. Participants were asked if they had ever been told by their doctor or another health professional that they had arthritis. If “yes”, they were asked to classify their arthritis diagnosis as osteoarthritis (OA), RA (rheumatoid arthritis), psoriatic arthritis, and others.
Assessment of thyroid function
The TT3, TT4, FT3 assay was a competitive binding immunoenzymatic assay. The TSH (mIU/L) level was detected by a two-site immunoenzymatic (“sandwich”) assay. The Tg (ng/mL) levels were assessed by a simultaneous one-step “sandwich” assay, while TgAb (IU/mL) and TPOAb (IU/mL) were assessed by a sequential two-step immunoenzymatic “sandwich” assay.
The following is the calculation method of sensitivity to thyroid hormone indices:
A high FT3/FT4 ratio indicates a higher peripheral sensitivity to thyroid hormone [17, 23].
The higher TSHI and TT4RI indices indicate that the central sensitivity to thyroid hormone is lower.
TFQI was calculated with FT4 and TSH values according to the empirical cumulative distribution function. The advantage of TFQI is that it will not produce extreme values in the case of thyroid dysfunction and is more stable than TT4RI and TSHI. The TFQI value ranges from − 1 to 1. Depending on the previous studies, TFQI was negative, suggesting that the HPT axis is more sensitive to changes in FT4. A positive value indicated that the HPT axis is insensitive to FT4. 0 indicated that the sensitivity of the HPT axis to FT4 changes is normal.
Covariates
The covariates are demographic information, laboratory data, and questionnaire data. Demographic information included age, gender, race, and education level. Laboratory data included HbA1c, albumin (g/dL), blood urea nitrogen (BUN, mg/dL), total calcium (mg/dL), creatinine (mg/dL), phosphorus (P, mg/dL), triglycerides (TG, mg/dL), and uric acid (UA, mg/dL). Finally, questionnaire data covered alcohol consumption, smoking behavior, physical activity, BMI, hypertension, and diabetes. Smoking behavior was defined as Current (smoked more than 100 cigarettes in the lifetime and currently still smoked), Past (smoked more than 100 cigarettes in the lifetime, but did not currently smoke), and Never (smoked less than 100 cigarettes in the lifetime). Alcohol consumption was classified as Non-drinker, low-to-moderate drinker (< 1 drink/day in women and < 2 drinks/day in men), and Heavy drinker (≥ 1 drink/day in women and ≥ 2 drinks/day in men). Physical activity was defined as Low (≤ twice a week), Moderate (< once a day and > twice a week), High (≥ once a day), and not recorded.
Statistical analyses
The complex survey design factors involved in NHANES, including weights, clustering, and stratification, were all considered as recommended according to the analytical guideline regulated by NCHS. All analysis data conforming to normal or skewed distributions were presented as mean ± standard deviation, while categorical variables were shown as the number and percentage of subjects. The weighted chi-squared test and a weighted linear regression model were used to compare various indexes of the population between the “Arthritis and Non-arthritis” groups. The statistically significant covariates were then incorporated into the multiple linear regression model. By adjusting covariates, we further analyzed the correlation between sensitivity to thyroid hormone indices and OA, and calculated the effect value β and its 95% confidence intervals (CI). Furthermore, we performed subgroup analyses stratified by gender and BMI to enhance the confidence of the data. To evaluate the performance of the indices, we examined the receiver operating characteristics curves (ROC), which plot sensitivity against 1-specificity, and calculated the cut-points from ROC results. In this study, R software and EmpowerStats were used for data analysis, and P < 0.05 was considered statistically significant. Missing values often occur during the data extraction and data collection processes. For avoiding potential survival bias, we used multiple interpolations to replace the missing values in our study.
Results
Baseline characteristics of the participants with or without arthritis
We included 8478 participants in our study. The demographic characteristics of the research population were presented in Table 1. Compared to the non-arthritis group, participants in the arthritis group were more likely to be older, female, more educated, and non-Hispanic white. Meanwhile, significant differences were also observed between the two groups for smoking behavior, alcohol consumption, physical activity, BMI, WC, albumin, blood urea nitrogen, total calcium, creatinine, phosphorus, triglycerides, and serum uric acid (P < 0.001). In addition, participants in this study were divided into four categories: OA, rheumatoid arthritis, psoriatic arthritis, and other types according to the type of arthritis. In arthritic patients, the levels of FT4, Tg, TSHI, TT4RI, and TFQI were significantly higher, while the levels of FT3 and FT3/FT4 were significantly lower (P < 0.001).
Associations between sensitivity to thyroid hormone indices and osteoarthritis
The adjusted correlation between OA and sensitivity to thyroid hormone indices was presented in Table 2. In the unadjusted model, we found that the TSHI, TT4RI, and TFQI were positively associated with the risks of OA, and FT3/FT4 was negatively associated with the prevalence of OA (P < 0.001). After adjusting for multiple covariates, FT3/FT4, TSHI, and TT4RI were not significantly associated with OA. However, the positive correlation between TFQI and the incidence of OA remained significant in the model 2 and 3. In addition, every 1 increase in TFQI was associated with a 26.1% increase in the risk of OA in the multivariable model 3 (OR = 1.261, 95% CI 1.079–1.623, P = 0.042). After converting TFQI to a categorical variable (quartiles), the TFQI levels of the Q2, Q4 groups were still positively correlated with the prevalence of OA in the multivariable model 3 compared with the lowest quartile of TFQI (Q1). In addition, the trend remained significant among different TFQI quartile groups (P < 0.05). The subgroup analyses stratified by gender and BMI were reported in Table 3. In both genders, those with higher TFQI levels had a higher incidence of OA than those with lower levels (OR = 1.491, 95% CI 1.070–2.077, P = 0.018) (OR = 2.548, 95% CI 1.929–3.365, P < 0.001). Furthermore, we discovered that higher TFQI levels were consistently associated with the increased risk of OA in the BMI (< 18.5 kg/m2) group after adjusting for different covariates, but not in other BMI groups.
ROC curves for optimal cut-points of sensitivity to thyroid hormone indices
Figure 2 shows that the areas under the curve (AUC) for FT3/FT4, TSHI, TT4RI, and TFQI were 0.563 (95% CI 0.542–0.584), 0.560 (95% CI 0.539–0.581), 0.555 (95% CI 0.533–0.576), 0639 (95% CI 0.619–0.659). TFQI performed better than FT3/FT4, TSHI, TT4RI on ROC analyses for OA prediction. The optimal cut-points of TFQI for OA prediction was 0.450, and sensitivity and specificity of this cutoff point were 0.705 and 0.505, respectively.
Discussion
In this study, we investigated the relationship between sensitivity to thyroid hormone and the prevalence of OA based on the NHANES 2007–2010 data. We demonstrated that both peripheral and central thyroid resistance indices were strongly correlated with the prevalence of OA. The higher levels of FT3/FT4 and lower levels of TSHI, TT4RI, and TFQI were associated with increased OA prevalence. In subgroup analyses stratified by BMI, the strongest relationship between TFQI and OA prevalence was observed in the BMI (< 18.5 kg/m2) group. The innovation of this study is that we used sensitivity to thyroid hormone indices rather than FT3, FT4, and TSH as predictors of bone loss for the first time. We directly linked resistance to thyroid hormone with chondrogenic differentiation, which provides a novel hypothesis for the early screening and treatment of OA.
We observed that higher FT3 and FT4 levels were associated with an increased risk of OA in this study. T4 is generally considered the primary hormone produced and released by the thyroid gland, and most T4 is thought to be converted to T3 by a cytoplasmic deiodination enzyme (D2) before it enters cell nuclei to bind to nuclear receptors and alter gene expression [24]. T3 could stimulate the synthesis of collagen X and the expression of matrix metalloproteinase 13 (MMP-13) and alkaline phosphatase, and promoted the progress of MMP-13, ultimately leading to cartilage mineralization and degradation [25,26,27]. Kim et al. indicated that both hypothyroid and hyperthyroid states were associated with the incidence of knee arthralgia and knee osteoarthritis [10]. According to Devrimsel et al., patients with hypothyroidism had thinner femurs and a higher risk of OA than healthy individuals at all measurement sites [28]. However, a prospective cohort study showed no association between hypothyroidism or hyperthyroidism measured by TSH and the risk of total hip replacement (THR) or total knee replacement (TKR) due to OA [29, 30]. In addition, several meta-analyses have shown that the presence of AITD not only increases the risk of clinically significant thyroid disease, but may also be associated with an increased prevalence of OA, RA, and other musculoskeletal diseases [31, 32]. Contrary to most previous studies, our result showed no relationship between TgAb, TPOAb, and the risk of OA. We considered that the discrepant results of these studies may be related to the different criteria for determining thyroid function and the composition of participants. In addition, TSH, FT4, and FT3 are highly interactive with each other, and there are complex interactions in the HPT axis [32]. Therefore, the measurement of individual parameters might be insufficient to explain the relationship between the thyroid system and chondrocyte differentiation. We began to use composite indices for further evaluation.
Our study observed that levels of sensitivity to thyroid hormone indices (central and peripheral) were associated with the prevalence of OA. The study by Nie et al. demonstrated a positive correlation between FT3/FT4 levels and serum osteocalcin levels, suggesting FT3/FT4 may affect serum osteocalcin levels in healthy thyroid subjects [33, 34]. Osteocalcin has been proposed as a biomarker for OA detection and monitoring. Its biologic function is considered to be related to the inhibition of bone matrix mineralization [35, 36]. In addition, multiple cross-sectional studies have found that FT3/FT4, TSHI, and TT4RI are significantly correlated with chronic kidney disease (CKD), non-alcoholic fatty liver disease (NAFLD), and coronary artery disease (CAD) [37,38,39]. As described in this article, we found that FT3/FT4 levels decreased with increasing risk of OA, whereas TSHI and TT4RI levels increased with increasing OA prevalence in the subjects. Taken all into consideration, these evidences showed the potential role of sensitivity to thyroid hormones in the prediction and evaluation of OA.
A previous study suggested that TFQI is more stable than TSH in evaluating sensitivity to thyroid hormone [20]. The study by Sun et al. demonstrated a close relationship between TFQI and obesity, metabolic syndrome, and diabetes-related mortality [40]. Consistent with these studies, our result showed that elevated TFQI was associated with an increased risk of OA and this positive association was still significant after adjusting for multiple covariates. Therefore, we hypothesized that chondrocyte genesis and differentiation may be related to the central sensitivity to thyroid hormone. In the subgroup analysis stratified by BMI, we found that the higher TFQI levels were consistently associated with a decreased risk of OA in the BMI (< 18.5 kg/m2) group, but not in other BMI groups. This suggested that thyroid hormone resistance may be affected by fat accumulation. A previous study by Zhou et al. found that obese patients had higher TSHI and TFQI levels compared to the control group, and TSHI and TFQI decreased significantly over time after bariatric surgery [41]. In addition, a cross-sectional analysis also showed that the secretory capacity of the thyroid gland (SPINA-GT) and TSHI were significantly negatively related to obesity indices, suggesting that obesity reduces levels of thyroid homeostasis indices [42].
Our study has some strengths. In our research, we used nationally representative NHANES data and combined sample weights for statistical analysis to ensure the authenticity and objectivity of the data. We employed innovative thyroid hormone indices and achieved significant results consistent with previous studies, but there were also some limitations. First, the anti-osteoarthritis treatment history of our participants was absent, which affects the objectivity of the results. In addition, the diagnosis of arthritis was based on patients’ self-report which may lead to bias. Finally, we used a cross-sectional approach in this study, thus we could not collect patient follow-up data to explore the causal relationship between STTH and OA.
Conclusions
Among our representative US population, our present study concluded that TFQI was negatively associated with the risk of OA after multiple adjustments. Therefore, we hypothesized that TFQI could be used as a new predictor of OA, providing help for early screening and preventive of OA.
Availability of data and materials
The survey data are publicly available on the internet for data users and researchers throughout the world http://www.cdc.gov/nchs/nhanes/.
References
Mobasheri A, et al. The role of metabolism in the pathogenesis of osteoarthritis. Nat Rev Rheumatol. 2017;13(5):302–11.
Kolasinski SL, et al. 2019 American college of rheumatology/arthritis foundation guideline for the management of osteoarthritis of the hand, hip, and knee. Arthritis Rheumatol. 2020;72(2):220–33.
Abramoff B, Caldera FE. Osteoarthritis: pathology, diagnosis, and treatment options. Med Clin N Am. 2020;104(2):293–311.
Hunter DJ, March L, Chew M. Osteoarthritis in 2020 and beyond: a lancet commission. Lancet. 2020;396(10264):1711–2.
He M, et al. Progress in the treatment of knee osteoarthritis with high tibial osteotomy: a systematic review. Syst Rev. 2021;10(1):56.
Vina ER, Kwoh CK. Epidemiology of osteoarthritis: literature update. Curr Opin Rheumatol. 2018;30(2):160–7.
Dieppe PA, Lohmander LS. Pathogenesis and management of pain in osteoarthritis. Lancet. 2005;365(9463):965–73.
Yunus M, Nordin A, Kamal H. Pathophysiological perspective of osteoarthritis. Medicina (Kaunas). 2020;56(11):614.
Liu B, et al. Sensitivity to thyroid hormones and risk of prediabetes: a cross-sectional study. Front Endocrinol (Lausanne). 2021;12:657114.
Kim BY, et al. Assessment of the relationship between knee ultrasound and clinical symptoms in patients with thyroid dysfunction. J Int Med Res. 2020;48(1):300060519897701.
Tagoe CE, et al. Rheumatic associations of autoimmune thyroid disease: a systematic review. Clin Rheumatol. 2019;38(7):1801–9.
Litwic A, et al. Epidemiology and burden of osteoarthritis. Br Med Bull. 2013;105:185–99.
Voloshyna L, Doholich O, Sithinska I. Hypothyroidism—a special comorbidity factor in patients with osteoarthrosis: clinical, pathophysiological and prognostic aspects. Georgian Med News. 2017;272:53–9.
Tagoe CE, Zezon A, Khattri S. Rheumatic manifestations of autoimmune thyroid disease: the other autoimmune disease. J Rheumatol. 2012;39(6):1125–9.
Yang S, et al. Thyroid feedback quantile-based index correlates strongly to renal function in euthyroid individuals. Ann Med. 2021;53(1):1945–55.
van der Spek AH, Fliers E, Boelen A. The classic pathways of thyroid hormone metabolism. Mol Cell Endocrinol. 2017;458:29–38.
Jostel A, Ryder WD, Shalet SM. The use of thyroid function tests in the diagnosis of hypopituitarism: definition and evaluation of the TSH Index. Clin Endocrinol (Oxf). 2009;71(4):529–34.
Susiarjo M, et al. Bisphenol a exposure disrupts metabolic health across multiple generations in the mouse. Endocrinology. 2015;156(6):2049–58.
Hoermann R, et al. The role of functional thyroid capacity in pituitary thyroid feedback regulation. Eur J Clin Invest. 2018;48(10):e13003.
Laclaustra M, et al. Impaired sensitivity to thyroid hormones is associated with diabetes and metabolic syndrome. Diabetes Care. 2019;42(2):303–10.
Liu ZM, et al. Increased central and peripheral thyroid resistance indices during the first half of gestation were associated with lowered risk of gestational diabetes-analyses based on huizhou birth cohort in South China. Front Endocrinol (Lausanne). 2022;13:806256.
Stepanek L, et al. Free triiodothyronine/free thyroxine (FT3/FT4) ratio is strongly associated with insulin resistance in euthyroid and hypothyroid adults: a cross-sectional study. Endokrynol Pol. 2021;72(1):8–13.
Nie X, et al. Increased serum adipocyte fatty acid-binding protein levels are associated with decreased sensitivity to thyroid hormones in the euthyroid population. Thyroid. 2020;30(12):1718–23.
Sachs LM, Buchholz DR. Frogs model man: in vivo thyroid hormone signaling during development. Genesis. 2017;55(1–2):e23000.
Varga F, et al. T3 affects expression of collagen I and collagen cross-linking in bone cell cultures. Biochem Biophys Res Commun. 2010;402(2):180–5.
Williams GR. Thyroid hormone actions in cartilage and bone. Eur Thyroid J. 2013;2(1):3–13.
Li L, et al. Triiodothyronine potentiates angiogenesis-related factor expression through PI3K/AKT signaling pathway in human osteoarthritic osteoblasts. Iran J Basic Med Sci. 2020;23(6):819–25.
Devrimsel G, et al. Ultrasonographic evaluation of the femoral cartilage thickness in patients with hypothyroidism. J Phys Ther Sci. 2016;28(8):2249–52.
Bayliss LE, et al. The effect of patient age at intervention on risk of implant revision after total replacement of the hip or knee: a population-based cohort study. Lancet. 2017;389(10077):1424–30.
Chaisson CE, et al. Lack of association between thyroid status and chondrocalcinosis or osteoarthritis: the Framingham osteoarthritis study. J Rheumatol. 1996;23(4):711–5.
Fallahi P, et al. The aggregation between AITD with rheumatologic, or dermatologic, autoimmune diseases. Best Pract Res Clin Endocrinol Metab. 2019;33(6):101372.
Botello A, et al. Prevalence of latent and overt polyautoimmunity in autoimmune thyroid disease: a systematic review and meta-analysis. Clin Endocrinol (Oxf). 2020. https://doi.org/10.1111/cen.14304.
Lai S, et al. Sensitivity to thyroid hormone indices are closely associated with NAFLD. Front Endocrinol (Lausanne). 2021;12:766419.
Nie X, et al. Suppressing effect of free triiodothyronine on the negative association between body mass index and serum osteocalcin levels in euthyroid population. Int J Endocrinol. 2021;2021:6624516.
Neofiti-Papi B, et al. Thyrotoxicosis involves beta2-adrenoceptor signaling to negatively affect microarchitecture and biomechanical properties of the femur. Thyroid. 2019;29(8):1060–72.
Zhou J, et al. Fall prevention and anti-osteoporosis in osteopenia patients of 80 years of age and older: a randomized controlled study. Orthop Surg. 2020;12(3):890–9.
Chen Y, et al. Thyroid parameters and kidney disorder in type 2 diabetes: results from the METAL study. J Diabetes Res. 2020;2020:4798947.
Turker F, et al. Does the FT3-to-FT4 ratio easily predict the progression of NAFLD and NASH cirrhosis? J Int Med Res. 2021;49(11):3000605211056841.
Yu N, et al. The association of thyroid hormones with coronary atherosclerotic severity in euthyroid patients. Horm Metab Res. 2022;54(1):12–9.
Sun Y, et al. Impaired sensitivity to thyroid hormones is associated with hyperuricemia, obesity, and cardiovascular disease risk in subclinical hypothyroidism subjects. Thyroid. 2022. https://doi.org/10.1089/thy.2021.0500.
Zhou Y, et al. Correlation between thyroid homeostasis and obesity in subclinical hypothyroidism: community-based cross-sectional research. Int J Endocrinol. 2021;2021:6663553.
Juiz-Valina P, et al. Central resistance to thyroid hormones in morbidly obese subjects is reversed after bariatric surgery-induced weight loss. J Clin Med. 2020;9(2):359.
Acknowledgements
We thank the staff and the participants of the NHANES study for their valuable contributions.
Funding
This work was supported by the Postgraduate Research and Practice Innovation Program of Jiangsu Province (No. SJCX20_0054), the Fundamental Research Funds of the Central Universities (322402109D) and the Second Affiliated Hospital of Nanjing University of Chinese Medicine (SEZ202107).
Author information
Authors and Affiliations
Contributions
All authors made a significant contribution to the work reported, and agreed to be accountable for all aspects of the work. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. All analyses were based on data of the National Health and Nutrition Examination Survey (NHANES). The study was approved by the ethics review board of the National Center for Health Statistics. The detailed information located on the NHANES website. Written informed consent was obtained from each participant before their inclusion on the NHANES database. Detailed information on the ethics application and written informed consent are provided on the NHANES website.
Consent for publication
All authors agree to publish this work.
Competing interests
Shuai Chen, Xiaohe Sun, Guowei Zhou, Jie Jin and Zhiwei Li declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Chen, S., Sun, X., Zhou, G. et al. Association between sensitivity to thyroid hormone indices and the risk of osteoarthritis: an NHANES study. Eur J Med Res 27, 114 (2022). https://doi.org/10.1186/s40001-022-00749-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40001-022-00749-1