Abstract
Background
Coronary artery calcification (CAC) is a potential risk marker of coronary atherosclerosis that has high specificity and sensitivity. However, the association between high-density lipoprotein cholesterol (HDL-C) concentration and CAC incidence and progression is controversial.
Methods
PubMed, Embase, Web of Science, and Scopus were systematically searched to identify relevant observational studies up to March 2023 and assessed the methodological quality using Newcastle-Ottawa Scale (NOS) scale. Random-effects meta-analysis was used to estimate pooled odds ratios (OR) and 95% confidence interval considering heterogeneity across studies.
Results
Of the 2,411 records, 25 cross-sectional (n = 71,190) and 13 cohort (n = 25,442) studies were included in the systematic review. Ten cross-sectional and eight cohort studies were not eligible and were omitted from the meta-analysis. A total of 15 eligible cross-sectional studies (n = 33,913) were included in the meta-analysis and pooled results revealed no significant association between HDL-C and CAC > 0, CAC > 10, or CAC > 100 [pooled OR: 0.99 (0.97, 1.01)]. Meta-analysis of the 5 eligible prospective cohort studies (n = 10,721) revealed no significant protective effect of high HDL-C against CAC > 0 [pooled OR: 1.02 (0.93, 1.13)].
Conclusions
According to this analysis of observational studies, high HDL-C levels were not found to predict protection against CAC. These results suggest HDL quality rather than HDL quantity is important for certain aspects of atherogenesis and CAC.
Registration number
CRD42021292077.
Similar content being viewed by others
Introduction
Atherosclerotic cardiovascular disease (CVD) is among the greatest cause of morbidity and mortality worldwide. Furthermore, ischemic heart disease (IHD) remains a major cause of CVD. Therefore, identifying the presence of coronary atherosclerosis prior to IHD incidence improves risk stratification and modification [1, 2].
Coronary artery calcification (CAC), a risk marker for atherosclerosis, indicates the presence of IHD, regardless of symptoms or risk factors [3]. CAC has been shown to be correlated with calcium deposition in the arterial wall, a very early stage of atherosclerosis and an established surrogate of the atherosclerotic total burden. Thus, CAC has been used as one of the validated and feasible markers of the presence and extent of subclinical coronary atherosclerosis. The presence of CAC can be detected by non-contrast computed tomography (CT) [4, 5]. High CAC scores (CAC > 0) were demonstrated to be linked with an increased risk of cardiovascular events and mortality. The Framingham Risk Score and other conventional risk stratification techniques have been shown to be inferior to the CAC score in terms of predicting future cardiac events and all-cause mortality [6]. As a result, American and European prevention guidelines recently assigned a class IIa recommendation for the use of CAC to further risk stratify and select individuals at borderline and intermediate risks of CVD events [7]. However, considering costs and radiation with CT, CAC evaluation is not a routine test in many health-centers. So, determining specific CAC risk factors could help develop better approaches to understand who needs to perform determination of CAC.
Several studies have shown that serum level of high-density lipoprotein cholesterol (HDL-C) is a biomarker of CAC. HDL delays the formation of atherosclerotic lesions and calcification by removing the cholesterol from macrophages within the coronary arterial wall and transports it to the hepatic cells [8]. It is postulated that lower HDL-C is associated with the presence and progression of CAC, whereas high HDL-C has a protective effect against CAC [9, 10]. However, several other research studies, failed to find this relationship [11, 12]. In the current systematic review and meta-analysis, we sought to investigate the relationship between HDL-C and the progression and incidence of CAC.
Methods
This meta-analysis was conducted in compliance with the recommendations of the Meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines [13]. The protocol of the present systematic review in the International Prospective Register of Systematic Reviews (PROSPERO) was documented (registration number: CRD42021292077).
Search strategy
Online databases, including PubMed, Embase, Web of Science (ISI), and Scopus were searched systematically until March 2023 without language restriction. To enhance the sensitivity and specificity, a combination of Medical Subject Headings (MESH) and non-MESH words was utilized to capture studies. The following keywords were used: (“Coronary Artery Disease”[Mesh] OR “Coronary Arteriosclerosis” OR “Cardiovascular Diseases”[Mesh] OR “Coronary Heart Disease”) AND (“Lipoproteins, HDL” [Mesh] OR “High Density Lipoprotein” OR HDL OR “Heavy Lipoproteins” OR “High-density lipoprotein cholesterol” OR HDL-C OR “High Density Lipoprotein Cholesterol”) AND (“Coronary Artery calcification” OR “Coronary Artery calcification score” OR “Coronary Artery calcium score” OR “Calcific Coronary Artery Disease” OR “calcific coronary disease”). Supplementary File Appendix 2 contains the entire search strategy. We also manually searched the reference lists of all retrieved articles and Google Scholar to identify any overlooked relevant publications.
Study selection and eligibility criteria
Three authors (FA, SS, NO) independently screened manuscripts to identify eligible studies and all manuscripts were included based on consensus among all authors. According to the PECOT (Population, Exposure, Comparison, Outcome, Type of study) template, the study inclusion criteria included (P) human samples, (E and C) examining the association of low or high levels of HDL-C, (O) on CAC score, (T) in observational studies (cohort, case-control, or cross-sectional). The following studies were excluded: studies with absence of HDL-C or its adjusted association with CAC, duplicate samples (studies based on the same sample/population), review articles, editorials, clinical guidelines, personal opinions, book chapters, conference abstracts, case reports, genetic studies, animal studies, and studies focusing on diseases other than coronary artery disease.
Data extraction and quality assessment
Data were independently extracted from included studies by three authors (FA, SS, NO) based on consensus among all authors. The following data was extracted: first authors, publication years, data source, study types, participants information (gender, age of patients, geographical location, sample size, and basic diseases), outcome definition, outcome measures including effect sizes and risk estimates (Odds Ratios) with their confidence intervals, stratification based on controlled variable in the multivariable model, duration of follow-up (for cohort studies), and quality assessment.
The methodological quality of evidence of each study was assessed using the Newcastle-Ottawa Scale (NOS) as previously established based on stringent criteria related to the 4 domains of selection, comparability, exposure (cross-sectional studies) or outcome (cohort studies) [14]. In general, an article score ≥ 7, is considered to be of good quality [14].
Statistical analysis
The observed relationship between HDL-C and CAC was estimated using Odds Ratios (ORs) as the effect size. Random effects meta-analysis was conducted to obtain the pooled OR and its 95% confidence intervals using the Der-Simonian and Laird method. A random-effects meta-analysis was used to account for conceptual and clinical heterogeneity between studies with a forest plot to demonstrate the ORs and respective 95% confidence intervals.
To assess study heterogeneity, the I2 statistic (I2 ≥ 50% indicates substantial heterogeneity) was utilized. Also, Cochran’s Q statistic was utilized with a significance level of P < 0.10 to indicate the variance among studies.
Sensitivity analysis with serial removal of a specific study or group of studies assessed the robustness of the pooled results. A subgroup meta-analysis on the association between HDL-C and CAC was conducted by HDL-C measurement scale (mg/dl vs. per 1 standard deviation increase).
To assess the publication bias, visual inspection of funnel plots was performed, so as log ORs were plotted against their standard errors (as study precision). Also, the Egger’s regression asymmetry test and Begg’s adjusted rank correlation test were performed. Statistical tests were two-tailed and significance levels were considered less than 0.10 for analyses. All statistical analyses were performed using the Stata version 14 software (Stata Corp., College Station, TX, USA).
Results
Search results
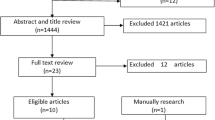
The flowchart of literature search and selection process is presented in Fig. 1. Using systematic database searching, 2,403 potentially relevant publications were identified in the first evaluation, as well as eight studies, through a manual search of the reference lists of these papers. Subsequently, 1,141 duplicates and 1,109 irrelevant articles after screening titles and abstracts. The full texts of 161 potentially relevant publications were evaluated to determine if they met the eligibility criteria. Of these, 123 publications were excluded for the reasons mentioned in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram. Finally, 38 articles met the eligibility criteria and were involved in the qualitative synthesis (systematic review) [9,10,11,12, 15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48] and 20 were included in the quantitative synthesis (meta-analysis) [9, 11, 12, 16, 20, 22,23,24, 26,27,28, 32, 34, 35, 39,40,41, 45, 47, 48].
Association between HDL-C and CAC in cross-sectional studies
Study characteristics
Twenty-five included cross-sectional studies [9, 11, 12, 15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35, 47] enrolled 71,190 participants and the sample size ranged from 104 to 16,493 participants. Populations varied in sex distribution and age, and also had mean or median HDL-C levels. Nineteen studies included both genders [9, 11, 12, 15,16,17,18,19,20,21,22, 25, 26, 28,29,30,31,32, 47], four included only men [23, 24, 27, 33], and two included only women [34, 35]. The average age of study participants varied (22–94 years). Of the 25 included cross-sectional studies, 14 were conducted in America [9, 11, 15,16,17,18, 21, 22, 25, 29,30,31, 34, 35], one in Europe [19], and ten in Asia [12, 20, 23, 24, 26,27,28, 32, 33, 47]. The sources of the three studies were the Multiethnic Study of Atherosclerosis (MESA) [15, 30, 31], three were from the Brazilian Longitudinal Study of Adult Health (ELSA-BRASIL) [9, 11, 22], three were from Shiga Epidemiological Study of Subclinical Atherosclerosis (SESSA) [23, 24, 27], and two studies were from the Study of Women’s Health Across the Nation (SWAN) [34, 35]. One study was a cross-sectional study of both MESA and ELSA-BRASIL [16], and four other studies were based on the Brazilian Study on Healthy Aging [21], Utrecht Patient Oriented Database (UPOD) [19], Study of Inherited Risk of Coronary Atherosclerosis (SIRCA) [29], and Mediators of Atherosclerosis in South Asians Living in America (MASALA) cohorts. All studies were published in English in the last 17 years (2005–2022). Nineteen studies reported an OR estimate for CAC, adjusted at least for age and sex [9, 11, 12, 16, 18, 20,21,22,23,24, 26,27,28, 31,32,33,34,35, 47]. In six other studies, the association between HDL-C and CAC was reported as relative risk (RR) [30], prevalence ratio (PR) [15], incident rate ratio (IRR) [25], coefficient β [17, 19], or tobit regression [29]. Many studies also adjusted for cardiovascular risk factors and other confounding factors. The presence of CAC was defined as a score greater than 0, 1, 10, or 100. Seventeen studies analyzed HDL-C as a quantitative variable of mg/dL [9, 12, 15, 17, 18, 20, 21, 23, 24, 28, 30,31,32,33,34,35, 47], two studies as mmol/L [19, 25] and six studies interpreted HDL-C in terms of one standard deviation increase [11, 16, 22, 26, 27, 29]. All included studies were assessed as having moderate to good quality according to the NOS scale (Supplementary Appendix 1). Details of each study are presented in Table 1.
Systematic review findings
Eleven cross-sectional studies reported an inverse relationship between HDL-C levels and CAC incidence [9, 15, 18, 21, 24,25,26, 29, 31,32,33]. In other words, higher HDL-C serum levels had a protective role against the presence of CAC. In a cross-sectional of MESA cohort, low HDL-C was associated with higher rates of multivessel CAC (PR: 1.20 (1.02, 1.40), < 0.01) [15]. In another cross-sectional study of the MESA cohort, HDL-C was associated with CAC presence (OR: 0.892 (0.854, 0.931), < 0.001) but not with CAC extent (OR: 0.006 (-0.038, 0.05), 0.791) [31]. In a cross-sectional study of the ELSA-Brasil cohort, HDL-C was associated with CAC ≥ 1 (OR: 0.99 (0.98, 0.99), ≤ 0.05) [9]. HDL-C level was associated with CAC > 0 (OR: 0.56 (0.34, 0.91)) in a cross-sectional study of the SESSA cohort [24]. In a cross-sectional study of the SIRCA cohort, HDL-C was inversely associated with CAC after adjusting for age and sex (tobit ratio: 0.72 (0.59, 0.88), 0.001), but after further adjustment for medications, blood pressure, lipids, tobacco and alcohol use, exercise, family history of premature IHD, body mass index (BMI), waist circumference, and high-sensitivity CRP got insignificant [29]. In a cross-sectional study of the Brazilian Study on Healthy Aging cohort in individuals aged 80 years or over, the association between HDL-C and CAC was significant (OR: 0.34 (0.15, 0.75), 0.008) [21]. In another cross-sectional study, individuals with low HDL-C had higher CAC scores and the adjusted correlation of HDL-C and CAC was significant in both men and women (OR: 0.92 (0.89, 0.95) and 0.93 (0.90, 0.96), respectively) [18]. HDL-C decreased the risk of CAC (IRR: − 0.04 (–0.07, 0.00), 0.0474) [25], and it was also associated with CAC in three other cross-sectional studies; (OR: 0.87 (0.82, 0.93), < 0.05) [26], (OR: 0.93 (0.87, 0.99), ≤ 0.0001) [32], and (OR: 0.78 (0.64, 0.94), 0.01) [33].
However, 11 studies failed to demonstrate the protective role of HDL-C against CAC incidence [11, 12, 16, 19, 20, 22, 23, 27, 28, 30, 47]. HDL-C level was not associated with CAC in a cross-sectional study of the MESA cohort (RR: 1.05 (0.98, 1.12)) [30]. HDL-C was also not independently associated with the presence or extent of CAC in a cross-sectionals of ELSA-Brasil cohort (OR: 1.041 (0.933, 1.161), and 0.940 (0.800, 1.105), respectively) [22]. In another cross-sectional study of ELSA-Brasil, lower HDL-C was not associated with CAC (OR: 1.02 (0.93, 1.13), 0.46) [11]. In a cross-sectional of MESA and ELSA-Brasil, adjusted prevalence OR for the association of HDL-C and CAC > 0 was insignificant (0.80 (0.60, 1.07)) [16]. Two cross-sectional studies of SESSA cohort also failed to demonstrate significant association between HDL-C and CAC; (OR: 1.03 (0.86, 1.22), 0.72) [23] and (OR: 0.98 (0.8, 1.2), < 0.955) [27]. In a cross-sectional study of UPOD, HDL-C was not associated with CAC (β coefficient: -0.05 (-0.17, 0.14), 0.559) [19]. The association between HDL-C and CAC was also insignificant in four other studies (OR: 0.98 (0.96, 1)) [12], (OR: 0.59 (0.27, 1.29) 0.49) [20], (OR: 0.99 (0.97, 1), < 0.094) [28], and (OR: 1.25 (0.97, 1.61), 0.09) [47].
In a recent cross-sectional of MASALA, HDL-C was directly associated with CAC density (β coefficient: 0.009 (0.001, 0.016)) but the reverse association between HDL-C and CAC volume was insignificant (β coefficient: -0.004 (-0.009, 0.001)) [17]. It is suggested that higher HDL-C probably results in denser coronary plaques that are more stable and therefore less likely to rupture.
Two cross-sectional studies investigated the relationship between HDL-C and CAC in menopausal women. In a cross-sectionals study of SWAN cohort, it is revealed that HDL-C is not protective against high CAC or any left main CAC in premenopausal or early perimenopausal (OR: 0.99 (0.95, 1.03) and 1.01 (0.96, 1.05), respectively). Moreover, in the late-perimenopausal or postmenopausal group, HDL-C was not protective against high CAC (0.99 (0.96, 1.02)), but was protective against left main CAC (1.08 (1.00, 1.16)) [35]. In a recent cross-sectional study of the SWAN cohort there was no association between HDL-C and CAC > 10 (OR: 1.04 (0.95, 1.12), 0.4) [34].
Meta-analysis findings
Ten cross-sectional studies were omitted from the meta-analysis because their data were not eligible for quantitative synthesis [15, 17,18,19, 21, 25, 29,30,31, 33]. Briefly, they have different statistic methodology for reporting association between HDL-C and CAC [15, 19, 25, 29, 31], different analyzing HDL-C variables [17, 18, 30, 33] and one study had included only elderly individuals aged ˃80 years [21].
Fifteen cross-sectional studies were eligible for the meta-analysis [9, 11, 12, 16, 20, 22,23,24, 26,27,28, 32, 34, 35, 47]. Ten studies analyzed HDL-C as a quantitative variable (mg/dL) [9, 12, 20, 23, 24, 28, 32, 34, 35, 47] and five studies measured and interpreted it in terms of one standard deviation increase [11, 16, 22, 26, 27]. The Pooled results revealed no significant association between HDL-C and CAC > 0, CAC > 10, or CAC > 100 (OR: 0.99 (0.97, 1.01)) as illustrated in Fig. 2.
Meta-analysis for cross-sectional studies
Forest plot of the association between HDL and CAC separated by HDL (mg/dl vs. per 1 standard deviation increase) and CAC measurement scale. Diamond represents the summary odds ratio (pooled OR) estimate and its width shows corresponding 95% CI with random effects estimate. The size of the square and its central point reflects the study specific statistical weight (inverse of variance) and point estimate of the OR and horizontal line reflects corresponding 95% CI of the study. I2 test and Cochran’s Q statistic were used to assessing the statistical heterogeneity (P < 0.10) across studies
Association between HDL-C and CAC in cohort studies
Study characteristics
Thirteen cohort studies [10, 36,37,38,39,40,41,42,43,44,45,46, 48] enrolled 25,442 participants with sample sizes ranging from 21 to 6,011 participants. The duration of follow-up was between 5 and 20 years. Populations varied in sex distribution and age, and also had mean or median HDL-C levels. Eleven cohorts included both genders [10, 36,37,38, 40, 41, 43,44,45,46, 48] and two included only women [39, 42]. The average age of the participants ranged from 29 to 84 years old. Of the 12 included cohort studies, ten were conducted in America [10, 36, 37, 39, 41,42,43,44, 46, 48], two in Europe [38, 40], and one in Asia [45]. All studies were published in English in the last 26 years (1996–2022). Seven studies reported an OR estimate for CAC adjusted at least for age and sex [36, 39,40,41,42,43,44]. In six other studies, the association between HDL-C and CAC was reported as hazard ratio (HR) [45, 48], RR [10], IRR [38], standardized β [37], or baseline and change in CAC [46]. Many studies also adjusted for cardiovascular risk factors and other confounding factors. The incidence, progression, and density of CAC were investigated in these studies. Eleven studies analyzed HDL-C as a quantitative variable (mg/dL) [10, 37, 39,40,41,42,43,44,45,46, 48], one study as mmol/L [38], and one study had interpreted HDL-C as one standard deviation increase [36]. All included studies were good quality according to the NOS scale (Supplementary Appendix 1). Details of each study are presented in Table 2.
Systematic review findings
In a Muscatine study cohort, decreased HDL-C measured during young adult life was associated with the presence of CAC in young adults (OR: 5.5 (2, 15.2), < 0.001) [43]. In the National Cholesterol Education Program, there was an association between higher HDL-C (> 60 mg/dl) and less progression of CAC volume (change in volume score: 203 (-52, 2,828) for HDL-C ≥ 60, 159 (-123, 3,872) for 40 < HDL-C ≤ 59, and 151 (0–2, 213) for HDL-C ≤ 40; P = 0.03) [46]. In another study, among subjects with a zero CAC score at baseline, there was an independent association between HDL-C and CAC progression (HR: 0.976 (0.953, 0.999), 0.043) [45]. In ELSA-Brasil cohort, lower HDL-C was associated with CAC incidence (OR: 0.83 (0.72, 0.96), 0.01) but not CAC progression (OR: 0.89 (0.74, 1.06), 0.19) [36]. However, in a recent study of MESA, HDL-C was associated with both a low risk of incident CAC development (RR: 0.92 (0.89, 0.96), < 0.001) and lower annual CAC progression (difference in average progression β: -0.92 (-1.74, -0.1), < 0.027) [10].
In the Coronary Artery Risk Development in Young Adults (CARDIA) cohort, non-optimal HDL-C during young adulthood was associated with CAC two decades later. HDL was independently associated with CAC in this study (OR for average exposure to HDL-C before age 35: 2.8 (1.1, 6.8), 0.03) [44]. In contrast, in a recent CARDIA cohort, there was no association between HDL-C and CAC progression (OR: 0.83 (0.647, 1.065), 0.143) [41]. Another recent cohort of both MESA and CARDIA showed that age might influence the relationship between HDL-C level and CAC. The results revealed a significant association between HDL-C and CAC incidence in middle-aged people between 46 and 64 years old (HR: 1.23 (1.12, 1.34)), but the association was insignificant in young individuals between 32 and 45 (HR: 1.07 (0.96, 1.19)) and older individuals between 65 and 84 years old (HR: 1.10 (0.97, 1.25)) [48].
A few cohorts also reported no association between HDL-C and CAC. In the Dallas Heart Study, HDL-C was not associated with prevalent CAC in adjusted models (P = 0.13) [37]. In Heinz Nixdorf Recall Study, HDL-C was not associated with CAC progression in either men or women (OR: 0.93 (0.7, 1.29) and 1.00 (0.78, 1.20), respectively) [40]. In another cohort, there was no association between HDL-C and the incidence (IRR: 0.84 (0.46, 1.54), 0.60) or progression of CAC (IRR: 0.94 (0.60, 1.48), 0.80) [38].
Two cohort studies investigated the relationship between HDL-C and CAC in menopausal women. In Healthy Women Study, HDL-C was associated with CAC progression in postmenopausal women. Adjusted OR for 10 mg/dL increase of HDL-C was 0.53 (0.03, 0.93) in women with CAC ≥ 101 [42]. However, in the SWAN cohort, there was no association between HDL-C and the progression of CAC after menopause (OR: 0.78 (0.51, 1.19), 0.60) or CAC density (OR: -1.48 (-10.76, 8.88), 0.77) in women transitioning through menopause [39].
Meta-analysis findings
Eight cohorts were omitted from meta-analysis as their data were not eligible for quantitative synthesis [10, 36,37,38, 42,43,44, 46]. They have different statistic methodology for reporting association between HDL-C and CAC [37, 38, 46], different analyzing HDL-C variables [10, 42,43,44] and one study had included patients with CAC > 0 at initiation of cohort [36]. Five cohorts were eligible for meta-analysis [39,40,41, 45, 48]. The pooled results revealed no significant association between HDL-C and CAC (OR: 1.02 (0.93, 1.13)) as illustrated in Fig. 3. A cumulative forest plot was also created (Fig. 4).
Meta-analysis for cohort studies
Forest plot of five cohort studies that investigated the association between HDL-C and CAC. Diamond represents the summary odds ratio (pooled OR) estimate and its width shows corresponding 95% CI with random effects estimate. I2 test and Cochran’s Q statistic were used to assessing the statistical heterogeneity (P < 0.10) across studies
Sensitivity analysis and publication bias
In cross-sectional studies, sensitivity analysis separated by subgroups was done and revealed that meta-analysis model was robust. Also, sensitivity analysis for cohort studies showed that the association between HDL-C and CAC was consistent (range of summary ORs: 0.99–1.04), indicating that the meta-analysis model was robust (Supplementary Appendix 3). To assess possible publication bias, the association between CAC and HDL-C are presented in a funnel plot (Fig. 5). There was no evidence of publication bias by visual inspection and assessment of statistical tests (P = 0.25, for Begg’s adjusted rank correlation test and P = 0.99 for Egger’s regression asymmetry test) (Supplementary Appendix 4).
Discussion
In this study, 25 cross-sectional (n = 71,190) and 13 cohort (n = 25,442) studies were systematically reviewed to characterize the link between CAC and HDL-C. Several studies have reported an independent inverse relationship between serum HDL-C and CAC incidence and progression. However, many other studies failed to reveal a statistically significant relationship between HDL-C and CAC incidence after adjustment for confounding factors. A meta-analysis of 15 eligible cross-sectionals (n = 33,913) and five eligible cohort studies (n = 10,721) demonstrated an insignificant association between HDL-C and CAC. Subsequently, HDL-C blood level might not be considered an independent risk factor for CAC.
Similarly, although previous epidemiological studies have been extensively demonstrated the inverse relationship between HDL-C plasma levels and IHD with a strong, graded and coherent pattern [49, 50], Mendelian randomization studies then revealed no causal association between HDL-C and the pathogenesis of IHD [51]. In a recent cohort study of 15.8 million Korean adults, both elevated and low HDL-C were associated with elevated mortality from CVD, and high HDL-C serum concentration was not necessarily a sign of better cardiovascular health [52]. Moreover, pharmacological increase in HDL-C with drugs such as fibrates, niacin, or cholesteryl ester transfer protein inhibitors failed to reduce IHD in several clinical trials [53]. Therefore, the HDL-C level is now considered a biomarker of cardiovascular health rather than a risk factor. This controversy might be the result of crude measurement of the total cholesterol content in HDL, while the entity of HDL is characterized by its structure and function [54, 55]. For instance, our previous studies demonstrated that increased HDL lipid peroxidation, which impairs the antioxidant function of HDL, is positively associated with cardiovascular events in the MASHAD cohort [56,57,58,59]. It is obvious that a simple measurement of cholesterol carried by HDL particles does not reflect HDL functionality or composition in the prediction or prevention of IHD. HDL subfractions can be classified according to their size, shape, charge, density, functionality, and biochemical composition. Although HDL-C is the only reproducible and standardized parameter available to estimate plasma concentration of HDL, there was an association between increased levels of small HDL and low proportions of large particles with an increased risk of coronary artery disease [60]. Hence, measurement of specific HDL subfractions would be a better biomarker than HDL-C level to evaluate the risk of IHD [61]. The same is true regarding the association between CAC and HDL-C levels. According to the results of the present meta-analysis, no clinically significant correlations were observed between HDL-C plasma levels and incidence or progression of CAC.
Interestingly, some observational clinical studies have indicated an association between different HDL subfractions and CAC. In cohort of Dallas Heart Study (9.3 years), HDL-particle (HDL-P) were inversely associated with prevalent CAC in fully adjusted models, including risk factors and HDL-C (standardized β= −0.06, P = 0.009). Furthermore, HDL-C was only associated with prevalent CAC after serial adjustment for HDL-P (standardized β = 0.07, P = 0.008) [37]. In a cross-sectional study, HDL-P and medium size HDL-P were protective against CAC (OR: 0.42 (0.22, 0.79), 0.002 and 0.36 (0.19, 0.69), 0.006, respectively), while large HDL-P and average size HDL-P were not (OR: 0.77(0.33, 1.83), 0.29 and 0.72(0.35, 1.48), 0.58, respectively) [20]. While high HDL-P was significantly negatively linked with CAC progress in the MESA cohort (9.6 0.6 years), this association was diminished when conventional lipids were taken into account. Low HDL-P levels were not linked to the development of CAC [62]. Cross-sectional SESSA demonstrated impaired antiatherogenic function of HDL in correlation with the binding capacity of dysfunctional HDL to lectin-like oxidized LDL receptor-1 (LOX-1). The adjusted OR of HDL-P for CAC was not significant in this study (0.92 (0.78, 1.08), 0.33) [23]. In another cross-sectional of SESSA, HDL-P concentrations and size also were not in association with the presence of CAC (OR 1.04 (0.62, 1.75) and 0.66 (0.40, 1.10), respectively) [24]. Similar results were obtained in a cross-sectional study by Mahajan et al. on age-adjusted PR of HDL-P concentrations and size with CAC [63].
HDL size is also a determinant of the anti-atherogenic properties of HDL. In a cross-sectional study of the Baptist Employee Healthy Heart Study (BEHHS) randomized trial, small HDL and large HDL offered modest protection against CAC (OR: 0.92 (0.89, 0.99) and 0.89 (0.83, 0.95), respectively) [64]. In another cross-sectional of Healthy Women Study, large HDL was inversely associated with CAC, but small HDL was not [65]. In a cross-sectional of MESA, the presence of proinflammatory protein apolipoprotein C-III on HDL was positively associated with CAC, whereas HDL lacking apolipoprotein C-III was inversely associated with CAC [66]. In some studies, HDL-C subclass 2 (HDL2-C), which is composed only of apolipoprotein A-I was more anti-atherogenic than subclass 3 (HDL3-C) which contains both apolipoprotein A-I and apolipoprotein A-II. In cohort of Healthy Women Study, the level of HDL2-C was strongly and inversely related to CAC (r= -0.31, < 0.001) and was much stronger in comparison with HDL3-C [42]. Decrease of HDL2-C was significantly associated with the increase of CAC prevalence and extent (OR: 3.45 (2.03, 50.1)), while in a cross-sectional of a preventive cardiology outpatient program cohort, HDL3-C was not (OR: 1.33 (0.07, 26.9)) [67]. But in a cross-sectional of ELSA-Brasil, neither HDL2-C and HDL3-C nor HDL2-C/HDL3-C ratio were independently associated with the presence or extent of CAC after adjustment for epidemiological variables and traditional CVD risk factors [22]. In cohort of SWAN HDL study (before and after menopause), longitudinal associations of the adjusted HDL metrics (total HDL-P, large HDL-P, medium HDL-P, small HDL-P, HDL size, HDL-phospholipid, HDL-triglyceride, HDL-C efflux capacity) with CAC incidence and density were investigated. CAC incidence was only associated with medium HDL-P (OR: 1.46 (1.12, 1.90), 0.006) and small HDL-P (OR: 0.76 (0.58, 1.01), 0.05). CAC density was not associated with any HDL metric in the adjusted model [39]. The HDL content of triglyceride, phospholipid, total cholesterol, and esterified cholesterol of different HDL subclasses were also evaluated in a cross-sectional of Genetics of Atherosclerosis Disease study. The findings showed that HDL subclasses might be CAC markers, however, they do not support an association between lipoproteins and CAC scores [68]. Thus, there is a controversy between the results of HDL particles and subfractions with CAC. This is probably due to the absence of a unique gold standard to measure the functional and physical characteristics of HDL subfractions among studies. Lack of standard and easily applicable methods to analyze and detect HDL particles and subfractions, limits their clinical usefulness for the assessment of cardiovascular risk. Therefore, further studies are needed to fully understand the impact of HDL subfractions and HDL-C in atherosclerotic CVD risk stratification in para clinics and different populations [69].
Strengths and limitations
This study had several strengths. First, this is the first systematic review and meta-analysis of HDL-C concentration and CAC in the literature. Second, it has replicable and extensive methods for searching the published literature. Third, a large sample size study was conducted, including 91,160 individuals from a wide region of Asia, Europe, and America in a cohort or cross-sectional design. Thus, a conclusive result with low bias and high precision was obtained for the general population in the current study. Finally, in addition to resolving the controversy between existing studies on the association between HDL-C level and CAC, this article reveals that HDL-C fails to predict the risk of CAC and should not be used as a biomarker or risk factor for CAC measurement and screening.
The present systematic review and meta-analysis has limitations. First, the included population were a wide range of healthy adults at baseline or a random sample of society with different ages and races. So, the heterogeneity among patients may affect how CAC is found to be linked with HDL-C. Moreover, 35 of the 38 studies were conducted in America and Asia, so the results of this meta-analysis may not be applied to other continents where different lifestyles and races would affect the association. Thus, it is uncertain whether the results are applicable to other ethnicities. Second, HDL-C levels and CAC measurements reported in each included study were measured at different laboratory centers using different assay methods, equipment and experimental kits. In addition, different cutoffs for HDL-C and CAC scores have been proposed. This may have caused inconsistencies in data interpretation. Third, most of the studies included in the meta-analysis were cross-sectional in design and temporal or causal relationships between HDL-C and CAC incidence cannot be determined. Only four cohorts were eligible for the meta-analysis and one study included only midlife women. Therefore, robust subgroup analysis was not feasible. Fourth, although multivariable adjustment was conducted in all included studies, they were not justified for identical confounding factors and may lead to discrepancies. However, we selected studies in meta-analysis that reported an estimate of OR for CAC adjusted at least for age and sex. In addition, all included studies are still subjected to bias, because many unexpected and unknown confounding factors may exist. Finally, as mentioned above, all included studies in meta-analysis measured HDL-C content but did not represent the functionality of HDL in preventing CAC and atherosclerosis.
Conclusions
The present meta-analysis findings indicate that high HDL-C levels have no significant protective effects against CAC in cohort and cross-sectional studies. Accordingly, this analysis did not reveal a major role for HDL-C level in CAC. As a result, HDL-C concentration cannot be used as a predictor or risk factor to estimate the need for CAC measurement and screening. One reason might be that crude measurement of total cholesterol content in HDL, such as HDL-C, does not represent the structure and function of HDL. This finding supports the concept that HDL quality, rather than quantity, is more important for certain aspects of atherogenesis and CAC. Standard measurement of HDL particles and subfractions and its association with CAC should be investigated in future large-scale prospective research to confirm these findings.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, Barengo NC, Beaton AZ, Benjamin EJ, Benziger CP. Global burden of cardiovascular diseases and risk factors, 1990–2019: update from the GBD 2019 study. J Am Coll Cardiol. 2020;76:2982–3021.
Herrington W, Lacey B, Sherliker P, Armitage J, Lewington S. Epidemiology of atherosclerosis and the potential to reduce the global burden of atherothrombotic disease. Circul Res. 2016;118:535–46.
Mori H, Torii S, Kutyna M, Sakamoto A, Finn AV, Virmani R. Coronary artery calcification and its progression: what does it really mean? JACC: Cardiovasc Imaging. 2018;11:127–42.
Greenland P, Blaha MJ, Budoff MJ, Erbel R, Watson KE. Coronary calcium score and cardiovascular risk. J Am Coll Cardiol. 2018;72:434–47.
Shemesh J. Coronary artery calcification in clinical practice: what we have learned and why should it routinely be reported on chest CT? Annals of Translational Medicine 2016, 4.
Zeb I, Budoff M. Coronary artery calcium screening: does it perform better than other cardiovascular risk stratification tools? Int J Mol Sci. 2015;16:6606–20.
Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, Himmelfarb CD, Khera A, Lloyd-Jones D, McEvoy JW. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;74:e177–e232.
Rye K-A, Bursill CA, Lambert G, Tabet F, Barter PJ. The metabolism and anti-atherogenic properties of HDL. J Lipid Res. 2009;50:195–S200.
Pedrosa JF, Ribeiro ALP, Santana PC, Araújo LF, Barreto SM. Relation of thoracic aortic and coronary artery calcium to Cardiovascular Risk factors (from the brazilian longitudinal study of Adult Health [ELSA-Brazil]). Am J Cardiol. 2019;124:1655–61.
Zeb I, Jorgensen NW, Blumenthal RS, Burke GL, Lloyd-Jones D, Blaha MJ, Wong ND, Nasir K, Budoff MJ. Association of inflammatory markers and lipoprotein particle subclasses with progression of coronary artery calcium: the multi-ethnic study of atherosclerosis. Atherosclerosis. 2021;339:27–34.
Bittencourt MS, Santos RD, Staniak H, Sharovsky R, Kondapally R, Vallejo-Vaz AJ, Ray KK, Bensenor I, Lotufo P. Relation of fasting triglyceride-rich lipoprotein cholesterol to coronary artery calcium score (from the ELSA-Brasil Study). Am J Cardiol. 2017;119:1352–8.
Chiu TY, Chen CY, Chen SY, Soon CC, Chen JW. Indicators associated with coronary atherosclerosis in metabolic syndrome. Clin Chim Acta. 2012;413:226–31.
Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA. 2000;283:2008–12.
Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–5.
Abd Alamir M, Goyfman M, Chaus A, Dabbous F, Tamura L, Sandfort V, Brown A, Budoff M. The Correlation of Dyslipidemia with the Extent of Coronary Artery Disease in the Multiethnic Study of Atherosclerosis. Journal of Lipids 2018, 2018.
Al Rifai M, Greenland P, Blaha MJ, Michos ED, Nasir K, Miedema MD, Yeboah J, Sandfort V, Frazier-Wood AC, Shea S, et al. Factors of health in the protection against death and cardiovascular disease among adults with subclinical atherosclerosis. Am Heart J. 2018;198:180–8.
Al Rifai M, Kanaya AM, Kandula NR, Patel J, Al-Mallah MH, Budoff M, Cainzos-Achirica M, Criqui MH, Virani SS. Association of coronary artery calcium density and volume with predicted atherosclerotic cardiovascular disease risk and cardiometabolic risk factors in South Asians: the Mediators of atherosclerosis in South Asians living in America (MASALA) study. Curr Probl Cardiol 2022:101105.
Allison MA, Wright CM. Age and gender are the strongest clinical correlates of prevalent coronary calcification (R1). Int J Cardiol. 2005;98:325–30.
den Harder AM, de Jong PA, de Groot MCH, Wolterink JM, Budde RPJ, Isgum I, van Solinge WW, ten Berg MJ, Lutgens E, Veldhuis WB, et al. Commonly available hematological biomarkers are associated with the extent of coronary calcifications. Atherosclerosis. 2018;275:166–73.
Ditah C, Otvos J, Nassar H, Shaham D, Sinnreich R, Kark JD. Small and medium sized HDL particles are protectively associated with coronary calcification in a cross-sectional population-based sample. Atherosclerosis. 2016;251:124–31.
Freitas WM, Quaglia LA, Santos SN, de Paula RC, Santos RD, Blaha M, Rivera JJ, Cury R, Blumenthal R, Nadruz-Junior W, et al. Low HDL cholesterol but not high LDL cholesterol is independently associated with subclinical coronary atherosclerosis in healthy octogenarians. Aging Clin Exp Res. 2015;27:61–7.
Generoso G, Bensenor IM, Santos RD, Staniak HL, Sharovsky R, Santos IS, Goulart AC, Jones SR, Kulkarni KR, Blaha MJ, et al. High-density lipoprotein-cholesterol subfractions and coronary artery calcium: the ELSA-Brasil Study. Arch Med Res. 2019;50:362–7.
Hirata A, Kakino A, Okamura T, Usami Y, Fujita Y, Kadota A, Fujiyoshi A, Hisamatsu T, Kondo K, Segawa H, et al. The relationship between serum levels of LOX-1 ligand containing ApoAI as a novel marker of dysfunctional HDL and coronary artery calcification in middle-aged japanese men. Atherosclerosis. 2020;313:20–5.
Hisamatsu T, Fujiyoshi A, Miura K, Ohkubo T, Kadota A, Kadowaki S, Kadowaki T, Yamamoto T, Miyagawa N, Zaid M, et al. Lipoprotein particle profiles compared with standard lipids in association with coronary artery calcification in the general japanese population. Atherosclerosis. 2014;236:237–43.
Kaplan H, Thompson RC, Trumble BC, Wann LS, Allam AH, Beheim B, Frohlich B, Sutherland ML, Sutherland JD, Stieglitz J, et al. Coronary atherosclerosis in indigenous south american tsimane: a cross-sectional cohort study. Lancet. 2017;389:1730–9.
Kim JD, Hwang YC, Ahn HY, Park CY. Validation of a newly developed equation for estimating serum apolipoprotein B: Associations with Cardiovascular Disease surrogate markers in Koreans. Yonsei Med J. 2017;58:975–80.
Kimani C, Kadota A, Miura K, Fujiyoshi A, Zaid M, Kadowaki S, Hisamatsu T, Arima H, Horie M, Ueshima H. Differences between coronary artery calcification and aortic artery calcification in relation to cardiovascular disease risk factors in japanese men. J Atheroscler Thromb. 2019;26:452–64.
Lee J, Lim JS, Chu Y, Lee CH, Ryu OH, Choi HH, Park YS, Kim C. Prediction of coronary artery calcium score using machine learning in a healthy population. J Personalized Med. 2020;10:1–10.
Martin SS, Qasim AN, Wolfe M, St Clair C, Schwartz S, Iqbal N, Schutta M, Bagheri R, Mehta NN, Rader DJ, Reilly MP. Comparison of high-density lipoprotein cholesterol to apolipoprotein A-I and A-II to Predict Coronary Calcium and the effect of insulin resistance. Am J Cardiol. 2011;107:393–8.
Paramsothy P, Knopp RH, Bertoni AG, Blumenthal RS, Wasserman BA, Tsai MY, Rue T, Wong ND, Heckbert SR. Association of Combinations of lipid parameters with carotid intima-media thickness and coronary artery calcium in the MESA (multi-ethnic study of atherosclerosis). J Am Coll Cardiol. 2010;56:1034–41.
Pletcher MJ, Sibley CT, Pignone M, Vittinghoff E, Greenland P. Interpretation of the coronary artery calcium score in combination with conventional cardiovascular risk factors: the multi-ethnic study of atherosclerosis (MESA). Circulation. 2013;128:1076–84.
Sharma A, Kasim M, Joshi PH, Qian Z, Krivitsky E, Akram K, Rinehart S, Vazquez G, Miller J, Rohman MS, Voros S. Abnormal lipoprotein(a) levels predict coronary artery calcification in southeast asians but not in caucasians: Use of noninvasive imaging for evaluation of an emerging risk factor. J Cardiovasc Transl Res. 2011;4:470–6.
Sung KC, Wild SH, Byrne CD. Controlling for apolipoprotein A-I concentrations changes the inverse direction of the relationship between high HDL-C concentration and a measure of pre-clinical atherosclerosis. Atherosclerosis. 2013;231:181–6.
Swabe G, Matthews K, Brooks M, Janssen I, Wang N, El Khoudary SR. High-density lipoprotein cholesterol and arterial calcification in midlife women: the contribution of estradiol and C-reactive protein. Menopause-the J North Am Menopause Soc. 2021;28:237–46.
Woodard GA, Brooks MM, Barinas-Mitchell E, Mackey RH, Matthews KA, Sutton-Tyrrell K. Lipids, menopause, and early atherosclerosis in study of Women’s Health across the Nation Heart women. Menopause-the J North Am Menopause Soc. 2011;18:376–84.
Cardoso R, Generoso G, Staniak HL, Foppa M, Duncan BB, Pereira AC, Blaha MJ, Blankstein R, Nasir K, Bensenor IM, et al. Predictors of coronary artery calcium incidence and progression: the brazilian longitudinal study of Adult Health (ELSA-Brasil). Atherosclerosis. 2020;309:8–15.
Chandra A, Neeland IJ, Das SR, Khera A, Turer AT, Ayers CR, McGuire DK, Rohatgi A. Relation of black race between high density lipoprotein cholesterol content, high density lipoprotein particles and coronary events (from the Dallas Heart Study). Am J Cardiol. 2015;115:890–4.
Diederichsen SZ, Grønhøj MH, Mickley H, Gerke O, Steffensen FH, Lambrechtsen J, Rønnow Sand NP, Rasmussen LM, Olsen MH, Diederichsen A. CT-Detected growth of coronary artery calcification in asymptomatic middle-aged subjects and Association with 15 biomarkers. JACC: Cardiovasc Imaging. 2017;10:858–66.
El Khoudary SR, Nasr A, Matthews KA, Orchard TJ, Brooks MM, Billheimer J, McConnell D, Janssen I, Everson-Rose SA, Crawford S. Associations of HDL metrics with coronary artery calcium score and density among women traversing menopause. J Lipid Res 2021, 62.
Erbel R, Lehmann N, Churzidse S, Möhlenkamp S, Moebus S, Mahabadi AA, Schmermund A, Stang A, Dragano N, Grönemeyer D, et al. Gender-specific association of coronary artery calcium and lipoprotein parameters: the Heinz Nixdorf Recall Study. Atherosclerosis. 2013;229:531–40.
Gao T, Wilkins JT, Zheng Y, Joyce BT, Jacobs DR, Schreiner PJ, Horvath S, Greenland P, Lloyd-Jones D, Hou L. Plasma lipid profiles in early adulthood are associated with epigenetic aging in the coronary artery Risk Development in Young adults (CARDIA) study. Clin epigenetics. 2022;14:1–10.
Kuller LH, Matthews KA, Sutton-Tyrrell K, Edmundowicz D, Bunker CH. Coronary and aortic calcification among women 8 years after menopause and their premenopausal risk factors - the healthy women study. Arterioscler Thromb Vascular Biology. 1999;19:2189–98.
Mahoney LT, Burns TL, Stanford W, Thompson BH, Witt JD, Rost CA, Lauer RM. Coronary risk factors measured in childhood and young adult life are associated with coronary artery calcification in young adults: the Muscatine study. J Am Coll Cardiol. 1996;27:277–84.
Pletcher MJ, Bibbins-Domingo K, Liu K, Sidney S, Lin F, Vittinghoff E, Hulley SB. Nonoptimal lipids commonly present in young adults and coronary calcium later in life: the CARDIA (coronary artery risk development in young adults) study. Ann Intern Med. 2010;153:137–46.
Shen YW, Wu YJ, Hung YC, Hsiao CC, Chan SH, Mar GY, Wu MT, Wu FZ. Natural course of coronary artery calcium progression in Asian population with an initial score of zero. BMC Cardiovasc Disord 2020, 20.
Wong ND, Kawakubo M, LaBree L, Azen SP, Xiang M, Detrano R. Relation of coronary calcium progression and control of lipids according to national cholesterol Education Program guidelines. Am J Cardiol. 2004;94:431–6.
Wang J-S, Chiang H-Y, Wang Y-C, Yeh H-C, Ting I-W, Liang C-C, Wang M-C, Lin C-C, Hsiao C-T, Shen M-Y. Dyslipidemia and coronary artery calcium: from association to development of a risk-prediction nomogram. Nutr Metabolism Cardiovasc Dis. 2022;32:1944–54.
Razavi AC, Allen NB, Dzaye O, Michos ED, Budoff MJ, Lima JA, Shikany JM, Liu K, Post WS, Blumenthal RS. Risk factors for incident coronary artery calcium in younger (Age 32 to 45 years) versus intermediate (46 to 64 years) versus older (65 to 84 years) persons. Am J Cardiol. 2022;184:14–21.
Boekholdt SM, Arsenault BJ, Hovingh GK, Mora S, Pedersen TR, LaRosa JC, Welch K, Amarenco P, DeMicco DA, Tonkin AM. Levels and changes of HDL cholesterol and apolipoprotein AI in relation to risk of cardiovascular events among statin-treated patients: a meta-analysis. Circulation. 2013;128:1504–12.
Toth PP, Barter PJ, Rosenson RS, Boden WE, Chapman MJ, Cuchel M, D’Agostino Sr RB, Davidson MH, Davidson WS, Heinecke JW. High-density lipoproteins: a consensus statement from the national lipid Association. J Clin Lipidol. 2013;7:484–525.
Voight BF, Peloso GM, Orho-Melander M, Frikke-Schmidt R, Barbalic M, Jensen MK, Hindy G, Hólm H, Ding EL, Johnson T. Plasma HDL cholesterol and risk of myocardial infarction: a mendelian randomisation study. The Lancet. 2012;380:572–80.
Yi S-W, Park H-B, Jung M-H, Yi J-J, Ohrr H. High-density lipoprotein cholesterol and cardiovascular mortality: a prospective cohort study among 15.8 million adults. Eur J Prev Cardiol. 2022;29:844–54.
Hafiane A, Genest J. HDL, atherosclerosis, and emerging therapies. Cholesterol 2013, 2013.
Rader DJ. Spotlight on HDL biology: new insights in metabolism, function, and translation. Volume 103. Oxford University Press; 2014. pp. 337–40.
Upadhyay RK. Emerging risk biomarkers in cardiovascular diseases and disorders. J lipids 2015, 2015.
Samadi S, Mehramiz M, Kelesidis T, Mobarhan MG, Sahebkar AH, Esmaily H, Moohebati M, Farjami Z, Ferns GA, Mohammadpour AH. High-density lipoprotein lipid peroxidation as a molecular signature of the risk for developing cardiovascular disease: results from MASHAD cohort. J Cell Physiol. 2019;234:16168–77.
Aghasizadeh M, Samadi S, Sahebkar A, Miri-Moghaddam E, Esmaily H, Souktanloo M, Avan A, Mansoori A, Ferns GA, Kazemi T, Ghayour-Mobarhan M. Serum HDL cholesterol uptake capacity in subjects from the MASHAD cohort study: its value in determining the risk of cardiovascular endpoints. J Clin Lab Anal. 2021;35:e23770.
Samadi S, Abolbashari S, Meshkat Z, Mohammadpour AH, Kelesidis T, Gholoobi A, Mehramiz M, Tabadkani M, Sadabadi F, Dalirfardouei R, et al. Human T lymphotropic virus type 1 and risk of cardiovascular disease: high-density lipoprotein dysfunction versus serum HDL-C concentrations. BioFactors. 2019;45:374–80.
Saberi-Karimian M, Safarian-Bana H, Mohammadzadeh E, Kazemi T, Mansoori A, Ghazizadeh H, Samadi S, Nikbakht-Jam I, Nosrati M, Ferns GA, et al. A pilot study of the effects of crocin on high-density lipoprotein cholesterol uptake capacity in patients with metabolic syndrome: a randomized clinical trial. BioFactors. 2021;47:1032–41.
Pérez-Méndez Ó, Pacheco HG, Martínez-Sánchez C, Franco M. HDL-cholesterol in coronary artery disease risk: function or structure? Clin Chim Acta. 2014;429:111–22.
Zhao Q, Wang J, Miao Z, Zhang NR, Hennessy S, Small DS, Rader DJ. A mendelian randomization study of the role of lipoprotein subfractions in coronary artery disease. Elife. 2021;10:e58361.
Ceponiene I, Li D, El Khoudary SR, Nakanishi R, Stein JH, Wong ND, Nezarat N, Kanisawa M, Rahmani S, Osawa K, et al. Association of Coronary Calcium, Carotid Wall Thickness, and carotid plaque progression with low-density lipoprotein and high-density lipoprotein particle concentration measured by Ion mobility (from multiethnic study of atherosclerosis [MESA]). Am J Cardiol. 2021;142:52–8.
Mahajan H, Zaid M, MacKey R, Kadota A, Vishnu A, Fujiyoshi A, Vasudha A, Hisamatsu T, Evans R, Okamura T et al. Lipoprotein particles and coronary artery calcium in middle-aged US-White and Japanese men. Open Heart 2019, 6.
Aneni EC, Osondu CU, De La Cruz J, Martin SS, Blaha MJ, Younus A, Feldman T, Agatston AS, Veledar E, Nasir K. Lipoprotein sub-fractions by ion-mobility analysis and its association with subclinical coronary atherosclerosis in high-risk individuals. J Atheroscler Thromb. 2019;26:50–63.
Mackey RH, Kuller LH, Sutton-Tyrrell K, Evans RW, Holubkov R, Matthews KA. Lipoprotein subclasses and coronary artery calcium in postmenopausal women from the healthy women study. Am J Cardiol. 2002;90:71I–6.
Aroner SA, Koch M, Mukamal KJ, Furtado JD, Stein JH, Tattersall MC, McClelland RL, Jensen MK. High-Density Lipoprotein Subspecies Defined by Apolipoprotein C-III and Subclinical Atherosclerosis Measures: MESA (The Multi-Ethnic Study of Atherosclerosis). J Am Heart Association 2018, 7.
Jug B, Papazian J, Lee R, Budoff MJ. Association of lipoprotein subfractions and coronary artery calcium in patient at intermediate cardiovascular risk. Am J Cardiol. 2013;111:213–8.
Garcia-Sanchez C, Posadas-Romero C, Posadas-Sanchez R, Carreon-Torres E, Rodriguez-Perez JM, Juarez-Rojas JG, Martinez-Sanchez C, Fragoso JM, Gonzalez-Pacheco H, Vargas-Alarcon G, Perez-Mendez O. Low concentrations of phospholipids and plasma HDL cholesterol subclasses in asymptomatic subjects with high coronary calcium scores. Atherosclerosis. 2015;238:250–5.
Fogacci F, Borghi C, Cicero AF. New evidences on the association between high-density lipoprotein cholesterol and cardiovascular risk: a never ending research story. Volume 29. Oxford University Press; 2022. pp. 842–3.
Acknowledgements
Not applicable.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study’s conception and design. Farshad Abedi and Javad Ramezani independently screened and extracted the data from the articles. The first draft of the manuscript was written by Farshad Abedi, Navid Omidkhoda and Theodoros Kelesidis. Masoumeh Sadeghi performed the statistical analysis and revised the manuscript. Sara Samadi and Amir Hooshang Mohammadpour resolved any discrepancies during screening and data extraction and revised the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Abedi, F., Sadeghi, M., Omidkhoda, N. et al. HDL-cholesterol concentration and its association with coronary artery calcification: a systematic review and meta-analysis. Lipids Health Dis 22, 60 (2023). https://doi.org/10.1186/s12944-023-01827-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12944-023-01827-x