Abstract
Purpose of Review
This review aimed to determine the association between statin use and coronary artery calcification (CAC), as detected by computed tomography in the general population, in previously published observational studies (OSs) and randomized controlled trials (RCTs).
Recent Findings
A systematic search until February 2022 identified 41 relevant studies, comprising 29 OSs and 12 RCTs. We employed six meta-analysis models, stratifying studies based on design and effect metrics. For cohort studies, the pooled β of the association with CAC quantified by the Agatston score was 0.11 (95% CI = 0.05; 0.16), with an average follow-up time per person (AFTP) of 3.68 years. Cross-sectional studies indicated a pooled odds ratio of 2.11 (95% CI = 1.61; 2.78) for the presence of CAC. In RCTs, the pooled standardized mean differences (SMDs) for CAC, quantified by Agatston score or volume, over and AFTP of 1.25 years were not statistically significant (SMD = − 0.06, 95% CI = − 0.19; 0.06 and SMD = 0.26, 95% CI = − 0.66; 1.19), but significantly different (p-value = 0.04). Meta-regression and subgroup analyses did not show any significant differences in pooled estimates across covariates.
Summary
The effect of statins on CAC differs across study designs. OSs demonstrate associations between statin use and higher CAC scores and presence while being prone to confounding by indication. Effects from RCTs do not reach statistical significance and vary depending on the quantification method, hampering drawing conclusions. Further investigations are required to address the limitations inherent in each approach.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cardiovascular disease (CVD) is the primary cause of morbidity and mortality worldwide [1,2,3]. Coronary artery disease, in particular, is the primary cause of disability-adjusted life years lost globally [3,4,5], and its risk increases with age [6, 7]. Coronary artery calcification (CAC) is a dynamic and complex biological process linked to aging and serves as an index of arteriosclerosis [8,9,10]. Recent studies have shown that CAC is a predictor of CVD and major adverse cardiac events [11,12,13] leading to the endorsement of computed tomography (CT) CAC scoring for risk decisions at the primary prevention level [14, 15•]. Consequently, CAC has emerged as one of the indications for starting statin therapy, given its well-established cardiovascular protective effects. Statins are inhibitors of HMG-CoA reductase and are considered the most effective class of drugs for reducing low-density lipoprotein cholesterol (LDL-c) levels [16, 17]. However, recent studies on the pharmacological mechanisms of statins have suggested that they have the potential to accelerate vascular calcification, implying the possibility of a paradoxical effect [18].
The increase in vascular calcification by statins can be mainly attributed to their pleiotropic, LDL-independent, effects. The concept of statin pleiotropy emerged after fully accounting for statin’s clinical influence on CVD, and it received substantial pharmacological and molecular investigations [19, 20••]. Notably, statins can inhibit the synthesis of vitamin K2, a key cofactor for matrix Gla-protein in blood vessels, crucial for protecting against calcification [20••, 21,22,23]. Additionally, statins can suppress various macrophage phenotypes, promoting calcium deposition [23,24,25]. Interestingly, the burden of calcified atherosclerotic plaques has been found to correlate with the overall coronary plaque burden [18, 26]. However the effect of statins on CAC in the general population remained uncertain.
Several previous human subject studies have been conducted to investigate the association of statin use and CAC, with major variations in design and methodology. A wide range of evidence from observational studies has suggested that statin use increases CAC [27,28,29,30,31,32]. Some others, however, have concluded that statins reduce CAC [33,34,35,36]. Randomized controlled trials (RCTs) have also shown contradictory results [37,38,39,40]. Consequently, the genuine effect remains disputed and inconclusive [20••, 41]. There are some drawbacks that limited the previous systematic literature reviews and meta-analyses on this association [42,43,44,45]. They mostly did not thoroughly consider the profound diversity of included studies in terms of study design, outcome metrics, imaging modality, and quantification methods of CAC. Furthermore, most had search strategy constraints, such as limiting the publishing period to after a certain date, focusing on those reporting CVD events, or limiting the source population to specific comorbidities.
Despite these extensive researches on the relationship between statin use and CAC, the genuine effect of statins on CAC in the general population remains unclear. Therefore, to provide a more comprehensive understanding of prior research and to reduce sources of uncertainty and conflicting results, we aimed to conduct a systematic literature review and meta-analysis. Our objective is to investigate the association between statin use and CAC in the general population while accounting for variations in study design and methods.
Methods
Protocol, Search Strategy, and Selection Criteria
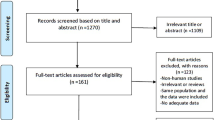
We developed a protocol submitted to PROSPERO on 10 June 2021 and registered with the number CRD42021254187. The selection process and reporting items were based on the preferred reporting items for systematic reviews and meta-analysis (PRISMA) flow diagram and checklist [46].
We formulated a comprehensive search strategy of Embase, Medline All via Ovid, Web of Science Core Collection, and Cochrane Central Register of Controlled Trials for publications without a language or time restriction up to 10 February 2022. A manual search to identify records through reference searches and gray, unpublished literature was conducted. Retrieved records were checked for inclusion and exclusion criteria after duplicates were removed. Observational studies and RCTs were eligible for inclusion if they investigated the association between statin use and CAC using a CT scan, the conventional imaging modality recommended for CAC scoring in clinical practice. The search strategy and inclusion and exclusion criteria were detailed in the supplementary material. In brief, any analytical observational study, comprising cross-sectional and longitudinal, that reported an effect estimate of the association between statin use and CAC was included. RCTs that measured CAC before and after the administration of statins were included. RCTs that only used statins in combination with non-statin medications in the same arm were excluded. The process was performed by two independent reviewers (M. N. S and S. M. J). Discrepancies over eligibility were resolved through consensus with a third reviewer (F. A.).
Data Selection and Extraction
Two study investigators (M. N. S and S. M. J) independently extracted the data from the included records. The studies’ characteristics, including design, participants, exposure, outcome, and summary of statistical analysis were retrieved. From observational studies, effect sizes and corresponding 95% confidence intervals (CI) were collected. Where the 95% CI of the available effect estimate was not reported, we obtained it from the reported p-value [47]. We extracted the adjusted effect size, where both adjusted and unadjusted effects were reported.
The mean and the corresponding standard deviation (SD) or the median and the corresponding interquartile range (IQR) of CAC at baseline and follow-up were extracted from the included RCTs. Extracted median and IQR converted into mean and SD [48] for a unified outcome. Using that, we calculated the mean change from the baseline and its corresponding SD by a correlation coefficient for change from baseline [49] where it was not reported. CAC scores from the first and the last scans were extracted from studies with multiple follow-up scans (> 2 CTs). When RCTs undertook multiple arms, statin and placebo arms were used. If there were no placebo arms and only two arms with different statins or different dosages of statins were given, higher and lower defined daily doses (DDD) were identified according to the values of the World Health Organization; afterward, data of the lower DDD arm was used as the control arm.
Quality and Risk of Bias Assessment
For quality and risk of bias assessment the Newcastle–Ottawa Scale [50] and Cochrane Risk of Bias assessment [51] tools were used.
Statistical Analysis
Data Synthesis and Meta-analysis
Observational studies were classified first by their design and then by their outcome metric as continuous or binary. The effect size metrics and the corresponding 95% CI were unified in each group with odds ratios (OR) for binary outcomes (such as the prevalence of CAC), and beta coefficients for continuous outcomes (representing the CAC score). In RCTs, the mean change and corresponding SD of each study were used to measure the standardized mean difference (SMD), as the effect size. We conducted a meta-analysis when there were more than two studies that utilized the same study design, belonged to the same effect metric category, and, in the case of continuous outcomes, employed the identical CAC quantification method.
To pool the effect estimates, the inverse variance-weighted method was used. Using pre-calculated ORs, all were log-transformed before meta-analysis. I2 statistics were measured to quantify the variability in effect estimate due to between-study heterogeneity rather than chance. Meta-analysis of random effect models was generally applied. Exclusively, a fixed random effect model was applied only in the case of low (I2 < 25%) heterogeneity. Prediction interval (95% PI) was incorporated into random effect models to quantify the distribution of effect estimates and provides a range within which future research effects are anticipated to fall [52,53,54]. Influence diagnostic and leave-one-out analysis was conducted to detect the outliers and influential cases. If any outlier or influential case were detected, the meta-analysis model was once recalculated after removing the detected case. Visual assessment of the symmetry of the funnel plot and Egger’s test was done to identify small-study effect and publication bias. P-values were from 2-tailed tests, and if the p-value was < 0.05, the results were deemed statistically significant. All statistical analyses were performed using R studio for Windows V2021 and {meta}, {metaphor}, and {dmetar} packages [55,56,57].
Subgroup and Meta-regression Analysis
We used subgroup and meta-regression analyses to identify effect modifiers and other sources of heterogeneity. When there were at least eight studies, continuous factors were assessed in meta-regression models. Categorical variables were tested using subgroup analysis when there were at least ten studies in the meta-analysis model [55]. Subgroup analyses were performed based on the quantifying unit of CAC, DDD of the control arm, industry source of funding, follow-up time (long or short), quality assessment results (QAR). Each of the following variables was assessed in a separate meta-regression model: publication year, male proportion, average age, percentage of known CVD, mean baseline CAC score, follow-up time, and (QAR) (when continuous).
Synthesis Without Meta-analysis (SWiM)
Where including a study in a meta-analysis model was not possible, we used SWiM guidelines in systematic reviews [58], which occurred, for instance, when the description of the outcome of the association was too diverse from others to yield a meaningful summary estimate. We used arrows to visually summarize the direction of effect estimates for each study’s results. An indication of study size and statistical significance was used for the arrows, using size and color [58, 59].
Grading of Recommendations Assessment, Development, and Evaluation (GRADE)
The GRADE approach was used as a systematic and transparent judgment tool to assess the quality of the body of evidence for each outcome reported in the systematic review [60], as suggested by the Cochrane Collaboration [49]. The findings from the synthesis, with or without meta-analysis, were graded based on study design and seven other criteria. Downgrading factors included within-study risk of bias, imprecision of effect estimates, inconsistency, and indirectness. Upgrading factors included dose–response gradients, a large enough effect, and no plausible confounding or obvious bias. The GRADE method specifies quality into four levels: high, moderate, low, and very low.
Results
Search Results and Study Details
From 2377 initially identified records by the search, 188 full-texts were assessed for eligibility, leaving 41 original articles for inclusion in this systematic review. The PRISMA flow diagram of the selection process is available in the Supplementary material, Fig. S1. There were 13 cohort studies [27,28,29, 33, 34, 61,62,63,64,65,66,67,68], 16 cross-sectional studies [30, 32, 35, 36, 69,70,71,72,73,74,75,76,77,78,79,80], and 12 RCTs [37,38,39,40, 81,82,83,84,85,86,87,88] in this systematic literature review. This involved 12,520, 7072, and 1791 individuals, respectively. The average follow-up time per person was longer in cohorts compared to RCTs (3.90 years (SD = 1.27) and 1.25 years (SD = 0.25) respectively). The summarized features of included articles such as the study sample size, comorbidities, age, sex, the prevalence of CVD and statin use, follow-up time, and quantification method of CAC are presented in Tables 1, 2, and 3. In the last column of each table, the overall results of each study’s quality assessment are included. Details of the assessment are available in Tables S2–4.
Meta-analyses
Six meta-analysis models were developed by using 32 studies (Figs. S2–7). Table 4 provides data on the pooled results before and after removing outliers and influential cases. Across the six models, two showed statistically significant associations. One of which is the model of cohort studies which showed statin use was significantly associated with CAC — quantified as Agatston score — (B-coefficient: 0.11, 95% CI: 0.05; 0.16). The model showed no heterogeneity (I2: 0.0%), and the included cohorts had an average follow-up per person of 3.68 years. The other model is the model of cross-sectional studies. The pooled odds of the presence of CAC in those who used vs did not use statins was 2.11 (95% CI: 1.61; 2.78). This model had high heterogeneity (I2: 69.7%), and a statistically significant prediction interval (95% PI: 1.00; 4.53). The available data were insufficient to standardize the beta coefficients before the meta-analysis. In RCTs, the pooled effect estimates with CAC quantification in Agatston score or volume were not statistically significant (SMD: − 0.06, 95% CI: − 0.19; 0.06, I2 = 0.0% and SMD: 0.26, 95% CI: − 0.66; 1.19, I2 = 77.6%; Table 4).
Subgroup and Meta-regression Analysis
The subgroup analyses showed a statistically significant difference in the pooled effect size based on whether CAC was quantified as Agatston score or volume (Table 5; 95% CI: [0.02 to 0.62]). Detailed results of other subgroup analyses are presented in Tables S6–9.
Meta-regression was conducted in two meta-analysis models, the model of cross-sectional studies with binary effect metrics and the model of RCTs which quantified CAC as Agatston score. Table 6 displays the estimated effect for each variable in meta-regression and the difference in the true effect size explained by each variable. None of the included variables had a statistically significant modification in the estimated effect.
Small-Study Effect and Publication Bias
Visual inspection of the funnel plots and the Eggers’ regression test results indicate small-study effects in the meta-analysis of RCTs quantified CAC in volume (Egger’s intercept: 3.78, 95% CI: 1.41; 6.16). The contour-enhanced funnel plot shows that the available small studies are more likely to have a larger effect size and a more significant result when compared to the larger available studies. The funnel plots and the details of the Egger regression tests are available in Figs. S14–20 and Table S10, respectively.
SWiM
Inclusion in the meta-analysis was precluded for nine observational studies. In one cross-sectional study with a binary outcome, statin use was significantly associated with the CAC score > 238 [73]. In the two other studies with a continuous outcome, statin use was significantly associated with rising calcium mass scores and absolute calcified plaque counts [31, 32]. One cohort study with a dichotomous outcome revealed that statin use was significantly associated with a rapid progression of CAC [64]. One out of the four studies with a continuous outcome showed statin use was significantly associated with a decrease in the median CAC progression [33]; the other three identified that statin use was associated with an increase in CAC [27, 67, 68], with only one reaching statistical significance. Tables S11–12 provide SWiM-detailed results accompanied by the reasons for exclusion from meta-analysis, according to McKenzie and Brennan [89].
GRADE
The confidence level of the body of evidence in RCT meta-analyses was high, while the confidence levels in observational studies, both with and without meta-analysis, were rated as low or very low. Detailed results of the GRADE approach are available in Table S13.
Discussion
The present study summarized quantitative evidence from previously published observational studies and RCTs on the association between statin use and CAC detected by CT scans in the general population. In observational studies, two meta-analysis models showed significant associations: one between statin use and increasing CAC, as quantified in the Agatston score, and the other with the presence of CAC. These results were inferred from cohort studies with an average follow-up time per person of 3.68 years and cross-sectional studies, respectively. Meta-analyses of RCTs did not reach statistical significance; however, the pooled effects differed significantly depending on whether CAC was quantified as Agatston score or volume.
Our findings from observational studies are supported by the results from in vivo investigations of the effect of statins on vascular calcification, which suggest an increase in calcification. These findings, potentially, contradict the expected cardiovascular protective effects of statins on CAC. Some explanations for this effect propose that statins may stabilize atherosclerotic plaques and prevent plaque rupture by increasing calcification. It is important to note that the beneficial effects of statins on coronary atherosclerotic plaque primarily manifest through their ability to slow down or reverse the progression of soft plaque components. Soft plaque components including lipid-rich core are particularly dangerous because such plaques are unstable and vulnerable to rupture [18, 90,91,92]. However, we would like to highlight that our findings from meta-analyses of observational studies were influenced by the inherent limitations of observational association studies which employed descriptive or etiological approaches. Among the most important ones is confounding and, in the case of our study, confounding by indication, which compromises the reliability of the conclusions regarding the genuine effect of statins. These inherent limitations also resulted in low or very low rates in the GRADE system used to assess the quality of evidence. As a result, it is uncertain how much of the observed effect estimate, suggesting an increase in CAC, would remain after accounting for confounding related to statin indication. Interestingly, the results from the two meta-analysis models of observational studies with statistically significant findings suggest a consistent pattern of association across included studies. This implication is supported by the use of a fixed-effect meta-analysis for cohort studies and the significant prediction intervals obtained from the random-effect meta-analysis for cross-sectional studies, after excluding the outliers. Therefore, there is a high probability that future observational studies employing similar designs and outcome metrics will yield similar effect estimates, showing an increase in CAC, in this level of multivariable-adjusted associations [53, 54]. Future observational studies are needed to address confounding using innovative causal inference methods. These methods can estimate causal effects without the need to measure all confounding factors, allowing for conclusions regarding the drug’s effects in the general population.
Our findings from RCTs in both quantification methods did not reach conventional levels of statistical significance after an average of 1.25-year period. The findings have a high-quality score, as indicated by the GRADE approach. They are also clinically relevant and promissings since an increased amount of CAC over serial scans has been closely associated with a higher risk of future cardiovascular events [11,12,13]. However, we believe that with this quite solid and yet limited available evidence obtained from RCTs, we cannot yet draw a comprehensive conclusion about the full scope of statin’s effect on CAC, since the drug’s effect on the CAC could be dynamic over time. Although the effect modification of follow-up time on the results from RCTs did not reach the statistical significance in both subgroup and meta-regression analysis, both analyses showed a trend toward smaller effect sizes as the follow-up was prolonged (range of 1–2 years) which aligns with our expectations. The pooled effects in cohorts and RCTs were assessed at the point in their follow-up period after the effect was detectable in clinical settings. This is inferred from statins’ in-clinic response time ranging from 4 to 12 weeks [93]. If the effect size were to continue increasing indefinitely, CAC would eventually reach a point where it obstructs blood flow, contradicting the established cardiovascular protective effect of statins. As a result, we expect the estimated effect size of statins on CAC to decline over time. This dilution of effect could possibly be affected in part by the natural aging-related increase in CAC. We think that a more significant effect size may be observed by assessing CAC immediately after and near the in-clinic response time. Considering the limited duration of time and degrees of freedom supplied by our data, which prevents drawing furthur conclusions about the effect in short-term vs long-term follow-up, future studies with tailored follow-up time customized to the pharmacological properties of statins are warranted.
The model of RTCs in volumetric units, one of our six meta-analysis models, was influenced by the small-study effect, which may indicate publication bias. This is despite our efforts to reduce the likelihood of this bias by undertaking the reference search and covering the gray and unpublished literature in our manual search. Since we observed that the small studies were more prone to publication bias and were more likely to have significant results, we think the missing studies are small studies with insignificant results. As a result, outcome reporting bias is probable, provided that RCTs mainly measure multiple outcomes of interest. It is likely that insignificant results in volumetric units, a less common method of quantification in clinics, were dropped.
The RCTs in this meta-analysis allowed us to compare the pooled effect estimates across the two quantification methods. Although the Agatston score and volume remain very closely correlated [94], the significant difference between the pooled effects could be attributed to what each scoring technique measures and the limitations inherent in each technique. The most commonly used method is the Agatston score, which is calculated by multiplying the total area of voxels (mm2) by an arbitrary density index based on the voxel with the highest density, ranging from one to four [94,95,96]. As a result, it is affected simultaneously by calcification area and density, and due to the weighted density index, it grows non-linearly as density increases. CAC volume score provides an alternate method of CAC scoring. It is calculated by multiplying the total number of calcium-containing voxels by the volume of 1 voxel [94, 95]. Volume score is considered more relevant for assessing CAC evolution over time since it allows for a linear augmentation when calcium rises [94, 95, 97]. By contrasting the pooled effect estimates between the two quantification techniques, we have highlighted the distinct potential effect of statin use on the density, area, and volume of CAC.
Recent data suggests that increased density in calcified coronary plaques could be protective against coronary artery diseases and major adverse events, consistent with the concept that it may increase the stability of atherosclerotic plaques and reduce the risk of plaque rupture [41, 98••]. The Agatston score, which was developed without specific histopathological data for correlation, assumes, by definition, that high-density CAC plaque is associated with a higher incidence of coronary artery diseases [94]. Recent evidence using coronary CT angiography and intravascular ultrasound (IVUS) has supported statin’s effect predominantly on increasing dense calcium [98••, 99]. Although the Agatston score is an independent predictor of cardiovascular risk, we hypothesize that by distinctly adding CAC density, area, and volume, the predictive value might be improved since the impact of statins on these measures could be different. Additionally, we believe there may be added value in using the inverse relation between CAC density and volume. This may help quantify what is already suggested as the stability and maturity of calcium within an atherosclerotic plaque.
Limitations and Strengths
We studied drug use as the exposure of the association under investigation; therefore, the duration and intensity of the exposure were not covered by the pooled results. Studying the general population, we designed to investigate the effect of the population’s risk profile on the association, which showed no modification in the effect. However, since few records were included in some meta-analysis models, we could not run subgroup or meta-regression analyses across all the models. We had limited data to provide a standardized measure of the beta coefficients in the analyses. However, it may not change the overall trend of the results considerably, given that the outcome data in most studies underwent the same transformation. Our meta-analysis employed study-level data, not individual patient-level data, which could have assisted in overcoming some of the already-mentioned limitations of this study.
Our study has a number of strengths. Statins have received the most extensive research attention in cardiovascular pharmacology. Our findings, building upon previous results, provided new insight into our understanding of the effect of these medicinal products on CAC in the general population. We assessed our findings’ robustness, inspected heterogeneity patterns, and discussed reasons why the effects differed. These approaches helped us develop informed hypotheses and conclusions from observational studies and RCTs in our systematic review and meta-analysis.
Conclusion
Drawing from our findings, the effects of statins on CAC demonstrate variations across different study designs and effect size metrics. Cohort and cross-sectional studies suggest a significant association between statin use and CAC score progression and CAC presence, respectively. Nevertheless, RCTs did not determine a significant effect, with the effects being different across the quantification unit of CAC, hindering forming a conclusion. Interpretation should consider limitations inherent in included studies, namely confounding by indication in observational studies, variations in CAC quantification method, and limited follow-up time points.
To gain a better understanding of this association, a large long-term RCT is required to consider the effect over customized follow-up times aligned with the pharmacological properties of statins. However, ethical, medical, and logistical aspects may restrict the feasibility of it. Observational studies with large sample sizes drawn from the general population could shed light on this association by taking one step towards addressing confounding by indication and establishing causality. Furthermore, future studies should independently assess the volume and density of the observed effects, thus providing a more detailed characterization of the effect.
Data Availability
The data are available upon reasonable requests made to the corresponding author.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Goff DC, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25 Part B):2935–59.
Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25):2889–934.
Mensah GA, Roth GA, Fuster V. The global burden of cardiovascular diseases and risk factors: 2020 and beyond. In American College of Cardiology Foundation Washington, DC. 2019;74:2529–32.
Vedanthan R, Seligman B, Fuster V. Global perspective on acute coronary syndrome: a burden on the young and poor. Circ Res. 2014;114(12):1959–75.
Ralapanawa U, Sivakanesan R. Epidemiology and the magnitude of coronary artery disease and acute coronary syndrome: a narrative review. J Epidemiol Glob Health. 2021;11(2):169.
Bauersachs R, Zeymer U, Brière J-B, Marre C, Bowrin K, Huelsebeck M. Burden of coronary artery disease and peripheral artery disease: a literature review. Cardiovasc Ther. 2019:8295054.
Ferreira-González I. The epidemiology of coronary heart disease. Rev Esp Cardiol (English Edition). 2014;67(2):139–44.
Pescatore LA, Gamarra LF, Liberman M. Multifaceted mechanisms of vascular calcification in aging. Arterioscler Thromb Vasc Biol. 2019;39(7):1307–16.
Garcia MR, Diaz MN, Andia JBC. Bone metabolism, vascular calcifications and mortality: associations beyond mere coincidence. J Nephrol. 2005;18(4):458.
Zhu D, Mackenzie NCW, Farquharson C, MacRae VE. Mechanisms and clinical consequences of vascular calcification. Front Endocrinol. 2012;3:95.
Hoffmann U, Massaro JM, D’Agostino RB Sr, Kathiresan S, Fox CS, O’Donnell CJ. Cardiovascular event prediction and risk reclassification by coronary, aortic, and valvular calcification in the Framingham Heart Study. J Am Heart Assoc. 2016;5(2):e003144.
Grandhi GR, Mirbolouk M, Dardari ZA, et al. Interplay of coronary artery calcium and risk factors for predicting CVD/CHD mortality: the CAC Consortium. Cardiovasc Imaging. 2020;13(5):1175–86.
Adelhoefer S, Uddin SMI, Osei AD, Obisesan OH, Blaha MJ, Dzaye O. Coronary artery calcium scoring: new insights into clinical interpretation—lessons from the CAC Consortium. Radiol: Cardiothorac Imaging. 2020;2(6):e200281.
Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;74(10):1376–414.
• Visseren FLJ, Mach F, Smulders YM, et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice: developed by the Task Force for cardiovascular disease prevention in clinical practice with representatives of the European Society of Cardiology and 12 medical societies With the special contribution of the European Association of Preventive Cardiology (EAPC). Eur Heart J. 2021;42(34):3227–337. (The article recommends using computed tomography (CT) CAC scoring to aid in making risk decisions for CVD prevention in clinical practice. This highlights the valuable application of CAC scoring from CT scans in clinical settings.)
Chou R, Dana T, Blazina I, Daeges M, Jeanne TL. Statins for prevention of cardiovascular disease in adults: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2016;316(19):2008–24.
Rodriguez F, Maron DJ, Knowles JW, Virani SS, Lin S, Heidenreich PA. Association between intensity of statin therapy and mortality in patients with atherosclerotic cardiovascular disease. JAMA Cardiol. 2017;2(1):47–54.
Ferencik M, Chatzizisis YS. Statins and the coronary plaque calcium “paradox”: insights from non-invasive and invasive imaging. Atherosclerosis. 2015;241(2):783–5.
Oesterle A, Laufs U, Liao JK. Pleiotropic effects of statins on the cardiovascular system. Circ Res. 2017;120(1):229–43.
•• Yu D, Liao JK. Emerging views of statin pleiotropy and cholesterol lowering. Cardiovasc Res. 2022;118(2):413–23. (This study showed statin pleiotropy plays a considrable role in the effect of this drug on CVD, as a clinical outcome. Therefore, a considerable amount of scientific evidence has explored and supported the pleiotropic effects of statins on different subclinical measures.)
Okuyama H, Langsjoen PH, Hamazaki T, et al. Statins stimulate atherosclerosis and heart failure: pharmacological mechanisms. Expert Rev Clin Pharmacol. 2015;8(2):189–99.
Hashimoto Y, Okuyama H. Statins cause lifestyle-related diseases-biochemical mechanism. Endocrinol Diabetes Res. 2017;3(2):10:2.
Almeida SO, Budoff M. Effect of statins on atherosclerotic plaque. Trends Cardiovasc Med. 2019;29(8):451–5.
Laufs U, La Fata V, Plutzky J, Liao JK. Upregulation of endothelial nitric oxide synthase by HMG CoA reductase inhibitors. Circulation. 1998;97(12):1129–35.
Huang K-C, Chen C-W, Chen J-C, Lin W-W. HMG-CoA reductase inhibitors inhibit inducible nitric oxide synthase gene expression in macrophages. J Biomed Sci. 2003;10(4):396–405.
Sangiorgi G, Rumberger JA, Severson A, et al. Arterial calcification and not lumen stenosis is highly correlated with atherosclerotic plaque burden in humans: a histologic study of 723 coronary artery segments using nondecalcifying methodology. J Am Coll Cardiol. 1998;31(1):126–33.
Smit JM, van Rosendael AR, El Mahdiui M, et al. Impact of clinical characteristics and statins on coronary plaque progression by serial computed tomography angiography. Circ: Cardiovasc Imaging. 2020;13(3):e009750.
Maréchal C, Coche E, Goffin E, et al. Progression of coronary artery calcification and thoracic aorta calcification in kidney transplant recipients. Am J Kidney Dis. 2012;59(2):258–69.
Elkeles RS, Godsland IF, Rubens MB, Feher MD, Nugara F, Flather MD. The progress of coronary heart disease in type 2 diabetes as measured by coronary calcium score from electron beam computed tomography (EBCT): the PREDICT study. Atherosclerosis. 2008;197(2):777–83.
Rodriguez K, Kwan AC, Lai S, et al. Coronary plaque burden at coronary CT angiography in asymptomatic men and women. Radiology. 2015;277(1):73–80.
Nguyen PTH, Coche E, Goffin E, et al. Prevalence and determinants of coronary and aortic calcifications assessed by chest CT in renal transplant recipients. Am J Nephrol. 2007;27(4):329–35.
Cheng VY, Wolak A, Gutstein A, et al. Low-density lipoprotein and noncalcified coronary plaque composition in patients with newly diagnosed coronary artery disease on computed tomographic angiography. Am J Cardiol. 2010;105(6):761–6.
Budoff MJ, Yu D, Nasir K, et al. Diabetes and progression of coronary calcium under the influence of statin therapy. Am Heart J. 2005;149(4):695–700.
Raggi P, Cooil B, Ratti C, Callister TQ, Budoff M. Progression of coronary artery calcium and occurrence of myocardial infarction in patients with and without diabetes mellitus. Hypertension. 2005;46(1):238–43.
Jung C-H, Lee W-Y, Kim S-Y, et al. The relationship between coronary artery calcification score, plasma osteoprotegerin level and arterial stiffness in asymptomatic type 2 DM. Acta Diabetol. 2010;47(1):145–52.
Hosseinsabet A, Mohebbi A, Almasi A. Association between C-reactive protein and coronary calcium score in coronary artery disease: cardiovascular topics. Cardiovasc J Afr. 2009;20(2):107–11.
Dichtl W, Alber HF, Feuchtner GM, et al. Prognosis and risk factors in patients with asymptomatic aortic stenosis and their modulation by atorvastatin (20 mg). Am J Cardiol. 2008;102(6):743–8.
Plazak W, Gryga K, Dziedzic H, et al. Influence of atorvastatin on coronary calcifications and myocardial perfusion defects in systemic lupus erythematosus patients: a prospective, randomized, double-masked, placebo-controlled study. Arthritis Res Ther. 2011;13(4):1–9.
Miyoshi T, Kohno K, Asonuma H, et al. Effect of intensive and standard pitavastatin treatment with or without eicosapentaenoic acid on progression of coronary artery calcification over 12 months-prospective multicenter study-. Circ J. 2018;82(2):532–40.
Terry JG, Carr JJ, Kouba EO, et al. Effect of simvastatin (80 mg) on coronary and abdominal aortic arterial calcium (from the coronary artery calcification treatment with zocor [CATZ] study). Am J Cardiol. 2007;99(12):1714–7.
Gill EA. Does statin therapy affect the progression of atherosclerosis measured by a coronary calcium score? Curr Atheroscler Rep. 2010;12(2):83–7.
Zhang X, Xiao J, Wang L, Xiaoping J. Statins accelerate coronary calcification and reduce the risk of cardiovascular events. Cardiol Rev. 2022.
Lai R, Ju J, Lin Q, Xu H. Coronary artery calcification under statin therapy and its effect on cardiovascular outcomes: a systematic review and meta-analysis. Front Cardiovasc Med. 2020;7:600497.
Andelius L, Mortensen MB, Nørgaard BL, Abdulla J. Impact of statin therapy on coronary plaque burden and composition assessed by coronary computed tomographic angiography: a systematic review and meta-analysis. Eur Heart J-Cardiovasc Imaging. 2018;19(8):850–8.
Henein MY, Owen A. Statins moderate coronary stenoses but not coronary calcification: results from meta-analyses. Int J Cardiol. 2011;153(1):31–5.
Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg. 2021;88:105906.
Altman DG, Bland JM. How to obtain the confidence interval from a P value. BMJ. 2011;343.
Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14(1):1–13.
Higgins JPT, Thomas J, Chandler J, et al. Cochrane handbook for systematic reviews of interventions: John Wiley & Sons. 2019.
Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Oxford. 2000.
Higgins JPT, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343.
Riley RD, Higgins JPT, Deeks JJ. Interpretation of random effects meta-analyses. BMJ. 2011;342.
Chiolero A, Santschi V, Burnand B, Platt RW, Paradis G. Meta-analyses: with confidence or prediction intervals? Eur J Epidemiol. 2012;27(10):823–5.
Wang CC, Lee WC. A simple method to estimate prediction intervals and predictive distributions: summarizing meta-analyses beyond means and confidence intervals. Res Synth Methods. 2019;10(2):255–66.
Schwarzer G, Carpenter JR, Rücker G. Meta-analysis with R. Springer. 2015;4784.
Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36(3):1–48.
Harrer M, Cuijpers P, Furukawa T, Ebert DD. Dmetar: companion R package for the guide ’Doing meta-analysis in R’. R package version 00. 2019;9000.
Campbell M, McKenzie JE, Sowden A, et al. Synthesis without meta-analysis (SWiM) in systematic reviews: reporting guideline. BMJ. 2020;368.
Thomson HJ, Thomas S. The effect direction plot: visual display of non-standardised effects across multiple outcome domains. Res Synth Methods. 2013;4(1):95–101.
Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–6.
Anand DV, Lim E, Darko D, et al. Determinants of progression of coronary artery calcification in type 2 diabetes: role of glycemic control and inflammatory/vascular calcification markers. J Am Coll Cardiol. 2007;50(23):2218–25.
Chen Z, Qureshi AR, Parini P, et al. Does statins promote vascular calcification in chronic kidney disease? Eur J Clin Invest. 2017;47(2):137–48.
Karpouzas GA, Ormseth SR, Hernandez E, Budoff MJ. Impact of cumulative inflammation, cardiac risk factors, and medication exposure on coronary atherosclerosis progression in rheumatoid arthritis. Arthritis Rheumatol. 2020;72(3):400–8.
Pechlivanis S, Jung D, Moebus S, et al. Pharmacogenetic association of diabetes-associated genetic risk score with rapid progression of coronary artery calcification following treatment with HMG-CoA-reductase inhibitors—results of the Heinz Nixdorf Recall Study. Naunyn Schmiedebergs Arch Pharmacol. 2021;394(8):1713–25.
Shemesh J, Koren-Morag N, Apter S, et al. Accelerated progression of coronary calcification: four-year follow-up in patients with stable coronary artery disease. Radiology. 2004;233(1):201–9.
Hsia J, Klouj A, Prasad A, Burt J, Adams-Campbell LL, Howard BV. Progression of coronary calcification in healthy postmenopausal women. BMC Cardiovasc Disord. 2004;4(1):1–6.
Hoffmann H, Frieler K, Schlattmann P, Hamm B, Dewey M. Influence of statin treatment on coronary atherosclerosis visualised using multidetector computed tomography. Eur Radiol. 2010;20(12):2824–33.
Zeb I, Li D, Nasir K, et al. Effect of statin treatment on coronary plaque progression–a serial coronary CT angiography study. Atherosclerosis. 2013;231(2):198–204.
Jeon G-H, Kim SH, Yun S-C, Chae HD, Kim C-H, Kang BM. Association between serum estradiol level and coronary artery calcification in postmenopausal women. Menopause. 2010;17(5):902–7.
Hamer M, Venuraju SM, Lahiri A, Rossi A, Steptoe A. Objectively assessed physical activity, sedentary time, and coronary artery calcification in healthy older adults. Arterioscler Thromb Vasc Biol. 2012;32(2):500–5.
Nakazato R, Gransar H, Berman DS, et al. Statins use and coronary artery plaque composition: results from the International Multicenter CONFIRM Registry. Atherosclerosis. 2012;225(1):148–53.
Greif M, Arnoldt T, von Ziegler F, et al. Lipoprotein (a) is independently correlated with coronary artery calcification. Eur J Intern Med. 2013;24(1):75–9.
Shikada T, Washio M, Nishizaki A, et al. Risk factors for coronary artery calcification in Japanese patients. J Cardiol. 2015;66(1):36–40.
Panh L, Ruidavets JB, Rousseau H, et al. Association between serum alkaline phosphatase and coronary artery calcification in a sample of primary cardiovascular prevention patients. Atherosclerosis. 2017;260:81–6.
Lee D, Joo HJ, Jung H-W, Lim D-S. Investigating potential mediator between statin and coronary artery calcification. PLoS One. 2018;13(9):e0203702.
Drouin-Chartier J-P, Tremblay AJ, Godbout D, et al. Correlates of coronary artery calcification prevalence and severity in patients with heterozygous familial hypercholesterolemia. CJC open. 2021;3(1):62–70.
Béland-Bonenfant S, Paquette M, Fantino M, et al. Montreal-FH-SCORE predicts coronary artery calcium score in patients with familial hypercholesterolemia. CJC open. 2021;3(1):41–7.
Elkeles RS, Feher MD, Flather MD, et al. The association of coronary calcium score and conventional cardiovascular risk factors in Type 2 diabetic subjects asymptomatic for coronary heart disease (The PREDICT Study). Diabet Med. 2004;21(10):1129–34.
Nguyen PTH, Coche E, Goffin E, et al. Prevalence and determinants of coronary and aortic calcifications assessed by chest CT in renal transplant recipients. Am J Nephrol. 2007;27(4):329–35.
Zhelyazkova-Savova MD, Yotov YT, Nikolova MN, et al. Statins, vascular calcification, and vitamin K-dependent proteins: is there a relation? Kaohsiung J Med Sci. 2021;37(7):624–31.
Houslay ES, Cowell SJ, Prescott RJ, et al. Progressive coronary calcification despite intensive lipid-lowering treatment: a randomised controlled trial. Heart. 2006;92(9):1207–12.
Petri MA, Kiani AN, Post W, Christopher-Stine L, Magder LS. Lupus atherosclerosis prevention study (LAPS). Ann Rheum Dis. 2011;70(5):760–5.
Lemos MM, Watanabe R, Carvalho AB, et al. Effect of rosuvastatin and sevelamer on the progression of coronary artery calcification in chronic kidney disease: a pilot study. Clin Nephrol. 2013;80(1):1–8.
Lo J, Lu MT, Ihenachor EJ, et al. Effects of statin therapy on coronary artery plaque volume and high-risk plaque morphology in HIV-infected patients with subclinical atherosclerosis: a randomised, double-blind, placebo-controlled trial. Lancet HIV. 2015;2(2):e52–63.
Yazbek DC, Carvalho ABd, Barros CS, Medina Pestana JO, Canziani MEF. Effect of statins on the progression of coronary calcification in kidney transplant recipients. PLoS One. 2016;11(4):e0151797.
Raggi P, Davidson M, Callister TQ, et al. Aggressive versus moderate lipid-lowering therapy in hypercholesterolemic postmenopausal women: beyond Endorsed Lipid Lowering with EBT Scanning (BELLES). Circulation. 2005;112(4):563–71.
Schmermund A, Achenbach S, Budde T, et al. Effect of intensive versus standard lipid-lowering treatment with atorvastatin on the progression of calcified coronary atherosclerosis over 12 months: a multicenter, randomized, double-blind trial. Circulation. 2006;113(3):427–37.
Auscher S, Heinsen L, Nieman K, et al. Effects of intensive lipid-lowering therapy on coronary plaques composition in patients with acute myocardial infarction: assessment with serial coronary CT angiography. Atherosclerosis. 2015;241(2):579–87.
McKenzie JE, Brennan SE. Synthesizing and presenting findings using other methods. Cochrane Handbook for Systematic Reviews of Interventions. 2019;321–47.
Nissen SE, Nicholls SJ, Sipahi I, et al. Effect of very high-intensity statin therapy on regression of coronary atherosclerosis: the ASTEROID trial. JAMA. 2006;295(13):1556–65.
Nicholls SJ, Ballantyne CM, Barter PJ, et al. Effect of two intensive statin regimens on progression of coronary disease. N Engl J Med. 2011;365(22):2078–87.
Dohi T, Maehara A, Moreno PR, et al. The relationship among extent of lipid-rich plaque, lesion characteristics, and plaque progression/regression in patients with coronary artery disease: a serial near-infrared spectroscopy and intravascular ultrasound study. Eur Heart J-Cardiovasc Imaging. 2015;16(1):81–7.
Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139(25):e1082–143.
Alluri K, Joshi PH, Henry TS, Blumenthal RS, Nasir K, Blaha MJ. Scoring of coronary artery calcium scans: history, assumptions, current limitations, and future directions. Atherosclerosis. 2015;239(1):109–17.
Willemink MJ, van der Werf NR, Nieman K, Greuter MJW, Koweek LM, Fleischmann D. Coronary artery calcium: a technical argument for a new scoring method. J Cardiovasc Comput Tomogr. 2019;13(6):347–52.
Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15(4):827–32.
Halliburton SS, Stillman AE, Lieber M, Kasper JM, Kuzmiak SA, White RD. Potential clinical impact of variability in the measurement of coronary artery calcification with sequential MDCT. Am J Roentgenol. 2005;184(2):643–8.
•• Van Rosendael AR, Van Den Hoogen IJ, Gianni U, et al. Association of statin treatment with progression of coronary atherosclerotic plaque composition. JAMA cardiology. 2021;6(11):1257–66. (These findings imply that statin treatment is associated with higher rates of coronary atherosclerosis transformation to high-density calcium, lending support to the concept of reduced atherosclerotic risk with increasing calcium densification.)
Ferencik M, Blankstein R, Nasir K. Unravelling the coronary artery calcium paradox: benefits of plaques of stone. Eur Heart J-Cardiovasc Imaging. 2019.
Acknowledgements
The authors wish to thank Dr. Wichor M. Bramer, PhD. from the Erasmus MC Medical Library for developing and updating the search strategies.
Author information
Authors and Affiliations
Contributions
All the authors contributed to the study conception and design. MNS: screening the records, retrieving data, building the dataset, analyzing the data, and writing the article; SMJ: screening the records, building the dataset, and revising the manuscript; DB: revising the manuscript and supervision; BHS: revising the manuscript, giving feedback on methodology, supervision, and final approval; FA: revising the manuscript and supervision. All the authors approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The institution where the work was performed: Erasmus Medical Center, Rotterdam, the Netherlands.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shahraki, M.N., Jouabadi, S.M., Bos, D. et al. Statin Use and Coronary Artery Calcification: a Systematic Review and Meta-analysis of Observational Studies and Randomized Controlled Trials. Curr Atheroscler Rep 25, 769–784 (2023). https://doi.org/10.1007/s11883-023-01151-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11883-023-01151-w