Abstract
Background
Insulin resistance, liver injury and dyslipidemia are reported in non-alcoholic fat liver disease (NAFLD) patients. Interleukin (IL)-38 may take part in the pathophysiology of insulin resistance. Nevertheless, the function of IL-38 in NAFLD is unknown. Herein, we determined whether serum IL-38 level might be utilised as a biochemical marker for diagnosing NAFLD.
Methods
NAFLD patients and healthy participants (n = 91 each) were enrolled. Circulating serum IL-38 levels were detected using enzyme-linked immunosorbent assay. Other metabolic and inflammatory indices related to NAFLD were also assessed.
Results
Patients with NAFLD had higher serum IL-38 levels than healthy individuals. Significantly higher serum IL-38 levels were found in patients with severe and moderate NAFLD than in patients with mild NAFLD. IL-38 showed a significant correlation with parameters of insulin resistance, inflammation, and liver enzyme in NAFLD cases. Anthropometric, insulin resistance, inflammatory parameters, lipids and frequency of NAFLD showed significant differences among the serum IL-38 level tertiles. Participants in the 2nd and 3rd tertiles of serum IL-38 levels had a greater risk of NAFLD than those in the 1st tertile. Furthermore, IL-38 ROC curve showed a high area under ROC with 0.861.
Conclusions
It is possible for serum IL-38 to be a biomarker for NAFLD.
Similar content being viewed by others
Background
Non-alcoholic fatty liver disease (NAFLD) is a frequent chronic liver condition that has been classified as an emerging epidemic and is generally seen as part of metabolic syndrome [1]. Changes in lifestyle and diet have led to the development of NAFLD as a global public health issue [2]. Data from current epidemiological studies and the Global Burden of Disease indicate that NAFLD incidence is growing and the disease will become more dangerous, and has already affected 25% of the adult population worldwide [3,4,5]. Moreover, in Western countries, NAFLD is the leading cause of liver transplantation and hepatocellular cancer [6]. Liver biopsy is not practical for screening the general population as it is invasive and risky [7]. Abdominal ultrasound is the most extensively used and recognised non-invasive technique for diagnosing NAFLD. However, accurate assessment of NAFLD using abdominal ultrasound is challenging, particularly given the difficulty in discriminating between steatosis and steatohepatitis, and it does not perform well in individuals with abdominal obesity [8]. A simple, objective, reproducible, non-invasive serological biomarker that can help in the early diagnosis and identification of the severity of NAFLD is therefore urgently required.
NAFLD is closely related to phenotypic changes in the hepatocytes. Peroxisome proliferator-activated receptor gamma, a critical adipogenic transcriptional factor, promotes adipogenic transformation and lipid accumulation in the hepatocytes of NAFLD patients [9]. Some signal molecules that regulate adipogenesis, including retinoic acid, adiponectin, and chemerin, have been referred as potential diagnostic markers for NAFLD [10, 11]. Furthermore, the development of NAFLD is accelerated by inflammation, and elevated pro-inflammatory cytokines are associated with progressive pathological fibrosis and cirrhosis [12, 13]. IL-38 is a recently discovered cytokine [14]. Evidence suggests that IL-38, like its family members, may involve in homeostasis of immune cells and exert anti- or pro-inflammatory actions [15]. Several researches have demonstrated the level of IL-38 is aberrant in diabetes mellitus patients and that it can suppress inflammatory responses, reduce liver fat, and attenuate insulin resistance [16,17,18,19]. IL-38 may participate in NAFLD since its correlation with insulin resistance and hepatic inflammation caused by fat deposition in the liver. Hence, we will investigate the correlation between IL-38 and NAFLD using.
Methods
Participants and study design
Ninety-one NAFLD patients (NFs) and 91 healthy controls (HCs) of matched age and sex who underwent annual health examinations from 1 October 2019 to 31 September 2020 were recruited as study participants. This cohort was obtained from Jiujiang No.1 People’s Hospital. The diagnosis of the 91 NAFLD cases was ascertained using radiological criteria. All NFs followed the guidelines for prevention and treatment of NAFLD (2018, China) [20]. The exclusion criteria for the NFs were as follows: (1) weekly ethanol consumption ≥140 g for men or ≥ 70 g for women; (2) cases with drug-induced liver disease, Wilson’s disease, or cancer; (3) cases with viral hepatitis or autoimmune liver disease; (4) cases with autoimmune diseases or infections; (5) cases with obstructive sleep apnoea syndrome or polycystic ovary syndrome; (6) usage of non-steroidal anti-inflammatory drugs within the last 3 months; (7) concurrent diabetes mellitus, cholestatic liver disease, pregnancy, substantial diseases of important organs, and hypothyroidism; and (8) cases with missing data or qualified blood samples. The exclusion criteria for the HCs were as follows: (1) abnormal ultrasound imaging of the liver or abnormal liver function tests in the past; and (2) other exclusion criteria applied to the NFs. In the fasting state, a 6 mL venous blood sample was collected. The separated specimens were maintained at − 80 °C until they were analyzed. This study was approved by the ethical standards of the patient-source institution and was in compliance with the Declaration of Helsinki. The informed written consent was given by all subjects.
Demographic, anthropometric, and laboratory evaluations
Participants’ demographic information such as age; sex; alcohol consumption; medical and medication history; and anthropometric variables; including weight and height, were collected. Counts of blood cells were obtained using the Sysmex XN2000 automatic haematology analyser. Biochemical indices were carried out by an Hitachi 7600 automatic biochemical analyzer, including alanine aminotransferase (ALT), fasting blood glucose (FBG), aspartate aminotransferase (AST), albumin (ALB), prealbumin (PA), urea, high-density lipoprotein cholesterol (HDL-C), creatinine (CREA), triglycerides (TRIG), cholesterol (CHOL), and low-density lipoprotein cholesterol (LDL-C). Serum insulin (INS), C-peptide (CpS), and IL-6 levels were detected by electrochemiluminescence immunoassay using the Cobas E602 system (Roche, Basel, Switzerland). Haemoglobin A1c (HbA1c) was measured using the MQ-6000PT HbA1c detection system with high-performance liquid chromatography. Concentration of 25 Hydroxy-vitamin D [25(OH)D] was quantified by chemiluminescent immunoassay in the MAGLUMI 4000 Plus Analyser (Snibe Co., Ltd., Shenzhen, China). C-reactive protein (CRP) was detected using the nephelometric immunoassay method on the IMMAGE 800 system. Next, for the determination of body mass index (BMI), NAFLD fibrosis score (NFS), homoeostasis model assessment of insulin resistance (HOMA-IR), and quantitative insulin sensitivity check index (QUICK), the following formulas were used: BMI = weight (kg)/height (m); NFS = ˗1.675 + 0.037 × age (years) + 0.094 × BMI (kg/m2) + 1.13 × Impaired fasting glucose or type 2 diabetes mellitus (yes = 1, no = 0) + 0.99 × AST/ALT ratio − 0.013 × PLT (× 109/L) − 0.66 × ALB (g/dL), with impaired fasting glucose defined as FBG ≥ 110 mg/dL; HOMA-IR = fasting glucose (mmol/L) × fasting insulin (mU/L)/22.5; and QUICK = 1/[log fasting insulin (mU/L) + log fasting glucose (mg/dL)].
NAFLD diagnosis
Upper abdominal ultrasonography was completed by two qualified radiologists. Ultrasonographic degrees of NAFLD were categorised into three types, mild, moderate, and severe, according to the Chinese standard [21].
Quantitation of serum IL-38 concentration
An enzyme-linked immunosorbent assay (ELISA) kit (DY9110–05, R&D Systems) was used to detect the level of serum IL-38 in all participants following the manufacturer’s instructions. The assay range was 31.2–2000 pg/mL. For each sample, the concentration of IL-38 in each sample was obtained according to a standard curve constructed using the appropriate recombinant IL-38.
Statistical analyses
Statistical analyses were completed by SPSS 23.0 software and R 4.1.2 Project for Statistical Computing. Normality was examined using the Shapiro–Wilk test. The independent t-test was used to compare the means of continuous variables when the data met the normal distribution; otherwise, Mann–Whitney U test was employed. χ2 test was applied for comparison of count data. And correlation analysis was analyzed by Spearman’s correlation. R was used to draw receiver operating characteristic (ROC) curves and performance assessment. p value < 0.05 was regarded as significance.
Results
Characteristics of the subjects
No statistical difference in subject age [32.00 (25.50,41.00) vs. 35.00 (28.00,41.50), p = 0.235], sex [56/35 vs. 59/32, p = 0.645], and serum levels of ALB [48.60 (47.50,50.65) vs. 49.20 (47.85,51.00), p = 0.199] was found between NFs and controls. Significant disparities in the anthropometric and laboratory data were shown in Table 1. As predicted, NF patients exhibited higher BMI than HCs (p < 0.05). Serum concentrations of AST, ALT, FBG, INS, CpS, HbA1c, PA, urea, CREA, TRIG, CHOL, and LDL-C were obviously higher in NFs than HCs (all p < 0.05). PLT and 25(OH) D were lower in NFs than in HCs (all p < 0.05). NFs exhibited higher levels of WBC, NEU, IL-6, and CRP than HCs (all p < 0.05). The two indicators of insulin resistance, HOMA-IR and QUICK, showed notable differences between the two groups. NFS, an internationally recognised biomarker for liver fibrosis degree, was also elevated in NFs.
IL-38 levels in NAFLD patients
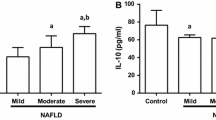
To characterise the status of IL-38 in NFALD cases, we detected the level of serum IL-38 using ELISA. Quantification analysis revealed circulating IL-38 in NFs was dramatically higher than HCs (p < 0.001, Fig. 1a). Based on the ultrasonographic degrees, NFs patients were categorized into three groups: mild group (n = 33), moderate group (n = 31), and severe group (n = 27). We further performed subgroup analysis within these grading groups, and the results showed that cases in the severe group had the highest levels of IL-38, followed by the moderate and mild groups (Fig. 1b). Furthermore, the IL-38 levels of patients with abnormal liver enzyme levels (ALT and/or AST) were higher than in those with normal liver enzyme levels (Fig. 1c). This result suggested that circulating IL-38 might involve in NAFLD pathogenesis.
Characteristics of participants according to serum IL-38 tertiles
Participants were classified into tertiles (low, medium, or high) based on serum IL-38 levels to further investigate the correlation these and NAFLD. Table 2 displayed the baseline characteristics of all subjects. Compared with individuals in the lower tertile of IL-38 (T1), those expressing higher levels of IL-38 had higher ALT, AST, FBG, HOMA-IR, CRP, INS, CpS, WBC, NEU, PA, TRIG, and BMI, but lower baseline levels of QUICK, HDL-C, and 25(OH)D. Furthermore, with increasing IL-38, the proportion of NAFLD patients in T1, T2, and T3 was 13.33, 48.39, and 88.33%, respectively, exhibiting a clear increasing trend (p < 0.0001 for the trend).
Serum IL-38 and anthropometric/biochemical parameters correlations in NAFLD patients
In NFs, the correlations between serum IL-38 and demographic or anthropometric parameters (age and BMI); liver enzymes (ALT and AST); lipids (CHOL, HDL-C, LDL-C, and TRIG); glucose metabolism (FBG, HbA1c, INS, CpS, HOMA-IR, and QUICK); inflammation indices (WBC, NEU, CRP, and IL-6); indicators of kidney function (urea and CREA); and 25(OH) D were further explored (Fig. 2). Significant positive associations of serum IL-38 levels with HOMA-IR, INS, and CpS were found, whereas QUICK was negatively associated with IL-38 level in glucose metabolism. Positive associations were observed between IL-38 and ALT or AST levels. When inflammation parameters were evaluated, a positive association between serum IL-38 and IL-6 was also observed. IL-38 and 25(OH) D were found to have a negative correlation.
Odd ratios (ORs) of NAFLD by IL-38 tertiles
The association between serum IL-38 and ORs of NAFLD was evaluated with a multivariable logistic regression analysis. The ORs for NAFLD in T2 or T3 vs. T1 for serum IL-38 were shown in Table 3. The OR of T2 vs. T1 was statistically significant after adjusting age, sex, BMI, ALT, and AST, but it was not significant after adjustment for HDL-C and LDL-C. In the multivariate analysis with adjustment for all the above explanatory variables, the OR of T3 vs. T1 remained a significantly positive determinant of NAFLD. These results indicated that higher serum IL-38 was linked to a higher risk of NAFLD.
ROC curve of IL-38
The ROC curve of serum IL-38 was plotted to predict NAFLD (Fig. 3.). The area under the ROC curve (AUC) was 0.859 (95% CI, 0.807–0.911, p < 0.0001). And at the cut-off value of 132.53 pg/mL for serum IL-38, the sensitivity and specificity were 79.12 and 74.73%, severally.
Variables related to serum IL-38
In all subjects, Spearman’s analysis revealed that serum IL-38 level was correlated with ALT, AST, PLT, FBG, HOMA-IR, QUICK, INS, CpS, HbA1c, WBC, PA, CREA, TRIG, HDL-C, BMI, IL-6, and 25(OH)D. Based on these results, we performed linear regression analysis using serum IL-38 level as the dependent variable and these above indexes as independent variables. AST, HOMA-IR, and TRIG were all independently linked to serum IL-38 in a multivariate linear regression model (Table 4).
Discussion
The current study demonstrated that serum IL-38 was considerably upregulated in NAFLD patients. Simultaneously, the frequency of NAFLD increased as serum IL-38 levels increased, as revealed by the analysis of serum IL-38 level tertiles. Furthermore, level of IL-38 was related with NAFLD severity, as measured by ultrasonography. Finally, correlation analyses revealed significant relationships between serum IL-38 levels and parameters related to liver injury, inflammation, and insulin resistance in individuals with NAFLD or in all participants. Indicators of liver injury, inflammation, lipids and insulin resistance were considerably higher in NAFLD cases than in controls. As we know, this is the first study to exhibit a link between serum IL-38 and NAFLD.
Investigations have showed that IL-38 participated in pathogenic processes of several inflammatory disorders [22,23,24]. Understanding IL-38 and its biological roles may bring about the exploration of new diagnostic and therapeutic strategies. A previous study on ischaemic stroke revealed that serum IL-38 levels might be an early predictor for this condition [25]. In a study of systemic lupus erythematosus, high level of circulating IL-38 was found to be correlated with high risk of renal complications [26]. Moreover, in osteoarthritis patients, higher IL-38 levels indicated severe disease activity [27]. In Graves’ disease and Hashimoto’s thyroiditis, two types of thyroid inflammatory diseases, low serum IL-38 levels were observed [28]. These results suggest that IL-38 may exhibit anti- or pro-inflammatory actions in different pathological states, similar to its family members [15]. Specifically, in metabolic diseases, researchers have found that plasma IL-38 increased in diabetes patients and was positively associated with markers of liver and kidney function, glucose metabolism, and serum lipids, which supported IL-38 as an associated factor of inflammation and/or altered metabolism [15, 19]. Recent studies have established associations between NAFLD, inflammatory responses, and insulin resistance [29,30,31,32]. IL-38 has anti-inflammatory effects, and it was found that IL-38 can increase the expression of GATA3 and glucose transporter type 4 mRNA in adipocytes and inhibit the production of IL-6 and IL-1β, suggesting that IL-38 can inhibit human adipocyte differentiation and inflammatory response [33]. The hydrodynamic delivery of recombinant IL-38 lowered liver fat content and the degree of insulin resistance in obese mice [18]. In addition, it attenuated the secretion of inflammatory mediators. NAFLD is charactered by abnormal liver fat deposition and insulin resistance, both of which have been proven to be closely linked to inflammation [34]. As observed in the present study, indicators related to these pathological processes exhibited abnormalities. Accordingly, we hypothesise that IL-38 may be related to the homeostasis of liver metabolism in NAFLD.
Our study revealed a higher concentration of anti-inflammatory IL-38 in NAFLD subjects, suggesting IL-38 could be part of a feedback loop to attenuate the observed inflammation. However, as the level of IL-38 upregulated in the tertiles, the frequency and OR of NAFLD also increased. Furthermore, serum levels of IL-38 showed a positive correlation with NAFLD when analysed using binary logistic regression and the ROC curve. Lastly, higher IL-38 levels were observed to be closely correlated with insulin resistance (INS, CpS, HOMA-IR, and QUICK), liver injury (AST and ALT), and inflammation [IL-6 and 25(OH)D]. Furthermore, the results also demonstrated AST, HOMA-IR, and TRIG were independently associated with IL-38 levels in all participants. Indeed, increased serum IL-38 has been found in chronic hepatitis B sufferers, indicating ongoing liver damage, as reflected by its positive correlation with IL-6 and/or AST [35]. Moreover, in a type 2 diabetes case-control research, IL-38 was also reported to be relevant for HOMA-IR and TRIG [19]. For the correlation between IL-38 and 25(OH) D, reports are scarce; however, NAFLD has been associated with reduced levels of 25(OH)D [36], which was confirmed to contribute to oxidative stress modulation, secretion of cytokines, and hepatocyte apoptosis [37,38,39]. These findings suggest that the level of IL-38 could be a measure of liver injury and/or insulin resistance; however, we were unable to determine whether a higher level of IL-38 was a provoker of insulin resistance or liver injury. Nevertheless, our results support the idea that IL-38 participates in NAFLD pathogenesis, possibly by modulating inflammation and insulin resistance. The identification of serum biomarkers associated with NAFLD is of clinical importance [40]. We investigated whether serum IL-38 level could be utilized as a biomarker of NAFLD. The ROC curve displayed a statistically significant AUC of 0.859. However, due to the relatively small sample size included in this study, the adequacy of serum IL-38 to distinguish NAFLD needs to be further approved in studies with large sample populations. Other limitations of the present research include the fact that all participants enrolled were from an urban area in China, which may have led to selection bias. Additionally, the gold standard for NAFLD diagnosis is liver biopsy instead of ultrasonography, the method selected for the present study. Moreover, on account of the nature of this investigation, we cannot build a causal link between serum IL-38 levels and NAFLD.
Conclusions
Circulating IL-38 concentration was associated with NAFLD. Furthermore, increased IL-38 was also accompanied by a higher NAFLD risk and correlated with indicators of insulin sensitivity and liver injury. This suggests that IL-38 could serve as a promising biomarker in NAFLD. However, further in-depth exploration is required to illuminate the mechanisms behind the link between IL-38 and NAFLD.
Availability of data and materials
The data of the current study are available from the corresponding author on reasonable request.
Abbreviations
- NAFLD:
-
Non-alcoholic fatty liver disease
- NFs:
-
NAFLD patients
- HCs:
-
Healthy controls
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- ALB:
-
Albumin
- PA:
-
Prealbumin
- TRIG:
-
Triglycerides
- HDL-C:
-
High density liptein cholesterol
- CREA:
-
Creatinine
- CHOL:
-
Total cholesterol
- LDL-C:
-
Low-density lipoprotein cholesterol
- INS:
-
Insulin
- CpS:
-
C-peptide
- IL-6:
-
Interleukin-6
- FBG:
-
Fasting blood glucose
- HbA1c:
-
Hemoglobin A1c
- CRP:
-
C-reactive protein
- 25(OH)D:
-
25 hydroxy-vitamin D
- BMI:
-
Body mass index
- NFS:
-
Non-alcoholic fatty liver disease fibrosis score
- HOMA-IR:
-
Homoeostasis model assessment of IR
- QUICK:
-
Quantitative insulin sensitivity check index
- WBC:
-
White blood cell
- NEU:
-
Neutrophil
- PLT:
-
Platelet
- ELISA:
-
Enzyme-linked immunosorbent assay
- ROC:
-
Receiver operating characteristic
- ORs:
-
Odd ratios
References
Petroni ML, Brodosi L, Bugianesi E, Marchesini G. Management of non-alcoholic fatty liver disease. BMJ. 2021;372:m4747.
Perdomo CM, Frühbeck G, Escalada J. Impact of nutritional changes on nonalcoholic fatty liver disease. Nutrients. 2019;11(3):677.
Murag S, Ahmed A, Kim D. Recent epidemiology of nonalcoholic fatty liver disease. Gut Liver. 2021;15(2):206–16.
Cotter TG, Rinella M. Nonalcoholic fatty liver disease 2020: the state of the disease. Gastroenterology. 2020;158(7):1851–64.
Paik JM, Golabi P, Younossi Y, Srishord M, Mishra A, Younossi ZM. The growing burden of disability related to nonalcoholic fatty liver disease: data from the global burden of disease 2007-2017. Hepatol Commun. 2020;4(12):1769–80.
Loomba R, Lim JK, Patton H, El-Serag HB. AGA clinical practice update on screening and surveillance for hepatocellular carcinoma in patients with nonalcoholic fatty liver disease: expert review. Gastroenterology. 2020;158(6):1822–30.
Harrison SA. NASH, from diagnosis to treatment: where do we stand? Hepatology. 2015;62(6):1652–5.
Hydes T, Brown E, Hamid A, Bateman AC, Cuthbertson DJ. Current and emerging biomarkers and imaging modalities for nonalcoholic fatty liver disease: clinical and research applications. Clin Ther. 2021;43(9):1505–22.
Lefere S, Puengel T, Hundertmark J, et al. Differential effects of selective- and pan-PPAR agonists on experimental steatohepatitis and hepatic macrophages. J Hepatol. 2020;73(4):757–70.
Liu Y, Chen H, Wang J, Zhou W, Sun R, Xia M. Association of serum retinoic acid with hepatic steatosis and liver injury in nonalcoholic fatty liver disease. Am J Clin Nutr. 2015;102(1):130–7.
Mohamed AA, Sabry S, Abdallah AM, et al. Circulating adipokines in children with nonalcoholic fatty liver disease: possible noninvasive diagnostic markers. Ann Gastroenterol. 2017;30(4):457–63.
Baselli GA, Dongiovanni P, Rametta R, et al. Liver transcriptomics highlights interleukin-32 as novel NAFLD-related cytokine and candidate biomarker. Gut. 2020;69(10):1855–66.
Fontes-Cal TCM, Mattos RT, Medeiros NI, et al. Crosstalk between plasma cytokines, inflammation, and liver damage as a new strategy to monitoring NAFLD progression. Front Immunol. 2021;12:708959.
van de Veerdonk FL, de Graaf DM, Joosten LA, Dinarello CA. Biology of IL-38 and its role in disease. Immunol Rev. 2018;281(1):191–6.
Han MM, Yuan XR, Shi X, et al. The pathological mechanism and potential application of IL-38 in autoimmune diseases. Front Pharmacol. 2021;12:732790.
Liu Y, Chen T, Zhou F, Mu D, Liu S. Interleukin-38 increases the insulin sensitivity in children with the type 2 diabetes. Int Immunopharmacol. 2020;82:106264.
Yu Z, Liu J, Zhang R, et al. IL-37 and 38 signalling in gestational diabetes. J Reprod Immunol. 2017;124:8–14.
Xu K, Sun J, Chen S, et al. Hydrodynamic delivery of IL-38 gene alleviates obesity-induced inflammation and insulin resistance. Biochem Biophys Res Commun. 2019;508(1):198–202.
Gurău F, Silvestrini A, Matacchione G, et al. Plasma levels of interleukin-38 in healthy aging and in type 2 diabetes. Diabetes Res Clin Pract. 2021;171:108585.
Fan JG, Wei L. Zhuang H; National Workshop on fatty liver and alcoholic liver disease, Chinese Society of Hepatology, Chinese Medical Association; fatty liver disease expert committee, Chinese medical doctor association. Guidelines of prevention and treatment of nonalcoholic fatty liver disease (2018, China). J Dig Dis. 2019;20(4):163–73.
Zeng MD, Fan JG, Lu LG, et al. Guidelines for the diagnosis and treatment of nonalcoholic fatty liver diseases. J Dig Dis. 2008;9(2):108–12.
Tsang MS, Sun X, Wong CK. The role of new IL-1 family members (IL-36 and IL-38) in atopic dermatitis, allergic asthma, and allergic rhinitis. Curr Allergy Asthma Rep. 2020;20(8):40.
Garraud T, Harel M, Boutet MA, Le Goff B, Blanchard F. The enigmatic role of IL-38 in inflammatory diseases. Cytokine Growth Factor Rev. 2018;39:26–35.
Boutet MA, Nerviani A, Pitzalis C. IL-36, IL-37, and IL-38 cytokines in skin and joint inflammation: a comprehensive review of their therapeutic potential. Int J Mol Sci. 2019;20(6):1257.
Zare Rafie M, Esmaeilzadeh A, Ghoreishi A, Tahmasebi S, Faghihzadeh E, Elahi R. IL-38 as an early predictor of the ischemic stroke prognosis. Cytokine. 2021;146:155626.
Rudloff I, Godsell J, Nold-Petry CA, et al. Brief report: Interleukin-38 exerts Antiinflammatory functions and is associated with disease activity in systemic lupus Erythematosus. Arthritis Rheumatol. 2015;67(12):3219–25.
Abassifard M, Khorramdelazad H, Rezaee S, Jafarzadeh A. Higher circulating concentration of Interleukin-38 in patients with knee osteoarthritis: its association with disease severity. Iran J Allergy Asthma Immunol. 2021;20(1):114–9.
Xu J, Huang G, Weng L, et al. Low serum interleukin-38 levels in patients with Graves' disease and Hashimoto's thyroiditis. J Clin Lab Anal. 2022;36(1):e24101.
Barbier L, Ferhat M, Salamé E, et al. Interleukin-1 family cytokines: keystones in liver inflammatory diseases. Front Immunol. 2019;10:2014.
Glass CK, Olefsky JM. Inflammation and lipid signaling in the etiology of insulin resistance. Cell Metab. 2012;15(5):635–45.
Barra NG, Henriksbo BD, Anhê FF, Schertzer JD. The NLRP3 inflammasome regulates adipose tissue metabolism. Biochem J. 2020;477(6):1089–107.
Tilg H, Moschen AR. Insulin resistance, inflammation, and non-alcoholic fatty liver disease. Trends Endocrinol Metab. 2008;19(10):371–9.
Li Y, Chen S, Sun J, Yu Y, Li M. Interleukin-38 inhibits adipogenesis and inflammatory cytokine production in 3T3-L1 preadipocytes. Cell Biol Int. 2020;44(11):2357–62.
Pafili K, Roden M. Nonalcoholic fatty liver disease (NAFLD) from pathogenesis to treatment concepts in humans. Mol Metab. 2021;50:101122.
Wang HJ, Jiang YF, Wang XR, Zhang ML, Gao PJ. Elevated serum interleukin-38 level at baseline predicts virological response in telbivudine-treated patients with chronic hepatitis B. World J Gastroenterol. 2016;22(18):4529–37.
Eliades M, Spyrou E, Agrawal N, et al. Meta-analysis: vitamin D and non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2013;38(3):246–54.
George N, Kumar TP, Antony S, Jayanarayanan S, Paulose CS. Effect of vitamin D3 in reducing metabolic and oxidative stress in the liver of streptozotocin-induced diabetic rats. Br J Nutr. 2012;108(8):1410–8.
Roth CL, Elfers CT, Figlewicz DP, et al. Vitamin D deficiency in obese rats exacerbates nonalcoholic fatty liver disease and increases hepatic resistin and toll-like receptor activation. Hepatology. 2012;55(4):1103–11.
Zhang A, Wang Y, Xie H, Zheng S. Calcitriol inhibits hepatocyte apoptosis in rat allograft by regulating apoptosis-associated genes. Int Immunopharmacol. 2007;7(8):1122–8.
Castera L, Friedrich-Rust M, Loomba R. Noninvasive assessment of liver disease in patients with nonalcoholic fatty liver disease. Gastroenterology. 2019;156(5):1264–1281.e4.
Acknowledgments
We thanks Editage (www.editage.cn) for language editing.
Funding
Funding was provided by grant from National Natural Science Foundation of China (No. 81860166).
Author information
Authors and Affiliations
Contributions
JC and GX designed the study. SZ, LH, JT, and FK contributed to enrollment of patients and collection of blood samples or clinical data. This study was conceived by GX. JC and GX wrote and revised the manuscript. The final version of the work was examined and approved by all authors, who also participated to the debate.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Ethics Committee of Jiujiang No.1 People’s Hospital and all patients provided signed informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that no conflict of interest existed.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Cao, J., Hua, L., Zhang, S. et al. Serum interleukin-38 levels correlated with insulin resistance, liver injury and lipids in non-alcoholic fatty liver disease. Lipids Health Dis 21, 70 (2022). https://doi.org/10.1186/s12944-022-01676-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12944-022-01676-0