Abstract
Background
Left ventricular hypertrophy (LVH) is an important risk factor for cardiovascular and all-cause mortality. Previous studies reported conflicting results concerning the relationship between serum lipid levels and left ventricular geometry pattern. We sought to explore the relationship between standard serum lipid profile measures with left ventricular geometry pattern in obese children.
Patients and methods
In this cross-sectional study, a total of 70 obese children were examined. Fasting blood samples were taken to measure total cholesterol, low density lipoprotein cholesterol (LDL-C), high density lipoprotein cholesterol (HDL-C), triglycerides (TGs), glucose, and insulin. Based on these values TG/HDL ratio, BMI and HOMA index were calculated. We also measured the average 24-h ambulatory systolic blood pressure (SBP) and two-dimensional (2/D) transthoracic echocardiography was performed to determine left ventricular mass index (LVMI) and relative wall thickness (RWT). Multiple regression analyses were conducted to explore relationships between study variables and the LVMI or RWT as outcome variables. The final model with LVMI included TG/HDL ratio, BMI, 24 h-average SBP, age and sex, while for the RWT we included BMI, insulin, age and sex.
Results
Our study included 70 children (65.71% boys and 34.29% girls) median age (14 years, IQR = 12–16)." We demonstrated independent and positive association of TG/HDL ratio, BMI and 24 h-average SBP with LVMI (effect = 3.65, SE = 1.32, p < 0.01; effect = 34.90, SE = 6.84, p < 0.01; effect = 0.32, SE = 0.12, p < 0.01, respectively). On the other hand, in model with RWT as outcome variable, only BMI and insulin were significantly linked (BMI: effect = 13.07, SE = 5.02, p = 0.01 Insulin: effect = 2.80, SE = 0.97).
Conclusion
Increased TG/HDL ratio in obese children is associated with the development of eccentric left ventricular hypertrophy while increased BMI and insulin were associated with concentric left ventricular hypertophy.
Similar content being viewed by others
Introduction
It is well established that the left ventricular hypertrophy (LVH) is an important risk factor for cardiovascular and all-cause mortality and morbidity [1,2,3]. A few cross-sectional studies have shown that male sex, age, genetics, hemodynamic overload, obesity, hormonal and metabolic factors correlate with LVH [4, 5]. Previous studies have reported conflicting results concerning the relationship between serum lipid levels and left ventricular geometry pattern, both in general population and patients with cardiovascular risk [6,7,8,9]. There are several proposed mechanisms through which hyperlipidemia may contribute to the left ventricular remodeling and hypertrophy These are a) accumulation of lipids in or around myocytes b) local and systemic inflammation c) insulin resistance d) oxidative stress, especially mitochondrial oxidative stress e) neuro-humoral effects including both autonomic-nervous-system dysfunction and other hormonal influences, mainly the renin-angiotensin-aldosterone system (RAAS) [10,11,12,13,14,15].
Left ventricular hypertrophy is a common echocardiographic abnormality in obese children which can’t be explained solely by hemodynamic mechanisms. On the other hand, altered lipid profile including elevated triglycerides (TG), VLDL (Very-Low-Density Lipoprotein) total cholesterol (TC) and low High-Density Lipoprotein (HDL) cholesterol is a frequent metabolic abnormality possible contributing to the development of cardiac hypertrophy in obese children [16, 17].
Recent studies suggest that besides direct accumulation of lipids in or around myocytes, other signaling pathways elicited by atherogenic dyslipidemia play a significant role in developing cardiac hypertrophy. It was shown that VLDL can promote aldosterone overproduction which in turn may induce LV remodeling independently of its hemodynamic effects on systemic BP [14, 18] In addition, the results of several cross-sectional and longitudinal studies reported that increased plasma aldosterone is associated with decreased HDL-C and increased triglycerides in patients with metabolic syndrome [19].
Related to mentioned researches this study aimed to explore the potential relationship between traditional cardiovascular risk factors and serum lipoprotein profile, with the left ventricular geometry pattern in obese children.
Material and methods
Study design
We conducted a cross-sectional survey on an initial sample of 70 obese children consecutively referred by the local and regional health units to our institution (Clinic of Pediatrics, Clinical Center Nis, Serbia) for further clinical evaluation of the obesity-related cardiovascular complication, from January 2016 to June 2018. The study inclusion criteria were that none of the children had positive history of antihypertensive drug treatment or other chronic diseases.
The study was approved by the Ethical Committee for Medical Research, (protocol number 01–9002-6). We obtained informed consent from the parents of children included in the study. Obesity was defined as a BMI at or above the 95th percentile for children and teens of the same age and sex according to the reference values published by the Centers for Disease Control and Prevention [20]. Body mass index (BMI) was calculated as weight (in kg) divided by height (in m) squared.
Covariates
In all study participants we measured: a) Standard lipid profile (Cholesterol, Triglycerides, LDL-C, and HDL-C) using UniCel DxC Systems (Beckman Coulter, Inc); b) Insulin level - using a chemiluminescent assay (AccessDxI800; Beckman Coulter Inc., Brea, CA); c) plasma glucose level. All blood specimens were taken after an overnight fasting. The ambulatory 24 h SBP was measured using the Scanlight measurement device (Scanlight III; I.E.M GmbH, Germany), which, according to certificate of equivalence DIN EN 9001:2000, 13,485, CMDCAS, is functionally and technically identical to the Mobil-O-Graph New Generation device that was validated in children.
The HOMA index was calculated as the product of the fasting plasma insulin level (microU/mL) and the fasting plasma glucose level (mmol/L) divided by 22.5. We further calculated the TG/HDL ratio in every participant.
Left ventricular structure
We studied left ventricular structure (geometry) through 2-D standard echocardiography and the left ventricular myocardial mass index (LVMI) was measured by M-mode echocardiography using the Devereux formula indexed by body height to the power of 2.7.
Relative wall thickness (RWT) was defined as LV wall thickness + septal thickness relative to the internal dimensions of the LV [21].
LV geometric patterns were defined based on whether RWT and/or LVM are normal versus increased: normal geometry (LVM and RWT are normal), concentric remodeling (increased RWT but normal LVM), concentric hypertrophy (LVM and RWT are increased) and eccentric hypertrophy (increased LVM with normal RWT). All echocardiographic measurements were performed in triplicate by the same specialized cardiologist, who was unaware of the subject’s BP, using the Acuson Sequoia 256 Cardiac Ultrasound Machine, 2,5–3,5 MHz probe.
Statistical analysis
We performed descriptive analyses of the demographic and clinical characteristics of the participants using mean, median, range and standard deviation for continuous and frequencies for the categorical variables. The assumption of normality was checked through the Shapiro-Wilk test and variables with a skewed distribution (age, BMI, insulin, TG, HDL-C, TG/HDL and HOMA) were logarithmically transformed to achieve normal distribution. The strength of correlation between different variables was evaluated using the Pearson’s linear correlation. Multiple regression analyses with stepwise selection were conducted to study relationships between age, sex, BMI, average 24 h - SBP, serum lipoprotein concentration and LVMI or RWT as outcome variables. Only variables attaining statistical significance in the univariate analysis at a 0.05 level as well as variables known from previous research to be associated with LVMI and RWT were kept in the final model. The p-value < 0.05 was considered statistically significant. All statistical analyses were performed using SPSS version 20 (SPSS, Chicago, IL, USA).
Results
Characteristics
Our study included 70 children (65.71% boys and 34.29% girls) with median age (14 years, IQR = 12–16) that were referred to our institution for further clinical assessment. Median BMI was 28.65 kg/m2 (IQR = 26.7–31.3) while 24 h average SBP was 123.77 mmHg (SD = 8.78). Median cholesterol was 4.4 mmol/L (IQR = 3.96–4.74), median triglycerides 1.19 mmol/L (IQR = 0.78–1.86) while LDL-C and HDL-C had median values of 2.4 mmol/L (IQR = 1.9–2.7) and 1.3 mmol/L (IQR = 1.08–1.41), respectively. From these values we calculeted the TG/HDL ratio which had median of 0.86 (IQR = 0.58–1.57). Insulin measurement was available for 58 (82.86%) children in which a median value was 25 μU/mL (IQR = 18–47). Left ventricular mass index was 46 g/m2.7 (IQR = 42–55) while the relative wall thickness was 36.5% (IQR = 31–41). Eccentric left ventricular hypertrophy (LVH) was found in 14 (20%) participants, concentric LVH in 10 (14.29%) and concentric remodeling in 10 (14.29%), while 36 (51.43%) had normal left ventricular geometry. The demographic and clinical characteristics of all participants are described in Table 1.
Correlations
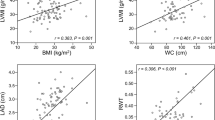
Based on the analysis on entire sample of children, triglycerides positively correlated with left ventricular mass index (r = 0.32, p < 0.01 while TG/HDL ratio positively correlated with left ventricular mass index and relative wall thickness (r = 0.33, p < 0.01; r = 0.24, p < 0.05, respectively). The other serum lipoprotein levels did not correlate with left ventricular mass index and left ventricular relative wall thickness.
Of other examined variables we found positive correlation of BMI and LVMI and RWT (r = 0.55 p < 0.01; r = 0.39, p < 0.01, respectively); 24 h ambulatory average SBP and LVMI (r = 0.35, p < 0.01) and RWT (r = 0.33, p < 0.01) while insulin and HOMA index positively correlated only with the RWT (r = 0.41, p < 0.01; r = 0.43, p < 0.01, respectively) (Table 2).
Regression analyses
Results from the linear regression analyses on the relationship between LVMI and RWT as an outcome variables and serum lipoproteins, BMI, Insulin, 24 h-average SBP and HOMA index are presented in Table 2.
Using multiple regression analyses with stepwise adjustment in a model with LVMI as a dependent variable and age, gender, BMI, 24 h-average SBP, TC, LDL-C, HDL-C, TG/HDL ratio and insulin as independent parameters, the TG/HDL ratio showed statistically significant independent positive association with LVMI (effect = 3.65, SE = 1.32, p < 0.01). Besides, BMI and the 24 h-average SBP also showed independent positive association with LVMI (BMI effect = 34.90, SE = 6.84, p < 0.01; 24 h-average SBP: effect = 0.32, SE = 0.12, p < 0.01), Table 3.
Since the TG also demonstrated positive correlation with the LVMI, we tried another analysis using TG instead of the TG/HDL ratio in the regression model and did not obtain stitisticaly significant result (results not shown).
On the other hand, in a model with RWT as a dependent variable, and age, gender, BMI, 24 h- average SBP, cholesterol, HDL-C, LDL-C, TG/HDL and insulin as independent parameters, only BMI and insulin showed statistically significant independent positive associations with RWT (BMI: effect = 13.07, SE = 5.02, p = 0.01; insulin: effect = 2.8, SE = 0.97, p < 0.01), as reported in Table 3.
Discussion
Based on the obtained results we showed that in obese children TG/HDL ratio was postively associated with eccentric left ventricular hypertrophy, and BMI and insulin levels are postively associated with concentric left ventricle hypertrophy.
To data only a few studies have been published focusing on the relationship between serum lipid and lipoprotein levels and left ventricular mass and geometry both in children and adults.
Sundstrom et al. showed that dyslipidaemia and unfavorable fatty acid profile at age 50 predicted the prevalence of LVH at age 70, to a similar degree as hypertension and obesity [6]. One autoptic study of human hearts indicated that the fat deposition in the left ventricle constitutes a direct risk of cardiac hypertrophy [22]. In addition, by using proton magnetic resonance spectroscopy Kankaanpää et al. demonstrated accumulation of TG in myocardium of moderately obese subjects. They reported that free fatty acid levels were a significant predictor of LV mass, whereas myocardial and epicardial fat were more strongly related to LV work and mechanical load [23]. Likewise, Kaminaga and others concluded that hypertrophic cardiomyopathy may be associated with the presence of myocardial fat in the LV with a thickened wall [24]. One preclinical study has recently shown that increased level of glycosphingolipids, may be an independent factor contributing to cardiac hypertrophy [8].
As for children, Dabas et al. reported no correlation between lipids and LV parameters in adolescents with type 1 diabetes [25]. On the other hand Di Bonito at al. identified a positive association between TG/HDL ratio and left ventricular mass and RWT independently of other cardiovascular (CV) risk factors in obese children which is partly in line with our results [26].
In this respect, our findings are also in agreement with those reported by Al-Daydamony and El-Tahlawi et al., who found a significant positive correlation between LVMI and TG levels in patients with metabolic syndrome without hypertension [27].
The disappearance of any relationship between TG/HDL ratio with RWT in multivariable regression equation correlation, indicate that BMI and 24 h-average SBP rather than insulin resistance (of which TG/HDL ratio is a surrogate marker) are associated with concentric left ventricular hypertrophy [9, 28,29,30].
Although the pathophysiological explanation of these results is not straightforward, and we didn’t measure aldosterone as well as VLDL levels in our patients, we can’t exclude the effects of potential signaling pathways activated by the VLDL which resulted in aldosterone overproduction and development of left ventricular hypertrophy. It is now appreciated that left ventricular hypertrophy (LVH) in hypertensive obese patients is not only mediated via clear-cut increase in blood pressure but also by various neuro-hormonal and metabolic factors that independently exert trophic effects on myocytes and non-myocytes in the heart [31, 32]. Experimental and clinical evidence clearly indicates that protracted exposure to inappropriately elevated aldosterone levels induces cardiac inflammation and interstitial fibrosis leading to a maladaptive remodeling in the heart and changes in left ventricular structure and function, regardless of the level of blood pressure. In particular, recent studies reported a possible involvement of aldosterone as a trigger of the activation of p38 mitogen-activated protein kinase (MAPK) and nicotinamide riboside kinase (nRK1/2) which have been involved in signal transduction pathways associated with cardiac hypertrophy [33, 34].
Recently it was shown that adipose tissue, via secretion of factors such as leptin, stimulates adrenal aldosterone secretion as well as that VLDL might induce aldosterone overproduction via induction of steroidogenic acute regulatory (StAR) protein and aldosterone synthase (CYP11B2) expressio n[35, 36]. Given the fact that VLDLs are major transporters of TG accounting for approximately 85% of their weight, as well as the strong positive correlation between TG levels and TG/HDL ratio (r = 0,885) in our study, we can’t exclude the possible link between atherogenic dyslipidemia (high TG/HDL ratio), TG, aldosterone and LVH or TG -VLDL - aldosterone - LVH continuity in obese children.
Furthermore, Goodfriend et al. described a negative association between aldosterone concentration and HDL-C and a positive correlation between aldosterone and triglycerides in a group of 30 volunteers. Inverse association between aldosterone levels with HDL-C in the general population was also confirme by Hannich et al. [19, 36] They speculated that HDL-C may inhibit adrenal aldosterone secretion by modulating the sensitivity of the adrenal zona glomerulosa [36].These results may possibly explain our finding that only TG/HDL ratio but not only TG levels were associated with eccentric LVH.
We would also like to point out the findings of Brady et al. who recently reported for the first time that serum aldosterone activity was higher in obese children with LVH when compared to those without LVH. Likewise, Somloova et al. found in patients with primary aldosteronism that HDL-C was markedly lower and BMI and TG were significantly higher in those having idiopathic hyperaldosteronism (IHA) than in patients with aldosterone-producing adenoma (APA). In addition, the metabolic syndrome was more prevalent in IHA than in APA [37].
In our opinion high TG/HDL ratio (indirectly high VLDL levels and low HLD levels) which has been shown to be associated with increased aldosterone concentration may be one possible explanation of these results. Since the magnitude of aldosterone elevation in obese children has not been object of our scientific research, we guess that further research, including direct measurement of serum aldosteron, VLDL levels and HDL-C levels may identify new subset of at risk obese patients for LVH, as well as novel targets for the treatment of obese children with LVH but without hypertension.
An alternative explanation of our results might be explained by facts that high TG levels (indirectly high VLDL levels) may affect the synthesis of pro-inflammatory cytokines TNF-α further promoting adverse cardiac remodeling, characterised by increased total are metalloproteases (MMP) activity and increased fibrosis [38]. The functional crosstalk between angiotensin II (Ang II) and tumor necrosis factor (TNF)-α has also been shown to cause adverse left ventricular remodeling and hypertrophy in hypertension [9, 39].
Our second finding that BMI and insulin levels are associated with developing concentric left ventricle geometry pattern in obese children was recently confirmed using magnetic resonance imaging in the multi-ethnic study of atherosclerosis and is accordance with results of Gubbio study in adults and results of Urbina et al. study in children [40].
Strengths and limitation
There are several limitations of this study including small number of participants as well as the, lack of control group of metabolically healthy obese children. Also, we cannot exclude the effects of some potential genetic determinants and environmental effects on left ventricular geometry that were not considered in our study which may have ultimately influenced the correlations reported here. However, this is only the first report suggesting a possible existence of TG -VLDL - aldosterone - LVH continuity in obese children, and a possible pathophysiological mechanism to explain very common practical clinical issue in pediatric cardiology such as “frequent finding of LVH in normotensive obese children”. Further studies with larger sample size are required to explore the nature of the changes in cardiac structure (geometry) as a result of atherogenic dyslipidemia and possible aldosterone overproduction.
Conclusions
To the best of our knowledge this is the first study to suggest the association of dyslipidemia asexpressed by the TG/HDL ratio with the development of eccentric left ventricular hypertrophy in obese children. The mechanisms, through which obesity causes left ventricular hypertorphy are still an area of research and require further investigations.
Availability of data and materials
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- TC:
-
Total cholesterol
- LDL:
-
Low Density Lipoprotein,
- HDL:
-
High Density Lipoprotein
- TG:
-
Triglycerides
- HOMA:
-
Homeostatic model assessment index
- SBP:
-
Systolic Blood Pressure
- BMI:
-
Body mass index
- LVMI:
-
Left ventricular mass
- RWT:
-
Relative wall thickness
- LVH:
-
Left ventricular hypertrophy
- VLDL:
-
Very-Low-Density Lipoprotein
References
Verdecchia P, Schillaci G, Borgioni C, et al. Adverse prognostic significance of concentric remodeling of the left ventricle in hypertensive patients with normal left ventricular mass. J Am Coll Cardiol. 1995;25(4):871–8. https://doi.org/10.1016/0735-1097(94)00424-O.
Koren MJ, Devereux RB, Casale PN, Savage DD, Laragh JH. Relation of left ventricular mass and geometry to morbidity and mortality in uncomplicated essential hypertension. Ann Intern Med. 1991;114(5):345. https://doi.org/10.7326/0003-4819-114-5-345.
Lorell BH, Carabello BA. Left ventricular hypertrophy: pathogenesis, detection, and prognosis. Circulation. 2000;102(4):470–9. https://doi.org/10.1161/01.CIR.102.4.470.
Bjelakovic B, Vukomanovic V, Saranac LJ, Krstic M, Jović M, Petrovski V. Blood pressure variability and left ventricular mass index ion children with true ambulatory hypertension. Prev Ped. 2016;2(1–2):45–9.
Sciacqua A, Cimellaro A, Mancuso L, et al. Different patterns of left ventricular hypertrophy in metabolically healthy and insulin-resistant obese subjects. Nutrients. 2020. https://doi.org/10.3390/nu12020412.
Sundström J, Lind L, Vessby B, Andrén B, Aro A, Lithell H. Dyslipidemia and an unfavorable fatty acid profile predict left ventricular hypertrophy 20 years later. Circulation. 2001;103(6):836–41. https://doi.org/10.1161/01.cir.103.6.836.
Levelt E, Mahmod M, Piechnik SK, et al. Relationship between Left Ventricular Structural and Metabolic Remodelling in Type 2 Diabetes Mellitus. Diabetes. 2015;65(1):db150627. https://doi.org/10.2337/db15-0627.
Mishra S, Bedja D, Amuzie C, Avolio A, Chatterjee S. Prevention of cardiac hypertrophy by the use of a glycosphingolipid synthesis inhibitor in ApoE−/− mice. Biochem Biophys Res Commun. 2015;465(1):159–64. https://doi.org/10.1016/j.bbrc.2015.07.159.
Ferrara AL, Vaccaro O, Cardoni O, Panarelli W, Laurenzi M, Zanchetti A. Is there a relationship between left ventricular mass and plasma glucose and lipids independent of body mass index? Results of the Gubbio study. Nutr Metab Cardiovasc Dis. 2003;13(3):126–32. https://doi.org/10.1016/S0939-4753(03)80171-0.
Ärnlöv J, Lind L, Sundström J, Andrén B, Vessby B, Lithell H. Insulin resistance, dietary fat intake and blood pressure predict left ventricular diastolic function 20 years later. Nutr Metab Cardiovasc Dis. 2005. https://doi.org/10.1016/j.numecd.2004.10.002.
Maulik SK, Kumar S. Oxidative stress and cardiac hypertrophy: a review. Toxicol Mech Methods. 2012. https://doi.org/10.3109/15376516.2012.666650.
Kotsis V, Stabouli S, Papakatsika S, Rizos Z, Parati G. Mechanisms of obesity-induced hypertension. Hypertens Res. 2010. https://doi.org/10.1038/hr.2010.9.
Manrique C, Lastra G, Sowers JR. New insights into insulin action and resistance in the vasculature. Ann N Y Acad Sci. 2014;1311:138–50. https://doi.org/10.1111/nyas.12395.
Catena C, Colussi G, Brosolo G, Novello M, Sechi LA. Aldosterone and left ventricular remodeling. Horm Metab Res. 2015;47(13):981–6. https://doi.org/10.1055/s-0035-1565055.
Piratello AC, Moraes-Silva I, Paulini J, et al. Renin angiotensin system and cardiac hypertrophy after sinoaortic denervation in rats. Clinics (Sao Paulo) 2010;65(12):1345–1350. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3020347&tool=pmcentrez&rendertype=abstract. Accessed 12 Sept 2015.
Cicero AFG, Fogacci F, Giovannini M, Bove M, Debellis G, Borghi C. Effect of quantitative and qualitative diet prescription on children behavior after diagnosis of heterozygous familial hypercholesterolemia. Int J Cardiol. 2019. https://doi.org/10.1016/j.ijcard.2019.05.069.
Cruz ML, Goran MI. The metabolic syndrome in children and adolescents. Curr Diab Rep. 2004;4(1):53–62. https://doi.org/10.1007/s11892-004-0012-x.
Tsai YY, Rainey WE, Johnson MH, Bollag WB. VLDL-activated cell signaling pathways that stimulate adrenal cell aldosterone production. Mol Cell Endocrinol. 2016;433:138–46. https://doi.org/10.1016/j.mce.2016.05.018.
Hannich M, Wallaschofski H, Nauck M, et al. Physiological aldosterone concentrations are associated with alterations of lipid metabolism: observations from the general population. Int J Endocrinol. 2018. https://doi.org/10.1155/2018/4128174.
Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, et al. CDC growth charts: United States. Adv Data 2000;(314):1–27. http://www.ncbi.nlm.nih.gov/pubmed/11183293. Accessed May 1, 2017.
Richey PA, Disessa TG, Somes GW, Alpert BS, Jones DP. Left ventricular geometry in children and adolescents with primary hypertension. Am J Hypertens. 2010;23(1):24–9. https://doi.org/10.1038/ajh.2009.164.
da Silva RMS, de Mello RJV. Fat deposition in the left ventricle: descriptive and observacional study in autopsy. Lipids Health Dis. 2017;16(1):86. https://doi.org/10.1186/s12944-017-0475-9.
Kankaanpää M, Lehto H-R, Pärkkä JP, et al. Myocardial triglyceride content and epicardial fat mass in human obesity: relationship to left ventricular function and serum free fatty acid levels. J Clin Endocrinol Metab. 2006;91(11):4689–95. https://doi.org/10.1210/jc.2006-0584.
Kaminaga T, Naitou H, Hamada S, Takamiya M. Detection of myocardial fatty components with ultrafast CT. Nippon Igaku Hoshasen Gakkai zasshi Nippon acta Radiol. 1993;53(1):28–34.
Dabas A, Yadav S, Gupta VK. Lipid profile and correlation to cardiac risk factors and cardiovascular function in type 1 adolescent diabetics from a developing country. Int J Pediatr. 2014;2014:513460. https://doi.org/10.1155/2014/513460.
Di Bonito P, Moio N, Scilla C, et al. Usefulness of the high triglyceride-to-HDL cholesterol ratio to identify cardiometabolic risk factors and preclinical signs of organ damage in outpatient children. Diabetes Care. 2012;35(1):158–62. https://doi.org/10.2337/dc11-1456.
Al-Daydamony MM, El-Tahlawi M. What is the effect of metabolic syndrome without hypertension on left ventricular hypertrophy? Echocardiography. 2016:1–6. https://doi.org/10.1111/echo.13247.
Singh B, Saxena A. Surrogate markers of insulin resistance: a review. World J Diabetes. 2010;1(2):36–47. https://doi.org/10.4239/wjd.v1.i2.36.
He J, He S, Liu K, Wang Y, Shi D, Chen X. The TG/HDL-C ratio might be a surrogate for insulin resistance in Chinese nonobese women. Int J Endocrinol. 2014;2014. https://doi.org/10.1155/2014/105168.
Paternostro G, Pagano D, Gnecchi-Ruscone T, Bonser RS, Camici PG. Insulin resistance in patients with cardiac hypertrophy. Cardiovasc Res. 1999;42(1):246–53.
Yan Y, Liu J, Wang L, et al. Independent influences of excessive body weight and elevated blood pressure from childhood on left ventricular geometric remodeling in adulthood. Int J Cardiol. 2017. https://doi.org/10.1016/j.ijcard.2017.05.085.
Tsai YY, Rainey WE, Bollag WB. Very low-density lipoprotein (VLDL)-induced signals mediating aldosterone production. J Endocrinol. 2017;232(2):R115–29. https://doi.org/10.1530/JOE-16-0237.
Weber KT, Brilla CG. Pathological hypertrophy and cardiac interstitium: fibrosis and renin-angiotensin-aldosterone system. Circulation. 1991. https://doi.org/10.1161/01.CIR.83.6.1849.
Unger T, Li J. The role of the renin-angiotensin-aldosterone system in heart failure JRAAS - J Renin-Angiotensin-Aldosterone Syst 2004. https://doi.org/10.3317/jraas.2004.024.
Vecchiola A, Lagos CF, Carvajal CA, Baudrand R, Fardella CE. Aldosterone production and signaling Dysregulation in obesity. Curr Hypertens Rep. 2016. https://doi.org/10.1007/s11906-016-0626-9.
Goodfriend TL, Egan B, Stepniakowski K, Ball DL. Relationships among plasma aldosterone, high-density lipoprotein cholesterol, and insulin in humans. Hypertension. 1995. https://doi.org/10.1161/01.hyp.25.1.30.
Somloova Z, Widimsky J Jr, Rosa J, et al. The prevalence of metabolic syndrome and its components in two main types of primary aldosteronism. J Hum Hypertens. 2010;24(10):625–30. https://doi.org/10.1038/jhh.2010.65.
Sivasubramanian N, Coker ML, Kurrelmeyer KM, et al. Left ventricular remodeling in transgenic mice with cardiac restricted overexpression of tumor necrosis factor. Circulation. 2001;104(7):826–31. https://doi.org/10.1161/hc3401.093154.
Sriramula S, Francis J. Tumor necrosis factor - Alpha is essential for angiotensin II-induced ventricular remodeling: Role for oxidative stress. PLoS One. 2015;10(9). https://doi.org/10.1371/journal.pone.0138372.
Turkbey EB, McClelland RL, Kronmal RA, et al. The Impact of Obesity on the Left Ventricle. The Multi-Ethnic Study of Atherosclerosis (MESA). JACC Cardiovasc Imaging. 2010. https://doi.org/10.1016/j.jcmg.2009.10.012.
Acknowledgments
This work was supported by a grant No 175092 from the Ministry of Science and Technology of the Republic of Serbia.
Funding
This work was supported by a grant No 175092 from the Ministry of Science and Technology of the Republic of Serbia.
Author information
Authors and Affiliations
Contributions
BB, NK, AK.SS, LS, BM Design of work. CS, MB, SL Have drafted the work or substantively revised it. VV, MJ, SP analyzed and interpreted the study data. All authors read and approved the final manuscript.”
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Ethical Committee for Medical Research, Medical Faculty, University of Nis, (protocol number 01–9002-6).
Consent for publication
I Bojko Bjelakovic give my consent for information about myself to be published in Lipids in Health and Disease, manuscript number LHAD-D-20-00057 and corresponding author Bojko Bjelakovic.
I understand that the information will be published without my name attached, but that full anonymity cannot be guaranteed. I understand that the text and any pictures or videos published in the article will be freely available on the internet and may be seen by the general public. I have been offered the opportunity to read the manuscript. Signing this consent form does not remove my rights to privacy.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Bjelakovic, B., Stefanutti, C., Vukovic, V. et al. Lipid profile and left ventricular geometry pattern in obese children. Lipids Health Dis 19, 109 (2020). https://doi.org/10.1186/s12944-020-01285-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12944-020-01285-9