Abstract
Background
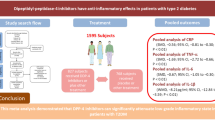
Dipeptidyl peptidase-4 inhibitors (DPP-4i) are emerging glucose-lowering agents through interacting with DPP-4 substrate, impact of which on systemic inflammation in type 2 diabetes mellitus (T2DM) remains unknown. This study aimed to evaluate the effect of DPP-4i on modulating serum levels of C-reactive protein (CRP) in T2DM.
Methods
PubMed, Cochrane library and Embase databases were searched. Randomized controlled trials (RCTs) with comparators were selected. A random-effects model was used for quantitative data analysis. Heterogeneity was evaluated with I2 index. Sensitivity analysis was performed using the one-study remove approach.
Results
Sixteen trials with 1607 patients with T2DM were included. Pooled analysis of DPP-4i demonstrated a significant decrease in serum CRP concentrations (− 0.86 mg/L, 95% CI, − 1.36 to − 0.36). No significant difference was found between DPP-4i and active comparators on serum CRP concentrations (0.64 mg/L, 95% CI, − 0.10 to 1.37). Pooled analysis proved to be stable and credible by sensitivity analysis. In subgroup analysis, changes in serum concentrations of CRP were significantly associated with short diabetes duration (− 0.23 mg/L, 95% CI, − 0.41 to − 0.05).
Conclusions
DDP-4i effectively reduced serum CRP levels and showed no stronger effect than traditional oral antidiabetic agents.
International Prospective Register for Systematic Review (PROSPERO) number: CRD42017076838.
Similar content being viewed by others
Background
Type 2 diabetes mellitus (T2DM) is a chronic metabolic disorder with decreased insulin action and hyperglycemia [1, 2]. T2DM and insulin resistance (IR) are also increasingly recognized as a chronic inflammatory state like atherosclerosis [3]. Meanwhile, persistent low-grade inflammation leads to peripheral IR and alleviating of inflammatory process improves IR and glucose handling [4].
As inflammation plays a key role in IR, T2DM and cardiovascular disease (CVD), a rational search for inflammatory markers was performed for a better prediction for CVD risks [5]. Among current downstream markers of inflammation, C-reactive protein (CRP) is a sensitive and dynamic protein for predicting systemic inflammation. It is an acute-phase protein synthesized by the liver, concentrations of which increase by up to 10,000-fold during acute responses to serious infection or major tissue damage. CRP was initially found to be negatively correlated with circulating insulin [6]. It was determined by high sensitivity ELISA kits and utilized to reflect acute inflammatory state in clinical practice. Whether or not CRP can be altered by dipeptidyl peptidase 4 inhibitors (DPP-4i) in T2DM remains uncertain.
Inflammation has been recognized as a major risk factor for T2DM. Systemic inflammation is often observed in overweight and obese subjects and elevated CRP concentrations are present in certain 27.6% among this group. Obese subjects are more likely faced with higher levels of CRP compared to normal-weight controls [7]. Serum baseline levels of CRP are markedly higher in patients with diabetes or glucose intolerance [8, 9]. Meta-analysis also shows that higher levels of CRP are significantly positively correlated with increased risk for T2DM [10].
In fact, CRP also plays a significant role in coronary heart disease. CRP binds to low-density lipoprotein cholesterol (LDL-C) and is present in atherosclerotic plaques. This acute-phase protein has been proved to serve as a better predictor for cardiovascular risk than LDL-C [11]. Drugs that reduce CRP concentrations effectively inhibit atherosclerosis progress, especially in patients with diabetes or IR. Thiazolidinediones (TZDs) have been recognized to reduce CRP levels at the molecular and serum levels as useful glucose-lowering agents with off-target or unwanted side effects [12, 13].
DPP-4i improve glycemic control via preventing the inactivation of incretins, such as glucagon-like peptide-1 and glucose dependent insulinotropic peptide [14, 15]. DPP-4i improve pancreatic β-cell function in both fasting and postprandial states in T2DM [16, 17]. In spite of the known efficacy on glucose metabolism, the shape of association between DPP-4i and CRP concentration in T2DM has not been well characterized. Based on the related risk of diabetes and coronary heart disease, CRP might be potentially recognized as a novel surrogate cardiovascular indicator and biomarker for antidiabetic agents. Therefore, the present meta-analysis aimed to help judge the extent to which DPP-4i modulated CRP concentrations in patients with T2DM.
Methods
Search strategy
A systematic review was performed according to PRISMA guidelines from Cochrane handbook. Multiple databases including PubMed, Cochrane Library and Embase databases were comprehensively searched. Medical subject heading terms and keywords used to identify studies included: (“C-reactive protein” OR CRP OR “high sensitivity C-reactive protein” OR hsCRP OR hs-CRP) AND (sitagliptin OR vildagliptin OR teneligliptin OR saxagliptin OR linagliptin OR anagliptin OR alogliptin). Randomized controlled trials published in English were identified up to December 31, 2017.
Study selection
Trials were combined and duplicates were discarded. Studies were first screened on the basis of title and abstract, after which total article was reviewed. Data from the published English languages were extracted. Trials must meet these inclusion criteria: (1) randomized controlled studies compared DPP-4i with current treatment; (2) results reporting CRP levels with DPP-4i treatment; (3) studies conducted in T2DM patients and not in healthy volunteers; and (4) studies were published as full-text articles. Studies were excluded if they were animal studies, narrative reviews, poorly described and only abstract papers. Studies were also excluded if they did not meet criteria listed before. The reference list of eligible articles was hand-searched and corresponding authors were contacted if missing information or clarification was relevant. For duplicate publications from the same study, only the most complete reports were identified. Inclusion and exclusion criteria were evaluated objectively by two reviewers.
Data extraction
Two reviewers entered the extracted data onto a standardized designed form and summarized the important information. Detailed data of first author, publication year, country origin, sample size, ratio of men and women, body mass index, mean age, diabetes duration, medication intervention, therapy duration and serum CRP concentrations at baseline in each included study were recorded. Studies with different treatment duration were extracted as the longest therapy duration. Related adverse events were summarized according to the information from the identified studies. Main investigators were contacted for missing data. Alterations of CRP concentrations before and after treatment were recorded for analysis. Standard errors and confidence intervals (CIs) were converted into standard deviations (SD) [18]. Otherwise, values would be imputed by assuming SDs of the missing outcome to be the mean of the SDs from the trials that reported relevant information.
Quality evaluation
The quality of studies was evaluated according to the Cochrane Reviewers’ Handbook [19].The parameters applied for the evaluation of each trial were as follows: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective outcome reporting, and other potential sources of bias. According to the Cochrane criteria, a judgment of ‘yes’ indicated low risk of bias, while ‘no’ indicated high risk of bias. An item of ‘unclear’ indicated an unknown or unclear risk of bias.
Statistical analysis
Statistical analysis was conducted using STATA 12.0 software. Change scores for serum concentrations of CRP were calculated as follows: value at the end of treatment period – value at baseline. According to whether or not significant heterogeneity of outcomes was present, the continuous variable was pooled as weighted mean difference (WMD) and 95% confidence interval (CI) with a fixed-effects or random-effects model. Heterogeneity among the studies was assessed using a Chi-squared test and quantified with I2 index. Sensitivity analysis was conducted with the leave-one-out method to assess the influence of each study on the overall effect size. Publication bias was examined by Begg’s test and Egger’s test if there were at least five studies for each outcome in the meta-analysis. Additionally, subgroup analysis was performed according to diabetes duration, race, dose, age, CRP and HbA1c at the baseline.
Results
Flow of included studies
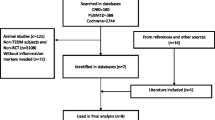
The initial literature search identified 189 records. After removal of inadequate studies, 16 randomized controlled trials with 1607 subjects were eligible for quantitative meta-analysis. Patients with CVD were not included for analysis on the basis of CRP fluctuation in pathological state. Flowchart of inclusion and exclusion was shown (Fig. 1).
Characteristics of included studies
Baseline characteristics of participants in identified studies were fully presented in Table 1. The largest study had a size of 341 subjects, while the smallest one recruited 25 subjects. Most patients among identified studies received DPP-4i treatment of sitagliptin and vildagliptin. Only two studies compared linagliptin and alogliptin with placebo and traditional antidiabetic agents, respectively. Therapy duration ranged from 3 to 26 months. Different trials conducted by the same researcher were analyzed respectively.
Quality evaluation
Study quality was critically evaluated based on the scheme suggested by the Cochrane criteria. All the studies were randomly designed and details on the items of bias criteria among included trials were summarized in Table 2. Four studies might have detection bias on the basis of blinding of outcome assessment. Additionally, eight trials had performance bias due to absence of implementation of blind methods.
Meta-analysis of the impact of DPP-4i treatment
Meta-analysis demonstrated that DPP-4i lowered CRP concentrations compared to placebo by − 0.86 mg/L (95% CI, − 1.36 to − 0.36, P = 0.001) (Fig. 2) and a significant heterogeneity was observed between these studies (I2 = 84.4%). Due to different chemical structure, sitagliptin and vildagliptin might have different efficacy on reducing serum CRP levels. Vildagliptin further reduced CRP concentrations by − 0.79 mg/L (95% CI, − 1.41 to − 0.17, I2 = 76%, P = 0.013) compared to sitagliptin (− 1.05 mg/L, 95% CI, − 2.86 to 0.76, I2 = 95.6%, P = 0.254). However, DPP-4i showed no stronger impact on reducing serum CRP levels than traditional antidiabetic agents and significant heterogeneity occurred between studies (0.64 mg/L, 95% CI, − 0.10 to 1.37, I2 = 95.1%, P = 0.090) (Fig. 3).
Sensitivity analysis was performed to validate these results, illustrating that the pooled results were stable and credible (Fig. 4). In the sensitivity analysis, the pooled effect estimates remained stable across all studies (WMD 0.01 mg/dL, 95% CI -0.50, 0.52, N = 15 studies, heterogeneity P = 0.962; Fig. 4). Subgroup analysis was performed according to regions, dose, age, baseline CRP and HbA1c, which showed no significant differences (see Additional file 1: Figure S1, Additional file 2: Figure S2; Additional file 3: Figure S3, Additional file 4: Figure S4 and Additional file 5: Figure S5). However, serum concentrations of CRP were significantly reduced in subgroup analysis stratified by diabetes duration (− 0.23 mg/L, 95% CI, − 0.41 to − 0.05) (see Additional file 6: Figure S6). Finally, there was no publication bias according to Begg’s test (p = 0.293) and Egger’s test (p = 0.332) among the seven sitagliptin studies (Fig. 5).
Discussion
In this meta-analysis, impact of DPP-4i on CRP concentrations was evaluated among 1607 participants with T2DM. Pooled analysis from extracted studies indicated that DPP-4i significantly decreased serum CRP concentrations compared to placebo. No significant effect on CRP was observed with treatment of DPP-4i compared to that of traditional antidiabetic drugs.
This meta-analysis revealed that vildagliptin and sitagliptin had a beneficial influence on reducing serum CRP concentrations. Mechanisms of which DPP-4i reduced serum CRP potentially explained these results. Soluble DPP4 has previously been identified as cytokine related to obesity. In vitro experiments reveal that soluble DPP4 could induce inflammatory reaction by activating MAPK and NF-κB pathway via protease-activated receptor 2-dependent mechanisms [36]. Soluble DPP4 is released from cell-surface to the circulation, increasing expression and secretion of pro-inflammatory cytokines like monocyte chemoattractant protein-1 (MCP-1) and interleukin-6 (IL-6). On the other hand, gemigliptin, one of the DPP-4i, improves lipopolysaccharide (LPS)-mediated pro-inflammatory effects in vascular endothelial cells by attenuating NF-κB and JNK signaling in an Akt-AMPK-dependent pathway [37]. Besides, evidence from animal studies also supported our results, in which DPP-4i presented favorable anti-inflammatory effect against diabetes and atherosclerosis via targeting proteins participating in inflammatory pathways [38].
It has been recognized that inflammation plays a key role in endothelial dysfunction in diabetes and atherosclerosis. Fortunately, sitagliptin has been demonstrated to improve endothelial dysfunction by increasing the release of endothelial progenitor cells (EPCs) through augmentation of number of stromal cell-derived factor-1α (SDF-1α) [39]. Another study also shows that sitagliptin reduces serum CRP levels via inhibiting the activation of NF-κB in T2DM, partially improving endothelial dysfunction in patients with uncontrolled diabetes and CVD [40]. Sitagliptin also inhibits the adhesion of inhibitory-κB kinase (IKKβ) to NF-κB within a short period and downregulates TLR-4 mRNA expression, indirectly resulting in a significant 20% decrease in CRP concentrations for 2 weeks. This inhibitory effect on CRP potentially indicates an antiatherogenic effect on CVD events following DPP-4i treatment [41].
In a recent meta-analysis, our team found that DPP-4i increased serum levels of adiponectin in T2DM, an adipose-specific protein which is negatively correlated with proatherogenic LDL-C and other cardiovascular risk factors for diabetes or IR [42]. In the current subgroup analysis, DPP-4i improved CRP concentrations in European participants, but not in Asian or American subjects, reasons of which might originate from different diets or other external factors. The impact of DPP-4i on CRP had not been modulated by potential variables of regions, dose, age, baseline CRP and HbA1c, except for diabetes duration. Further researches would have to explore the exact mechanism of favorable anti-inflammatory aspects of gliptins on inhibiting the secretion of CRP. Immuno-inflammatory response correlated with hyperglycemia could promote metabolic disorder and end-stage renal disease. The effects of diabetes treatment on inflammatory reaction also suggested that patients would benefit from these drugs beyond simple control of glucose homeostasis [43].
Given that CVD is a major cause of mortality in T2DM, CRP has been proven to be a better predictor for coronary heart disease than other inflammatory markers, such as tumor necrosis factor alpha (TNF-α) and IL-6. It is crucial to explore the safety and efficacy of current glucose-lowering agents, especially, the novel class of DPP-4i. It was the first meta-analysis that assesses and demonstrates the impact of chronic treatment with DPP-4i on inflammatory markers that are known to be associated with T2DM and obesity. Patients from Europe and Asia mostly received vildagliptin and sitagliptin treatment. Adverse events and safety were evaluated and recorded in Table 3. DPP-4i were well tolerated and associated with low risks of gastrointestinal disorders and hypoglycemia (FPG < 60 mg/dL) when used alone, and all of adverse events were mild to moderate and transient. Episodes of hypoglycemia were commonly associated with precipitating factors, such as skipped meals, delay in eating and enhanced activity [23]. DPP-4i limited the breakdown of gastrointestinal hormones of glucagon-like peptide 1 (GLP-1) and glucose-dependent insulinotropic peptide (GIP), which might partially result in gastrointestinal discomfort. None of records produced severe adverse events, results of which were in line with other analysis [44, 45].
The present study is the first meta-analysis evaluating the impact of DDP-4i on serum CRP concentrations in T2DM patients. It is suggested that DPP-4i possess favorable effect against atherosclerosis and CVD events. It also provides insights into the therapeutic implications in diabetic-related atherosclerotic disease in humans for the potentially protective effects on inflammation. Secondly, results suggest that CRP might potentially serve as cardiovascular biomarker in T2DM. Thirdly, subgroup analysis has been performed to explore the influence of diabetes duration, race, dose and age.
However, this meta-analysis also has some limitations. Firstly, smaller number of patients and smaller number of trials were identified, and these studies only reported relatively short-term effect of DPP-4i on CRP levels. Secondly, only studies published in English were extracted, which inevitably resulted in potential publication bias. Finally, some heterogeneity was present in some of pooled results, although measures had been taken to overcome it by performing a sensitivity analysis.
Conclusions
DPP4i could effectively reduce serum CRP concentrations, which might prevent exacerbation of cardiovascular events in diabetes progress. Long-term effects and cardiovascular endpoints should be studied to better guide clinicians to integrate resolutions in patients with T2DM.
Availability of data and materials
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
Abbreviations
- CI:
-
Confidence intervals
- CRP:
-
C-reactive protein
- CVD:
-
Cardiovascular disease
- DPP-4i:
-
Dipeptidyl peptidase-4 inhibitors
- EPCs:
-
Endothelial progenitor cells
- GIP:
-
Glucose-dependent insulinotropic peptide
- GLP1:
-
Glucagon-like peptide 1
- IKKβ:
-
Inhibitory-κB kinase
- IL-6:
-
Interleukin-6
- IR:
-
Insulin resistance
- LPS:
-
Lipopolysaccharide
- MCP-1:
-
Monocyte chemoattractant protein-1
- RCTs:
-
Randomized controlled trials
- SD:
-
Standard deviations
- SDF-1α:
-
Stromal cell-derived factor-1α
- T2DM:
-
Type 2 diabetes mellitus
- TZDs:
-
Thiazolidinediones
- WMD:
-
Weighted mean difference
References
ter Horst KW, Gilijamse PW, Koopman KE, de Weijer BA, Brands M, Kootte RS, Romijn JA, Ackermans MT, Nieuwdorp M, Soeters MR, Serlie MJ. Insulin resistance in obesity can be reliably identified from fasting plasma insulin. Int J Obes. 2015;39:1703–9.
Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, Hadden D, Turner RC, Holman RR. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321:405–12.
Fernandez-Real JM, Ricart W. Insulin resistance and chronic cardiovascular inflammatory syndrome. Endocr Rev. 2003;24:278–301.
Svenson KL, Pollare T, Lithell H, Hallgren R. Impaired glucose handling in active rheumatoid arthritis: relationship to peripheral insulin resistance. Metabolism. 1988;37:125–30.
Lee S, Norheim F, Langleite TM, Gulseth HL, Birkeland KI, Drevon CA. Effects of long-term exercise on plasma adipokine levels and inflammation-related gene expression in subcutaneous adipose tissue in sedentary dysglycaemic, overweight men and sedentary normoglycaemic men of healthy weight. Diabetologia. 2019;62:1048–64.
Juhan-Vague I, Thompson SG, Jespersen J. Involvement of the hemostatic system in the insulin resistance syndrome. A study of 1500 patients with angina pectoris. The ECAT angina pectoris study group. Arterioscler Thromb. 1993;13:1865–73.
Visser M, Bouter LM, McQuillan GM, Wener MH, Harris TB. Elevated C-reactive protein levels in overweight and obese adults. JAMA. 1999;282:2131–5.
Festa A, D'Agostino R Jr, Tracy RP, Haffner SM. Elevated levels of acute-phase proteins and plasminogen activator inhibitor-1 predict the development of type 2 diabetes: the insulin resistance atherosclerosis study. Diabetes. 2002;51:1131–7.
McMillan DE. Increased levels of acute-phase serum proteins in diabetes. Metabolism. 1989;38:1042–6.
Wang X, Bao W, Liu J, Ouyang YY, Wang D, Rong S, Xiao X, Shan ZL, Zhang Y, Yao P, Liu LG. Inflammatory markers and risk of type 2 diabetes: a systematic review and meta-analysis. Diabetes Care. 2013;36:166–75.
Ridker PM, Rifai N, Rose L, Buring JE, Cook NR. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med. 2002;347:1557–65.
Mohanty P, Aljada A, Ghanim H, Hofmeyer D, Tripathy D, Syed T, Al-Haddad W, Dhindsa S, Dandona P. Evidence for a potent antiinflammatory effect of rosiglitazone. J Clin Endocrinol Metab. 2004;89:2728–35.
Pfutzner A, Schondorf T, Hanefeld M, Forst T. High-sensitivity C-reactive protein predicts cardiovascular risk in diabetic and nondiabetic patients: effects of insulin-sensitizing treatment with pioglitazone. J Diabetes Sci Technol. 2010;4:706–16.
Nauck MA, Meininger G, Sheng D, Terranella L, Stein PP. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor, sitagliptin, compared with the sulfonylurea, glipizide, in patients with type 2 diabetes inadequately controlled on metformin alone: a randomized, double-blind, non-inferiority trial. Diabetes Obes Metab. 2007;9:194–205.
Brazg R, Xu L, Dalla Man C, Cobelli C, Thomas K, Stein PP. Effect of adding sitagliptin, a dipeptidyl peptidase-4 inhibitor, to metformin on 24-h glycaemic control and beta-cell function in patients with type 2 diabetes. Diabetes Obes Metab. 2007;9:186–93.
Xu L, Man CD, Charbonnel B, Meninger G, Davies MJ, Williams-Herman D, Cobelli C, Stein PP. Effect of sitagliptin, a dipeptidyl peptidase-4 inhibitor, on beta-cell function in patients with type 2 diabetes: a model-based approach. Diabetes Obes Metab. 2008;10:1212–20.
Derosa G, Maffioli P. Dipeptidyl peptidase-4 inhibitors: 3 years of experience. Diabetes Technol Ther. 2012;14:350–64.
Higgins J, Green SE. Cochrane handbook for systematic reviews of interventions version 5.1.0. The Cochrane collaboration (Eds). Naunyn Schmiedebergs Arch Exp Pathol Pharmakol. 2011;5:S38.
Brazg R, Xu L, Dalla MC, Cobelli C, Thomas K, Stein PP. Effect of adding sitagliptin, a dipeptidyl peptidase-4 inhibitor, to metformin on 24-h glycaemic control and ?-cell function in patients with type 2 diabetes. Diabetes Obes Metab. 2010;9:186–93.
Derosa G, Carbone A, Franzetti I, Querci F, Fogari E, Bianchi L, Bonaventura A, Romano D, Cicero A, Maffioli P. Effects of a combination of sitagliptin plus metformin vs metformin monotherapy on glycemic control, beta-cell function and insulin resistance in type 2 diabetic patients. Diabetes Res Clin Pract. 2012;98:51–60.
Satoh-Asahara N, Sasaki Y, Wada H, Tochiya M, Iguchi A, Nakagawachi R, Odori S, Kono S, Hasegawa K, Shimatsu A. A dipeptidyl peptidase-4 inhibitor, sitagliptin, exerts anti-inflammatory effects in type 2 diabetic patients. Metabolism. 2013;62:347–51.
Suzuki K, Tanaka S, Aoki C, Kato K, Jojima T, Aso Y. Greater efficacy and improved endothelial dysfunction in untreated type 2 diabetes with liraglutide versus sitagliptin. Dokkyo J Med Sci. 2014;41:211–20.
Liu SC, Chien KL, Wang CH, Chen WC, Leung CH. Efficacy and safety of adding pioglitazone or sitagliptin to patients with type 2 diabetes insufficiently controlled with metformin and a sulfonylurea. Endocr Pract. 2013;19:980–8.
Derosa G, Maffioli P, Salvadeo S, Ferrari I, Ragonesi P, Querci F, Franzetti I, Gadaleta G, Ciccarelli L, Piccinni M, et al. Effects of sitagliptin or metformin added to pioglitazone monotherapy in poorly controlled type 2 diabetes mellitus patients. Metabolism. 2010;59:887–95.
Nakamura K, Oe H, Kihara H, Shimada K, Fukuda S, Watanabe K, Takagi T, Yunoki K, Miyoshi T, Hirata K. DPP-4 inhibitor and alpha-glucosidase inhibitor equally improve endothelial function in patients with type 2 diabetes: EDGE study. Cardiovasc Diabetol. 2014;13:110.
Derosa G, Ragonesi PD, Carbone A, Fogari E, Bianchi L, Bonaventura A, Romano D, Cicero AFG, Maffioli P. Vildagliptin added to metformin on β-cell function after a euglycemic hyperinsulinemic and hyperglycemic clamp in type 2 diabetes patients. Diabetes Technol Ther. 2012;14:475–84.
Strozik A, Steposz A, Basiak M, Drozdz M, Okopien B. Multifactorial effects of vildagliptin added to ongoing metformin therapy in patients with type 2 diabetes mellitus. Pharmacol Rep. 2014;67:24–31.
Zografou I, Sampanis C, Gkaliagkousi E, Iliadis F, Papageorgiou A, Doukelis P, Vogiatzis K, Douma S. Effect of vildagliptin on hsCRP and arterial stiffness in patients with type 2 diabetes mellitus. Hormones (Athens). 2015;14:118–25.
Derosa G, Bonaventura A, Bianchi L, Romano D, Fogari E, D'Angelo A, Maffioli P. Comparison of vildagliptin and glimepiride: effects on glycaemic control, fat tolerance and inflammatory markers in people with type 2 diabetes. Diabet Med. 2014;31:1515–23.
Kim NH, Kim DL, Kim KJ, Kim NH, Choi KM, Baik SH, Kim SG. Effects of vildagliptin or pioglitazone on glycemic variability and oxidative stress in patients with type 2 diabetes inadequately controlled with metformin monotherapy: a 16-week, randomised, open label, pilot study. Endocrinol Metab. 2017;32:241–7.
Mita T, Katakami N, Yoshii H, Onuma T, Kaneto H, Osonoi T, Shiraiwa T, Kosugi K, Umayahara Y, Yamamoto T, et al. Alogliptin, a dipeptidyl peptidase 4 inhibitor, prevents the progression of carotid atherosclerosis in patients with type 2 diabetes: the study of preventive effects of Alogliptin on diabetic atherosclerosis (SPEAD-A). Diabetes Care. 2016;39:139–48.
de Boer SA, Heerspink HJL, Juarez Orozco LE, van Roon AM, Kamphuisen PW, Smit AJ, Slart R, Lefrandt JD, Mulder DJ. Effect of linagliptin on pulse wave velocity in early type 2 diabetes: a randomized, double-blind, controlled 26-week trial (RELEASE). Diabetes Obes Metab. 2017;19:1147–54.
Yamada H, Tanaka A, Kusunose K, Amano R, Matsuhisa M, Daida H, Ito M, Tsutsui H, Nanasato M, Kamiya H, et al. Effect of sitagliptin on the echocardiographic parameters of left ventricular diastolic function in patients with type 2 diabetes: a subgroup analysis of the PROLOGUE study. Cardiovasc Diabetol. 2017;16:63.
Koren S, Shemesh BL, et al. The effect of Sitagliptin versus Glibenclamide on arterial stiffness, blood pressure, lipids, and inflammation in type 2 diabetes mellitus patients. Diabetes Technol Ther. 2012;14:561–7.
Nogueira KC, Furtado M, Fukui RT, Correia MR, Dos Santos RF, Andrade JL, Rossi da Silva ME. Left ventricular diastolic function in patients with type 2 diabetes treated with a dipeptidyl peptidase-4 inhibitor- a pilot study. Diabetol Metab Syndr. 2014;6:103.
Wronkowitz N, Romacho T, Villalobos LA, Sell H, Eckel J. Soluble DPP4 induces inflammation and proliferation of human smooth muscle cells via protease-activated receptor 2. Biochim Biophys Acta. 2014;1842:1613–21.
Hwang HJ, Chung HS, Jung TW, Ryu JY, Hong HC, Seo JA, Kim SG, Kim NH, Choi KM, Choi DS. The dipeptidyl peptidase-IV inhibitor inhibits the expression of vascular adhesion molecules and inflammatory cytokines in HUVECs via Akt- and AMPK-dependent mechanisms. Mol Cell Endocrinol. 2015;405:25–34.
Omar BA, Vikman J, Winzell MS, Voss U, Ekblad E, Foley JE, Ahren B. Enhanced beta cell function and anti-inflammatory effect after chronic treatment with the dipeptidyl peptidase-4 inhibitor vildagliptin in an advanced-aged diet-induced obesity mouse model. Diabetologia. 2013;56:1752–60.
Shinjo T, Nakatsu Y, Iwashita M, Sano T, Sakoda H, Ishihara H, Kushiyama A, Fujishiro M, Fukushima T, Tsuchiya Y. DPP-IV inhibitor anagliptin exerts anti-inflammatory effects on macrophages, adipocytes, and mouse livers by suppressing NF-κB activation. Am J Physiol Endocrinol Metab. 2015;309. https://doi.org/10.1152/ajpendo.00553.2014.
Matsubara J, Sugiyama S, Akiyama E, Iwashita S, Kurokawa H, Ohba K, Maeda H, Fujisue K, Yamamoto E, Kaikita K. Dipeptidyl peptidase-4 inhibitor, sitagliptin, improves endothelial dysfunction in association with its anti-inflammatory effects in patients with coronary artery disease and uncontrolled diabetes. Circ J. 2013;77:1337–44.
Makdissi A, Ghanim H, Vora M, Green K, Abuaysheh S, Chaudhuri A, Dhindsa S, Dandona P. Sitagliptin exerts an antinflammatory action. J Clin Endocrinol Metab. 2012;97:3333.
Liu X, Peng M, Wang Y, Zhai S, Liu G. Impact of dipeptidyl peptidase-4 inhibitors on serum adiponectin: a meta-analysis. Lipids Health Dis. 2016;15:204.
Bonacina F, Baragetti A, Catapano AL, Norata GD. The interconnection between Immuno-metabolism, diabetes, and CKD. Curr Diab Rep. 2019;19:21.
Karagiannis T, Paschos P, Paletas K, Matthews D, Tsapas A. Dipeptidyl peptidase-4 inhibitors for treatment of type 2 diabetes mellitus in the clinical setting: systematic review and meta-analysis. BMJ. 2012;344:e1369.
Hemmingsen B, Sonne DP, Metzendorf M-I, Richter B. Dipeptidyl-peptidase (DPP)-4 inhibitors and glucagon-like peptide (GLP)-1 analogues for prevention or delay of type 2 diabetes mellitus and its associated complications in people at increased risk for the development of type 2 diabetes mellitus. Cochrane Database Syst Rev. 2017;5:CD012204.
Acknowledgments
We would like to thank Dr. Xunde Xian of the Department of Molecular Genetics, UT Southwestern Medical Center in Dallas, TX for his advice and suggestions relating to this manuscript.
Funding
This work is supported in part by Beijing Hospitals Authority Youth Programme (No. QML20170507) and Cooperative Basic-Clinical Research Program (No. 17JL67) of China awarded to X. Liu and Beijing Hospitals Authority Sail Programme (No. ZYLX201827) of China awarded to Z. Zhao.
Author information
Authors and Affiliations
Contributions
XL, PM, BW, GC and ZZ participated in the design, conduct and collection of this meta-analysis. XL and PM searched the literature, extracted the data and evaluated risk of bias. All the authors took part in writing the manuscript. GC and ZZ have full access to all the data in the study, and take full responsibility for the integrity of the data analysis. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional files
Additional file 1:
Figure S1. Forest plot for the impact of DDP-4i treatment versus active comparator on serum concentrations of CRP in subgroups of trials with regions of Asian and European. (TIF 522 kb)
Additional file 2:
Figure S2 Forest plot for the impact of DDP-4i treatment versus active comparator on serum concentrations of CRP in subgroups of trials with agents of low and normal dose. (TIF 545 kb)
Additional file 3:
Figure S3. Forest plot for the impact of DDP-4i treatment versus active comparator on serum concentrations of CRP in subgroups of trials with ages of <= 60 years and > 60 years. (TIF 549 kb)
Additional file 4:
Figure S4. Forest plot for the impact of DDP-4i treatment versus active comparator on serum concentrations of CRP in subgroups of trials with baseline CRP levels of <= 2 mg/L and > 2 mg/L. (TIF 775 kb)
Additional file 5:
Figure S5. Forest plot for the impact of DDP-4i treatment versus active comparator on serum concentrations of CRP in subgroups of trials with HbA1c levels of <= 8.0% and > 8.0%. (TIF 773 kb)
Additional file 6:
Figure S6. Forest plot for the impact of DDP-4i treatment versus active comparator on serum concentrations of CRP in subgroups of trials with diabetes durations of <= 12 months and > 12 months. (TIF 808 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Liu, X., Men, P., Wang, B. et al. Effect of dipeptidyl-peptidase-4 inhibitors on C-reactive protein in patients with type 2 diabetes: a systematic review and meta-analysis. Lipids Health Dis 18, 144 (2019). https://doi.org/10.1186/s12944-019-1086-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12944-019-1086-4