Abstract
Background
This study evaluated the separate and combined associations of physical activity and obesity with blood lipids in Chinese adults with and without diabetes.
Methods
Data of 17,535 participants aged 18 to 78 years old were collected. Physical activity was categorized as inactive (low) or active (moderate or high) according to the International Physical Activity Questionnaire. Linear and logistic regression analyses were performed to investigate the associations of physical activity and obesity with lipid-related indices.
Results
Compared with physically active participants, inactive participants had higher triglyceride (TG) level, lower high-density lipoprotein cholesterol (HDL-C) level, and higher odds ratios for abnormal TG and HDL-C. Compared with non-obese participants, obese participants had higher levels of total cholesterol (TC), TG and low-density lipoprotein cholesterol (LDL-C), lower HDL-C level, and higher odds ratios for the four abnormal lipid indices. Inactive obese participants had highest levels of TC, TG and LDL-C, lowest HDL-C level, and highest odds ratios compared to the other groups. No significant associations were found between obesity and TC, LDL-C in patients with diabetes.
Conclusions
Irrespective of diabetes, physical inactivity and obesity were associated with the presences of abnormal TG and HDL-C. Moreover, there were additive effects on blood lipids when physical inactivity and obesity co-occur.
Similar content being viewed by others
Background
Dyslipidemia contributes to the increased risk of cardiovascular morbidity and mortality [1]. In recent decades, the prevalence of dyslipidemia has increased dramatically in China [2, 3]. People with type 2 diabetes (T2D) had worse blood lipids than those without T2D [4].
Physical activity could bring benefits to the serum lipid levels to some extent [5]. Previous studies indicated that higher physical activity level would cause a decrease in triglyceride (TG) level and an increase in high-density lipoprotein cholesterol (HDL-C) level [6,7,8]. Physical inactivity led to an increase in total cholesterol (TC) and low-density lipoprotein cholesterol (LDL-C) [8]. Ritti-Dias’s study found that physical activity level was negatively associated with non-HDL concentrations in overweight and obese individuals [9]. Moreover, it was reported that sedentary behavior time was unfavourably associated with TC, TG and LDL-C [10, 11].
Obesity has been regarded as a risk factor for dyslipidemia, which consisted of increased TG, decreased HDL-C with HDL dysfunction and normal or slightly increased LDL-C with increased small dense LDL [12, 13]. It was indicated that body mass index (BMI) correlated positively with the levels of TC, TG and LDL-C, but negatively with HDL-C level [14]. It was also reported that increasing BMI increased risks for hypertriglyceridemia, high LDL-C and low HDL-C [15].
Most studies have examined associations of physical activity or obesity alone with lipid-related indices, including one single factor’s effect among two groups of subjects for comparison. Nevertheless, there are few studies to investigate the joint effects of physical activity and obesity on lipid-related indices [16, 17]. For example, Anderson et al. found that physical activity could attenuate some of the risk for metabolic syndrome associated with a higher BMI [17]. Furthermore, it is unclear whether the relationships of physical activity and obesity with blood lipids in diabetic patients differ from those in non-diabetic patients. Therefore, we intended to examine the individual and combined associations of physical activity and obesity with lipid-related indices in cohorts of non-diabetic and diabetic Chinese adults.
Methods
Participants and groups
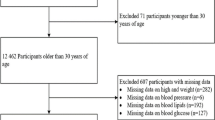
A total of 18,000 potential participants without statins aged 18 years old or older from the physical examination center of Chinese PLA General Hospital in Beijing were screened. We excluded 465 individuals for missing values on relevant variables. Finally, 17,535 participants aged from 18 to 78 (48.6 ± 7.8) years old were included in our analyses. A flow chart of participants is shown in Fig. 1. Informed consent was obtained from all participants after detailed experimental procedures were explained. This study was approved by the Institutional Ethics Committee of Chinese PLA General Hospital and was performed in accordance with the ethical standards.
Based on estimated physical activity and the diagnosis of obesity and T2D, participants were grouped into one of the following eight groups: non-diabetic active and non-obese (n = 6041), non-diabetic inactive and non-obese (n = 6993), non-diabetic active and obese (n = 1159), non-diabetic inactive and obese (n = 1489), diabetic active and non-obese (n = 790), diabetic inactive and non-obese (n = 607), diabetic active and obese (n = 243), diabetic inactive and obese (n = 213).
Data collection and measurements
In the physical examination center, a questionnaire was administered among participants by well-trained investigators via face-to-face interviews. The following data were collected: characteristics of social demography, medical history, and information about lifestyle including physical activity, cigarette smoking and alcohol consumption.
After the interview, participants were taken through a medical examination. Anthropometric measurements (body weight, height and waist circumference (WC)) were measured by trained nurses, according to standardized procedures [18]. BMI was then calculated as body weight (unit: kg) divided by the square of height (unit: m) using Quetlet’s index. After a period of 5-min rest in a quiet room, the systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured on the right upper arm in sitting position, using an automatic machine (AND TM-2655P, Japan). Venous blood samples were collected after an overnight fast of at least 10 h. All measurements were performed in air-conditioned room (22–26 °C) of the examination center, between 8:00 and 11:00 in the morning. Fasting plasma glucose (FPG), TC, TG, HDL-C and LDL-C levels were assayed by using a fully automated biochemistry analyzer (Roche Cobas 6000, Germany). Hemoglobin A1c (HbA1c) was assayed with an automated glycohemoglobin analyzer (TOSOH HLC-723G8, Japan).
Physical activity
The overall physical activity level of a person was assessed by using the International Physical Activity Questionnaire (IPAQ short form) [19]. Participants were asked to indicate the frequency (number of days) and duration (minutes) that they spent in three types of activity (walking, moderate and vigorous activities) in the past seven days. According to the protocol for IPAQ short form, we computed a metabolic equivalents of energy (MET)·minutes/week for each activity (3.3 × minutes × days for walking, 4.0 × minutes × days for moderate activity and 8.0 × minutes × days for vigorous activity). Total physical activity was the sum of walking, moderate (≥3 MET and < 6 MET) and vigorous (≥6 MET) activities in MET·minutes/week. Overall physical activity for each participant was classified as low, moderate or high level, which was defined in IPAQ short form. The participants with low level of physical activity were defined to be inactive, otherwise they were active.
Diagnostic criteria and definition
T2D was defined as HbA1c at least 6.5%, FPG at least 7.0 mmol/L and/or currently under diabetes treatment [20]. Obesity was defined as having a BMI at least 28.0 kg/m2 [21]. Hypertension was defined as SBP/DBP at least 140/90 mmHg and/or currently under medical treatment for hypertension [22]. Cut-off values that were often used for high TC, TG and LDL-C were 5.18 mmol/L, 1.70 mmol/L, 3.37 mmol/L, and for low HDL-C was 1.04 mmol/L, according to the clinical definition of metabolic syndrome by the National Cholesterol Education Program Adult Treatment Panel III [23]. Ischemic cardiovascular disease (ICVD) was defined as having prior physician-diagnosis of coronary heart disease, angina or myocardial infarction.
Statistical analysis
Statistical analyses were performed with SPSS version 21.0 (SPSS Inc., IBM, USA). Continuous variables were shown as mean ± standard deviation (SD). And differences between study groups were evaluated by one-way analysis of variance (ANOVA). Categorical variables were presented as percentage and compared using Chi-square test. Stepwise multiple linear regression analysis was used to estimate the relationships between lipid-related indices (dependent variables) and BMI or WC (independent variables), adjusted for age, gender, smoking and drinking (covariates). The participants were divided into active and inactive, and regression coefficients for BMI or WC were calculated separately within each stratum. To examine whether the regression coefficients differed significantly by physical activity levels, we evaluated the interactions of physical activity with BMI and WC. In the forward binary logistic regression analysis, TC, TG, HDL-C and LDL-C were transformed into binary variables according to the abnormal cut-off values. Then, the abnormal lipid indices were set as the dependent variables separately, other variables including age, gender, smoking and drinking were set as covariate, physical activity and obesity were set as independent variables. For both the non-diabetic condition and the diabetic condition, with active non-obese group as reference, odds ratios (ORs) in the other groups were calculated. Two-sided P-values < 0.05 were considered to be statistically significant.
Results
Basic clinical characteristics
The basic characteristics of the study participants are given in detail in Table 1. Among the total participants, 75.1% (13,161/17,535) were male, 10.6% (1853/17,535) had T2D. Diabetic participants were older and had a lower percentage of women than non-diabetic participants within the same group. Among non-obese groups, the proportion of participants with smoking or drinking habits was higher in diabetic participants than that in non-diabetic participants. Diabetic participants had higher prevalence of hypertension and ICVD than non-diabetic participants within the same group.
Comparison of mean levels of blood lipid-related indices between groups
Figure 2 shows the mean levels of TC, TG, HDL-C and LDL-C. In Fig. 2a and d, obese participants had significantly higher levels of TC and LDL-C compared to non-obese participants, in non-diabetic condition, but not in diabetic condition. The levels of TC and LDL-C were lower in diabetic participants than those in non-diabetic participants within the same group (all P < 0.01). Diabetic participants had a higher TG level and a lower HDL-C level compared with non-diabetic participants in each group except active obese group (Fig. 2b and c). Inactive participants had significantly higher TG level and significantly lower HDL-C level compared to active participants. Obese participants had significantly higher TG level and significantly lower HDL-C level compared to non-obese participants. TG level was highest in inactive obese group, whereas HDL-C level was lowest.
Mean levels of blood lipid-related indices. a TC (mmol/L), b TG (mmol/L), c HDL-C (mmol/L), d LDL-C (mmol/L). Error bars represented as mean ± SE. *P < 0.05, **P < 0.01, ***P < 0.001 vs. same group, different condition; ǂP < 0.05, ǂǂP < 0.01, ǂǂǂP < 0.001 vs. active and non-obese group, same condition; ×P < 0.05, ××P < 0.01, × × ×P < 0.001 vs. inactive and non-obese group, same condition; †P < 0.05, ††P < 0.01, †††P < 0.001 vs. active and obese group, same condition
Correlations of BMI or WC with blood lipid-related indices
Table 2 presents regression slopes of TC, TG, HDL-C and LDL-C levels versus BMI and WC, stratified by physical activity. In non-diabetic participants, we observed that BMI was positively correlated with TC, TG and LDL-C, whereas negatively correlated with HDL-C. In diabetic participants, BMI was positively correlated with TG, whereas negatively correlated with HDL-C. Similar relationships were observed between WC and the four lipid indices. Table 2 also shows the significance of the interaction between physical activity level and body size on blood lipid-related indices. Higher physical activity level significantly suppressed the relationships of BMI and WC with TC, TG and LDL-C in non-diabetic participants. The reduced BMI and WC effects on TG with physical activity were also observed in diabetic participants.
Associations of physical activity and obesity with abnormal TC, TG, LDL-C and HDL-C
The ORs with 95% confidence intervals (CIs) for the four abnormal lipid indices in the groups are presented in Table 3. In non-diabetic condition, obese participants had higher ORs for high TC, high TG, low HDL-C and high LDL-C compared to non-obese participants. Physically inactive participants had higher ORs for high TG and low HDL-C compared to non-obese participants. ORs for high TG and low HDL-C were highest in inactive obese group, were 2.23 (1.98–2.51) and 2.17 (1.91–2.47) respectively (P < 0.001 for all). In diabetic condition, physically inactive and obese participants had higher ORs for high TG and lower HDL-C which were 1.70(1.23–2.33) and 1.70(1.23–2.35) respectively (p<0.01 for all).
Discussion
To our knowledge, this was the first study reporting combined associations of physical activity and obesity with blood lipids in non-diabetic and diabetic patients. The major findings from this study could be summarized as follows: (1) Physical inactivity was significantly associated with the presences of abnormal TG and HDL-C in non-diabetic and diabetic participants (Fig. 2b and c); (2) Obesity was significantly associated with the presences of the four abnormal lipid indices in non-diabetic participants (Fig. 2), while the associations disappeared in diabetic participants between obesity and the presences of abnormal TC and LDL-C (Fig. 2a and d); (3) Physical inactivity and obesity may have additive effects on TG and HDL-C in non-diabetic and diabetic participants (Table 3).
Diabetic participants had higher TG and lower HDL-C, compared to non-diabetic participants. This was consistent with the result of previous studies, which had demonstrated that the most typical lipoprotein pattern in T2D, consisted of moderate elevation in TG concentrations, and decrease in HDL-C concentrations [24]. It has been proposed that insulin deficiency or resistance, adipocytokines and hyperglycemia contributed to the risk increase of dyslipidemia, which was present even before the onset of T2D [25, 26]. While in obese group, those alterations of TG and HDL-C were not statistically significant in non-diabetic versus diabetic in our study. It may imply that obesity was more critical than diabetes for worsening TG and HDL-C levels.
In the present study, we found that obesity was positively associated with the presences of the four abnormal lipid indices, which confirmed previous reports [12, 13]. It was reported that abdominal subcutaneous fat accumulation was closely related to dyslipidemia [27]. There were significantly positive associations between physical inactivity and the presences of abnormal TG and HDL-C. The current findings were consistent with those observed in previous cross-sectional studies [6, 8] and were confirmed by prospective study [5, 28,29,30]. For example, Kraus et al. found that greater amount of physical activity was closely associated with improved lipids, but the intensity of exercise was less important [28]. Wang and Xu reported that physical activity promoted the reverse cholesterol transport process and lipid peroxide transport clearing, this means that physical activity not only has a positive effect on individuals with dyslipidemia, but can also help improve lipids profile [31]. Saltin and Helge pointed that physical activity increased the ability of muscles to better burn fat instead of glycogen by activation of enzymes for lipid turnover [32]. TC and LDL-C levels were not significantly different among physically active participants and inactive participants, which agreed with the result of previous studies [33, 34]. Norregaard et al. reported that six weeks of physical activity training reduced LDL-C level [30]. Pedersen Saltin pointed that high-volume/high-intensity physical activity reduced small LDL particles and increased the size of the LDL particles [35]. The relationship between physical activity and LDL-C remains unclear and complex.
It was verified that physical inactivity and obesity could bring dyslipidemia risks. The joint effects of physical inactivity and obesity had varied impact on different lipid-related indices. In obese participants, physical inactivity was related to increased TG level, decreased HDL-C level and increased ORs of high TG and low HDL-C. These findings indicated that the joint effect of physical inactivity and obesity was associated with deeper exacerbation of TG and HDL-C compared to their individual effect. It confirmed that physical inactivity could amplify some of the risk for low HDL-C associated with a higher BMI [16].
There was strong experimental evidence that physical activity could decrease the risk of developing dyslipidemia through weight loss and the current study showed that physical activity, independent of obesity, may have a beneficial effect on the lipid profile [35]. The mechanism of physical activity-induced lipid changes is still unclear, though may be partly explained by weight loss. The study described by Earnest et al. pointed that physical activity may increase blood lipid utilization hence to decrease lipids levels [36]. It was also reported that physical activity could significantly increase activity of lipoprotein lipase [37], which was responsible for chylomicrons and TG hydrolysis in granules [38]. Venkatraman et al. reported that physical activity reduce interleukin-1β, tumor Necrosis Factor-α levels in men, which may be the cause of dyslipidemia [39]. Further studies are required to decisively ascertain the mechanisms and dose-response relationship between physical activity and blood lipids.
There were some limitations that need to be considered when interpreting the findings from this study. First, the assessment of physical activity level in the present study was not based on an objective measurement by motion sensors but was derived from a physical activity questionnaire. Hence, misclassification of physical activity level may be introduced due to the recall bias. Second, although some common confounders (age, gender, smoking and drinking) had been adjusted in our analyses, the possibility still existed that unmeasured confounders might have some impact on the results. Finally, the cross-sectional study did not have a strict follow-up design. Thus, the current findings could not infer cause-and-effect relationships.
Conclusions
The findings of this current study suggested that both physical inactivity and obesity were significantly associated with increased risks for abnormal TG and HDL-C irrespective of diabetes. Furthermore, there was an additive interaction between physical inactivity and obesity. Longitudinal studies are needed to investigate the effects and their mechanisms.
Abbreviations
- ANOVA:
-
Analysis of variance
- BMI:
-
Body mass index
- CIs:
-
Confidence intervals
- DBP:
-
Diastolic blood pressure
- FPG:
-
Fasting plasma glucose
- HbA1c :
-
Hemoglobin A1c
- HDL-C:
-
High-density lipoprotein cholesterol
- ICVD:
-
Ischemic cardiovascular disease
- IPAQ:
-
International physical activity questionnaire
- LDL-C:
-
Low-density lipoprotein cholesterol
- ORs:
-
Odds ratios
- SBP:
-
Systolic blood pressure
- T2D:
-
Type 2 diabetes
- TC:
-
Total cholesterol
- TG:
-
Triglyceride
- WC:
-
Waist circumference
References
Katsiki N, Tentolouris N, Mikhailidis DP. Dyslipidaemia in type 2 diabetes mellitus: bad for the heart. Curr Opin Cardiol. 2017;32:422–9.
Zhang FL, Xing YQ, Wu YH, Liu HY, Luo Y, Sun MS, et al. The prevalence, awareness, treatment, and control of dyslipidemia in Northeast China: a population-based cross-sectional survey. Lipids Health Dis. 2017;16:1–13.
Yang WY, Xiao JZ, Yang ZJ, Ji LN, Jia WP, Weng JP, et al. Serum lipids and lipoproteins in Chinese men and women. Circulation. 2012;125:2212–21.
Haffner SM. Dyslipidemia management in adults with diabetes. Diabetes Care. 2004;27(Suppl 1):S68–71.
Monda KL, Ballantyne CM, North KE. Longitudinal impact of physical activity on lipid profiles in middle-aged adults: the atherosclerosis risk in communities study. J Lipid Res. 2009;50:1685–91.
Hamasaki H, Noda M, Moriyama S, Yoshikawa R, Katsuyama H, Sako A, et al. Daily physical activity assessed by a triaxial accelerometer is beneficially associated with waist circumference, serum triglycerides, and insulin resistance in Japanese patients with prediabetes or untreated early type 2 diabetes. J Diabetes Res. 2015;2015:1–6.
Loprinzi PD, Joyner C. Relationship between objectively measured physical activity, cardiovascular disease biomarkers, and hearing sensitivity using data from the National Health and nutrition examination survey 2003-2006. Am J Audiol. 2017;26:163–9.
Hu B, Liu XY, Zheng Y, Fan HM, Yin SF, Guo CY, et al. High physical activity is associated with an improved lipid profile and resting heart rate among healthy middle-aged Chinese people. Biomed Environ Sci. 2015;28:263–71.
Ritti-Dias RM, Cucato GG, do Prado WL, RDO C, Santos RD, Bittencourt MS. Self-initiated changes in physical activity levels improve cardiometabolic profiles: A longitudinal follow-up study. Nutr Metab Carbiovasc Dis. 2017;27:48–53.
Crichton GE, Alkerwi A. Physical activity, sedentary behavior time and lipid levels in the observation of cardiovascular risk factors in Luxembourg study. Lipids Health Dis. 2015;14:1–9.
Brocklebank LA, Falconer CL, Page AS, Perry R, Cooper AR. Accelerometer-measured sedentary time and cardiometabolic biomarkers: a systematic review. Prev Med. 2015;76:92–102.
Jung UJ, Choi MS. Obesity and its metabolic complications: the role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int J Mol Sci. 2014;15:6184–223.
Klop B, Elte JWF, Cabezas MC. Dyslipidemia in obesity: mechanisms and potential targets. Nutrients. 2013;5:1218–40.
Cong L, Zhan JQ, Yang L, Zhang W, Li SG, Chen C, et al. Overweight and obesity among low-income Muslim Uyghur women in far western China: correlations of body mass index with blood lipids and implications in preventive public health. PLoS One. 2014;9:1–7.
Yang Z, Ding X, Liu J, Duan P, Si L, Wan BH, et al. Associations between anthropometric parameters and lipid profiles in Chinese individuals with age ≥ 40 years and BMI < 28kg/m2. PLoS One. 2017;12:1–11.
Mora S, Lee IM, Buring JE, Ridker PM. Association of physical activity and body mass index with novel and traditional cardiovascular biomarkers in women. JAMA-J Am Med Assoc. 2006;295:1412–9.
Anderson AA, Yoo H, Franke WD. Associations of physical activity and obesity with the risk of developing the metabolic syndrome in law enforcement officers. J Occup Environ Med. 2016;58:946–51.
Haftenberger M, Lahmann PH, Panico S, Gonzalez CA, Seidell JC, Boeing H, et al. Overweight, obesity and fat distribution in 50-to 64-year-old participants in the European prospective investigation into Cancer and nutrition (EPIC). Public Health Nutr. 2002;5:1147–62.
Craig CL, Marshall AL, Sjostrom M, Bauman AE, Booth ML, Ainsworth BE, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35:1381–95.
Association AD. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37:S81–90.
Chen C, Lu FC. The guidelines for prevention and control of overweight and obesity in Chinese adults. Biomed Environ Sci. 2004;17(Suppl):1–36.
Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, et al. Seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension. 2003;42:1206–52.
Cleeman JI, Grundy SM, Becker D, Clark LT, Cooper RS, Denke MA, et al. Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III). JAMA-J Am Med Assoc. 2001;285:2486–97.
Srikanth S, Deedwania P. Management of dyslipidemia in patients with hypertension, diabetes, and metabolic syndrome. Curr Hypertens Rep. 2016;18:1–10.
Taskinen MR, Boren J. New insights into the pathophysiology of dyslipidemia in type 2 diabetes. Atherosclerosis. 2015;239:483–95.
Haffner SM, Stern MP, Hazuda HP, Mitchell BD, Patterson JK. Cardiovascular risk-factors in confirmed prediabetic individuals - does the clock for coronary heart-disease start ticking before the onset of clinical diabetes. JAMA-J Am Med Assoc. 1990;263:2893–8.
Brown CD, Higgins M, Donato KA, Rohde FC, Garrison R, Obarzanek E, et al. Body mass index and the prevalence of hypertension and dyslipidemia. Obes Res. 2000;8:605–19.
Kraus WE, Houmard JA, Duscha BD, Knetzger KJ, Wharton MB, McCartney JS, et al. Effects of the amount and intensity of exercise on plasma lipoproteins. N Engl J Med. 2002;347:1483–92.
Shakil-Ur-Rehman S, Karimi H, Gillani SA. Effects of supervised structured aerobic exercise training program on high and low density lipoprotein in patients with type II diabetes mellitus. Pak J Med Sci. 2017;33:96–9.
Norregaard J, Gram M, Vigelso A, Wiuff C, Kuhlman AB, Helge JW, et al. The effect of reduced physical activity and retraining on blood lipids and body composition in young and older adult men. J Aging Phys Act. 2015;23:489–95.
Wang YT, Xu DY. Effects of aerobic exercise on lipids and lipoproteins. Lipids Health Dis. 2017;16:1–8.
Saltin B, Helge JW. Metabolic capacity of skeletal muscles and health. Ugeskr Laeger. 2000;162:2159–64.
Kwasniewska M, Kostka T, Jegier A, Dziankowska-Zaborszczyk E, Leszczynska J, Rebowska E, et al. Regular physical activity and cardiovascular biomarkers in prevention of atherosclerosis in men: a 25-year prospective cohort study. BMC Cardiovasc Disord. 2016;16:1–9.
da Silva RC, Diniz M, Alvim S, Vidigal PG, Fedeli LMG, Barreto SM. Physical activity and lipid profile in the ELSA-Brasil study. Arq Bras Cardiol. 2016;107:10–8.
Pedersen BK, Saltin B. Exercise as medicine - evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand J Med Sci Sports. 2015;25:1–72.
Earnest CP, Artero EG, Sui XM, Lee DC, Church TS, Blair SN. Maximal estimated cardiorespiratory fitness, cardiometabolic risk factors, and metabolic syndrome in the aerobics center longitudinal study. Mayo Clin Proc. 2013;88:259–70.
Ferguson MA, Alderson NL, Trost SG, Essig DA, Burke JR, Durstine JL. Effects of four different single exercise sessions on lipids, lipoproteins, and lipoprotein lipase. J Appl Physiol. 1998;85:1169–74.
Calabresi L, Franceschini G. Lecithin: cholesterol acyltransferase, high-density lipoproteins, and atheroprotection in humans. Trends Cardiovasc Med. 2010;20:50–3.
Venkatraman JT, Rowland JA, Denardin E, Horvath PJ, Pendergast D. Influence of the level of dietary lipid intake and maximal exercise on the immune status in runners. Med Sci Sport Exer. 1997;29:333–44.
Acknowledgements
None.
Funding
This research was supported by the Science and Technology Service Network Program of Chinese Academy of Sciences, China (No. KFJ-STS-ZDTP-033).
Availability of data and materials
The datasets analysed during the current study are not publicly available due the research project has not been completed, but are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Contributions
All of the authors contributed to the design and analysis of this article. YLZ, JY, YNS and QZ conceived and designed the research study. YLZ obtained funding for the study. JY, QG and WMW collected all data, and YLZ and JY analyzed the data. JY wrote the manuscript. YLZ, QG, WMW, YNS and QZ provided critical revision of the manuscript for important intellectual content. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval
Ethics approval was obtained from the Institutional Ethics Committee of Chinese PLA General Hospital (Beijing, China), and written informed consent was obtained for all participants.
Consent for publication
All authors agree to publish this article in the journal of Lipids in Health and Disease.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Zhang, Y., Yang, J., Ye, J. et al. Separate and combined associations of physical activity and obesity with lipid-related indices in non-diabetic and diabetic patients. Lipids Health Dis 18, 49 (2019). https://doi.org/10.1186/s12944-019-0987-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12944-019-0987-6