Abstract
Metastasis has been one of the primary reasons for the high mortality rates associated with tumours in recent years, rendering the treatment of current malignancies challenging and representing a significant cause of recurrence in patients who have undergone surgical tumour resection. Halting tumour metastasis has become an essential goal for achieving favourable prognoses following cancer treatment. In recent years, increasing clarity in understanding the mechanisms underlying metastasis has been achieved. The concept of premetastatic niches has gained widespread acceptance, which posits that tumour cells establish a unique microenvironment at distant sites prior to their migration, facilitating their settlement and growth at those locations. Neutrophils serve as crucial constituents of the premetastatic niche, actively shaping its microenvironmental characteristics, which include immunosuppression, inflammation, angiogenesis and extracellular matrix remodelling. These characteristics are intimately associated with the successful engraftment and subsequent progression of tumour cells. As our understanding of the role and significance of neutrophils in the premetastatic niche deepens, leveraging the presence of neutrophils within the premetastatic niche has gradually attracted the interest of researchers as a potential therapeutic target. The focal point of this review revolves around elucidating the involvement of neutrophils in the formation and shaping of the premetastatic niche (PMN), alongside the introduction of emerging therapeutic approaches aimed at impeding cancer metastasis.
Similar content being viewed by others
Introduction
Although many therapies have been developed for cancer treatment, metastasis remains a major cause of cancer mortality. Metastasis occurs when tumour cells spread from the primary site to distant organs via the bloodstream [1]. With new discoveries concerning the tumour environment, cancer metastasis to particular sites can be explained as a process in which a specific microenvironment plays a key role in trapping tumour cells [2]. This finding allowed the concept of the premetastatic niche (PMN) to be proposed, which specifically refers to circumstances in the future sites of metastasis [3]. The premetastatic niche (PMN) is a complex environment formed by various bone marrow-derived cells (BMDCs) and molecular factors. Both the cellular constituents and molecular elements collaborate in reshaping the microenvironment, priming distant organs for the metastasis of tumour cells. After the formation of the premetastatic niche, this particular microenvironment is characterized by immunosuppression, vascular permeability and angiogenesis, which are all vital for the settlement and growth of tumour cells [4].
Neutrophils, the most abundant type of leukocyte in peripheral blood, are stimulated to undergo massive proliferation after tumorigenesis. The precise timing of neutrophil migration to the premetastatic niche and the roles of these cells throughout the metastatic cascade remain elusive. The role of neutrophils in metastasis has long been unclear, and studies on both pro- and antimetastatic effects have been conducted [5, 6]. With the rapid advancement of high-throughput sequencing technology, the recognition of neutrophils has become evident, confirming their accumulation and functional roles within the premetastatic niche.
Here, we broadly summarize recent progress in investigating the specific role of neutrophils in the premetastatic niche and relevant therapies for the premetastatic niche.
Basic information on the premetastatic niche
Evolution of the premetastatic niche theory
Tumour metastasis is a significant driver of cancer-related mortality, and the intricacies of this process are increasingly being recognized. The understanding of the process by which tumour cells metastasize to distant organs has undergone protracted evolution. In 1889, a pivotal hypothesis was presented by Steven Paget, who suggested that metastases are dominated by interactions between “seeds” (circulating tumour cells) and “soil” (host microenvironment) [7]. This theory is gaining increasing support, as studies about the host microenvironment in a distant organ have experienced exponential growth. In the 1980s, researchers began to focus on the interplay between tumour cells and their surrounding stroma, vasculature, and immune cells, among other factors, laying the groundwork for future investigations into the role of the microenvironment in distant metastatic sites [8]. The concept of the premetastatic niche was first proposed in 2005 and refers to the microenvironment with a specific organization that generates a series of adaptations and changes, laying the foundation for the settlement and clonal expansion of circulating tumour cells [3]. Moreover, studies have indicated that tumour cells alter the microenvironment of distant tissues by releasing extracellular vesicles, which serve as triggers for the formation of premetastatic niches at distant sites [9]. In the next decade, a deeper understanding of premetastatic niches was obtained, encompassing the intricate involvement of the entire immune system, fibroblasts, and specific miRNAs within extracellular vesicles in shaping the formation of these niches. Specific biomarkers of premetastatic niches and their organ tropism were also comprehensively elucidated during this period, and research on premetastatic niches is evolving towards clinical explorations for the prevention and treatment of tumour metastasis [10,11,12,13] (Fig. 1). In the following discussion of this section, we focus on two main questions: what constitutes the premetastatic niche and how it is formed.
Summary of the timeline of premetastatic niche concept development. Since the introduction of the “seed and soil” hypothesis in 1889, no significant breakthroughs in understanding metastasis have occurred throughout the 20th century. In the 21st century, a surge of studies focusing on the tumour microenvironment emerged, leading to the proposal of the premetastatic niche concept. The understanding of the composition of the microenvironment within these niches has rapidly advanced. As we moved into the 2020s, an increasing number of therapeutic strategies targeting the premetastatic niche were proposed and validated, providing a clear direction for preventing tumour metastasis
Cellular components of the premetastatic niche
Immune cells
Immunosuppressive cells play crucial roles in the premetastatic niche. Both innate and adaptive immune cells interact with stromal cells to reshape the distant microenvironment. Early changes in the premetastatic niche lead to the recruitment and activation of immunosuppressive cells, which could create a metastasis-friendly microenvironment [14].
Neutrophils
Neutrophils, the predominant leukocytes in peripheral blood, consistently act as the vanguards of the immune system by eliminating pathogens and inflammatory debris [15]. With the rapid development of high-throughput sequencing technology, subsets of neutrophils have been divided into two sharply contrasting groups [16]. The protumor subpopulation “N2” is deemed significant in influencing the immunosuppressive environment in the premetastatic niche, as it possesses the ability to secrete arginase 1 (ARG1) for the breakdown of arginine, which is crucial for the tumour-killing efficacy of T cells [17]. In particular, in lung metastases, neutrophils promote tumour cell proliferation in distal organs, resulting in a poor prognosis [18]. The functional phenotype of N1 neutrophils, which favours tumour suppression, clearly contradicts the conducive nature of the premetastatic niche for the circulation and settlement of tumour cells. Research indicates that within the premetastatic microenvironment, factors such as transforming growth factor-\(\:\beta\:\) (TGF-β) impede the emergence of the N1 phenotype, thereby preventing the extensive killing of tumour cells [19, 20].
Macrophages
Macrophages, which are profoundly vital immune cells, congregate around the site of a lesion once inflammation has occurred. Like neutrophils, macrophages also exhibit distinct functional M1/M2 subsets [21]. M2 macrophages promote tumour progression by expressing high levels of transforming growth factor-\(\:\beta\:\) (TGF-\(\:\beta\:\)) and C-C motif chemokine 22 (CCL22), both of which can recruit Treg cells to suppress the function of T cells [22]. However, in reality, macrophages exist on a spectrum of opposing phenotypes, and the mechanisms underlying their involvement in the premetastatic niche have not yet been sufficiently elucidated [15]. Similar to neutrophils, the tumour-suppressive phenotype of M1 macrophages is also influenced by the unique microenvironment of premetastatic sites, prompting a transition towards the protumoral M2 phenotype to mitigate the cytotoxic effects within the premetastatic niche [23].
T cells
T cells, pivotal adaptive immune cells, play crucial roles in shaping the immune microenvironment. According to current research, the involvement of T cells in the formation of the premetastatic niche involves primarily helper T cells (Th cells) and regulatory T cells (Treg cells), which, upon receiving tumour-derived signals, secrete a plethora of cytokines to establish an immunosuppressive microenvironment, thereby creating a conducive setting for the seeding of circulating tumour cells. In the context of metastatic breast cancer models, S100A4 in the peripheral environment can facilitate the differentiation of Th cells towards Th2 cells, which subsequently secrete downstream cytokines that upregulate the expression of complement C3 via a STAT6-dependent pathway, thereby recruiting neutrophils [24, 25]. In a lung cancer model, tumours induce fibroblasts to secrete increased levels of CCL1, which, through the activation of CCR8, induces the differentiation of Tregs, ultimately contributing to the formation of an immunosuppressive premetastatic niche [26].
Stromal cells
In addition to immune cells, native stromal cells also serve as mediators of the initiation signal. In response to signals secreted by tumours or immune cells, stromal cells undergo a series of alterations to reshape the surrounding microenvironment, transforming it into a conducive environment for metastasis. Notably, normal stromal cells include not only endothelial cells and fibroblasts but also a diverse array of tissue-specific cells from various organs. In the lung, a subset of cells expressing cyclooxygenase 2 (COX-2) can produce PGE2, which can remodel the microenvironment by suppressing the function of dendritic cells [27]. Another type of stromal cell in the lung, endothelial cells, can be stimulated by exosomal RNAs produced by tumours to secrete proinflammatory molecules, attracting neutrophils to participate in the formation of the premetastatic niche [28]. In another organ, hepatic sinusoidal endothelial cells, which serve as stromal cells in the liver, exhibit upregulated expression of fibronectin, which facilitates adhesion to tumour cells and progression, by tumour-secreted extracellular vesicles (EVs) containing TGF-β1[29]. Stromal cells exhibit specificity in shaping niches, displaying distinct alterations when confronted with various metastatic cancer types.
Molecular components of the premetastatic niche
Tumour-derived secreted factors (TDSFs): Tumours secrete various signalling molecules (i.e., TDSFs) that influence surrounding tissues and cells. A significant quantity of TDSFs has been found to play a role in the formation of the premetastatic niche, with the function of TDSFs varying according to the specificity of the tumour. In a study on bone metastasis, vascular endothelial growth factor (VEGF) was shown to recruit vascular endothelial growth factor receptor+ (VEGFR+) cells, which can capture CXCR4 + tumour cells [30]. However, in a study on lung metastasis, VEGF was shown to induce the expression of S100A8 and S100A9 to promote the formation of the premetastatic niche [31]. Research on this topic has become increasingly detailed, with greater systematic recognition of various TDSFs, such as nuclear factor kB (NF-kB) and hypoxia-inducible factor-1 (HIF-1). TDSFs can directly interact with immune cells or stromal cells to reshape their phenotypes and prepare the surrounding microenvironment for tumour metastasis through different signalling pathways.
Extracellular vesicles (EVs): Complex communication occurs between the premetastatic niche and circulating tumour cells, and EVs have been recently discovered as conduits of intercellular information interchange [32]. EVs are lipid bilayer-encased entities that are extruded from the cell membrane and transport nucleic acids and proteins. EVs are commonly categorized into different subtypes: exosomes, microvesicles, apoptotic bodies, oncosomes, and megasomes [33]. In the microenvironment of the premetastatic niche, we focus on tumour-derived exosomes and microvesicles, which are generated via the inwards budding of the tumour cell plasma membrane. Exosomes, which are membranous structures, are capable of secreting a multitude of cellular factors into their surroundings, including cytokines that recruit MDSCs, angiogenic factors, and microRNAs, all of which can promote the formation of premetastatic niches [14, 34, 35]. Furthermore, recent research has revealed that RNA fragments within exosomes can be recognized by Toll-like receptor 3 to initiate the recruitment of neutrophils [28]. Microvesicles are believed to be more likely to mediate communication and capture between circulating tumour cells and stromal cells. Research on the contents of microvesicles revealed that microvesicles are rich in metastasis-related proteases, such as matrix metalloproteinase (MMP)-2 and MMP-9, which participate in the degradation of the surrounding extracellular matrix, consequently bolstering tumour metastasis [36].
The formation of the premetastatic niche
Tumour-derived EVs play a crucial role in initiating premetastatic niche formation, although the exact mechanisms remain unclear. In recent studies, the formation of the premetastatic niche has been shown to involve four primary parts: changes in vascular permeability, activation of stromal cells, reprogramming of the extracellular matrix (ECM), and recruitment of immune cells [13].
Enhancing vascular permeability
Alterations in vascular permeability manifest as hyperpermeability of the vascular endothelium, aberrant morphologies, and destruction of the vascular basement membrane. Induced by TDSFs such as cyclooxygenase 2 (Cox-2) and the EGF receptor (EGFR) ligand epiregulin, vascular permeability at distal metastatic sites increases, facilitating the extravasation of various cells and collectively constructing the premetastatic niche microenvironment [37]. Cox-2 catalyses the synthesis of prostaglandins, and among cerebral prostaglandins, PGE2 is capable of binding to EP2 and EP4 receptors on vascular endothelial cells, thereby activating the downstream cAMP/PKA signalling pathway, phosphorylating occludin, and consequently enhancing vascular permeability, which facilitates cellular extravasation [38].
Activation of stromal cells
Studies on stromal cells have shown that upon the induction of EVs, resident stromal cells of distant organs exhibit elevated expression levels of the S100 protein family, which can increase bone marrow-derived cell recruitment and tumour cell adhesion [39]. Fibroblasts in the matrix serve as a classic example of cells that upregulate the expression of the S100 protein family upon stimulation with exosomes. Different initiating cancer cells lead to the upregulation of distinct proteins. Among them, the S100 family protein with the most well-studied mechanism is S100A4, which has been found to be abundant in the premetastatic niche. S100A4 has been shown to activate the JAK-STAT3 signalling pathway, leading to an increase in osteopontin gene expression, thereby conferring a greater propensity for metastasis in affected individuals [40]. S100A8 and S100A9 promote cell proliferation and inhibit apoptosis, laying the groundwork for the successful colonization of tumour cells [41].
ECM remodelling
ECM remodelling is a key step in the preparation of distant organs for metastasis, and one of the most noteworthy features of this process is organ specificity, indicating that different organs exhibit unique adaptive changes. The vital components of ECM remodelling, fibronectin and proteoglycans, can play roles in tumour cell capture and obstruction of immune cell function [42]. Matrix metalloproteinases play crucial roles in the remodelling of the extracellular matrix via diverse mechanisms. For example, MMPs can degrade the surrounding fibrous collagen, affecting tumour cells by downregulating p27kip1 and thus fostering proliferation. Additionally, they can degrade surrounding mechanical components, releasing growth factors bound within the ECM to stimulate the proliferation of nearby cells [43, 44].
Recruitment of immune cells
The last step involves creating a unique immune landscape. The cytokines and exosomes secreted by surrounding cells can both contribute to the recruitment of immune cells, orchestrating a complex interplay of signals that ultimately dictates the trafficking and localization of immune cells within the premetastatic niche. Exosomal RNA, enriched in small nuclear RNA from primary tumours, activates lung epithelial cells, which in turn induces the secretion of chemokines in the lungs to increase the recruitment of neutrophils [45]. The distinct premetastatic microenvironment not only attracts aggregated immune cells but also induces phenotypic alterations in response to diverse signalling molecules, thereby modulating their behaviours and functions. One of the most renowned features is immunosuppression, which is closely associated with the chemotaxis and polarization of immune cells [46]. Representative examples include the premetastatic niche recruiting immunosuppressive MDSCs and inducing neutrophils to differentiate towards a protumor phenotype, the N2 phenotype [47, 48]. This differentiation process is believed to be associated with the secretion of G-CSF by tumour cells, and N2 neutrophils are known to express high levels of matrix metalloproteinase 9, which can modify the surrounding stromal environment to facilitate tumour cell metastasis [49]. Recent studies have revealed that tumour-derived exosomes play a critical role in this process.
Roles of neutrophils in the formation of the premetastatic niche
Chemotaxis
Neutrophils are pivotal both in terms of quantity and function at distant metastatic sites. The factors that recruit neutrophils from circulating blood are primarily classified into three major categories: chemotactic factors, extracellular vesicles (EVs) and other bioactive factors [50]. The most well-studied chemotactic factors are the C-X-C motif chemokine ligand (CXCL) family. CXCL8 (IL-8)/CXCR2 is likely the most common chemotactic axis that functions abundantly at distal sites, and IL-8 is secreted by tumour cells and stromal cells in various cancers, such as breast cancer, colorectal cancer, cervical cancer, and acute myeloid leukaemia [51]. Following the activation of CXCR2, GTPase-activating protein 1 (IQGAP1) and vasodilator-stimulated phosphoprotein (VASP) adhere to this receptor. F-Actin binds to CXCR2 via IQGAP1 and VASP, initiating filament contraction and consequent chemotaxis [52]. Notably, in Yu’s study focusing on murine models deficient in CXCR2 expression, fewer infiltrating neutrophils and metastases in distant organs were observed [53]. Extracellular vesicles (EVs) are single-membrane vesicles with pivotal roles in facilitating long-range intercellular communication through their cargo, including DNA, RNA and certain proteins. In particular, tumour-derived EVs have emerged as critical components in the orchestration of the premetastatic niche. In a recent investigation, Toll-like receptor 3 (TLR3) expressed on lung epithelial cells was identified as the catalyst instigating neutrophil recruitment and the establishment of lung premetastatic niches. This process occurs through the detection of tumour-derived exosomal RNA by TLR3, thereby stimulating the secretion of CXCL5 and CXCL12[28]. Prominent representatives of bioactive molecules include myeloid-related proteins such as S100A8 and S100A9, which are abundantly expressed at metastatic sites and serve as potent chemoattractants for neutrophils [54]. Nevertheless, the precise mechanism underlying MRP-mediated neutrophil mobilization remains elusive, and more in-depth explorations are warranted. Another crucial bioactive molecule is the downstream product of vascular endothelial growth factor (VEGF), which can recruit neutrophils and mediate their adhesion to postcapillary venules [55].
Adaptive alterations in neutrophils
Upon attraction to distant metastatic organs, neutrophils undergo a series of phenotypic alterations influenced by the surrounding microenvironment to facilitate premetastatic niche formation. Neutrophils in cancer exhibit heterogeneity, manifesting both an antitumorigenic phenotype (N1) and a protumorigenic phenotype (N2) [16]. The intricate microenvironment surrounding the niche during its formation significantly influences the differentiation of neutrophils [56]. Type I interferons (IFNs) play a crucial role in shaping immune responsiveness by directing the polarization of neutrophils towards the N1 phenotype [57]. In recent investigations of murine models, the absence of type I interferons often not only results in significant aggregation of neutrophils and their transition towards the N2 phenotype but also leads to the secretion of substantial amounts of neutrophil-derived immunosuppressive molecules such as S100A8, S100A9, and Bv8[31, 54, 59]. Not only does the decrease in IFNs play a role in the formation of the premetastatic niche, but the secretion of IL-6, IL-10, and G-CSF by tumour cells also induces the transition of neutrophils towards the N2 phenotype upon arrival at premetastatic sites via the bloodstream to create an immunosuppressive environment [28]. However, this finding does not imply that all neutrophils in the metastatic environment convert to the N2 phenotype. In most cases, neutrophils in the premetastatic niche predominantly exist in coexisting N1 and N2 phenotypes.
Neutrophil extracellular traps (NETs), newly discovered structures originating from neutrophils, are also believed to be involved in the metastasis of tumour cells. NETs are large, adhesive networks composed of decondensed chromatin filaments and protein molecules, including histones and neutrophil elastase [59]. Unique molecular constituents within the premetastatic microenvironment prompt NET generation, with pivotal instigators encompassing IL-8 from other inflammatory cell reservoirs, high mobility group box protein 1 (HMGB1) and G-CSF originating from tumour cells [60]. IL-8 not only functions as a chemoattractant for neutrophils, as discussed earlier, but also serves as a critical inducer in the process of NET generation. Upon recognition by CXCR2, neutrophils initiate the PI3K/AKT/reactive oxygen species (ROS) signalling cascade, which catalyses the production of NETs [61]. G-CSF secreted by tumour cells can reshape the haematopoietic function of the bone marrow and promote the differentiation of neutrophils towards the protumor N2 phenotype [47]. The specific mechanisms by which platelets promote the formation of NETs have not been fully elucidated; however, studies have confirmed that platelets also release HMGB1, which is recognized by TLR4 receptors on the surface of neutrophils, thereby inducing NET formation. Notably, the net-like structure of NETs is particularly conducive to capturing free-floating platelets, potentially resulting in a positive feedback regulatory effect [62, 63]. Moreover, complement 3 (C3) within the complement system has been shown to promote the formation of NETs [64]. NETs have been demonstrated to facilitate tumour cell survival and proliferation through various mechanisms, such as suppressing apoptosis and increasing the expression of activation markers in tumour cells or increasing ATP production in tumour cell mitochondria to promote proliferation [61, 65].
Metabolic alterations are another important change in neutrophils to facilitate the successful metastasis and growth of tumours. The metabolic changes include the upregulation of fatty acid transport protein 2 (FATP2) and increased levels of Arg1, both of which inhibit the tumoricidal activity of CD8 + T cells to some extent [66,67,68]. The increased expression of FATP2 induces neutrophils to engulf lipids and metabolize them into prostaglandin E2 (PGE2), which could cause T-cell suppression via the PGE2-EP2/EP4 signalling pathway [69]. ARG1 impedes T-cell proliferation and functionality by depleting the indispensable amino acid L-arginine, which is crucial for T-cell activation and proliferation and promotes the polarization of macrophages towards an immunosuppressive M2-like phenotype [67]. Another altered metabolic pathway is glucose metabolism, and studies have shown that tumour-associated neutrophils exhibit elevated rates of glycolysis and oxidative phosphorylation compared to neutrophils in normal tissues [70]. In the future, elucidating the precise mechanisms underlying accelerated glycolysis will be imperative.
Neutrophils prepare the surroundings for tumour metastasis
After undergoing chemotaxis and being shaped by the surrounding milieu, neutrophils also prepare the environment around them to facilitate successful colonization by circulating tumour cells.
The protumor neutrophil phenotype, N2, is shaped by the premetastatic microenvironment and can promote tumour cell seeding and progression through the secretion of various molecular components. Some of the most crucial molecular components are proinflammatory factors, specifically TNF-α, which alters the surrounding environment and participate in the constitution of one of the premetastatic niche characteristics—inflammation. Leukotrienes, products upregulated on neutrophils separated from premetastatic sites, have been found to induce tumour cell proliferation and metastasis. Moreover, the converse approach of inhibiting the enzyme responsible for leukotriene synthesis, arachidonate 5-lipoxygenase (ALOX-5), has also been discovered to modulate T-cell aggregation, thus reducing tumour metastasis [6, 71]. The factors secreted by tumours also aid in tumour cell growth. A recent study revealed that the iron transport protein secreted by ly6g + neutrophils can promote the mitosis of tumour cells. Moreover, the generation of iron transport proteins is induced by tumour cell-secreted GM-CSF through the activation of the neutrophil Janus kinase (Jak)-Stat5β pathway [72].
Neutrophils are also capable of directly interacting with circulating tumour cells, thereby facilitating their metastatic colonization. Research has shown that neutrophils aid in the retention of human melanoma cells in the lungs by facilitating the tethering of circulating tumour cells (CTCs) to the endothelium [73]. Despite longstanding research on direct interactions between neutrophils and circulating tumour cells, investigations into their precise mechanisms remain relatively limited. Currently, the majority of studies predominantly focus on the classical interactions between integrins on neutrophils and intercellular adhesion molecule 1 (ICAM-1) on tumour cells [74, 75]. ICAM-1 interacts with MAC-1 on neutrophils, forming a complex that subsequently migrates towards the vascular wall, facilitating transendothelial movement, and ultimately seeding at premetastatic sites [76]. Furthermore, studies have indicated that direct contact between neutrophils and circulating tumour cells can also facilitate the binding of tumour cells to vascular endothelial cells. The analysis of direct interactions between neutrophils and tumour cells is still in its nascent stage, representing a highly promising avenue of research.
NETs formed through the stimulation of primary tumours can act as scaffolds for circulating tumour cells, providing a favourable environment for their colonization and growth, and this particular role has been confirmed in the omental metastasis of ovarian cancer and liver metastasis of colon cancer [77, 78]. The capture of circulating tumour cells by NETs can be mediated by β-integrins [79]. They have been demonstrated to facilitate tumour cell survival and proliferation through various mechanisms, such as suppressing apoptosis and increasing the expression of activation markers in tumour cells or increasing ATP production in tumour cell mitochondria to promote proliferation [61, 65]. In addition to their direct capture function, many components of NETs can also indirectly influence the metastasis of tumour cells. For example, one of the crucial constituents of NETs, the DNA‒histone complex, can recognize and bind to the transmembrane protein CCDC25 on breast cancer cells, thereby activating the downstream ILK‒paxillin pathway to increase the motility of tumour cells [80]. In colorectal cancer, NE may trigger the TLR-4 signalling pathway in tumour cells, leading to the upregulation of proteins involved in tumour mitochondrial biogenesis and growth [81]. NETs can also perform barrier functions.
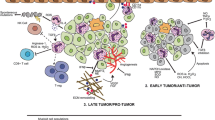
We may conclude that upon migrating to the premetastatic niche, neutrophils engage in significant interactions with the local milieu. These interactions facilitate the transformation of the surrounding environment into one that is conducive to metastasis, laying the groundwork for the colonization of circulating tumour cells (Fig. 2).
Upon entering the premetastatic niche through chemotaxis, interactions occur between the microenvironment and neutrophils. Influenced by the microenvironment, neutrophils undergo a phenotypic transformation into a protumorigenic state, stimulating the production of neutrophil extracellular traps (NETs) and immunosuppressive factors. Activated neutrophils lead to the progression of local inflammation and the entrapment of circulating tumour cells, thereby facilitating tumour progression
Although the aforementioned description details how neutrophils enter premetastatic organs and prepare for the colonization of circulating tumour cells, the mechanisms of successful premetastatic niche formation differ depending on the target organ. The following table 1 categorizes different organs and succinctly summarizes the key molecules and specific mechanisms mediating neutrophil migration and function in various organs.
Characteristics of premetastatic niches formed by neutrophil involvement
Due to the intricate structural components and distinctive physiological niches, many of features, such as immunosuppression, angiogenesis, lymphangiogenesis, and organotropism, have been elucidated with remarkable clarity. In the following discourse, we shall specifically delve into which characteristics entail the involvement of neutrophils and expound upon their roles in these processes.
Inflammation
The relationship between inflammation and cancer is not new [97]. The microenvironment dominated by inflammatory cells plays an indispensable role in the progression and metastasis of tumours [98]. As discussed in the preceding section, one of the pivotal pathways through which neutrophils shape the surrounding microenvironment to prepare for tumour metastasis involves the secretion of proinflammatory factors, which include not only specific cytokines but also various proteases. One of the representative proinflammatory cytokines is TNF-α, which has been found to participate in oedema formation, regulate blood coagulation, recruit immune cells and promote oxidative stress at sites of inflammation [99]. The involvement of inflammatory cells in orchestrating the tumour microenvironment is deemed an indispensable step in the progression and metastasis of tumours, where neutrophils, as secretors of proinflammatory factors such as IL-12, induce inflammation in the surrounding microenvironment, thereby activating adjacent endothelial and stromal cells to increase the adhesion of circulating tumour cells [6, 100]. Other significant inflammatory factors, the proteases, predominantly comprise neutrophil elastase (NE), myeloperoxidase (MPO), and matrix metalloproteinases (MMPs). NE exhibits strong hydrolytic activity and is capable of hydrolysing fibronectin, proteoglycans, type IV collagen, and other extracellular matrix proteins. This process exacerbates damage to the basement membrane and surrounding tissues, worsening the inflammatory condition of the surrounding environment [101]. MMPs can degrade endothelial calmodulin, leading to compromised endothelial integrity and promoting the leakage of circulating tumour cells [102].
The establishment of an inflammatory milieu does not rely solely on neutrophils. The interplay among regulatory immune cells, inflammation, cancer growth, and metastasis involves complicated molecular components and signalling pathways. For example, improper activation of Toll-like receptor 4 (TLR4) on tumour cells within premetastatic niches can lead to increased invasiveness and resistance to apoptosis, whereas inappropriate activation of TLR4 on other immune cells can result in pronounced inflammatory responses [103].
Immunosuppression
Tumours, as targets for immune surveillance and elimination by the immune system, face hindrances in terms of their progression and metastasis within the body. However, tumours possess unique immunosuppressive mechanisms to shield themselves from immune system assault. Within the premetastatic niche of the disseminated tumour, immunosuppression is a well-established facilitator of metastatic traits. Evidence indicates the pivotal role of neutrophils in orchestrating immunosuppression within the premetastatic niche. First, a unique form of neutrophil death discovered in recent years, ferroptosis, plays a significant role in immunosuppression. Ferroptosis is a nonapoptotic form of regulated cell death triggered by the dysregulation of redox mechanisms, ultimately leading to the release of large quantities of peroxidized polyunsaturated phospholipids into the surrounding microenvironment that subsequently restrict the proliferation and function of T cells [104, 105].
In addition, neutrophils within the premetastatic niche can undergo phenotypic transitions, thereby inhibiting immune responses. Within the premetastatic microenvironment, numerous factors induce neutrophils to differentiate into a protumor phenotype known as N2. N2 neutrophils employ various mechanisms to suppress the normal functions of the surrounding immune system, thereby promoting the survival of tumour cells. N2 neutrophils are capable of secreting immunosuppressive factors; for example, type 1 arginase (ARG1) is a protease released by neutrophils that degrades arginine, which is important for maintaining T-cell function through the expression of the T-cell coreceptor CD3ζ[108]. The immunosuppressive function of neutrophils also manifests as the upregulation of programmed death ligand 1 (PD-L1), an important coinhibitory molecule that recognizes programmed death 1 (PD1) on T cells to restrain their activity [107]. The specific underlying mechanism is now quite clear: GM-CSF released by tumour cells can activate neutrophils via the Jak-Stat3 signalling pathway, inducing the expression of PD-L1 on neutrophils [108].
NETs also play a role in establishing the immunosuppressive characteristics of the surrounding environment [109]. In experiments conducted by Christof et al., a majority of CD4 + and CD8 + TILs expressed various inhibitory receptors in NET-rich microenvironments. Additionally, these cells exhibited a phenotype of functional and metabolic exhaustion. The subsequent targeted degradation of NETs in vivo through DNase treatment in mice led to suppressed tumour growth, with the functions of surrounding T cells restored to normal levels [110]. Another crucial function of NETs is their barrier capabilities; their distinctive mesh-like structures not only provide anchorage points for tumour cells but also serve as a physical barrier, shielding tumour cells from contact and damage by cytotoxic cells [111]. Intravital microscopy has provided evidence that neutrophil extracellular traps (NETs) interfere with the contact between cytolytic T lymphocytes (CTLs) and NK cells with tumour cells [112].
Indeed, immunosuppression within premetastatic niches is not solely attributed to the functions of neutrophils alone. MDSCs are inevitably pertinent; they can hinder the functions of immune cells within the local environment through the generation of reactive oxygen species (ROS) [113]. Additionally, the aggregation of myeloid cells impedes dendritic cell activation and prompts macrophage differentiation towards the immunosuppressive M2 phenotype. This mechanism is orchestrated by the secretion of GRP78 by breast cancer cells [114]. Immunosuppression undeniably emerges as a significant hallmark facilitating the successful dissemination of tumours.
Angiogenesis
One notable feature of tumours is the intricate vasculature developed within the tumour tissue. Premetastatic niches, which serve as target sites for tumour metastasis, also exhibit preexisting angiogenesis. Neutrophils are significant promoters of angiogenesis. Within the human body, neutrophils are considered rich in VEGFA. Upon exposure to TNF, these cells rapidly degranulate and release VEGFA into the surrounding microenvironment, where it targets VEGFR2 on endothelial cells, thereby facilitating angiogenesis. Furthermore, G-CSF, which is recognized by G-CSFR, initiates the JAK/STAT3 pathway, increasing the expression of proangiogenic factor 2 and thereby increasing the proliferation of vascular endothelial cells [115].
The discovery and elucidation of the role of NETs in promoting angiogenesis are still in their nascent stages. In a recent study conducted by Yang et al., the researchers discovered that one of the primary components of NETs, NET-DNA, can bind to the ccdc25 receptor on human umbilical vein endothelial cells (HUVECs), activating AKT/mTOR signal transduction. This activation facilitates HUVEC proliferation, thus initiating angiogenesis and promoting tumour metastasis and progression [116]. A notable characteristic of angiogenesis is its potential for self-perpetuation through internal loops. Specifically, angiogenesis induced by NETs can recruit additional neutrophils, which, when stimulated, contribute to further NET formation. The underlying mechanism involves the recognition and binding of Notch1 receptors on endothelial cells by tumour cells. This interaction increases the expression of VCAM1 receptors, consequently promoting the recruitment and infiltration of neutrophils [117]. Moreover, studies have indicated that neutrophil extracellular traps (NETs) can facilitate vascular rejuvenation and generation by eliminating the senescent vasculature [118].
In contrast to the incomplete understanding of neutrophil involvement in angiogenesis, mechanisms involving other cellular and molecular components have long been elucidated and shown to promote vascular formation. For example, recruited TIE2 + monocytes have the capacity to secrete high levels of VEGF, thereby fostering the formation of a perivascular niche conducive to metastasis [119]. Tumour-derived extracellular vesicles also play pivotal roles in promoting angiogenesis. For example, extracellular vesicles originating from colorectal cancer cells harbour miR-25-3p, which selectively binds to KLF2 and KLF4, thereby orchestrating the expression of VEGFR2, ZO-1, occludin, and Claudin5 and consequently fostering vascular permeability and angiogenesis [120].
Extracellular matrix remodelling
The extracellular matrix (ECM) is a component of the microenvironment that is essential for tumour cell survival. The transformation of the intracellular microenvironment during premetastatic niche formation is comprehensive, extending beyond cellular components to influence extracellular matrix constituents as well.
The extracellular matrix (ECM) is composed of chromatin and granule proteins, with various components, such as metalloproteinases, myeloperoxidase (MPO), neutrophil elastase (NE), and proteinase 3, involved in its remodelling [121]. Metalloproteinases secreted by neutrophils play a significant role in reshaping the surrounding environment [122]. Among the matrix metalloproteinase family, MMP-9 has been extensively studied. It can degrade the existing matrix to generate VEGF-α, thus promoting endothelial proliferation within blood vessels [123]. An analysis of MMTV-PyMT transgenic breast cancer model mice revealed that MMP-9 is overexpressed in the lungs, indicating its importance in the metastatic process [124]. Another substance pivotal in the remodelling of the pericellular matrix is neutrophil elastase (NE), which degrades proteins within the pericellular matrix, consequently influencing intercellular signalling. The tumour suppressor protein EMILIN1 serves as a substrate for neutrophil elastase. In sarcomas and ovarian cancers, notable degradation of EMILIN1 within the pericellular matrix by neutrophil elastase has been observed, leading to a shift in the pericellular environment in a protumorigenic direction [125]. Recently, a novel enzyme secreted by neutrophils, cathepsin-g, has been identified. Cathepsin G can degrade endothelial calcium-binding protein and fibronectin within the surrounding microenvironment, activate MMPs, and increase the strength of MCF-7 cell adhesion mediated by E-calcium-binding protein, thus promoting the implantation of tumour cells [126, 127].
Fibrosis of the extracellular matrix is an unavoidable topic in the remodelling of premetastatic niches. A confirmed mechanism of fibrosis involves the uptake of extracellular vesicles mediated by CD44v6/C1QBP in haematopoietic stem cells. In this PDAC model, vesicle uptake leads to the phosphorylation of insulin-like growth factor 1 and downstream signalling, culminating in the activation of haematopoietic stem cells and fibrosis [128]. Many studies have linked fibrosis in the premetastatic niche with the recruitment of immune cells and the progression of metastasis. However, further exploration is needed to definitively establish how fibrosis precisely initiates these downstream effects.
Treatments targeting neutrophils in the premetastatic niche
The preceding discussion underscores the importance of neutrophils as integral constituents of the premetastatic niche, as they are architects of many pivotal features. The formation of and physiological alterations in neutrophil function constitute mutually influential processes that are independent of each other. As the premetastatic niche has emerged as a crucial destination for cancer metastasis, it has become a focal point in efforts to inhibit cancer dissemination. Existing therapies related to neutrophils can be broadly categorized into four classes: (1) inhibition of bioactive substance secretion by neutrophils; (2) engineering of neutrophil membranes; (3) induction of the pseudopremetastatic niche in vivo to misguide neutrophils; and (4) targeting NETs within the premetastatic niche (Fig. 3).
Inhibition of bioactive substance secretion by neutrophils
Upon attraction by chemotactic factors to premetastatic sites, neutrophils undergo phenotypic alterations and secrete a series of bioactive substances through the induction of the surrounding environment, thereby constructing essential features of premetastatic niches.
One prominent feature is inflammation, and neutrophils, crucial inflammatory cells, are frequently observed to upregulate the expression of IL-6, which could promote tumour metastasis by suppressing the function of CD8 + T cells through the overexpression of PD-L1[131, 132]. In a Gprc5a-knockout mouse model of cancer, the use of anti-IL-6 antibodies as a control treatment revealed the restoration of both innate and adaptive immune responses within the microenvironment. Moreover, this approach resulted in the successful suppression of pulmonary metastasis [130]. In another study focusing on ovarian cancer, investigators reported potent anticancer activity through the concurrent administration of neutralizing IL-6 antibodies and the epidermal growth factor (EGFR) inhibitor gefitinib [131].
Another pivotal therapeutic target is the matrix metalloproteinase (MMP) family. A therapeutic strategy for inhibiting matrix metalloproteinases has long been proposed, yet the absence of selective inhibitors has impeded successful research outcomes. With advancements in biotechnology, treatment regimens involving MMPs have once again emerged at the forefront of attention. For example, the utilization of MMP-9-specific antibodies in MMTV-PyMT breast cancer models has revealed notable suppression of lung cancer metastasis [132]. Moreover, another member of the matrix metalloproteinase family, MMP-14, is also regarded as a potential therapeutic target, and researchers have discovered an antiangiogenic immunotherapy that inhibits MMP-14 that could overcome therapeutic resistance in colorectal cancer [133].
Among all biologically active substances secreted by neutrophils, inhibitors of NE may represent one of the most extensively studied experimental directions in clinical research. Endogenous inhibitors of neutrophil elastase, such as elafin, exist naturally within the human body. In breast cancer cells, the expression of elafin has been found to trigger cell cycle arrest [134]. Sivelestat, a neutrophil elastase inhibitor, has already emerged on the market due to its anti-inflammatory properties. Research has shown promising results in reducing the metastatic burden in the liver and lungs of rats with colon adenocarcinoma and mice bearing human lung tumours, respectively [135, 136].
Engineering of neutrophil membranes
Exploiting the abundant presence of neutrophils in premetastatic niches, the membrane structure of neutrophils allows them to travel unhindered. Many researchers have capitalized on this characteristic, focusing on the membrane of neutrophils to identify therapeutic avenues for tumour metastasis.
First, we introduce a sponge-like neutrophil membrane-coated nanosystem (NM/PPcDG/D). Its synthesis involves the self-assembly and crosslinking of a synthesized redox-responsive polymer (PPDG) with nanocores loaded with doxorubicin (D) (PPcDG/D). This process yields PPcDG/D nanoparticles (NPs). An activated neutrophil membrane is subsequently coated to prepare biomimetic NM/PPcDG/D [139]. This system exhibits remarkable premetastatic niche tropism, and the nanosystem serves as a nanosponge to neutralize inflammatory factors associated with MDSC recruitment. MDSCs are classical drivers of premetastatic niche formation, and the ultimate outcome of this nanosystem is the inhibition of premetastatic niche formation [138]. Another nanosystem exploits adhesion molecules on neutrophil membranes for targeted delivery to the circulating tumour cell niche. Researchers have generated this nanosystem by encapsulating neutrophil membranes onto the surface of poly(lactic-co-glycolic acid) nanoparticles (NPs) [139]. NM-NPs augmented cellular interactions in 4T1 cell models under shear flow conditions in vitro, indicating increased CTC capture efficiency and reduced recruitment towards the premetastatic niche.
In addition to nanosystems, neutrophil membranes can also be modulated through bacterial magnetosome processing to regulate premetastatic niche formation [140]. In this process, live neutrophils undergo rapid cryopreservation and transform into cryopreserved neutrophils (CNEs). Subsequently, bacterial magnetosomes (Mag) are further engineered onto the surface of the CNE, forming CNE-Mag. As crucial components of premetastatic niches, macrophages consistently exhibit remarkable plasticity. Our engineered CNE-Mag precisely facilitates their repolarization from the M2 phenotype to the M1 phenotype, inducing a localized environmental disruption and consequently impeding metastasis formation [140, 141].
Induction of the pseudopremetastatic niche in vivo
Research indicates that neutrophils accumulate in the tumour microenvironment or in metastatic niches to facilitate tumour metastasis [47, 142,143,144]. The premetastatic site can attract neutrophils and alter their phenotypes and physiological functions. Activated neutrophils play crucial roles in the formation of the premetastatic niche and the capture of circulating tumour cells [28].
A recent emerging therapy involves creating a synthetic premetastatic niche within the body to attract various immune cells and molecular components, including neutrophils. These sites are supposed to lack adequate biological materials and chemical factors, thereby inhibiting the migration of tumour cells to specific locations. CXCL12 is a crucial chemotactic factor, and its receptor, CXCR4, is expressed not only on neutrophils but also on numerous cancer cells. The authors developed a CXCL12-loaded gel (CLG) to investigate its therapeutic efficacy in inhibiting tumour metastasis. The authors selected hyaluronic acid gel as the carrier because previous studies have already documented the spatial colocalization of CXCL12 and hyaluronic acid within the ECM. The authors addressed the high instability of CXCL12 by incorporating polymeric hydrogels, which can conceal and protect chemokines from enzymatic degradation, into the system. Glycosaminoglycans (GAGs) were employed to immobilize and increase the local concentration of chemokines, promoting their oligomerization and improving their presentation to receptors. C57BL/6 mice were subcutaneously injected with CLGs or empty gels (EGs), followed by intravenous inoculation of B16-hCXCR4-GFP cells five days later. Compared with EGs, CLGs have a greater capacity to recruit a greater quantity of B16-hCXCR4-GFP cells, concurrently reducing pulmonary metastasis in mice harbouring CLGs, and CLGs are infiltrated by a greater number of CD45-positive leukocytes, mainly neutrophils CD11b + Ly6G + cells[147]. The specific mechanism likely involves artificially creating a CXCL12-enriched environment at a localized site. CXCL12 attracts and binds to CXCR4-overexpressing cells, including tumour cells and various immune cells, capturing them within a pseudoniche where they ultimately die within two weeks. Through this process, the critical number of metastasis-initiating cells may be reduced.
Compared with existing therapies, this gel is less harmful for preventing tumour metastasis. Conventional radiotherapy and chemotherapy often result in significant damage to normal cells due to their lack of specificity. In contrast, this therapy, which specifically targets CXCR4-overexpressing cells, markedly reduces the impact on normal cells, thereby minimizing harm to the body. However, the feasibility of this treatment in humans requires further investigation. For example, whether the injection of large amounts of this gel could trigger an immune response or whether the pseudopremetastatic niche, after attracting CXCR4 cells, might induce angiogenesis and lymphangiogenesis at the gel site, making it difficult to remove and potentially leading to unintended tumour metastasis, remain to be investigated.
Targeting NETs
As previously discussed, NETs engage in stromal reprogramming within the premetastatic niche microenvironment, participating in the capture of circulating tumour cells. With recent advancements in understanding the specific mechanisms and components contributing to NET formation, inhibiting NET generation and impeding their physiological functions have emerged as novel therapeutic avenues.
Numerous distinct targets have emerged for therapeutic interventions aimed at inhibiting NET formation. An RNA-seq study suggested that the complement component C3 plays a significant role in neutrophil recruitment and NET formation, with its abundant expression induced by Th2 cell cytokines and STAT6. Consequently, the authors propose targeting the inhibition of the Th2 cell cytokine-STAT6-C3-NET axis as a therapeutic approach to suppress tumour metastasis [146]. Another complement component implicated in inhibiting NET formation is C5a, which can facilitate neutrophil generation of NETs by binding to C5aR1 on MDSCs, promoting their migration to premetastatic sites. Furthermore, in subsequent studies, both targeting tissue C5aR1 and eliminating C5a have been shown to result in reduced NET formation [147]. The formation of NETs, which is intricately tied to chromatin decondensation and DNA release, is strongly associated with histone proteolysis. Researchers have observed a significant reduction in pulmonary metastases in the MMTV-PyMT model via the inhibition of arginine deiminase 4, which is responsible for histone protein hydrolysis. However, this target has yet to be explored in human tissues [148].
Additionally, therapeutic approaches have been directed towards degrading preexisting NETs, with deoxyribonuclease I (DNase I) identified as an enzyme capable of degrading NETs. However, its short half-life has hindered its practical application. A novel research avenue involves encapsulating DNase I within nanoparticles, which not only overcomes the short half-life limitation in the bloodstream but also preserves its efficacy in inhibiting tumour metastasis [149].
Discussion
The premetastatic niche hypothesis is an emerging concept concerning metastasis, primarily involving alterations in vascular permeability, activation of stromal cells, remodelling of the extracellular matrix (ECM), and recruitment of immune cells. Neutrophils are recruited into the premetastatic niche to prepare the surrounding environment for tumour cell dissemination, providing novel insights into therapeutic strategies for combating cancer metastasis. However, the roles of neutrophils in the premetastatic niche and therapeutic approaches still have certain limitations.
Due primarily to the exceedingly short lifespan of neutrophils within the body, the precise mechanisms underlying their contributions to premetastatic niche formation remain inadequately understood. In particular, with the utilization of single-cell sequencing technology, neutrophils account for less than 1.5% of the single-cell sequencing outcomes across various prevalent sequencing platforms, making it challenging for us to fully elucidate their precise distribution and functions.
Moreover, many studies on therapeutic approaches remain in the theoretical and animal testing phases; namely, they are conducted in preclinical models. Consequently, research focusing on neutrophils within the premetastatic niche should be performed in models with greater clinical relevance, such as patient-derived tumour xenografts and tumour-derived organoids, and should also encompass a broader range of clinical tissue samples. Research adopting neutrophils as a therapeutic entry point is anticipated to become increasingly abundant.
With the continual advancement of single-cell sequencing technologies in recent years, the heterogeneity inherent in human cells has increasingly come into focus. Just as neutrophils exhibit diametrically opposed physiological N1 and N2 phenotypes, many cells, including macrophages and B cells, have been discovered to be composed not of a single phenotype but of multiple phenotypes. Future therapeutic strategies aimed at preventing tumour metastasis are expected to be tailored to specific cellular subtypes, necessitating further research into the tumour microenvironment and the premetastatic niche.
Data availability
No datasets were generated or analysed during the current study.
Abbreviations
- PMN:
-
Pre-metastatic Niche
- BMDCs:
-
Bone Marrow Derived Cells
- ARG1:
-
Arginase 1
- TGF-β:
-
Transforming Growth Factor-β
- CCL22:
-
C-C motif Chemokine 22
- MDSC:
-
Myeloid-Derived-Suppressor-Cells
- GM-CSF:
-
Granulocyte-Macrophage Colony Stimulating Factor
- PGE2:
-
Prostaglandin E2
- ROS:
-
Reactive Oxygen Species
- NK cell:
-
Natural Killer cell
- VEGF:
-
Vascular Endothelial Growth Factor
- VEGFR:
-
Vascular Endothelial Growth Factor Receptor
- NF-kB:
-
Nuclear Factor kB
- HIF-1:
-
Hypoxia-Inducible Factor-1
- EV:
-
Extracellular Vesicles
- MMP:
-
Matrix Metalloproteinase
- CXCL:
-
C-X-C motif Chemokine Ligand
- IL-8:
-
Interleukin 8
- IQGAP1:
-
IQ Motif Containing GTPase Activating Protein 1
- VASP:
-
Vasodilator-Stimulated Phosphoprotein
- TLR3:
-
Toll-Like Receptor 3
- FATP2:
-
Fatty Acid Transport Protein 2
- TNF-α:
-
Tumor Necrosis Factor-α
- ALOX-5:
-
Arachidonate 5-Lipoxygenase
- Jak:
-
Janus Kinase
- ICAM-1:
-
Intercellular Adhesion Molecule 1
- CTCs:
-
Circulating Tumor Cells
- NETs:
-
Neutrophil Extracellular Traps
- HMGB1:
-
High Mobility Group Box Protein 1
- MPO:
-
Myeloperoxidase
- PD-L1:
-
Programmed Death Ligand 1
- PD1:
-
Programmed Death 1
- IFNs:
-
Interferons
- CTLs:
-
Cytolytic T Lymphocytes
- TDSF:
-
Tumor-Derived Secreted Factors
- EMT:
-
Epithelial-Mesenchymal Transition
- ECM:
-
Extracellular Matrix
- S100A8:
-
S100 Calcium Binding Protein A8
- S100A9:
-
S100 Calcium Binding Protein A9
- KLF:
-
Kruppel Like Factor
- VEGFA:
-
Vascular Endothelial Growth Factor A
- EGF:
-
Epidermal Growth Factor
- EGFR:
-
Epidermal Growth Factor Receptor
- NM:
-
Neutrophil Membrane
- PPcDG/D:
-
Redox-Responsive Polymer Loaded with Doxorubicin
- CNE:
-
Cryopreserved Neutrophils
- CLG:
-
CXCL12-Loaded Gel
- CXCR4:
-
C-X-C Chemokine Receptor Type 4
- HA:
-
Hyaluronic Acid
- DNase I:
-
Deoxyribonuclease I
References
Massagué J, Obenauf AC. Metastatic colonization by circulating tumour cells. Nature. 2016;529:298–306.
Gu Y, et al. Tumor-educated B cells selectively promote breast cancer lymph node metastasis by HSPA4-targeting IgG. Nat Med. 2019;25:312–22.
Kaplan RN, et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438:820–7.
Liu Y, Cao X. Characteristics and significance of the pre-metastatic niche. Cancer Cell. 2016;30:668–81.
Granot Z, et al. Tumor entrained neutrophils inhibit seeding in the premetastatic lung. Cancer Cell. 2011;20:300–14.
Wculek S, Malanchi I. Neutrophils support lung colonization of metastasis-initiating breast cancer cells. Nature. 2015;528:413–7.
Paget S. The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastasis Rev. 1989;8:98–101.
Folkman J. How is blood vessel growth regulated in normal and neoplastic tissue? G.H.A. Clowes memorial award lecture. Cancer Res. 1986;46:467–73.
Peinado H, et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med. 2012;18:883–91.
Joyce J, Pollard J. Microenvironmental regulation of metastasis. Nat Rev Cancer. 2009;9:239–52.
Valastyan S, Weinberg R. Tumor Metastasis: Molecular insights and Evolving paradigms. Cell. 2011;147:275–92.
Hoshino A, et al. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527:329–35.
Peinado H, et al. Pre-metastatic niches: organ-specific homes for metastases. Nat Rev Cancer. 2017;17:302–17.
Patras L, Shaashua L, Matei I, Lyden D. Immune determinants of the pre-metastatic niche. Cancer Cell. 2023;41:546–72.
Ormseth B, Onuma A, Zhang H, Tsung A. The hepatic pre-metastatic niche. Cancers. 2022;14:3731.
Hedrick CC, Malanchi I. Neutrophils in cancer: heterogeneous and multifaceted. Nat Rev Immunol. 2022;22:173–87.
Tyagi A et al. Nicotine promotes breast cancer metastasis by stimulating N2 neutrophils and generating pre-metastatic niche in lung. Nat Commun 12 (2021).
Ching-Fang W, et al. The lack of type I interferon induces neutrophil‐mediated pre‐metastatic niche formation in the mouse lung. Int J Cancer. 2015;137:837–47.
Giese MA, Hind LE, Huttenlocher A. Neutrophil plasticity in the tumor microenvironment. Blood. 2019;133:2159–67.
Jablonska J, Lang S, Sionov RV, Granot Z. The regulation of pre-metastatic niche formation by neutrophils. Oncotarget. 2017;8:112132–44.
Tacke F, Zimmermann H. Macrophage heterogeneity in liver injury and fibrosis. J Hepatol. 2014;60:1090–6.
Olkhanud PB, et al. Tumor-evoked regulatory B cells promote breast cancer metastasis by converting resting CD4⁺ T cells to T-regulatory cells. Cancer Res. 2011;71:3505–15.
Seif F, Sharifi L, Khoshmirsafa M, Mojibi Y, Mohsenzadegan M. A review of preclinical experiments toward targeting M2 macrophages in prostate Cancer. CDT. 2019;20:789–98.
Zheng Z et al. Lung mesenchymal stromal cells influenced by Th2 cytokines mobilize neutrophils and facilitate metastasis by producing complement C3. Nat Commun 12 (2021).
Grum-Schwensen B et al. S100A4-neutralizing antibody suppresses spontaneous tumor progression, pre-metastatic niche formation and alters T-cell polarization balance. BMC Cancer 15 (2015).
Wang M, et al. Tumor-derived exosomes drive pre-metastatic niche formation in lung via modulating CCL1 + fibroblast and CCR8 + Treg cell interactions. Cancer Immunol Immunother. 2022;71:2717–30.
Zheng G, et al. Lung fibroblasts facilitate pre-metastatic niche formation by remodeling the local immune microenvironment. Immunity. 2022;55:1483–e15001489.
Yanfang L, et al. Tumor exosomal RNAs promote lung pre-metastatic niche formation by activating alveolar epithelial TLR3 to Recruit neutrophils. Cancer Cell. 2016;30:243–56.
Junyoung K, et al. Three-Dimensional Human Liver-Chip emulating Premetastatic Niche formation by breast Cancer-derived extracellular vesicles. ACS Nano. 2020;14:14971–88.
Kaplan R, et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438:820–7.
Sachie H, Watanabe A, Aburatani H, Maru Y. Tumour-mediated upregulation of chemoattractants and recruitment of myeloid cells predetermines lung metastasis. Nat Cell Biol. 2006;8:1369–75.
Ryan S et al. The emerging roles of Extracellular vesicles as Communication vehicles within the Tumor Microenvironment and Beyond. Front Endocrinol 8 (2017).
Niel G, D’angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018;19:213–28.
Bardi G, Smith MA, Joshua LH. Melanoma exosomes promote mixed M1 and M2 macrophage polarization. Cytokine. 2018;105:63–72.
Haiou Y, et al. Exosome-derived miR-130a activates angiogenesis in gastric Cancer by targeting C-MYB in vascular endothelial cells. Mol Ther. 2018;26:2466–75.
Doeuvre L, Angles-Cano E. Cell-derived microparticles unveil their fibrinolytic and proteolytic function. Med Sci (Paris). 2009;25:37–44.
Farnsworth R, et al. Vascular remodeling in cancer. Oncogene. 2014;33:3496–505.
Rodríguez-Lagunas MJ, Martín-Venegas R, Moreno JJ, Ferrer R. PGE2 promotes Ca2+-mediated epithelial barrier disruption through EP1 and EP4 receptors in Caco-2 cell monolayers. Am J Physiology-Cell Physiol. 2010;299:C324–334.
Lukanidin E, Sleeman J. Building the niche: the role of the S100 proteins in metastatic growth. Sem Cancer Biol. 2012;22:216–25.
Sun H et al. Exosomal S100A4 derived from highly metastatic hepatocellular carcinoma cells promotes metastasis by activating STAT3. Sig Transduct Target Ther 6 (2021).
Paolillo M, Schinelli S. Extracellular matrix alterations in metastatic processes. IJMS. 2019;20:4947.
Sunhwa K, et al. Carcinoma-produced factors activate myeloid cells through TLR2 to stimulate metastasis. Nature. 2009;457:102–6.
Gonzalez-Avila G, et al. Matrix metalloproteinases participation in the metastatic process and their diagnostic and therapeutic applications in cancer. Crit Rev Oncol/Hematol. 2019;137:57–83.
Apte RS, Chen DS, Ferrara N. VEGF in Signaling and Disease: Beyond Discovery and Development. Cell. 2019;176:1248–64.
Liu Y, et al. Tumor exosomal RNAs promote lung pre-metastatic niche formation by activating alveolar epithelial TLR3 to Recruit neutrophils. Cancer Cell. 2016;30:243–56.
Hannah HY, et al. Gr-1 + CD11b + myeloid cells tip the balance of immune protection to tumor promotion in the premetastatic lung. Cancer Res. 2010;70:6139–49.
Casbon A, et al. Invasive breast cancer reprograms early myeloid differentiation in the bone marrow to generate immunosuppressive neutrophils. Proc Natl Acad Sci U S A. 2015;112:E566–75.
Jablonska J, Lang S, Ronit Vogt S, Granot Z. The regulation of pre-metastatic niche formation by neutrophils. Oncotarget. 2017;8:112132–44.
Martin KR, Wong H, Witko-Sarsat V, Wicks I. G-CSF - A double edge sword in neutrophil mediated immunity. Semin Immunol. 2021;54:101516.
Enli C, Jing Y. The role and metabolic adaptations of neutrophils in premetastatic niches. Biomark Res 11 (2023).
Xie K. Interleukin-8 and human cancer biology. Cytokine Growth Factor Rev. 2001;12:375–91.
Korbecki J, et al. CXCR2 receptor: regulation of expression, Signal Transduction, and involvement in Cancer. IJMS. 2022;23:2168.
Yu PF, et al. TNFα-activated mesenchymal stromal cells promote breast cancer metastasis by recruiting CXCR2 + neutrophils. Oncogene. 2017;36:482–90.
Wu C-F, et al. The lack of type I interferon induces neutrophil-mediated pre-metastatic niche formation in the mouse lung. Int J Cancer. 2015;137:837–47.
Christoffersson G, et al. VEGF-A recruits a proangiogenic MMP-9-delivering neutrophil subset that induces angiogenesis in transplanted hypoxic tissue. Blood. 2012;120:4653–62.
Que H, Fu Q, Lan T, Tian X, Wei X. Tumor-associated neutrophils and neutrophil-targeted cancer therapies. Biochim et Biophys Acta (BBA) - Reviews Cancer. 2022;1877:188762.
Yu R, Zhu B, Chen D. Type I interferon-mediated tumor immunity and its role in immunotherapy. Cell Mol Life Sci. 2022;79:191.
Ohms M, Möller S, Laskay T. An attempt to Polarize Human neutrophils toward N1 and N2 phenotypes in vitro. Front Immunol. 2020;11:532.
Ravindran M, Khan M, Palaniyar N. Neutrophil extracellular trap formation: Physiology, Pathology, and Pharmacology. Biomolecules. 2019;9:365.
Papayannopoulos V. Neutrophil extracellular traps in immunity and disease. Nat Rev Immunol. 2017;18:134–47.
Podaza E et al. Neutrophils from chronic lymphocytic leukemia patients exhibit an increased capacity to release extracellular traps (NETs). Cancer Immunol Immunotherapy 66, 77–89.
Zhang Y, et al. Neutrophil extracellular traps induced by activated platelets contribute to procoagulant activity in patients with colorectal cancer. Thromb Res. 2019;180:87–97.
Meo MD, Spicer J. The role of neutrophil extracellular traps in cancer progression and metastasis. Semin Immunol. 2022;57:101595.
Zheng Z, et al. Lung mesenchymal stromal cells influenced by Th2 cytokines mobilize neutrophils and facilitate metastasis by producing complement C3. Nat Commun. 2021;12:6202.
Podaza E, et al. Neutrophils from chronic lymphocytic leukemia patients exhibit an increased capacity to release extracellular traps (NETs). Cancer Immunol Immunother. 2016;66:77–89.
Junjie J, Haiyue T, Peishan L. Lipid metabolism and neutrophil function. Cell Immunol. 2022;377:104546.
Canè S, Bronte V. Detection and functional evaluation of arginase-1 isolated from human PMNs and murine MDSC. Method Enzymol. 2020;632:193–213.
Karim M, et al. Relief of tumor hypoxia unleashes the tumoricidal potential of neutrophils. J Clin Investig. 2019;130:389–403.
Behnia A et al. PGE2-EP2/EP4 signaling elicits mesoCAR T cell immunosuppression in pancreatic cancer. Front Immunol 14 (2023).
Patel S, et al. Unique pattern of neutrophil migration and function during tumor progression. Nat Immunol. 2018;19:1236–47.
Poczobutt JM, et al. Deletion of 5-Lipoxygenase in the Tumor Microenvironment promotes Lung Cancer Progression and Metastasis through regulating T cell recruitment. J Immunol. 2016;196:891–901.
Liang W, Qin L, Ferrara N. Metastatic growth instructed by neutrophil-derived transferrin. Proc Natl Acad Sci U S A. 2018;115:11060–5.
Sung Jin H, Liang S, Arati S, Cheng D, Gavin PR. Transiently entrapped circulating tumor cells interact with neutrophils to facilitate lung metastasis development. Cancer Res. 2010;70:6071–82.
Mccourt M, Jiang-huai W, Sookhai S, Redmond H. Proinflammatory mediators stimulate neutrophil-directed angiogenesis. Arch Surg. 1999;134:1325–discussion13311331.
Auguste P, Lemiere S, Larrieu-Lahargue F, Bikfalvi A. Molecular mechanisms of tumor vascularization. Crit Rev Oncol/Hematol. 2005;54:53–61.
Spicer J, et al. Neutrophils promote liver metastasis via Mac-1-mediated interactions with circulating tumor cells. Cancer Res. 2012;72:3919–27.
WonJae L, et al. Neutrophils facilitate ovarian cancer premetastatic niche formation in the omentum. J Exp Med. 2019;216:176–94.
Yang L, et al. IL-8 mediates a positive loop connecting increased neutrophil extracellular traps (NETs) and colorectal cancer liver metastasis. J Cancer. 2020;11:4384–96.
O’Brien XM, Reichner JS. Neutrophil integrins and matrix ligands and NET release. Front Immunol. 2016;7:363.
Linbin Y, et al. DNA of neutrophil extracellular traps promotes cancer metastasis via CCDC25. Nature. 2020;583:133–8.
Yazdani HO, et al. Neutrophil Extracellular traps Drive mitochondrial homeostasis in tumors to augment growth. Cancer Res. 2019;79:5626–39.
Zeng Z, et al. HAO1-mediated oxalate metabolism promotes lung pre-metastatic niche formation by inducing neutrophil extracellular traps. Oncogene. 2021;41:3719–31.
Qi M et al. Lin28B-high breast cancer cells promote immune suppression in the lung pre-metastatic niche via exosomes and support cancer progression. Nat Commun 13 (2022).
Charan M, et al. Tumor secreted ANGPTL2 facilitates recruitment of neutrophils to the lung to promote lung pre-metastatic niche formation and targeting ANGPTL2 signaling affects metastatic disease. Oncotarget. 2020;11:510–22.
Moresco M et al. Enzymatic inactivation of oxysterols in breast tumor cells constraints metastasis formation by reprogramming the metastatic lung microenvironment. Front Immunol 9 (2018).
Fusella F, Seclì L, Brancaccio M. Escaping NK cells and recruiting neutrophils: how Morgana/NF-κB signaling promotes metastasis. Mol Cell Oncol. 2018;5:e1432258.
Su X et al. Tumour extracellular vesicles induce neutrophil extracellular traps to promote lymph node metastasis. J Extracell Vesicle 12 (2023).
Zhang Q et al. ETV4 mediated Tumor-Associated Neutrophil Infiltration facilitates lymphangiogenesis and lymphatic metastasis of bladder Cancer. Adv Sci 10 (2023).
Xie M, et al. FGF19/FGFR4-mediated elevation of ETV4 facilitates hepatocellular carcinoma metastasis by upregulating PD-L1 and CCL2. J Hepatol. 2023;79:109–25.
Li C et al. FGF19-Induced Inflammatory CAF promoted Neutrophil Extracellular trap formation in the liver metastasis of Colorectal Cancer. Adv Sci 10 (2023).
Wang H, et al. KIAA1199 drives immune suppression to promote colorectal cancer liver metastasis by modulating neutrophil infiltration. Hepatology. 2022;76:967–81.
Luckett T, et al. Mesothelin Secretion by Pancreatic Cancer cells co-opts macrophages and promotes metastasis. Cancer Res. 2024;84:527–44.
Liu Y, et al. c-Met mediated Cytokine Network promotes brain metastasis of breast Cancer by remodeling neutrophil activities. Cancers. 2023;15:2626.
Safarulla S, Madan A, Xing F, Chandrasekaran A. CXCR2 mediates distinct Neutrophil Behavior in Brain metastatic breast tumor. Cancers. 2022;14:515.
Deng J et al. DDR1-induced neutrophil extracellular traps drive pancreatic cancer metastasis. JCI Insight 6 (2020).
Bellomo G, et al. Chemotherapy-induced infiltration of neutrophils promotes pancreatic cancer metastasis via Gas6/AXL signalling axis. Gut. 2022;71:2284–99.
Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–45.
Coussens L, Werb Z. Inflammation and cancer. Nature. 2002;420:860–7.
Hana Z, Hošek J. TNF-α signalling and inflammation: interactions between old acquaintances. Inflamm Res. 2013;62:641–51.
Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–7.
Huang H, Zhang H, Onuma AE, Tsung A. Neutrophil elastase and Neutrophil Extracellular traps in the Tumor Microenvironment. Adv Exp Med Biol. 2020;1263:13–23.
Bastien H, et al. Identification of proteases involved in the Proteolysis of Vascular Endothelium cadherin during Neutrophil Transmigration*. J Biol Chem. 2003;278:14002–12.
Xuetao C. Self-regulation and cross-regulation of pattern-recognition receptor signalling in health and disease. Nat Rev Immunol. 2015;16:35–50.
Kim R, et al. Ferroptosis of tumour neutrophils causes immune suppression in cancer. Nature. 2022;612:338–46.
Flemming A. Neutrophil ferroptosis causes immunosuppression. Nat Rev Immunol. 2022;23:6.
Sippel TR et al. Neutrophil Degranulation and Immunosuppression in Patients with GBM: Restoration of Cellular Immune Function by Targeting Arginase I. Clinical Cancer Research.
Tseng S-Y, et al. B7-Dc, a New Dendritic Cell Molecule with Potent Costimulatory properties for T cells. J Exp Med. 2001;193:839–46.
Ting-ting W, et al. Tumour-activated neutrophils in gastric cancer foster immune suppression and disease progression through GM-CSF-PD-L1 pathway. Gut. 2017;66:1900–11.
Wang H, et al. Regulatory T cell and neutrophil extracellular trap interaction contributes to carcinogenesis in non-alcoholic steatohepatitis. J Hepatol. 2021;75:1271–83.
Kaltenmeier C et al. Neutrophil Extracellular traps promote T cell exhaustion in the Tumor Microenvironment. Front Immunol 12 (2021).
Shahzad MH, et al. Neutrophil Extracellular traps in Cancer Therapy Resistance. Cancers. 2022;14:1359.
Teijeira Á, et al. CXCR1 and CXCR2 chemokine receptor agonists produced by tumors induce Neutrophil Extracellular traps that interfere with Immune cytotoxicity. Immunity. 2020;52:856–e871858.
Robert ST, et al. Myeloid suppressor cells induced by hepatitis C virus suppress T-cell responses through the production of reactive oxygen species. Hepatology. 2012;55:343–53.
Lu C et al. Tumor-secreted GRP78 promotes the establishment of a pre-metastatic niche in the liver microenvironment. Front Immunol 11 (2020).
De Palma M, Biziato D, Petrova TV. Microenvironmental regulation of tumour angiogenesis. Nat Rev Cancer. 2017;17:457–74.
Shifeng Y et al. Neutrophil extracellular traps promote angiogenesis in gastric cancer. Cell Commun Signal 21 (2023).
Wieland E, et al. Endothelial Notch1 activity facilitates metastasis. Cancer Cell. 2017;31:355–67.
Binet F et al. Neutrophil extracellular traps target senescent vasculature for tissue remodeling in retinopathy. Science 369 (2020).
Saharinen P, Eklund L, Pulkki K, Bono P, Alitalo K. VEGF and angiopoietin signaling in tumor angiogenesis and metastasis. Trends Mol Med. 2011;17:347–62.
Zhicheng Z et al. Cancer-derived exosomal mir-25-3p promotes pre-metastatic niche formation by inducing vascular permeability and angiogenesis. Nat Commun 9 (2018).
Zhu Y, et al. Interplay between Extracellular Matrix and neutrophils in diseases. J Immunol Res. 2021;2021:1–11.
Jan ES, Pietro G, Erler J. NEUTROPHIL INFLUENCE ON EXTRACELLULAR MATRIX IN CANCER PROGRESSION. Am J Physiology-Cell Physiol. 2022;323:C486–93.
Deryugina E, et al. Tissue-infiltrating neutrophils constitute the major in vivo source of angiogenesis-inducing MMP-9 in the Tumor Microenvironment12. Neoplasia. 2014;16:771–88.
Mark O, et al. MMP9 modulates the metastatic cascade and immune landscape for breast cancer anti-metastatic therapy. Life Sci Alliance. 2019;2:e201800226.
Pivetta E, et al. Neutrophil elastase-dependent cleavage compromises the tumor suppressor role of EMILIN1. Matrix Biol. 2014;34:22–32.
Strøbech JE, Giuriatti P, Erler J. NEUTROPHIL INFLUENCE ON EXTRACELLULAR MATRIX IN CANCER PROGRESSION. Am J Physiology-Cell Physiol. 2022;323:C486–93.
Son E, et al. Cathepsin G increases MMP expression in normal human fibroblasts through fibronectin fragmentation, and induces the conversion of proMMP-1 to active MMP-1. J Dermatol Sci. 2009;53:150–2.
Zhibo X, et al. Exosome-delivered CD44v6/C1QBP complex drives pancreatic cancer liver metastasis by promoting fibrotic liver microenvironment. Gut. 2021;71:568–79.
Wu J, et al. MAOA-Dependent activation of Shh-IL6-RANKL Signaling Network promotes prostate Cancer metastasis by engaging tumor-stromal cell interactions. Cancer Cell. 2017;31:368–82.
Bo J, et al. Interleukin-6/STAT3 signaling orchestrates pre-metastatic niche formation and immunosuppressive traits in lung. Cancer Res. 2019;80:784–97.
Milagre C, et al. Adaptive upregulation of EGFR limits attenuation of Tumor Growth by neutralizing IL6 antibodies, with implications for combined therapy in Ovarian Cancer. Cancer Res. 2015;75:1255–64.
Radisky E, Raeeszadeh-Sarmazdeh M, Radisky D. Therapeutic potential of Matrix Metalloproteinase inhibition in breast Cancer. J Cell Biochem. 2017;118:3531–48.
Simone R, et al. Antiangiogenic immunotherapy suppresses desmoplastic and chemoresistant intestinal tumors in mice. J Clin Investig. 2020;130:1199–216.
Caruso JA, Hunt KK, Keyomarsi K. The Neutrophil elastase inhibitor elafin triggers Rb-Mediated growth arrest and caspase-dependent apoptosis in breast Cancer. Cancer Res. 2010;70:7125–36.
Doi K, et al. Neutrophil elastase inhibitor reduces hepatic metastases induced by ischaemia-reperfusion in rats. Eur J Surg. 2002;168:507–10.
Inada M, Yamashita J, Nakano S, Ogawa M. Complete inhibition of spontaneous pulmonary metastasis of human lung carcinoma cell line EBC-1 by a neutrophil elastase inhibitor (ONO-5046.Na). Anticancer Res. 1998;18:885–90.
Xia C, et al. Sponge-like Nano-system suppresses Tumor recurrence and metastasis by restraining myeloid-derived suppressor cells-mediated immunosuppression and formation of pre-metastatic niche. Acta Biomater. 2023;158:708–24.
Zheng Y, et al. Chronic psychological stress promotes breast cancer pre-metastatic niche formation by mobilizing splenic MDSCs via TAM/CXCL1 signaling. J Exp Clin Cancer Res. 2023;42:129.
Ting K, et al. Nanoparticles coated with neutrophil membranes can effectively treat Cancer Metastasis. ACS Nano. 2017;11:1397–411.
Xiaoqing H et al. Bacterial magnetosome-hitchhiked Quick‐Frozen neutrophils for targeted Destruction of Pre‐Metastatic Niche and Prevention of Tumor Metastasis. Adv Healthc Mater 12 (2023).
Samantha MM et al. Tumor-derived exosomes drive immunosuppressive macrophages in a pre-metastatic niche through glycolytic dominant metabolic reprogramming. Cell Metabol 33, 2040–58.e2010 (2021).
Yu P, et al. TNFα-activated mesenchymal stromal cells promote breast cancer metastasis by recruiting CXCR2 + neutrophils. Oncogene. 2016;36:482–90.
Zhen W et al. CD62Ldim neutrophils specifically migrate to the lung and participate in the formation of the pre-metastatic niche of breast Cancer. Front Oncol 10 (2020).
Zhicheng Z, et al. HAO1-mediated oxalate metabolism promotes lung pre-metastatic niche formation by inducing neutrophil extracellular traps. Oncogene. 2021;41:3719–31.
Ieranò C et al. CXCL12 loaded-dermal filler captures CXCR4 expressing melanoma circulating tumor cells. Cell Death Dis 10 (2019).
Zhiyuan Z et al. Lung mesenchymal stromal cells influenced by Th2 cytokines mobilize neutrophils and facilitate metastasis by producing complement C3. Nat Commun 12 (2021).
Ortiz-Espinosa S, et al. Complement C5a induces the formation of neutrophil extracellular traps by myeloid-derived suppressor cells to promote metastasis. Cancer Lett. 2021;529:70–84.
Á T, et al. CXCR1 and CXCR2 chemokine receptor agonists produced by tumors induce Neutrophil Extracellular traps that interfere with Immune cytotoxicity. Immunity. 2020;52:856–e871858.
Hao W et al. Characteristics of pre-metastatic niche: the landscape of molecular and cellular pathways. Mol Biomed 2 (2021).
Acknowledgements
Thanks to Biorender.com for the figure-making.
Funding
This study was supported by grants from the National Natural Science Foundation of China (824011555,82170211, 32100698), the Natural Science Foundation of Henan Province (222300420068), Funding for Scientific Research and Innovation Team of The First Affiliated Hospital of Zhengzhou University, Joint Co-construction Project of Henan Medical Science and Technology Research Plan (LHGJ20220301 LHG120230294), The China Postdoctoral Science Foundation(2023M743201), National funded postdoctoral researcher program (GZB20230671).
Author information
Authors and Affiliations
Contributions
SXY conceptualized the idea for the Review. Drafts were written and revised by YHW , JCJ and MJL. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Jia, J., Wang, Y., Li, M. et al. Neutrophils in the premetastatic niche: key functions and therapeutic directions. Mol Cancer 23, 200 (2024). https://doi.org/10.1186/s12943-024-02107-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12943-024-02107-7