Abstract
Background
Despite the clinical benefits of external ventricular drains (EVD), these devices can lead to EVD-related infections (EVDRI). The drainage insertion technique and standardized guidelines can significantly reduce the risk of infection, mainly caused by gram-positive bacteria. However, gram-negative microorganisms are the most frequent causative microorganisms of EVDRI in our hospital. We aimed to determine whether a new bundle of measures for the insertion and maintenance of a drain could reduce the incidence of EVDRI. This cohort study of consecutive patients requiring EVD from 01/01/2015 to 12/31/2018 compared the patients’ characteristics before and after introducing an updated protocol (UP) for EVD insertion and maintenance in 2017.
Results
From 204 consecutive patients, 198 requiring EVD insertion were included (54% females, mean age 55 ± 15 years). The before-UP protocol included 87 patients, and the after-UP protocol included 111 patients. Subarachnoid (42%) and intracerebral (24%) hemorrhage were the main diagnoses at admission. The incidence of EVDRI fell from 13.4 to 2.5 episodes per 1000 days of catheter use. Gram-negative bacteria were the most frequent causative microorganisms. Previous craniotomy remained the only independent risk factor for EVDRI. EVDRI patients had increased mechanical ventilation durations, hospital and ICU stays, and percutaneous tracheostomy requirements.
Conclusions
A care bundle focusing on fewer catheter sampling and more accurate antiseptic measures can significantly decrease the incidence of EVDRI. After implementing the management protocol, a decreased incidence of infections caused by gram-negative and gram-positive bacteria and reduced ICU and hospital lengths of stay were observed.
Similar content being viewed by others
Background
External ventricular drains (EVD) are often used in modern neurosurgical practice for the management of acute hydrocephalus, monitoring intracranial pressure, or draining intraventricular blood. Despite their clinical benefits, complications such as EVD-related infections (EVDRI) are outstanding [1,2,3,4,5,6,7,8].
The clinical manifestations of EVDRI are often subtle since patients often present neurological symptoms due to the underlying pathology or sedative medications. Given that cerebrospinal fluid (CSF) leukocyte, glucose, and protein values are not specific to infection, trends in values are more important than absolute values [1]. The main risk factors for EVDRI are patient-related (e.g., male, age, concurrent infections, high intracranial pressure), hospital-related (e.g., length of stay, use of steroids, insertion site), and catheter-related (e.g., duration, manipulation, leaks) [9,10,11,12,13]. Intraventricular hemorrhage, intracranial hypertension, craniotomy, the average time of EVD, and concurrent infections, are the most common conditions associated with EVDRI [14].
Improvement of drainage insertion technique and implementation of standardized guidelines can significantly reduce the risk of EVDRI [2, 14]. However, there is no standard consensus for the management of EVD. Some recent protocols consider chlorhexidine patches to minimize bacterial colonization of catheters [15] and the incidence of ventriculitis [16]. Despite their promising clinical applications, chlorhexidine patches offer limited protection against gram-negative microorganisms [17], the most frequent etiology of EVDRI in our working environment. In this observational study, we aimed to determine whether a new bundle of measures focused on the insertion and maintenance of EVD could reduce the incidence of EVDRI.
Methods
Study design and participants
A cohort study was conducted in a 700-bed university hospital for adults in Spain, covering 500,000 people and serving as a referral center for advanced procedures for 2.5 million inhabitants (30% of Catalonia’s population) from the southern Barcelona metropolitan area.
Consecutive patients were included if they were admitted to the neurocritical intermediate care area (6 beds) or intensive care unit (ICU) (36 beds) from 01/01/2015 to 12/31/2018 and required an EVD. A multidisciplinary team (e.g., infectious diseases, neurosurgery, intensive medicine, anesthesiology, and microbiology) performed patient decision-making. We excluded patients < 18 years, with previous EVDRI, or refusing the EVD informed consent. The Clinical Research Ethics Committee of Bellvitge University Hospital (HUB) approved the study (reference PR317/18).
Protocol, definitions, and variables
Analyses of the incidence of EVDRI and associated risk factors were performed after updating the EVD management protocol (updated protocol, UP) in 2017. We compared patients before (pre-UP) and after (post-UP) the protocol update. The care bundle added to the updated protocol in 2017 is detailed in Table 1 (highlighted in bold). It included staff training and introduced a checklist for protocol systematization. Our unit routinely places conventional drainages first, reserving antibiotic- and silver-covered drainages for patients with suspected infection or at significant risk for developing an EVDRI. The implementation of the updated care bundle coincided with a local campaign to raise awareness of the importance of drainage care and its complications. We held workshops to explain the new measures to improve acceptance and protocol adherence, targeting nursing and medical staff. This was accomplished using a checklist to enhance support for protocol recommendations and ensure rigorous application. Increasing awareness of possible complications and introducing the need to record practices may have contributed to decreasing the number of unnecessary manipulations and sampling while improving adherence to aseptic measures.
EVDRI was defined as the presence of positive CSF cultures, microorganisms on Gram stain, and suggestive symptoms. Cultures were considered for EVDRI if positivity was detected from 24 h after implantation to 5 days after removal [6]. In cases of suspected or unconfirmed EVDRI, patients were evaluated by the multidisciplinary team and the treating team. The criteria used to rule out EVDRI were: (1) absence of symptoms; (2) delayed growth in the CSF culture (e.g., Corynebacterium or gram-negative bacilli based on a negative Gram stain); and (3) subsequent negative cultures within 24 h after the initial culture in the absence of empirical or targeted treatment. Given the diagnostic challenge in these cases, patients were followed up until discharge. If EVDRI patient presented another superinfection EVDRI, only the first was analyzed.
We included the following variables: demographic (age, gender, and comorbidities), admission diagnosis, EVD indication, EVD risk factors, EVDRI, ICU and hospital length of stay, mortality, and morbidity. The Glasgow Coma Scale (GCS) was used to assess the neurological status, and the APACHE III (Acute Physiology and Chronic Health Evaluation III) and SOFA (Sequential Organ Failure Assessment Score) scores were used to assess illness severity. Complications of EVD insertion included hematoma, catheter occlusion, or catheter misplacement.
Statistical analysis
To determine the sample size, we estimated an EVDRI rate of 15 infections per 1000 days of catheter use, expecting the new protocol to reduce this by at least 50%. We accepted an α value of 0.05 to prove a significant reduction in EVDRI. By including 100 patients before (pre-UP) and 100 after (post-UP) the new protocol, the statistical power to detect a significant reduction in EVDI would exceed 80%, indicating the need for a minimum sample size of 200 patients.
We calculated means and standard deviations (SD) or medians and interquartile ranges for quantitative variables and expressed categorical data as frequencies and percentages, as appropriate. Categorical data and proportions were compared with the chi-square test, and continuous variables were compared with Student t-tests, Mann–Whitney U tests, or Kruskal–Wallis tests. Multivariate logistic regression was performed with significant variables, reporting odds ratios (ORs) and 95% confidence intervals (CIs). An α value of 0.05 was used to determine statistical significance. We analyzed all data in the present study using IBM SPSS statistics 27 (SPSS Inc©, Chicago, USA).
Results
Participants and demographic characteristics
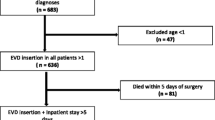
The study sample comprised 204 consecutive patients (54% females) with a mean age of 55 (SD 15) years who required EVD insertion. In total, 94% of the patients required ICU admission, and 6% required admission to an intermediate care unit, while 65% had undergone neurointervention (39% craniotomy and 26% aneurysm embolization). We excluded one patient with craniotomy infection and five with ventriculoperitoneal drain infection. The final sample included 198 patients.
The demographic characteristics and diagnoses of the patients in the pre-UP group (n = 87) and post-UP group (n = 111) were broadly similar. However, the pre-UP group had fewer cases of intracranial hemorrhage (at admission), craniotomy, or complications during EVD insertion. The pre-up group had an increased number of intracranial tumors and hospital length of stay. Also, they presented different reasons for EVD removal, and worse GCS and SOFA scores. After logistic regression analyses, differences in the SOFA score, complications during EVD insertion, and placement of tunneled catheters were the only independent variables (Table 2).
Occurrence of EVDRI
During the study period, 261 EVD catheters were placed; 198 were first placements and 63 relocations. The incidence of EVDRI decreased from 13.4 per 1000 days of catheter use in the pre-UP group to 2.5 per 1000 days in the post-UP group (Table 3). Of the 198 patients, 138 had negative results in all follow-up cultures, and 60 had at least one positive culture (CSF, EVD catheter, or underlying skin). Among the 60 patients with positive cultures, 37 were not considered as having EVDRI, given that symptoms, CSF, or cultures were not suggestive (17 only had a positive EVD catheter result, and 18 had a negative CSF culture < 24 h after the first or delayed growth with other negative CSF cultures). Thus, 23 patients were considered to have EVDRI, 18/87 (21%) in the pre-UP group and 5/111 (4%) in the post-UP group (Chi-square, < 0.001). Five of 23 EVDRI patients presented superinfection EVDRI (4 Pre-UP and 1 post-UP). Mortality did not change significantly according to infection status (30% vs. 25%) or before and after protocol implementation (24% vs. 28%).
Among EVDRI patients, first catheters were most commonly involved (17/198, 9%; 13 pre-UP and 4 post-UP), the second catheter in 5 patients (5/47, 12%; 4 pre-UP and 1 post-UP), and the third catheter in one patient (1/13, 8%). Diagnosis relied on CSF alone in most cases (70%), followed by CSF plus EVD catheter culture (26%), and few relied on the EVD catheter alone (4%). EVDRI patients had CSF values showing decreased glucose (80%), increased protein (30%), and increased cell counts (55%). Fever was present in 70%, and neurological impairment was present in 35%. Gram-negative bacteria caused 61% of EVDRI, gram-positive bacteria in 30%, and Candida albicans in 9%. In the pre-UP group, 67% were caused by gram-negative bacteria, 22% by gram-positive bacteria and 11% by Candida albicans. In the post-UP, 40% were caused by gram-negative bacteria and 60% by gram-positive bacteria (Table 4). Isolated micro-organisms were susceptible to the usual antibiotics with the exception of 1 carbapenem-resistant Pseudomonas aeruginosa, 1 Pseudomonas aeruginosa resistant to aztreonam, antipseudomonal cephalosporins and penicillin, 1 Escherichia coli BLEE, 1 Acinetobacter baumannii only susceptible to colistin and amikacin and 1 methicillin-resistant Staphylococcus aureus.
Risk factors for EVDRI
We conducted a bivariate analysis of risk factors. Patient characteristics associated with increased risk of infection were previous craniotomy, insertion site infection, concomitant systemic infection, and rectal colonization (Table 5). In the logistic regression with significant variables, the previous craniotomy remained the only independent risk factor, having an OR of 2.7 (95% CI, 1.1–6.8). The characteristics of the 198 first catheters (17 EVDRI) are shown in Table 6.
EVDRI patients had adverse outcomes, such as increased EVD and mechanical ventilation duration, tracheostomy requirement, and prolonged ICU and hospital stay (Table 7). Yet, mortality did not increase significantly.
Discussion
The updated EVD management protocol was associated with a decrease in the number of EVDRI. Gram-negative bacteria were the most frequent causative microorganisms. Demographic and diagnostic features of patients were similar before and after the protocol update. Moreover, the only independent risk factor for EVDRI was a previous craniotomy. Patients with EVDRI required more days of mechanical ventilation, tracheostomy requirement, and increased ICU and hospital length of stay.
EVDRI is widely defined as a positive CSF culture with or without evidence of microorganisms on Gram stain and associated with high fever and signs of meningitis [6]. Most authors recommend dynamic CSF analysis [5, 18, 19]. The complexity of these patients also hampers diagnosis because their symptoms could be explained by deterioration secondary to the underlying disease [20,21,22]. The Center for Disease Control and Prevention does not specify definitions for contamination and colonization, as with other devices. Indeed, CSF inflammation can be secondary to non-infectious causes, such as intraventricular hemorrhage or the neurosurgery itself [2, 6, 8, 20,21,22,23]. The lack of consensus makes diagnosis difficult, with an incidence of 10%, ranging from 2 to 27% [6, 12, 24,25,26,27]. EVDRI may increase mortality to 15%–20% and lengths of ICU and hospital stay, contributing to increased healthcare costs [9, 28].
Several authors have implemented protocols that achieved decreases in the incidence of EVDRI by 0.4% to 18% [29, 30]. This study found a significant decline in the EVDRI incidence from 23 to 4%, from 2015 to 2018, after implementing an updated EVD management protocol. The 2017 guidelines of the Neurocritical Care Society and Infectious Diseases Society of America recommend the systematic use of care bundles. However, as with other studies, there is no consensus on which bundle elements are essential [2, 25, 31,32,33]. The main changes implemented in our updated protocol concerned hygiene during EVD insertion, routine maintenance, and proper technique for CSF sampling (e.g., changing gloves after field preparation for catheter insertion, the use of dressings with chlorhexidine patches, a reduced number of sample collections, and head washing every 4 days). Also, fewer EVD manipulations and the implementation of staff re-education were implemented, using a checklist to ensure protocol systematization.
Different interventions have been studied to reduce the incidence of EVDRI. The EVD insertion site contamination can be prevented using an aseptic technique and antibiotic prophylaxis [1, 34, 35]. According to Tunkel et al., no prophylactic EVD changes were made [1] in the absence of infection in this study. The updated care bundle considered the need for continuous reassessment of prolonged antibiotic treatment to prevent multi-resistance [36, 37].
Routine daily sample extraction is not widely accepted, although recommended by some authors [5]. Most studies recommend CSF sampling when infection is suspected [2, 19, 30, 35, 38]. Considering our microbiology patterns, gram-negative bacteria accounted for the most frequent cause of EVDRI. Thus, most infections probably emerged from manipulation. In the updated protocol, routine CSF sampling is performed at the time of placement, on day 7, and every 4 days thereafter, unless EVDRI was suspected or confirmed. This differs from the pre-UP protocol, which considered routine CSF sampling every 48 h and upon suspicion of infection. Decreasing unnecessary sampling leading to excessive manipulation of the EVD, along with the implementation of strict hygiene measures, should be considered essential.
When a dressing becomes loose or soiled, the UP (Table 1) recommends dressing exchange using sterile barriers, cleaning the surrounding area with an antiseptic solution, disinfecting catheter connections, wrapping with sterile gauze, and placing a chlorhexidine patch over the catheter exit site, as reported elsewhere [14]. Following the recommendations of Flint et al., chlorhexidine patches (3M™ Tegaderm™ CHG kit, 3M©, Minnesota, United States) were introduced in the UP to cover the catheter exit site after tunneling. These patches are safe and reduce the incidence of infections related to central lines and central nervous system catheters [15, 17, 25], offering protection, especially against gram-positive bacteria [39].
The effectiveness and cost-effectiveness of antibiotic-impregnated EVD are controversial, although some studies have shown they are effective at reducing infection [2, 14, 25, 40, 41]. Silver-coated or antibiotic-impregnated catheters are most effective against gram-positive bacteria [2, 25, 39, 42]. Our protocol included tunneling (3–5 cm from the insertion site) and the systematic use of simple silicone drainages, prioritizing drainage care to decrease EVDRI [43, 44]. This result has significant implications for overall costs, given that antibiotic-impregnated catheters are 3–5 times more expensive than silicone catheters [43].
Over recent years, several studies have shown EVD care bundles reduce EVDRI, though most reports have focused on infection caused by gram-positive bacteria [5, 7, 25, 29, 45, 46]. Still, relatively few studies showed a reduction in EVDRI in areas where gram-negative bacteria are responsible for most cases, as in this study [27, 30].
Other studies have typically associated different risk factors with the development of EVDRI, including a previous craniotomy, intracranial pressure > 20 mmHg, coexisting systemic infection, depressed cranial fractures, CSF fistulas, longer EVD use, frequent device manipulation, intraventricular hemorrhage, and some clinical settings [6, 7, 12,13,14]. However, this study found that a previous craniotomy was the only statistically significant independent risk factor. EVD-related insertion and maintenance complications (e.g., obstruction, misplacement, hematoma, accidental removal, and dysfunction) may reflect patient neurological complexity, thereby increasing the incidence of EVDRI. Still, no other independent associations were observed in this study. Duration of EVD has been related with higher risk of infection, but in this sample it has not being statistically significant. Although mortality did not differ significantly between patients according to infection status, the higher morbidity was associated with an increased need for mechanical ventilation and prolonged ICU and hospital lengths of stay. Consequently, significant increases in hospitalization costs can be inferred.
Limitations
Although patient characteristics were similar between the groups, the lack of randomization could have hindered the detection of differences. We did not individually evaluate each protocol measure, which precluded attributing the observed success to a specific intervention. Further, we used retrospective data for the pre-UP group, which may have resulted in missing data.
Conclusion
An updated care bundle for EVD management, centered on less sampling and strict antiseptic measures during catheter insertion, maintenance, and manipulations, was associated with a reduced incidence of EVDRI caused by gram-negative and gram-positive bacteria. The implementation of the bundle was associated with reductions in ICU and hospital length of stay when staff were adequately trained on protocol adherence.
Availability of data and materials
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- EVD:
-
External ventricular drains
- EVDRI:
-
EVD-related infection
- GCS:
-
Glasgow Coma Scale
- HUB:
-
Hospital Universitari de Bellvitge
- ICU:
-
Intensive care unit
- SD:
-
Standard deviations
- UP:
-
Updated protocol
References
Tunkel AR, Hasbun R, Bhimraj A, et al. 2017 Infectious Diseases Society of America’s Clinical Practice Guidelines for Healthcare-Associated Ventriculitis and Meningitis. Clin Infect Dis. 2017;64(6):34–65. https://doi.org/10.1093/cid/ciw861.
Fried HI, Nathan BR, Rowe AS, et al. The insertion and management of external ventricular drains: an evidence-based consensus statement: a statement for healthcare professionals from the Neurocritical Care Society. Neurocrit Care. 2016;24(1):61–81. https://doi.org/10.1007/s12028-015-0224-8.
Cinibulak Z, Aschoff A, Apedjinou A, Kaminsky J, Trost HA, Krauss JK. Current practice of external ventricular drainage: a survey among neurosurgical departments in Germany. Acta Neurochirurg. 2016. https://doi.org/10.1007/s00701-016-2747-y.
Park YG, Woo HJ, Kim E, Park J. Accuracy and safety of bedside external ventricular drain placement at two different cranial sites: kocher’s point versus forehead. J Korean Neurosurg Soc. 2011;50(4):317–21. https://doi.org/10.3340/jkns.2011.50.4.317.
Champey J, Mourey C, Francony G, et al. Strategies to reduce external ventricular drain–related infections: a multicenter retrospective study. J Neurosurg. 2018;130:1–6. https://doi.org/10.3171/2018.1.JNS172486.
Lozier AP, Sciacca RR, et al. Ventriculostomy-related infections: acritical review of the literature. Neurosurgery. 2002;51(1):170–82. https://doi.org/10.1227/01.NEU.0000017465.78245.6C.
Ramanan M, Lipman J, Shorr A, Shankar A. A meta-analysis of ventriculostomy-associated cerebrospinal fluid infections. BMC Infect Dis. 2015;15(1):3. https://doi.org/10.1186/s12879-014-0712-z.
Gozal YM, Farley CW, Hanseman DJ, et al. Ventriculostomy-associated infection: a new, standardized reporting definition and institutional experience. Neurocrit Care. 2014;21(1):147–51. https://doi.org/10.1007/s12028-013-9936-9.
Cabellos C, Navas E, Martinez Lacasa J, Gatell J. Infecciones del sistema nervioso central. In: Fernández-Viladrich P, ed. Protocolos Clínicos SEIMC. Published online 2000:4–22. http://www.seimc.org/contenidos/documentoscientificos/procedimientosclinicos/seimc-procedimientoclinicoii.pdf. Accessed 7 Jan 2017.
Beer R, Pfausler B, Schmutzhard E. Management of nosocomial external ventricular drain-related ventriculomeningitis. Neurocrit Care. 2009;10(3):363–7. https://doi.org/10.1007/s12028-008-9155-y.
Mounier R, Lobo D, Cook F, et al. Clinical, biological, and microbiological pattern associated with ventriculostomy-related infection: a retrospective longitudinal study. Acta Neurochirurg. 2015. https://doi.org/10.1007/s00701-015-2574-6.
Beer R, Lackner P, Pfausler B, Schmutzhard E. Nosocomial ventriculitis and meningitis in neurocritical care patients. J Neurol. 2008;255(11):1617–24. https://doi.org/10.1007/s00415-008-0059-8.
Camacho EF, Boszczowski Í, Basso M, et al. Infection rate and risk factors associated with infections related to external ventricular drain. Infection. 2011;39(1):47–51. https://doi.org/10.1007/s15010-010-0073-5.
Whyte C, Alhasani H, Caplan R, Tully AP. Impact of an external ventricular drain bundle and limited duration antibiotic prophylaxis on drain-related infections and antibiotic resistance. Clin Neurol Neurosurg. 2020;190:105641. https://doi.org/10.1016/j.clineuro.2019.105641.
Ho KM, Litton E. Use of chlorhexidine-impregnated dressing to prevent vascular and epidural catheter colonization and infection: a meta-analysis. J Antimicrob Chemother. 2006;58(2):281–7. https://doi.org/10.1093/jac/dkl234.
Scheithauer S, Schulze-Steinen H, Höllig A, et al. Significant reduction of external ventricular drainage-associated meningoventriculitis by chlorhexidine-containing dressings: a before-after trial. Clin Infect Dis. 2016;62(3):404–5. https://doi.org/10.1093/cid/civ887.
Scheithauer S, Möller M, Höllig A, et al. Are chlorhexidine-containing dressings safe for use with ventricular drainages? Infection. 2014;42(3):545–8. https://doi.org/10.1007/s15010-014-0596-2.
Mounier R, Lobo D, Cook F, et al. From the skin to the brain: pathophysiology of colonization and infection of external ventricular drain, a prospective observational study. PLoS ONE. 2015. https://doi.org/10.1371/journal.pone.0142320.
Dorresteijn KRIS, Jellema K, van de Beek D, Brouwer MC. Factors and measures predicting external CSF drain-associated ventriculitis: a review and meta-analysis. Neurology. 2019;93(22):964–72. https://doi.org/10.1212/WNL.0000000000008552.
Lewis A, Wahlster S, Karinja S, Czeisler BM, Kimberly WT, Lord AS. Ventriculostomy-related infections: the performance of different definitions for diagnosing infection. Br J Neurosurg. 2016;30(1):49–56. https://doi.org/10.3109/02688697.2015.1080222.
Woo PYM, Wong HT, Pu JKS, et al. Moving the goalposts: a comparison of different definitions for primary external ventricular drain infection and its risk factors: a multi-center study of 2575 patients. J Clin Neurosci. 2017;45:67–72. https://doi.org/10.1016/j.jocn.2017.05.042.
CDC, Ncezid, DHQP. CDC/NHSN Surveillance Definitions for Specific Types of Infections. Published online 2022. https://www.cdc.gov/nhsn/pdfs/pscmanual/17pscnosinfdef_current.pdf. Accessed 31 Jul 2022.
van de Beek D, Drake JM, Tunkel AR. Nosocomial bacterial meningitis. N Engl J Med. 2010;362(2):146–54. https://doi.org/10.1056/nejmra0804573.
Schade RP, Schinkel J, Roelandse FWC, et al. Lack of value of routine analysis of cerebrospinal fluid for prediction and diagnosis of external drainage-related bacterial meningitis. J Neurosurg. 2006;104(1):101–8. https://doi.org/10.3171/jns.2006.104.1.101.
Flint AC, Rao VA, Renda NC, Faigeles BS, Lasman TE, Sheridan W. A simple protocol to prevent external ventricular drain infections. Neurosurgery. 2013. https://doi.org/10.1227/NEU.0b013e31828e8dfd.
Lyke KE, Obasanjo OO, Williams MA, O’Brien M, Chotani R, Perl TM. Ventriculitis complicating use of intraventricular catheters in adult neurosurgical patients. Clin Infect Dis. 2001;33(12):2028–33. https://doi.org/10.1086/324492.
Walek KW, Leary OP, Sastry R, Asaad WF, Walsh JM, Mermel L. Decreasing external ventricular drain infection rates in the neurocritical care unit: 12-year longitudinal experience at a single institution. World Neurosurg. 2021;150:e89–101. https://doi.org/10.1016/J.WNEU.2021.02.087.
Hagel S, Bruns T, Pletz MW, Engel C, Kalff R, Ewald C. External ventricular drain infections: risk factors and outcome. Interdiscip Perspect Infect Dis. 2014;2014:1–6. https://doi.org/10.1155/2014/708531.
Kubilay Z, Amini S, Fauerbach LL, Archibald L, Friedman WA, Layon AJ. Decreasing ventricular infections through the use of a ventriculostomy placement bundle: experience at a single institution. J Neurosurg. 2013;118(3):514–20. https://doi.org/10.3171/2012.11.JNS121336.
Chatzi M, Karvouniaris M, Makris D, et al. Bundle of measures for external cerebral ventricular drainage-associated ventriculitis. Crit Care Med. 2014;42(1):66–73. https://doi.org/10.1097/CCM.0b013e31829a70a5.
Camacho EF, Boszczowski Í, Freire MP, et al. Impact of an educational intervention implanted in a neurological intensive care unit on rates of infection related to external ventricular drains. PLoS ONE. 2013;8(2):e50708. https://doi.org/10.1371/journal.pone.0050708.
Leverstein-Van Hall MA, Hopmans TEM, van der Sprenkel JWB, et al. A bundle approach to reduce the incidence of external ventricular and lumbar drain-related infections: clinical article. J Neurosurg. 2010;112(2):345–53. https://doi.org/10.3171/2009.6.JNS09223.
Darrow DP, Quinn C, Do TH, Hunt M, Haines S. Creation of an external ventricular drain registry from a quality improvement project. World Neurosurg. 2018;114:84–9. https://doi.org/10.1016/j.wneu.2018.03.018.
Bratzler DW, Dellinger EP, Olsen KM, et al. Clinical practice guidelines for antimicrobial prophylaxis in surgery. Surg Infect (Larchmt). 2013;14(1):73–156. https://doi.org/10.1089/SUR.2013.9999.
Williams TA, Leslie GD, Dobb GJ, Roberts B, van Heerden PV. Decrease in proven ventriculitis by reducing the frequency of cerebrospinal fluid sampling from extraventricular drains: clinical article. J Neurosurg. 2011;115(5):1040–6. https://doi.org/10.3171/2011.6.JNS11167.
Gopal RG. Risk factors for the spread of antibiotic-resistant bacteria. Drugs. 1998;55(3):323–30. https://doi.org/10.2165/00003495-199855030-00001.
Ribeiro B, Bishop P, Jalili S. When a stroke is not just a stroke: Escherichia coli meningitis with ventriculitis and vasculitis: a case report. J Crit Care Med. 2020;6(1):65. https://doi.org/10.2478/JCCM-2020-0002.
Jamjoom B, Joannides AJ, Poon MTC, et al. Prospective, multicentre study of external ventricular drainage-related infections in the UK and Ireland. J Neurol Neurosurg Psychiatry. 2018;89:120–6. https://doi.org/10.1136/jnnp-2017-316415.
del Río-Carbajo L, Vidal-Cortés P. Tipos de antisépticos, presentaciones y normas de uso. Med Intensiva. 2019;43:7–12. https://doi.org/10.1016/j.medin.2018.09.013.
Root BK, Barrena BG, Mackenzie TA, Bauer DF. Antibiotic impregnated external ventricular drains: meta and cost analysis. World Neurosurg. 2016;86:306–15. https://doi.org/10.1016/j.wneu.2015.09.032.
Stenehjem E, Armstrong WS. Central nervous system device infections. Infect Dis Clin N Am. 2012;26:89. https://doi.org/10.1016/j.idc.2011.09.006.
Atkinson RA, Fikrey L, Vail A, Patel HC. Silver-impregnated external-ventricular-drain-related cerebrospinal fluid infections: a meta-analysis. J Hosp Infect. 2016;92(3):263–72. https://doi.org/10.1016/j.jhin.2015.09.014.
Zhou YJ, Wu JN, Chen LJ, Zhao HY. Comparison of infection rate with tunneled vs standard external ventricular drainage: a prospective, randomized controlled trial. Clin Neurol Neurosurg. 2019;184:105416. https://doi.org/10.1016/J.CLINEURO.2019.105416.
Lord AS, Nicholson J, Lewis A. Infection prevention in the neurointensive care unit: a systematic review. Neurocrit Care. 2019;31(1):196–210. https://doi.org/10.1007/s12028-018-0568-y.
Hong B, Apedjinou A, Heissler HE, et al. Effect of a bundle approach on external ventricular drain-related infection. Acta Neurochir. 2021;163(4):1135–42. https://doi.org/10.1007/s00701-020-04698-8.
Sader E, Moore J, Cervantes-Arslanian AM. Neurosurgical infections. Semin Neurol. 2019;39(4):507–14. https://doi.org/10.1055/s-0039-1693107.
Acknowledgements
We thank Universitat de Barcelona for their help in the open access publishing.
Funding
This work was not supported by any foundation.
Author information
Authors and Affiliations
Contributions
LC, MR and CC were responsible of the conception and design of the study, drafted the manuscript, reviewed and analyzed the literature and were responsible for the manuscript’s revision. MR, IZC, PLO VFM, IRP, AP and CLB collected data. MR, LC, EPM, JS and CC made all data and made a signifcant intellectual contribution. All authors read and approved the fnal manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Clinical Research Ethics Committee of Bellvitge University Hospital (HUB) with the reference PR317/18.
Consent for publication
Not applicable.
Competing interests
MR, LC, ILC, PLO, VFM, IRP, CLB, EPM, AP, JS and CC report no competing interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Rojas-Lora, M., Corral, L., Zabaleta-Carvajal, I. et al. External ventriculostomy-associated infection reduction after updating a care bundle. Ann Clin Microbiol Antimicrob 22, 59 (2023). https://doi.org/10.1186/s12941-023-00612-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12941-023-00612-z