Abstract
Background
The emergence of Acquired Immunodeficiency Syndrome has highlighted the increased incidence and importance of the disease caused by Non-tuberculous Mycobacteria (NTM). While disease due to M. avium-intracellulare complex is apparently common throughout the world, other Non-tuberculous mycobacterial species have been isolated from both immunocompromised and immunocompetent individuals. The increasing number of infections caused by these organisms has made it clinically important to quickly identify mycobacterial species. The diagnosis of a pathogenic versus a non-pathogenic species not only has epidemiological implications but is also relevant to the demands of patient management. Since antibiotic treatment varies according to the species encountered, species identification would reduce the burden of some of these emerging opportunistic pathogens especially in immunocompromised patients and improve their quality of life.
Findings
A total of 91 NTM suspected isolates from four regions of Zambia were included in the study. These isolates were identified using the sequence analysis of the 16S-23S rRNA intergenic transcribed spacer (ITS) region of Mycobacteria.
Fifty-four of the 91 (59%) isolates were identified as NTM and these included M. intracellulare (27.8%), M. lentiflavum (16.7%), M. avium (14.8%), M. fortuitum (7.4%), M. gordonae (7.4%), M. kumamotonense (3.7%), M. indicus pranii (3.7%), M. peregrinum (3.7%), M. elephantis (1.85%), M. flavescens (1.85%), M. asiaticum (1.85%), M. bouchedurhonense (1.85%), M. chimaera (1.85%), M. europaeum (1.85%), M. neourum (1.85%), M. nonchromogenicum (1.5%).
Conclusion
The study has shown that DNA sequencing of the ITS region may be useful in the preliminary identification of NTM species. All species identified in this study were potentially pathogenic.
Similar content being viewed by others
Findings
Members of the genus Mycobacterium are important causes of respiratory disease, thereby posing an important public health threat to people and animals worldwide. Recently, there has been increased cognisance of a variety of diseases that have been caused by Non-tuberculous Mycobacteria (NTM) [1]. The current unprecedented high level of interest in NTM infections is mainly the result of the association of NTM infection with immune-suppression [2] and the recognition that NTM pulmonary infections are encountered with increasing frequency in the immune-competent patients. Another major factor contributing to the increased awareness of the importance of NTM as human pathogens is the improvement in the mycobacteriology laboratory techniques, resulting in enhanced isolation and more rapid and accurate identification of NTM from clinical specimens [3]. Consistent with advances in mycobacteriological laboratory techniques is the emphasis on the identification of individual NTM species and the clinical disease-specific syndromes they produce [4]. The number of NTM species has been steadily increasing [5] and currently there are more than 160 NTM species [6].
Although the reservoir of infection in most cases remains unclear, there is a general notion that NTM infections are derived mainly from the environment. NTM are widely distributed in nature and have been isolated from water and soil with water being the major reservoir [7]. There are a variety of situations where human and mycobacterial geographical and environmental distributions can overlap leading to exposure of humans. A major overlap occurs with water where humans are exposed to mycobacteria in water through drinking, swimming and bathing [8]. Aerosols generated during some of these activities can also lead to human exposure [9]. The presence of NTM in water, coupled with their disinfectant resistance, leads to their presence in hot tubs, solutions used in medical treatments and water–oil emulsions used to cool metal working tools [10]. It is however, generally believed that the majority of human-mycobacterial interactions are transient, self-curing colonisations [11,12]. These subclinical human-mycobacterial interactions may give a transient stimulation of certain pathways that may set the stage for manifestation of other diseases [4].
Non-tuberculous Mycobacteria are often involved in nosocomial outbreaks [13], although there is little or no evidence for person-to-person transmission of these organisms [3]. However, the significance of isolation of these organisms in clinical samples remains unclear since the number of diseases they cause is difficult to assess and no system for notification exists as in the case of M. tuberculosis. In addition, treatment and infection control measures vary according to the aetiological species [3]. Therefore, rapid and accurate identification of mycobacteria to the species level is essential to facilitate early treatment of mycobacterioses.
Zambia is a high burden country for tuberculosis and patients with chronic pneumonia, lymphadenitis, pyrexia of unknown origin and other chronic infections are evaluated for tuberculosis through microbiological cultures of various clinical specimens. In the process of isolating M. tuberculosis, NTM are also isolated from these specimens, without any attempt to identify them to species level. Therefore this study was initiated to identify NTM to species level for ease of managing such suspect conditions.
Materials and methods
This was a retrospective study of 91 isolates stored over a period of three and half years from January 2009 to June 2012 from four regions of Zambia (Eastern, Lusaka, Southern and Western). The stored isolates were revived using Lowestein Jensen (LJ) and Mycobacterium Growth Indicator Tube (MGIT) by standard microbiological procedures [14]. The cultures were then subjected to PCR identification and DNA sequencing of the 23S rRNA (ITS) region with primers Sp1 (5′-ACC TCC TTT CTA AGG AGC ACC-3′) and Sp2 (5′-GAT GCT CGC AAC CAC TAT CCA-3′) [15]. The obtained sequences were compared with those available in GenBank by BLAST searches. Sequences that displayed at least 98% sequence identity when compared to those in the GenBank were preliminary considered as identified species [16].
Results
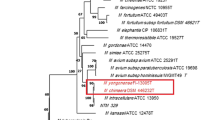
Sequence analysis and identification of the ITS region of the 91 strains showed: NTM species (68), Mycobacterium tuberculosis complex (17), Rodococcus equi (3), Tsukamurella pulmonis (1), Norcadia carnea (1) and Paenibacillus species (1) as shown in Figure 1 and Additional file 1: Table S1. Of the 68 NTM isolates, 54 were identified to species level as shown in Table 1, while 14 could not be identified. The 54 NTM species identified belonged to 16 different species with M. intracellulare exhibiting the highest frequency of identity (Additional file 2). Furthermore, M. intracellulare was the only NTM specie identified in the four regions of Zambia under study, with Lusaka region having a higher frequency (10), Southern (3), Western (1) and Eastern (1). M. fortuitum was identified in the Eastern and Lusaka regions, with one and three isolates respectively. All the other 14 species identified were from the region of Lusaka. A map of Zambia showing regions of distribution of various NTM identified in this study is shown in Figure 2.
Map of Zambia showing regions of various NTM identified. The regions are indicated in bold with the identified NTM. MI (M. intracellulare), ML (M. lentiflavum), MA (M. avium), MF (M. fortuitum), MG (M. gordonae), MK (M. kumamotonense), ME (M. elephantis), MIP (M. indicus pranii), MFL (M. flavescens), MP (M. peregrinum), MAS (M. asiaticum), MB (M. bouchedurhonense), MC (M. chimaera), MEU (M. europaeum), MN (M. neoaurum), MNO (M. nonchromogenicum).
Discussion
Non-tuberculous Mycobacteria have gained a lot of clinical significance in the last couple of decades in immunocompromised and immunocompetent individuals or patients [2]. Their ubiquitous distribution in nature put them at an advantage of having hosts close to ecological niches compounded by human activities. This might be the first study in Zambia to identify NTM species using PCR and DNA sequencing of the ITS region. This study has provided a range of NTM species which are potentially pathogenic. A total of 64 isolates were initially identified as NTM species. On sequencing and GenBank comparison, only 54 were identified to species level using the preliminary identification strategy which has been previously described [16]. The most prevalent species was M. intracellulare followed by M. lentiflavum and M. avium. This was in partial agreement with the findings of the study conducted by Buijtels and others [17] in the Eastern region of Zambia where sputum Mycobacterial culture isolates were identified by 16S rRNA gene sequencing. In this study M. fortuitum, was isolated from a clinical case. The other studies conducted in the Western and Northern regions of Zambia [18] and other parts of the world [19,20] were in contrast with these findings. The reason for this difference is that NTM species distribution differs from one geographical region to another [21].
M. intracellulare has been identified as the important species of the Mycobacterium avium complex. It has been identified together with M. avium as a complex because of their close similarities. M. intracellulare has been found to be more pathogenic than M. avium [22] and have been reported to cause disease not only in immunocompromised but also in immunocompetent subjects [23]. Other NTM species such as M. lentiflavum and M. avium have been implicated in clinical disease of immunocompromised as well as immunocompetent individuals [24,25]. M. lentiflavum has been isolated from various human specimens including pleural effusions, ascites and lung tissue [26,27] and have mainly been associated with causing an array of infections in immunocompromised patients [28]. Unlike M. intracellulare, most M. avium species do not multiply in monocytes of healthy individuals [29]. M. fortuitum infrequently cause a variety of diseases including bone and soft tissue infections, lymphadenitis and post-surgical infections and lung disease [30]. M. kumamotonense, M. indicus pranii, M. flavescens, M. bouchedurhonense, M. chimaera, M. europaeum and M. nonchromogenicum were identified and reported for the first time in Zambia. Some of these NTM have been associated with clinical disease [31,32] while M. indicus pranii is an atypical saprophytic bacterium that has raised a lot of research interest in leprosy immunotherapeutic [33]. M. flavescens has been isolated from the synovial fluid of an AIDS patient [34], whereas M. bouchedurhonense and M. chimaera have been documented in some respiratory tract infections [35]. M. europaeum was isolated from the sputum samples of an Iranian human immunodeficiency virus-infected patient and a cystic fibrosis patient with chronic pulmonary disease [36] while M. nonchromogenicum has been associated with sarcoidosis [37].
Other organisms which are not NTM that were identified include Mycobacterium tuberculosis complex species, Rodococcus equi, Nocardia carnea, Tsukamura pulmonis and Paenibacillus species. Of significance is the identification of Rodococcus equi from a clinical specimen in Zambia. This is the second time Rodococcus equi has been reported in Zambia [38]. The organisms: Rodococcus equi, Nocardia carnea, Tsukamura pulmonis and Paenibacillus species have been known to cause pulmonary diseases that are similar to tuberculosis [39-41]. Management of infections by these agents is different from that of tuberculosis. Therefore species identification of NTM remains of great importance as it provides an opportunity to develop a database that may help increase the scope of mycobacterioses.
Availability of supporting data
The data supporting the results of this study are included within this article.
Abbreviations
- NTM:
-
Non-tuberculous mycobacteria
- MGIT:
-
Mycobacteria growth indicator tube
- PCR:
-
Polymerase chain reaction
- LJ:
-
Lowestein Jensen
- ITS:
-
Intergenic transcribed spacer
References
Brown-Elliott B, Wallace B, Tichindelean RJ, Sarria C, McNulty JC, Vasireddy S, et al. Five-year outbreak of community- and hospital-acquired Mycobacterium porcinum infections related to public water supplies. J Clin Micro. 2011;49:4231–8.
Sexton P, Harrison AC. Susceptibility to Non-tuberculous Mycobacterial lung disease. Eur Respir J. 2008;31:1322–33.
Griffith DE, Aksamit T, Brown-elliott BA, Catanzaro A, Daley C, Gordin F, et al. American Thoracic Society documents an official ATS/IDSA statement: diagnosis, treatment, and prevention of non-tuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367–417.
Primm TP, Lucero CA, Falkinham III JO. Health impacts of environmental mycobacteria. Clin Microbiol Rev. 2004;17:98–106.
Gutierrez MC, Supply P, Brosch R. Pathogenomics of mycobacteria. Genome Dyn. 2009;6:198–210.
Lindsay AH, Jeffrey RS. Common Presentations of Nontuberculous Mycobacterial Infections. Pediatr Infect Dis J. 2014;33(1):89–91.
van Ingen J, Blaak H, de Beer J, Husman AMR, van Soolingen D. Rapidly growing Non-tuberculous Mycobacteria cultured from home tap and shower water. Appl Environ Microbiol. 2010;76:6017–9.
Falkinham III JO. Surrounded by mycobacteria: Non-tuberculous Mycobacteria in the human environment. J App Micro. 2009;107:356–67.
Feazel LM, Baumgartner LK, Peterson KL, Frank DN, Harris JK, Pace NR. Opportunistic pathogens enriched in showerhead biofilms. Proc Natl Acad Sci U S A. 2009;106:16393–9.
Thomson RM, Carter R, Tolson C, Coulter C, Huygens F, Hargreaves M. Factors associated with the isolation of Nontuberculous Mycobacteria (NTM) from a large municipal water system in Brisbane, Australia. BMC Microbiol. 2013;13:89.
Mahayiddin A. Mycobacterial infections. Malaysian Jour Path. 1996;18:17–9.
Al Majid F. Peritonitis due to Mycobacterium fortuitum following gastric banding. Saudi J Ent. 2010;16:113–5.
Set R, Shastri J. Laboratory aspects of clinically significant rapidly growing mycobacteria. Indian J Med Microbiol. 2011;29:343–52.
Koneman EW, Allen SD, Janda WM, et al. Color atlas and textbook of diagnostic microbiology. 5th ed. Philadelphia: Lippincott-Raven Publishers; 2006. p. 1064–124.
Roth A, Reischl U, Streubel A, Naumann L, Kroppenstedt RM, Habicht M, et al. Novel diagnostic algorithm for identification of mycobacteria using genus-specific amplification of the 16S-23S rRNA gene spacer and restriction endonucleases. J Clin Microbiol. 2000;38:1094–104.
Turenne YC, Tschetter L, Wolfe J, Kabani AA. Necessity of quality-controlled 16S rRNA gene sequence databases: ıdentifying nontuberculous mycobacterium species. J Clin Microbiol. 2001;39:3637–48.
Buijtels PCAM, van der Sande MAB, de Graaff CS, Parkinson S, Verbrugh HA, Petit PLC, et al. Nontuberculous Mycobacteria, Zambia. Emerg Inf Dis. 2009;15:242.
Buijtels PCAM, Iseman MD, Parkinson S, de Graaff CS, Verbrugh HA, Petit PLC, et al. Misdiagnosis of tuberculosis and the clinical relevance of Nontuberculous Mycobacteria in Zambia. Asian Pac Jour Trop Med. 2010;3:386–91.
Corbett ELM, Hay GJ, Churchyard P, Herselman T, Clayton BG, Williams R, et al. Mycobacterium kansasii and M. scrofulaceum isolates from HIV-negative South African gold miners: incidence, clinical significance and radiology. Int J Tuber Lung Dis. 1999;3:501–7.
Hoefsloot W, van Ingen J, Andrejak C, Angeby K, Bauriaud R, Bemer P, et al. The geographic diversity of Nontuberculous Mycobacteria isolated from pulmonary samples: A NTM-NET collaborative study. Eur Respir J. 2013;42:1604–13.
Van der Werf MJ, Ködmön C, Katalinić-Janković V, Kummunik T, Soini H, Richter E, et al. Inventory Study of Nontuberculous Mycobacteria in European Union. BMC Infect Dis. 2014;14:62.
Koh WJ, Jeong BH, Jeon K, Lee NY, Lee KS, Woo SY, et al. Clinical Significance of the Differentiation Between Mycobacterium avium and Mycobacterium intracellulare in M. avium Complex Lung Disease. Chest. 2012;142:1482–8.
Han XY, Tarrand JJ, Infante R, Jabson KL, Truong M. Clinical Significance and Epidemiologic Analyses of Mycobacterium avium and Mycobacterium intracellulare among Patients without AIDS. J Clin Microbiol. 2005;43:4407–12.
Molteni C, Gazzola L, Cesari M, Lombardi A, Salerno F, Tortoli E, et al. Mycobacterium lentiflavum infection in immunocompetent patient. Emerg Infect Dis. 2005;11:119–22.
Shamaei M, Marjani M, Farnia P, Tabarsi P, Mansouri D. Human infections due to Mycobacterium lentiflavum: first report in Iran. Iran J Microbiol. 2010;2:27–9.
Safdar A, Han XY, Haase G, Kentrup H, Skopnik H, Springer B, et al. Mycobacterium lentiflavum: an etiologic agent of cervical lymphadenitis. Clin Infect Dis. 1997;25:1245–6.
Tortoli E, Mattei R, Russo C, Scarparo C. Mycobacterium Lentiflavum an emerging pathogen. J Infect. 2005;52:185–7.
Niobe SN, Bebear CM, Clerc M, Pellegrin JL, Bebear C, Maugein J. Disseminated Mycobacterium lentiflavum infection in a Human Immunodeficiency Virus-infected patient. J Clin Microbiol. 2001;39:2030–2.
Toba H, Crawford JT, Ellneri JJ. Pathogenicity of Mycobacterium avium for Human Monocytes: Absence of Macrophage-Activating Factor Activity of Gamma Interferon. Infect Immun. 1989;57:239–44.
Wallace Jr RJ, Swenson JM, Silcox VA, Good RC, Tschen JA, Stone MS. Spectrum of disease due to rapidly growing mycobacteria. Rev Infect Dis. 1983;5:657–79.
García-Agudo L, García-Martos P. Clinical significance and antimicrobial susceptibility of rapidly growing mycobacteria. In: Mende-Vilas A, editor. Science against microbial pathogens: communicating current research and technological advances. 2011. p. 363–77.
Simons S, van Ingen J, Hsueh PR, Hung NV, Dekhuijzen PNR, Boeree MJ, et al. Nontuberculous Mycobacteria in Respiratory Tract Infections, Eastern Asia. Emerg Infect Dis. 2011;17:343–9.
Ahmed N, Saini V, Raghuvanshi S, Khurana JP, Tyagi AK, Tyagi AK, et al. Molecular analysis of a leprosy immunotherapeutic bacillus provides insights into Mycobacterium evolution. PLoS One. 2007;2:e968.
Tortoli E, Rindi L, Bartoloni A, Garzelli C, Manfrin V, Mantella A, et al. Isolation of a novel sequevar of Mycobacterium flavescens from the synovial fluid of an AIDS patient. Clin Microbiol Infect. 2004;10:1017–9.
Ben SI, Cayrou C, Raoult D, Drancourt M. Mycobacterium marseillense sp. nov., Mycobacterium timonense sp. nov. and Mycobacterium bouchedurhonense sp. nov., members of the Mycobacterium avium complex. Int J Syst Evol Microbiol. 2009;59:2803–8.
Pourahmad F, Shojaei H, Heidarieh P, Khosravi A, Hashemi A. Report of two cases of Mycobacterium europaeum from Iran. Jpn J Infect Dis. 2012;65:539–41.
Toda S, Suematsu R, Inoue H, Koarada S, Tada Y, Aoki Y, et al. A case of cutaneous Mycobacterium nonchromogenicum infection suggesting sarcoidosis association. Kansenshogaku Zasshi. 2010;84:300–4.
Takai S, Syakalima M, Yasuda J, Sasaki Y, Tsutsumi H, Miyagawa E, et al. Isolation of Rhodococcus equi from the feces of indigenous animals and soil from the Lower Zambezi National Park and Lochinvar National Park, Zambia. J Vet Med Sci. 2008;66:743–6.
Sakar MM, Gopinath K, Singh R, Singh S. In vitro antimicrobial drug susceptibility testing of nontubercular mycobacteria by tetrazolium microplate assay. Ann Clin Microbiol Antimicrob. 2008;7:15.
Ménard A, Degrange S, Peuchant O, Nguyen TD, Dromer C, Maugein J. Tsukamurella tyrosinosolvens-an unusual report of bacteremic pneumonia afterlung transplantation. Ann Clin Microbiol Antimicrob. 2009;8:30.
Mehta YB, Goswami R, Bhanot N, Mehta Z, Simonelli P. Tsukamurella infection: a rare cause of community-acquired pneumonia. Am J Med Sci. 2011;341:500–3.
Acknowledgements
This work was supported by the Ministry of Education, Science, Vocational Training and Early Education of Zambia and Hokkaido University Research Center for Zoonosis Control, Global COE Program, Kita-ku, Sapporo, Japan.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
MG and KG conceived and designed the experiments. MG, HBM and SE performed the experiments. MG, KG, HBM, SE and SS analysed and interpreted the data. KT, NS and SY helped in study design, coordinated the study and reviewed the manuscript. MG and HBM wrote the manuscript. All authors have read and approved the final manuscript.
Additional files
Additional file 1: Table S1.
Species with the highest degree of nucleotide sequence identity to isolates from Zambia.
Additional file 2:
Nucleotide sequences of Zambian isolates.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Mwikuma, G., Kwenda, G., Hang’ombe, B.M. et al. Molecular identification of non-tuberculous mycobacteria isolated from clinical specimens in Zambia. Ann Clin Microbiol Antimicrob 14, 1 (2015). https://doi.org/10.1186/s12941-014-0059-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12941-014-0059-8