Abstract
It is a growing problem around the world to deal with nontuberculous mycobacteria infection (NTM), but its clinical significance is still largely unknown. This study aims to investigate the epidemiology of NTM infections from various clinical samples and determine their clinical significance. From December 2020 to December 2021, 6125 clinical samples were collected. In addition to phenotypic detection, genotypic detection through multilocus sequence typing (hsp65, rpoB, and 16S rDNA genes) and sequencing was also conducted. Records of patients were consulted for clinical information, such as symptoms and radiological findings. Of the 6,125 patients, 351 (5.7%) were positive for acid-fast bacteria (AFB). Out of 351 AFB, 289 (82.3%) and 62 (17.7%) subjects were identified as M. tuberculosis complex (MTC) and NTM strains, respectively. Isolates of Mycobacterium simiae and M. fortuitum were the most frequent, followed by isolates of M. kansasii and M. marinum. We also isolated M. chelonae, M. canariasense, and M. jacuzzii, which are rarely reported. Symptoms (P = 0.048), radiographic findings (P = 0.013), and gender (P = 0.039) were associated with NTM isolates. M. Fortuitum, M. simiae, and M. kansasii presented with bronchiectasis, infiltration, and cavitary lesions most frequently, while cough was the most common symptom. In conclusion, Mycobacterium simiae and M. fortuitum were presented in seventeen and twelve NTM isolates from the collected samples. There is evidence that NTM infections in endemic settings may contribute to the dissemination of various diseases and the control of tuberculosis. In spite of this, further research is needed to evaluate the clinical significance of NTM isolates.
Similar content being viewed by others
Introduction
Increasing numbers of nontuberculous mycobacteria (NTM) have been isolated from environmental and clinical samples worldwide (Mbeha et al. 2019). NTM agents are ubiquitous environmental saprophytes that are frequently isolated from water, plants, and soil samples (Jeon 2019). Over 200 NTM species have been identified, and new species are being discovered every year (Falkinham 2021). Depending on their growth rate, NTM can be classified as ‘‘slow’’ or ‘‘rapid.’’ Different NTM species are split based on the growth rate, rapid (e.g., M. abscessus, M. chelonae, M. fortuitum), and slow growers (e.g., M. avium complex, M. kansasii). Besides, M. marinum was introduced as an intermediate category between rapid and slow growers (Huang et al. 2020; Mortazavi et al. 2019). As opportunistic pathogens, NTM agents often cause serious infections such as pulmonary disorders, soft tissue infections, and skin infections. Therefore, it can be regarded as one of the most alarming sources of infection in the healthcare system for both immune-compromised and immune-competent people (Abubakar et al. 2018). Despite surviving in diverse environmental conditions, these microaerobic organisms are extremely antibiotic and disinfectant resistant. Based on these characteristics, NTMs cause a wide variety of infections throughout the world, and hence their epidemiological surveillance is crucial (Kaelin et al. 2020). It is possible to ignore such infections and treat them ineffectively because of misdiagnoses (Ratnatunga et al. 2020). Because NTM infections have similar symptoms to tuberculosis, diagnosis can be challenging (Gharbi et al. 2019; Hirabayashi et al. 2019). It has been reported in several studies from developed countries that the prevalence of NTM infections is increasing (Chunfang et al. 2021; Harada et al. 2021; Winthrop et al. 2020). As a result of the development of molecular methods for detecting NTM infections causality, such as 16SrDNA sequencing, the reporting rate of NTM infections has gone up in such areas. A lack of laboratory facilities and misdiagnosis may be the cause of this issue in many economically challenged countries (Nasiri et al. 2015; Shafipour et al. 2021). There is still a challenge to detect NTM infections among tuberculosis (TB) suspected samples and to control them in some of these developing countries like Iran. Meanwhile, conducting comprehensive epidemiological studies have been still required across Iran's various regions. To more clearly identify the causative NTM species in this region, epidemiological data were collected from Iranian patients suffering from various NTM infections.
Materials and methods
Sample collection and preparation
We conducted a cross-sectional study from December 2020 to December 2021 on 6125 clinical samples referred to the Pasteur Institute of Iran that were suspected to be infected with TB. American Thoracic Society and Infectious Diseases Society of America (ATS/IDSA) guidelines were used to identify NTM isolates (Aksamit et al. 2007). As clinically relevant to define NTM pulmonary disease, we included patients with at least two positive cultures from sputum samples and/or bronchoalveolar lavages (BALs). Only those that had three adequate sputum samples collected in consecutive mornings were included in this study. The other samples were including skin, urine, pus, joints, lymph nodes, and soft tissues. Löwenstein-Jensen (LJ) medium was used to culture all samples after decontamination with N-acetyl-L-cysteine and sodium hydroxide (Kent 1985).
Identification of NTM strains using phenotypic and genotypic tests
Based on Centers for Disease Control (CDC) procedures, phenotypic tests for isolation of NTM strains included macroscopic and microscopic morphological characteristics, growth rate on LJ medium, growth at 25 °C, 32 °C, 37 °C, and 42 °C, Tween-80 hydrolysis, arylsulfatase, urease, tellurite reduction, nitrate reduction, semiquantitative catalase production, and salt tolerance (Kent 1985). Following the manufacturer's instructions, DNA was isolated from bacteria using a Proba-NK DNA extraction kit (DNA-Technology Company, Moscow, Russia). In positive cultures, insertion sequence 6110 (IS6110)-PCR (123 bp) was applied to differentiate MTC and NTM species (McKibben et al. 2016). As a primary method of detection, heat shock protein 65 (hsp65) fragments of 441 bp, followed by 16S rDNA and rpoB, were used as multilocus sequence typing (MLST) (Telenti et al. 1993). The ABI Automated Sequencer (Applied Biosystems, Foster City, CA, USA) was used to sequence two highly conserved genes, 16S rDNA (nearly 1500 bp) and rpoB (750 bp) genes from NTM isolates detected up to Mycobacterial species level (Adékambi et al. 2003; Kämpfer et al. 1991).
Drug susceptibility testing (DST)
According to the guidelines from the Clinical and Laboratory Standards Institute (CLSI), the broth microdilution method was used to determine the minimum inhibitory concentrations (MICs) of each drug. A wide range of drugs were tested including isoniazid, rifampicin, ethambutol, streptomycin, ethionamide, amikacin, ofloxacin, ciprofloxacin, and capreomycin. The drug was diluted in Mueller–Hinton broth with 5% oleic acid, albumin, dextrose and catalase and serially double diluted between 0.06 and 512 mg/L. Each culture was incubated aerobically at 37 °C after inoculation. The growth of rapidly growing mycobacteria (RGM) was evaluated on days 3 and 7 and that of slowly growing mycobacteria (SGM) was evaluated weekly for up to four weeks. MICs are defined as the lowest concentration of an antimicrobial agent that inhibits visible growth. The CLSI guidelines were used to assign susceptible, moderately susceptible, and resistant breakpoints (Woods et al. 2011).
Statistical analysis
The analyses of all clinical data and demographic characteristics were carried out using SPSS version 24.0 (2016; IBM Corp., Armonk, NY, USA). Statistics were considered significant at a P-value of 0.05 with two tailed tests. The Shapiro–Wilk test was initially used to verify the normality of continuous variables. For determining significant associations between qualitative and continuous variables, Fisher’s exact test/χ2 and Mann–Whitney U-test were used, respectively. Means were reported for variables with continuous distributions.
Results
Typical characteristics of the patients
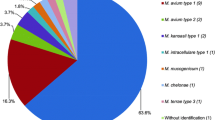
An analysis of 6125 clinically samples of TB-suspected patients was conducted in this study by the culture method and 351 (5.7%) yielded acid-fast bacteria. By evaluating phenotypic and molecular data, 289 (82.3%) and 62 (17.7%) subjects were identified as M. tuberculosis complex (MTC) and NTM strains, respectively (Fig. 1). A summary of the baseline demographic characteristics of NTM patients is shown in Table 1. In brief, 36 (61.3%) of the samples were sputum, and 15 (17.7%) were BALs collected from pulmonary sites. Further, 13 (21.0%) samples of suspected lesions were biopsied. An overview of the types of samples used to collect different isolates of NTM is presented in Fig. 2. Symptoms included coughing, sputum, fever, weight loss, and night sweating. Among the NTM patients, the average age was 53.2 ± 11.6 years old and the number of men and women was 35 (56.5%), 27 (43.5%). The smear was positive in 38 (21.3%) samples. A total of 14 (22.6%), 10 (16.1%), 8 (12.9%), 6 (9.8%), 5 (8.1%), and 4 (6.5%) had obesity, gastrointestinal diseases, human immunodeficiency virus (HIV), cystic fibrosis (CF), diabetes mellitus, and asthma, respectively.
Identification of NTM isolates by phenotypic tests
Twenty-seven (43.5%) and 35 (56.5%) of the 62 isolates were RGMs and SGMs, respectively. A phenotypic analysis revealed that M. simiae (17 isolates) and M. fortuitum (12 isolates) were the most prevalent strains, followed by M. kansasii (9 isolates), M. marinum (9 isolates), and, M. chelonae (5 isolates). The phenotypic tests identified 52 (83.9%) strains of 62 isolates, while the rest could not be identified.
Identification of NTM isolates by molecular tests
A MLST molecular test (hsp65, rpoB, and 16S rDNA) determined that M. simiae (18 isolates), M. fortuitum (13 isolates), M. kansasii (9 isolates), M. marinum (9 isolates), M. chelonae (6 isolates), M. mucogenicum (3 isolates), M. canariasense (2 isolates), M. bacteremicum (1 isolates), and M. jacuzzii (1 isolates) were among NTM isolates (Table 1).
The clinical significance of pulmonary NTM isolates
Table 2 reports patients’ characteristics of pulmonary NTM isolates. NTM isolates were not significantly associated with mean age, smoking history, and underlying disease. However, there was a correlation between NTM isolates and gender and radiographic findings. The M. simiae group had 9 (50.0%), 4 (22.2%), 3 (16.6%), 1 (5.6%), and 1 (5.6%) patients with obesity, HIV, CF, diabetes mellitus, and asthma, respectively. In this group, there was a high frequency of infiltrates. The mean age of patients with M. simiae infection was older and they were mostly female (69.2%). Isoniazid, rifampicin, ethambutol, and streptomycin were resistant to the majority of M. simiae strains, while amikacin, ofloxacin, and ciprofloxacin were susceptible.
Among the M. fortuitum positive group, chest radiography was also most frequently interpreted as having bronchiectasis (84.6%). Among the patients, there were 1 (7.7%), 9 (69.2%), 1 (7.7%), 1 (7.7%), and 1 (7.7%) patients with obesity, gastrointestinal diseases, HIV, diabetes mellitus, and asthma, respectively. It was found that 69.2% of the patients who were infected suffered from gastroesophageal disease, including chronic vomiting and achalasia. There were several major symptoms observed in these patients, including cough, sputum, fever, and weight loss. There was a high level of resistance to isoniazid, rifampicin, ethambutol, and streptomycin among the M. fortuitum strains, while amikacin was susceptible to most of them.
There were 2 (22.2%), 1 (11.1%), 1 (11.1%), 1 (11.1%), 2 (22.2%), and 2 (22.2%) patients with obesity, gastrointestinal diseases, HIV, CF, diabetes mellitus, and asthma, respectively, among the M. kansasii group. Chest radiography results showed cavitary lesions in all patients. In this group of patients, hemoptysis was more common. It was found that most of the strains of M. kansasii were susceptible to isoniazid, rifampicin, ethambutol, and streptomycin, and resistant to amikacin, ofloxacin, and ciprofloxacin.
Discussion
A key component of controlling and preventing tuberculosis is the isolation and detection of mycobacteria. We enrolled 6125 presumptive pulmonary tuberculosis patients in our study over one year, and 62 of them (17.7%) tested positive for NTM. In a similar study conducted in Iran during 2019, 53 (11.1%) out of 478 suspected pulmonary tuberculosis patients were found to be infected with NTM, which is comparable to our findings (Mortazavi et al. 2019). This study showed that the most common NTM isolates were M. fortuitum, followed by M. simiae, M. kansasii, M. gordonae, and M. conceptense. Aside from that, a variety of studies have been conducted on the prevalence of NTM infections, in particular M. avium-intracellulare (MAC) infection, M. abscessus, M. chelonae, M. fortuitum, and M. kansasii (Chiang et al. 2021; Zhou et al. 2021). Our study revealed that in the majority of cases, M. simiae, M. fortuitum, M. kansasii and M. marinum were detected. Our isolates also include M. chelonae, M. canariasense, and M. jacuzzii, all of which are rarely reported.
A real infection or colonization could be indicated by presence of M. simiae in respiratory samples (Nasiri et al. 2018). M. simiae infections were most common in females and older patients than other NTM isolates in the current study. In general, men are more likely than women to suffer pulmonary disease from NTM species except for M. abscessus, M. chelonae, and M. simiae, a finding in line with ours (Coolen-Allou et al. 2018; Jabbour et al. 2020). We found that the M. simiae positive cases in our study had obesity, HIV, cystic fibrosis, diabetes mellitus, and asthma as underlying diseases. There was a 21% incidence of clinical disease among M. simiae isolates, according to early surveillance reports, but other reports have shown a much lower rate (Griffith et al. 2007). There may be clinical and radiologic similarities between M. simiae and tuberculosis (Dezhkhi et al. 2021). As part of the present study, M. simiae isolated from TB patients receiving anti-TB drugs were originally diagnosed as multidrug-resistant. As a consequence, DST should be performed on every M. simiae isolate (Dezhkhi et al. 2021). The present study noted that M. simiae isolates had a wide range of susceptibility to amikacin, ofloxacin, and ciprofloxacin, unlike Van Ingen et al. who reported susceptibility to amikacin (14–40%) and ciprofloxacin (33–62%) (van Ingen et al. 2012). Several geographical regions show different susceptibility profiles for M. simiae, emphasizing the need to test susceptibility before starting treatment in these regions. Despite this, little is known about in vitro susceptibility and treatment response (Hamieh et al. 2018).
In our study, M. fortuitum were also frequent isolated NTMs from respiratory samples. M. fortuitum is the most frequently isolated RGM in Iran’s environmental and clinical samples (Arfaatabar et al. 2021; Ayoubi et al. 2021). In accordance with other studies, most patients had an underlying disease such as gastrointestinal diseases, HIV, cystic fibrosis, or diabetes mellitus. Additionally, the radiographic findings of these patients showed a tendency toward bronchiectasis. Structured lung disease appears to be the most common cause of M. fortuitum pulmonary infection (Irandoost et al. 2018). The ATS/IDSA guideline states that M. fortuitum is occurring in 15% of patients with pulmonary disease due to RGM, which is often observed as a pathogen in conditions such as chronic vomiting, achalasia, and exogenous lipoid pneumonia. As a result of most research on NTM pulmonary disease as well as esophageal disorders, it is discovered that most of the patients had achalasia and lung infection by M. fortuitum (Hadjiliadls et al. 1999; Jamal and Hammer 2022). As seen in the current study, most M. fortuitum infected patients had gastroesophageal disease and achalasia, which contributes significantly to M. fortuitum pulmonary disease. A minimum of two positive cultures were obtained by each M. fortuitum positive patient (Abraham 2007). M. fortuitum lung disease is rare, but it could potentially be the cause of disease in two of three respiratory sample cultures, according to several studies observing M. fortuitum isolation. As a result of our study, M. fortuitum was found to be one of highly pathogenic in respiratory samples. As shown by DST results against M. fortuitum isolates, this isolate was susceptible to amikacin as well as intermediate to ofloxacin, ciprofloxacin, and capreomycin. Drugs have generally been more effective against the M. fortuitum group than other RGM species. There is usually a high susceptibility or intermediate susceptibility to doxycycline, fluoroquinolones, sulfonamides, and macrolides in these bacteria (Brown-Elliott and Philley 2017). Nevertheless, it is suggested that treatment with antibiotics for most M. fortuitum positive patients may not be necessary (Mortazavi et al. 2019). It may be appropriate to use less invasive treatment strategies for this group of patients because M. fortuitum infection can be seen as colonization or a transient infection.
This study found M. kansasii to be the third most frequently occurring pulmonary NTM. As a result of its clinical and antigenic properties, it is similar to M. tuberculosis, whose prevalence is high in polluted cities (Nour-Neamatollahie et al. 2017). There were a variety of radiographic findings for M. kansasii pulmonary disease in this study. The most common radiographic feature was a cavitary lesion. The prevalence of M. kansasii pulmonary disease cavitation has been reported to be 75–96% in several studies (Bakuła et al. 2018). In radiographic terms, M. kansasii pulmonary disease has characteristics that are similar to tuberculosis. It is recommended to treat M. kansasii pulmonary disease with a combination of isoniazid, rifampin, and ethambutol for at least 12 months after a negative sputum result. Our study also found the same results with DST for M. kansasii pulmonary disease as other studies. Ciprofloxacin showed the highest resistance to M. kansasii isolates, followed by isoniazid and rifampin (Davari et al. 2019). As in our study, Shitrit et al. also showed that M. kansasii isolates had the strongest resistance to ciprofloxacin and the strongest sensitivity to rifampin and ethambutol (Shitrit et al. 2007).
A higher rate of hemoptysis was observed in our group patients with M. kansasii than in other studies. Hemoptysis in lung infections may be associated with endobronchial disease and may cause bronchial vessel disintegration by cavitation (Bakuła et al. 2018; Griffith et al. 2007). The cavitation rate associated with the M. kansasii pulmonary infection is reported at 57%, which is similar to our study in terms of the severity of the disease. However, there is no practical information regarding the relative incidence of endobronchial disease. In patients with other types of NTM infections, cavitations were far less common (Davari et al. 2019; Liu et al. 2019; Shitrit et al. 2008). A higher number of patients had hemoptysis, dyspnea, and cough, whereas fewer had fever or night sweats. The results in this study were inconsistent with those found in the Shitrit et al. report (Shitrit et al. 2007). The short period between the onset of symptoms and diagnosis may have caused these discrepancies.
One more isolated NTM from TB-suspected patients is M. marinum. As a closely related species to M. tuberculosis, M. marinum causes extrapulmonary mycobacterial infections ranging from cutaneous lesions to disseminated infections in immunocompromised individuals (Canetti et al. 2022; Tarashi et al. 2022). In our study, this bacterial agent was found in cutaneous biopsies of patients. It is most common for cutaneous bacterial infections to be caused by ubiquitous potential pathogens, while NTMs are rarely involved. It is reported, however, that NTM infections are becoming more prevalent over time (Sander et al. 2018). Cutaneous infections caused by NTMs were most prevalent with MAC (68%) and M. marinum (24%) (Lee et al. 2010). In Canada, M. marinum was found to occur in 0.08 cases per 100,000 people (Sander et al. 2018). As compared with France where there are 0.04 cases per 100,000 and the United States where there are 0.05 to 0.27 cases per 100,000, this is a lower incidence (Yu et al. 2016). M. ulcerans and M. marinum nearly always cause cutaneous infection through direct inoculation, unlike most NTMs that cause disseminated diseases (Kothavade et al. 2013; Lee et al. 2010). Among the factors that increase the risk of M. marinum infection is exposure to freshwater and saltwater, swimming in pools that are unchlorinated and owning and handling fish tanks (Hashish et al. 2018; Sia et al. 2016). According to our results, occupational exposure and fish tank cleaning are the most common sources of exposure. Adults with upper extremity involvement are typically affected (Sander et al. 2018). Among the antibiotics tested, ciprofloxacin showed the highest resistance to M. marinum isolates and the susceptibility to rifampin and ethambutol.
It is rare to find M. chelonae, M. canariasense, and M. jacuzzii in various samples of TB-suspected patients. The M. chelonae species are ubiquitous in the environment and have been found in soil, water, and aquatic animals (Akram et al. 2017). Infections of the extremities, such as cellulitis and abscesses, are commonly associated with this bacterial agent. M. chelonae also causes catheter-related infections and post-surgical infections after implant placement, transplantation, and sclerotherapy injections (Jones et al. 2019; Nakamura et al. 2019). Our results show that 50% of M. chelonae is isolated from cutaneous biopsy samples of tattoo. Despite the limited literature on mycobacterial infections complicating tattoos, tattoo-associated infections occur frequently (Drage et al. 2010). In an evaluation in 2003, an unclassified mycobacterial species was identified in an erythematous nodule associated with a tattoo (Wolf and Wolf 2003). De Quatrebarbes et al. described an outbreak of M. chelonae associated with tattoos that resulted in multiple, pruritic papules and pustules (De Quatrebarbes et al. 2005). A number of antibiotics have demonstrated in vitro susceptibility to M. chelonae, including clarithromycin, amikacin, tobramycin, linezolid, and tigecycline (Borek et al. 2022). For localized infections caused by M. chelonae while waiting for susceptibility results, clarithromycin and azithromycin are useful oral agents. Nevertheless, it has been well documented that patients with M. chelonae are resistant to clarithromycin (Jhaveri et al. 2020; Wallace et al. 1993). Amikacin demonstrated the resistance to M. chelonae isolates, whereas rifampin, isoniazid, and ethambutol showed the lowest susceptibility.
It is also interesting to note that M. canariasense has also been isolated from respiratory samples in the present study. Patients with immunocompromised immune systems were reported to develop skin infections and subcutaneous fat infections from M. canariasense isolates (Michienzi et al. 2019). There have also been few studies that confirm that this isolate is a pulmonary pathogen (Kim et al. 2012; Lee et al. 2014). The presence of M. conceptionense was only detected in two patients with pulmonary disease in one study in Iran (Shojaei et al. 2011). The second case of M. conceptionense in Iran was found in a 37 year-old male with HIV infection and 51 year-old male with diabetes mellitus (Mortazavi et al. 2019). It was interesting to note that both patients had underlying diseases, and that DST showed that they were susceptible to ofloxacin, ciprofloxacin, and amikacin, as well as intermediate to isoniazid, rifampicin, and ethionamide, which is in line with our findings. The results concur with Kim et al. s study, which suggested M. conceptionense can cause pulmonary disease, despite being a rare NTM (Kim et al. 2012).
Additionally, we detected M. jacuzzii in biopsy samples which is a very rare NTM. NTM tenosynovitis most commonly affects the hand and wrist due to the redundancy of tissues and synovial fluid. In most cases, these conditions are caused by mycobacteria, especially M. marinum, which are slowly growing. In Israel, breast implants first showed evidence of M. jacuzzii isolation in 2003 (Rahav et al. 2006). Using phenotypic and genotypic tests on wrist synovial samples, we described our experience in detecting M. jacuzzii in Iran (Sakhaee et al. 2020). The antimycobacterial agents that are active against M. jacuzzii are limited. There were three antibiotics that were susceptible to this bacterium including amikacin, levofloxacin, and ethambutol. Generally, our study was limited by inadequate follow-up information. In addition, chest radiography was the main focus of the radiologic evaluation, not a CT scan.
In conclusion, the presence of nine NTM species was detected in samples taken from patients suspected of having TB. Most commonly found NTM species were M. simiae and M. fortuitum, followed by M. kansasii and M. marinum with a variety of radiographic findings, clinical symptoms, and drug resistance. Additionally, we isolated M. chelonae, M. canariasense, and M. jacuzzii, all of which have seldom been reported before. Further research is needed on the epidemiology of NTM infections in Iran with the aim of increasing Iranian physicians’ knowledge regarding the diagnosis and treatment of NTM isolates.
Availability of data and materials
The data used to support the findings of this study are included within the article and supplementary file. The nucleotide sequence data are available in the GenBank databases under the accession numbers OP580654 to OP580841 for the 16S rDNA, OP793506 to OP793567 for hsp68, and OP793568 to OP793629 for rpoB genes.
References
Abraham E (2007) Erratum: ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med 175(7):744b–7745
Abubakar I, Gupta RK, Rangaka MX (2018) Update in tuberculosis and nontuberculous mycobacteria 2017. Am J Respir Crit Care Med. 197(10):1248–1253
Adékambi T, Colson P, Drancourt M (2003) rpoB-based identification of nonpigmented and late-pigmenting rapidly growing mycobacteria. J Clin Microbiol 41(12):5699–5708
Akram SM, Rathish B, Saleh D. 2017. Mycobacterium chelonae.
Aksamit T, Brown-Elliott B, Catanzaro A, Daley C, Gordin F, Holland S, Horsburgh R, Huitt G, Iademarco M, Iseman M (2007) ATS Mycobacterial diseases subcommittee american thoracic society, infectious disease society of america an official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med 175(4):367416
Arfaatabar M, Karami P, Khaledi A (2021) An update on prevalence of slow-growing mycobacteria and rapid-growing mycobacteria retrieved from hospital water sources in Iran–a systematic review. Germs 11(1):97
Ayoubi S, Farnia P, Farnia P, Aghajani J, Ghanavi J, Velayati AA (2021) Prevalence of non-tuberculosis mycobacteria among samples deposited from the national tuberculous reference laboratory of Iran (2011–2018). Asian Pac J Trop Med 14(10):451
Bakuła Z, Kościuch J, Safianowska A, Proboszcz M, Bielecki J, van Ingen J, Krenke R, Jagielski T (2018) Clinical, radiological and molecular features of Mycobacterium kansasii pulmonary disease. Respir Med 139:91–100
Borek A, Zabost A, Głogowska A, Filipczak D, Augustynowicz-Kopeć E (2022) New RAPMYCOI SensititreTM antimicrobial susceptibility test for atypical rapidly growing mycobacteria (RGM). Diagnostics 12(8):1976
Brown-Elliott B, Philley J (2017) Rapidly growing mycobacteria. Microbiol Spectr. https://doi.org/10.1128/microbiolspec
Canetti D, Riccardi N, Antonello RM, Nozza S, Sotgiu G. (2022). Mycobacterium marinum: a brief update for clinical purposes. European J Internal Med.
Chiang C-H, Tang P-U, Lee GH, Chiang T-H, Chiang C-H, Ma KS-K, Fang C-T (2021) Prevalence of Nontuberculous mycobacterium infections versus Tuberculosis among autopsied HIV patients in Sub-Saharan Africa: a systematic review and meta-analysis. Am J Trop Med Hyg 104(2):628
Chunfang W, Jihong R, Yu W, Yunhong Z, Xuejuan S, Xiuyun J, Chunfeng W (2021) Prevalence of Nontuberculous mycobacterial disease in the Changchun district of China. Curr Microbiol 78(4):1643–1647
Coolen-Allou N, Touron T, Belmonte O, Gazaille V, Andre M, Allyn J, Picot S, Payet A, Veziris N (2018) Clinical, radiological, and microbiological characteristics of Mycobacterium simiae infection in 97 patients. Antimicrob Agents Chemother 62(7):e00395-e318
Davari M, Irandoost M, Sakhaee F, Vaziri F, Sepahi AA, Rahimi Jamnani F, Siadat SD, Fateh A (2019) Genetic diversity and prevalence of nontuberculous mycobacteria isolated from clinical samples in Tehran. Iran Microbial Drug Resis 25(2):264–270
De Quatrebarbes J, Pestel-Caron M, Duval-Modeste A, Abboud P, Etienne M, Caron F, Joly P (2005) P251-Épidémie à Mycobacterium chelonae chez un tatoueur. Elsevier Masson, Amesterdam
Dezhkhi H, Farnia P, Haddadi A, Farnia P, Velayati AA (2021) Characterization of clinical isolates of Mycobacterium simiae using drug susceptibility tests and molecular analyses. Curr Microbiol 78(6):2324–2331
Drage LA, Ecker PM, Orenstein R, Phillips PK, Edson RS (2010) An outbreak of mycobacterium chelonae infections in tattoos. J Am Acad Dermatol 62(3):501–506
Falkinham JO (2021) Ecology of nontuberculous Mycobacteria. Microorganisms 9(11):2262
Gharbi R, Mhenni B, Fraj SB, Mardassi H (2019) Nontuberculous mycobacteria isolated from specimens of pulmonary tuberculosis suspects, Northern Tunisia: 2002–2016. BMC Infect Dis 19(1):1–11
Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, Holland SM, Horsburgh R, Huitt G, Iademarco MF (2007) An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med 175(4):367–416
Hadjiliadls D, Adlakha A, Prakash UB (1999) Rapidly growing mycobacterial lung infection in association with esophageal disorders. Elsevier, Amsterdam. https://doi.org/10.4065/74.1.45
Hamieh A, Tayyar R, Tabaja H, Zein EL, Bou S, Khalil P, Kara N, Kanafani ZA, Kanj N, Bou AKL, Araj G (2018) Emergence of Mycobacterium simiae: a retrospective study from a tertiary care center in Lebanon. PLoS ONE 13(4):0195390
Harada K, Hagiya H, Funahashi T, Koyama T, Kano MR, Otsuka F (2021) Trends in the Nontuberculous Mycobacterial disease mortality rate in Japan: a nationwide observational study, 1997–2016. Clin Infect Dis 73(2):e321–e326
Hashish E, Merwad A, Elgaml S, Amer A, Kamal H, Elsadek A, Marei A, Sitohy M (2018) Mycobacterium marinum infection in fish and man: epidemiology, pathophysiology and management; a review. Vet Q 38(1):35–46
Hirabayashi R, Nakagawa A, Takegawa H, Tomii K (2019) A case of pleural effusion caused by Mycobacterium fortuitum and Mycobacterium mageritense coinfection. BMC Infect Dis 19(1):1–3
Huang W-C, Yu M-C, Huang Y-W (2020) Identification and drug susceptibility testing for nontuberculous mycobacteria. J Formos Med Assoc 119:S32–S41
Irandoost M, Ghanbari MZ, Sakhaee F, Vaziri F, Jamnani FR, Siadat SD, Fateh A (2018) High rates of Mycobacterium fortuitum isolation in respiratory samples from Iranian patients with suspected tuberculosis: is it clinically important? J Med Microbiol 67(9):1243–1248
Jabbour J-F, Hamieh A, Sharara SL, Kanj SS (2020) Mycobacterium simiae: Harmless colonizer or deadly pathogen? PLoS Pathog 16(4):e1008418
Jamal F, Hammer MM (2022) Nontuberculous Mycobacterial Infections. Radiologic. Clinics 60(3):399–408
Jeon D (2019) Infection source and epidemiology of nontuberculous mycobacterial lung disease. Tuberc and Respir Dis 82(2):94–101
Jhaveri VV, Singhal D, Riedel S, Rowley CF, Nathavitharana RR (2020) Surgical cure of clarithromycin resistant Mycobacterium chelonae breast implant infection: a case report and review of the literature. J Clin Tuberc Mycobact Dis 21:100183
Jones RS, Shier KL, Master RN, Bao JR, Clark RB (2019) Current significance of the Mycobacterium chelonae-abscessus group. Diagn Microbiol Infect Dis 94(3):248–254
Kaelin M, Kuster S, Hasse B, Schulthess B, Imkamp F, Halbe M, Sander P, Sax H, Schreiber P (2020) Diversity of non-tuberculous mycobacteria in heater-cooler devices: results from prospective surveillance. J Hosp Infect 105(3):480–485
Kämpfer P, Kroppenstedt RM, Dott W (1991) A numerical classification of the genera Streptomyces and Streptoverticillium using miniaturized physiological tests. Microbiology 137(8):1831–1891
Kent PT. (1985). Public health mycobacteriology: a guide for the level III laboratory: US Department of Health and Human Services, Public Health Service, Centers
Kim SY, Kim MS, Chang HE, Yim J-J, Lee J-H, Song SH, Park KU, Song J, Kim E-C (2012) Pulmonary infection caused by Mycobacterium conceptionense. Emerg Infect Dis 18(1):174
Kothavade R, Dhurat R, Mishra S, Kothavade U (2013) Clinical and laboratory aspects of the diagnosis and management of cutaneous and subcutaneous infections caused by rapidly growing mycobacteria. Eur J Clin Microbiol Infect Dis 32(2):161–188
Lee WJ, Kang SM, Sung H, Won CH, Chang SE, Lee MW, Kim MN, Choi JH, Moon KC (2010) Non-tuberculous mycobacterial infections of the skin: a retrospective study of 29 cases. J Dermatol 37(11):965–972
Lee KH, Heo ST, Choi S-W, Park DH, Kim YR, Yoo SJ (2014) Three cases of postoperative septic arthritis caused by Mycobacterium conceptionense in the shoulder joints of immunocompetent patients. J Clin Microbiol 52(3):1013–1015
Liu C-J, Huang H-L, Cheng M-H, Lu P-L, Shu C-C, Wang J-Y, Chong I-W (2019) Outcome of patients with and poor prognostic factors for Mycobacterium kansasii-pulmonary disease. Respir Med 151:19–26
Mbeha B, Mine M, Motswaledi MS, Dewar J (2019) Nontuberculous Mycobacteria, Botswana, 2011–2014. Emerg Infect Dis 25(7):1401
McKibben RA, Haberlen SA, Post WS, Brown TT, Budoff M, Witt MD, Kingsley LA, Palella FJ Jr, Thio CL, Seaberg EC (2016) A cross-sectional study of the association between chronic hepatitis C virus infection and subclinical coronary atherosclerosis among participants in the Multicenter AIDS Cohort Study. J Infect Dis 213(2):257–265
Michienzi SM, Burgos RM, Novak RM (2019) Mycobacterium conceptionense Pneumonitis in Patient with HIV/AIDS. Emerg Infect Dis 25(10):1986
Mortazavi Z, Bahrmand A, Sakhaee F, Doust RH, Vaziri F, Siadat SD, Fateh A (2019) Evaluating the clinical significance of nontuberculous mycobacteria isolated from respiratory samples in Iran: an often overlooked disease. Infection Drug Res 12:1917
Nakamura Y, Yoshioka D, Miyagawa S, Yoshikawa Y, Hata H, Nakae M, Toda K, Sawa Y (2019) A case of Mycobacterium chelonae mediastinitis and acute humoral rejection after heart transplantation. J Card Surg 34(4):205–207
Nasiri MJ, Dabiri H, Darban-Sarokhalil D, Hashemi SA (2015) Prevalence of non-tuberculosis mycobacterial infections among tuberculosis suspects in Iran: systematic review and meta-analysis. PLoS ONE 10(6):e0129073
Nasiri MJ, Dabiri H, Fooladi AAI, Amini S, Hamzehloo G, Feizabadi MM (2018) High rates of nontuberculous mycobacteria isolation from patients with presumptive tuberculosis in Iran. New Microbes New Infect 21:12–17
Nour-Neamatollahie A, Ebrahimzadeh N, Siadat SD, Vaziri F, Eslami M, Sepahi AA, Khanipour S, Masoumi M, Sakhaee F, Jajin MG (2017) Distribution of non-tuberculosis mycobacteria strains from suspected tuberculosis patients by heat shock protein 65 PCR–RFLP Saudi. J Biol Sci 24(6):1380–1386
Rahav G, Pitlik S, Amitai Z, Lavy A, Blech M, Keller N, Smollan G, Lewis M, Zlotkin A (2006) An outbreak of Mycobacterium jacuzzii infection following insertion of breast implants. Clin Infect Dis 43(7):823–830
Ratnatunga CN, Lutzky VP, Kupz A, Doolan DL, Reid DW, Field M, Bell SC, Thomson RM, Miles JJ (2020) The rise of non-tuberculosis mycobacterial lung disease. Front Immunol 11:303
Sakhaee F, Masoumi M, Vaziri F, Siadat SD, Fateh A (2020) A case report of wrist synovial infection due to Mycobacterium jacuzzii Iran. BMC Infect Dis 20(1):1–3
Sander MA, Isaac-Renton JL, Tyrrell GJ (2018) Cutaneous nontuberculous mycobacterial infections in Alberta, Canada: an epidemiologic study and review. J Cutan Med Surg 22(5):479–483
Shafipour M, Shirzad-Aski H, Ghaemi EA, Sohrabi A, Taziki M, Kochkaksaraei MB, Rahimi S (2021) Occurrence and risk factors of nontuberculous mycobacteria in tuberculosis-suspected patients in the north of Iran Iranian. J Microbiol 13(2):190–198
Shitrit D, Priess R, Fox B, Amital A, Kramer MR (2007) Pulmonary mycobacterium kansasii infection: comparison of the clinical features, radiological appearance, and outcome with pulmonary tuberculosis. Chest 132(4):434A
Shitrit D, Peled N, Bishara J, Priess R, Pitlik S, Samra Z, Kramer MR (2008) Clinical and radiological features of Mycobacterium kansasii infection and Mycobacterium simiae infection. Respir Med 102(11):1598–1603
Shojaei H, Hashemi A, Heidarieh P, Ataei B, Naser AD (2011) Pulmonary and extrapulmonary infection caused by Mycobacterium conceptionense: the first report from Iran. JRSM Short Rep 2(4):1–3
Sia TY, Taimur S, Blau DM, Lambe J, Ackelsberg J, Yacisin K, Bhatnagar J, Ritter J, Shieh W-J, Muehlenbachs A (2016) Clinical and pathological evaluation of Mycobacterium marinum group skin infections associated with fish markets in New York City. Clin Infect Dis 62(5):590–595
Tarashi S, Siadat SD, Fateh A. (2022). Nontuberculous Mycobacterial Resistance to Antibiotics and Disinfectants: Challenges Still Ahead. BioMed Research International 2022.
Telenti A, Marchesi F, Balz M, Bally F, Böttger E, Bodmer T (1993) Rapid identification of mycobacteria to the species level by polymerase chain reaction and restriction enzyme analysis. J Clin Microbiol 31(2):175–178
van Ingen J, Totten SE, Heifets LB, Boeree MJ, Daley CL (2012) Drug susceptibility testing and pharmacokinetics question current treatment regimens in Mycobacterium simiae complex disease. Int J Antimicrob Agents 39(2):173–176
Wallace RJ, Tanner D, Brennan PJ, Brown BA (1993) Clinical trial of clarithromycin for cutaneous (disseminated) infection due to Mycobacterium chelonae. Ann Intern Med 119(6):482–486
Winthrop KL, Marras TK, Adjemian J, Zhang H, Wang P, Zhang Q (2020) Incidence and prevalence of nontuberculous mycobacterial lung disease in a large US managed care health plan, 2008–2015. Ann Am Thorac Soc 17(2):178–185
Wolf R, Wolf D (2003) A tattooed butterfly as a vector of atypical Mycobacteria. J Am Acad Dermatol 48(5):S73–S74
Woods GL, Brown-Elliott BA, Conville PS, Desmond EP, Hall GS, Lin G, Pfyffer GE, Ridderhof JC, Siddiqi SH, Wallace Jr RJ. (2011). Susceptibility testing of mycobacteria nocardiae and other aerobic actinomycetes
Yu X, Liu P, Liu G, Zhao L, Hu Y, Wei G, Luo J, Huang H (2016) The prevalence of non-tuberculous mycobacterial infections in mainland China: systematic review and meta-analysis. J Infect 73(6):558–567
Zhou W, Zhao H, Yuan H, Zhang G, Liu Q, Yang J. (2021) Prevalence and antimicrobial susceptibility of non-tuberculous mycobacteria isolated from sputum samples of patients with pulmonary infections in China Jundishapur. J Microbiol 14(1)
Acknowledgements
The authors thank all the personnel of Mycobacteriology and Pulmonary Research Department, Pasteur Institute of Iran for their assistance in this project.
Funding
None.
Author information
Authors and Affiliations
Contributions
MM and FS: Sample collection and performed the experiments; ST and MGJ: Data acquisition and manuscript preparation; SDS: analyzed data and interpreted data; AF: designed and supervised study, interpreted data, read and approved manuscript. All authors reviewed the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The Declaration of Helsinki (1975) and local regulations were followed during this study. Informed consent was obtained from all individuals. The project was approved by the institutional review board at Pasteur Institute of Iran (IR.PII.REC.1394.54).
Consent for publication
Not Applicable.
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tarashi, S., Sakhaee, F., Masoumi, M. et al. Molecular epidemiology of nontuberculous mycobacteria isolated from tuberculosis-suspected patients. AMB Expr 13, 49 (2023). https://doi.org/10.1186/s13568-023-01557-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13568-023-01557-4