Abstract
Background
Existing evidence on long-term ambient air pollution (AAP) exposure and risk of cardio-respiratory diseases in China is mainly on mortality, and based on area average concentrations from fixed-site monitors for individual exposures. Substantial uncertainty persists, therefore, about the shape and strength of the relationship when assessed using more personalised individual exposure data. We aimed to examine the relationships between AAP exposure and risk of cardio-respiratory diseases using predicted local levels of AAP.
Methods
A prospective study included 50,407 participants aged 30–79 years from Suzhou, China, with concentrations of nitrogen dioxide (NO2), sulphur dioxide (SO2), fine (PM2.5), and inhalable (PM10) particulate matter, ozone (O3) and carbon monoxide (CO) and incident cases of cardiovascular disease (CVD) (n = 2,563) and respiratory disease (n = 1,764) recorded during 2013–2015. Cox regression models with time-dependent covariates were used to estimate adjusted hazard ratios (HRs) for diseases associated with local-level concentrations of AAP exposure, estimated using Bayesian spatio–temporal modelling.
Results
The study period of 2013–2015 included a total of 135,199 person-years of follow-up for CVD. There was a positive association of AAP, particularly SO2 and O3, with risk of major cardiovascular and respiratory diseases. Each 10 µg/m3 increase in SO2 was associated with adjusted hazard ratios (HRs) of 1.07 (95% CI: 1.02, 1.12) for CVD, 1.25 (1.08, 1.44) for COPD and 1.12 (1.02, 1.23) for pneumonia. Similarly, each 10 µg/m3 increase in O3 was associated with adjusted HR of 1.02 (1.01, 1.03) for CVD, 1.03 (1.02, 1.05) for all stroke, and 1.04 (1.02, 1.06) for pneumonia.
Conclusions
Among adults in urban China, long-term exposure to ambient air pollution is associated with a higher risk of cardio-respiratory disease.
Similar content being viewed by others
Background
Ambient air pollution (AAP) is a major risk factor for many diseases, with ambient fine particulate matter (PM2.5) and ozone (O3) estimated to account for 4.5 million deaths worldwide in 2019, including 1.5 million in China [1]. These estimates were largely based on the dose–response relationships of ambient PM2.5 with cardio-respiratory diseases and O3 with chronic obstructive pulmonary disease (but not other conditions), derived from prospective cohort studies mostly conducted in western high-income populations. Meanwhile, the associations of other key air pollutants, such as sulphur dioxide (SO2), nitrogen dioxide (NO2), and carbon monoxide (CO) with health outcomes are less well-understood due to the lack of reliable prospective evidence [2].
In recent decades, China has undergone rapid economic growth, urbanisation, and industrialisation, with pollution levels far exceeding both international [3] and national guidelines [4]. While the short-term health effects of AAP exposure have been well-documented across China, especially for cardio-respiratory morbidity [5, 6] and mortality [7, 8], evidence is more limited on associations of AAP exposure with longer term health outcomes in China. For a few such studies in China, they mainly relied on spatially and temporally averaged concentrations from fixed-site monitors as proxies for personal exposure [9,10,11,12,13,14]. Recent studies have modelled exposure based on satellite remote sensing data, but at a relatively crude spatial and temporal resolution and/or for a limited number of pollutants [15,16,17]. We therefore aimed to examine the relationship between AAP exposure and cardio-respiratory disease incidence using exposure estimates from spatio–temporal modelling applied to individuals in a prospective Chinese cohort for all six criteria air pollutants.
Methods
Study design
We used data from the prospective China Kadoorie Biobank (CKB) study. Details of the study design, objectives, and methodology are described elsewhere [18]. In brief, 512,713 participants aged 30 to 79 from 10 diverse areas of China were recruited between June 2004 and July 2008 (overall response rate 28%). The study population, identified from public registry records, was not designed to be representative of China as a whole but cover a wide variation in risk factors and diseases. At local assessment centres participants underwent a laptop-based interview providing information on socio-demographic characteristics, aspects of lifestyle (smoking, alcohol intake, diet, physical activity), exposure to passive smoking and household air pollution, and medical history. Trained health professionals undertook physical examination using standard protocols. The Chinese Center for Disease Control and Prevention and the University of Oxford gave ethics clearance for the CKB study. Written informed consent was obtained from all participants. For this report we included participants in the urban region of Suzhou only (n = 53,259), for which detailed air pollution data were available. We excluded one assessment centre and its participants (n = 257) located outside the urban area of Suzhou. The included assessment centres are spread across an area of approximately 30 km by 15 km.
Air pollution exposure
Daily 24 h-averaged measurements of inhalable (PM10) and fine (PM2.5) particulate matter, SO2, NO2, CO and O3 were obtained from 10 fixed-site monitors situated in Suzhou for years 2013 to 2015. We also obtained daily meteorological variables (ground temperature, total precipitation, wind speed, and relative humidity) from five local weather stations. Geographic covariates of elevation, distance to nearest major road, distance to nearest motorway, total length of major roads and motorways in a 1 km radius, and land use (urban or non-urban) were also obtained.
Predictions for pollutant levels at assessment centre locations for each month from January 2013 to December 2015 were derived using Bayesian models. Details of the modelling methodology can be found elsewhere [19]. A two-stage approach was used, applying spatio–temporal models for the meteorological variables then using these predictions (in addition to the five geographic covariates) in spatio–temporal models for each pollutant. Bayesian inference was via integrated nested Laplace approximation (INLA) and the SPDE approach, using the R-INLA package for R software [20]. In these models, predicted values for a random sample of fifty observations showed high correlation with observed values (r = 0.80 for CO and 0.87–0.98 for other pollutants).
Monthly pollutant levels were predicted for 77 baseline assessment centres matched to 53,002 participants, each living within 1 km of their respective centre at baseline. The two long-term exposures used in analyses were annual exposure (mean pollutant levels in calendar years 2013, 2014, and 2015) and cumulative exposure (mean pollutant levels from January 2013 to a given month).
Follow-up for morbidity and mortality
Deaths were identified by electronic linkage to local mortality records, supplemented by annual confirmation of survival through street committees or village administrators and standardised verbal autopsies for mortality without medical attention before death (< 5%). Non-fatal events and any episodes of hospitalisation were captured by disease registers (for cancer, ischaemic heart disease, stroke, and diabetes) and health insurance records. The underlying causes of death and hospital diagnoses were coded in accordance with the International Classification of Diseases, 10th Revision (ICD-10). By 31st December 2015, 1,467 participants (2.8%) had died, and 15 (< 0.1%) were lost to follow-up. The primary outcomes in this analysis were the first occurrence of either non-fatal or fatal cardiovascular (ICD-10: I00-I99) and respiratory (J00-J99) disease from 1st January 2013 to 31st December 2015 when AAP data are available. We also examined specific cardiovascular and respiratory diseases, including ischemic heart disease (IHD) (I20-I25), all-type stroke (I60-I61, I63-I64, I69.0, I69.1, I69.3, I69.4), ischemic stroke (I63), intracerebral haemorrhage (I61), chronic obstructive pulmonary disease (COPD) (J41-J44), and pneumonia (J12-J18). Endpoints capturing other cardiovascular diseases (I00-I16, I27-I52, I62, I65-I89, I95-I99), and other respiratory diseases (J00-J06, J20-J22, J30-J40, J80-J86, J90-J96, J98-J99) were also included (details can be found in eTable 1).
Statistical analysis
We excluded all participants censored prior to 2013 (n = 1482) in addition to participants with the respective cardio-respiratory endpoint(s) of interest recorded prior to 1st January 2013. We restricted our study population to participants with no history of cancer at baseline or incident cancer prior to the start of follow-up. In analyses of all cardiovascular disease and other cardiovascular diseases, participants with history of stroke/transient ischemic attack (TIA) or IHD (n = 907) at baseline were excluded. In analyses of stroke, participants with history of stroke/TIA (n = 402) were excluded, and in analyses of IHD participants with history of IHD (n = 522) were excluded. In analyses of respiratory disease, participants with history of asthma, tuberculosis, or emphysema/bronchitis at baseline (n = 2,955) were excluded. Baseline characteristics of the study population and annual levels of individual air pollutants were summarised by means and standard deviations (SDs) or proportions.
Cox proportional hazard models were used to estimate adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) for disease incidence per 10 µg/m3 increase in pollutant exposures, or per 100 µg/m3 increase for CO exposure. Pollutant exposure was included as a time-varying covariate. In all models time from start of study period was used as the time scale, and models were stratified for baseline age group (5-year groups) and sex, and adjusted for active smoking (never, occasional, ex-, or current smoker), exposure to passive smoking (never, previously, or currently lives with smoker), self-rated health status (excellent, good, fair, or poor), body mass index (kg/m2), physical activity level (metabolic equivalent of task per days), alcohol consumption (never, ex-, occasional, monthly, reduced, or weekly drinker), highest education level (no formal schooling, primary school, middle school, high school, college, or university), solid-fuel use for cooking (always clean fuels, switched from solid to clean fuels, always solid fuels, never cooked regularly, other), ambient mean temperature, and prior cardiovascular/respiratory disease (incident respiratory disease prior to the cardiovascular endpoint, or incident cardiovascular disease prior to a respiratory endpoint). Highest education level was chosen as it has been shown to be the best available measure for socioeconomic status in the CKB study. Temperature was included as a time-varying covariate on the same time scale as the pollutant exposure. Additional potential confounders hypothesised a priori to confound the relationship between AAP exposure and cardio-respiratory disease incidence were included. For cardiovascular diseases, these were consumption of fresh fruit and preserved vegetables, current use of hypertensive medication, and systolic blood pressure (SBP) at baseline. For respiratory diseases, these were consumption of fresh fruit, and preserved vegetables respectively, and current use of diabetes medication. We also used a robust variance estimator to account for spatial correlation of participant characteristics and disease incidence within assessment centres. For cardiovascular disease and respiratory disease, further analyses included adjustment variables sequentially and additionally adjusted for household income (6 groups).
We carried out subgroup analyses for pollutant exposures significantly associated with all cardiovascular disease or all respiratory disease, and tested for heterogeneity (or trend, if appropriate) of associations using annual exposures. Additionally, for these pollutant exposures we fit models adjusting for other pollutant exposures. To assess linearity of the associations between these pollutants and cardiovascular and respiratory diseases, we also fit models using natural cubic splines (with 4 degrees of freedom) for annual pollutant exposure.
To ensure the reliability of estimates of associations between pollutant exposures and cardio-respiratory disease, we also estimated the associations with certain infectious and parasitic disease incidence as a “negative control”. This encompassed a limited range of diseases (eTable 1) unlikely to be linked to ambient air pollution exposure but only to potential confounders (e.g. socioeconomic status).
Results
Mean pollutant concentrations and long-term trends through the study period (2013–2015) varied considerably between pollutants (Table 1, Fig. 1 and eFigure 1). Mean levels of particulate matter declined 15.5% and 21.9% between 2013 and 2015 for PM10 and PM2.5, respectively. Gaseous pollutant concentrations showed less consistent long-term trends and concentrations varied substantially between assessment centres. Mean levels of SO2 fell by 39.9% between 2013 and 2015. Annual levels of PM2.5 were strongly positively correlated with PM10 and NO2, while annual O3 levels were negatively correlated with PM10 and PM2.5 (eFigure 2).
Baseline characteristics for men and women included in analyses are presented in Table 2. The mean age of participants was 51.9 years for men and 51.5 years for women. While current regular smoking was highly prevalent (68.7%) among men, it was rare in women (0.4%). Similarly, regular alcohol consumption was common (41.1%) in men but not in women (0.6%). Self-rated health was slightly better among men (33.4% excellent and 7.1% poor) than women (26.6% excellent and 11.9% poor).
During the study period (including a total of 135,199 person-years of follow-up for CVD and 130,917 person-years for respiratory disease), 2,563 participants had a fatal or non-fatal cardiovascular event, and 1,764 had a respiratory disease event. Rates of both first cardiovascular event and first respiratory disease event were slightly higher in men (20.0 cardiovascular and 14.5 respiratory events per 1,000 person-years) compared to women (18.6 cardiovascular and 12.8 respiratory events per 1,000 person-years).
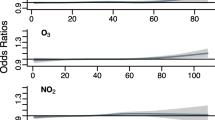
Adjusted HRs per 10 µg increase in long-term pollutant exposures (per 100 µg for CO) for cardiovascular diseases are shown in Fig. 2. Increased SO2 exposure is significantly associated with all cardiovascular disease (HR (95%CI): 1.07 (1.02, 1.12)) and other cardiovascular disease (1.11 (1.03, 1.19)). O3 exposure is moderately positively associated with all cardiovascular disease, all-type stroke, and ischemic stroke (1.02 (1.01, 1.03), 1.03 (1.02, 1.05), and 1.04 (1.01, 1.06)). PM10 and PM2.5 are moderately inversely associated with all cardiovascular disease (0.97 (0.95, 0.99)), in particular ischaemic stroke for PM2.5, and similarly NO2 with ischaemic stroke (0.97 (0.95, 0.99)).
Adjusted HRs for respiratory diseases are shown in Fig. 3. Increased SO2 exposure is significantly associated with all respiratory disease (1.11 (1.04, 1.19)), COPD (1.25 (1.08, 1.44)), pneumonia (1.12 (1.02, 1.23)), and other respiratory disease (1.09 (1.01, 1.19)). O3 exposure is associated with increased risk of pneumonia (1.04 (1.02, 1.06)). PM10 and PM2.5 are inversely associated with pneumonia (0.94 (0.90, 0.98) and 0.95 (0.90, 1.00), respectively), and CO is inversely associated with COPD (0.89 (0.83, 0.97)).
Analyses using cumulative pollutant exposures from January 2013 show similar results to analyses using annual exposures. Analyses of all cardiovascular disease and all respiratory disease with sequential adjustment for potential confounders show minimal change in estimates after adjustment for age, sex, and selected lifestyle and environmental factors (eFigure 8).
After adjustment for NO2 or CO, SO2 exposure remains significantly associated with cardiovascular disease, however adjustment for PM2.5, PM10 or O3 attenuates the association (eFigure 3). Adjustment for other pollutants does not attenuate the associations between O3 exposure and all-type stroke and ischemic stroke. After adjustment for other pollutants, SO2 exposure is significantly associated with respiratory disease, but not pneumonia (eFigure 4).
Results of stratified analyses for SO2 and O3 are presented in eTables 2 and 3. Heterogeneous associations are observed for SO2 and cardiovascular disease between different exposures to passive smoking (p = 0.04), and for O3 and respiratory disease between different smoking (p < 0.01).
Associations between SO2 and O3 and cardiovascular diseases are not significantly non-linear (p = 0.45 and p = 0.46, respectively). However, associations with respiratory diseases show significantly non-linearity (p = 0.002 and p < 0.001), with approximately linear associations above 30 µg/m3 SO2 and above 100 µg/m3 O3 as shown in eFigure 5.
Increased SO2 exposure is also associated with a higher risk of all-type and ischemic stroke when using monthly pollutant exposures (eFigure 6). However, the associations with all cardiovascular disease and other cardiovascular disease are not significant, as seen when using annual SO2 exposure. Monthly O3 is also associated with cardiovascular disease, all-type stroke, and ischemic stroke. For respiratory diseases, monthly SO2 exposure remains associated only with increased risk of pneumonia (eFigure 7).
Associations between annual and monthly pollutant exposures and infectious and parasitic disease incidence (467 events) are not significant (eTable 4).
Discussion
In this prospective cohort study in the urban region Suzhou in China we found some evidence for associations between long-term exposure to ambient SO2 and O3 pollution and increased risk of cardio-respiratory events. In particular, higher SO2 exposure is associated with increased risk of COPD and pneumonia incidence, and higher O3 with increased risk of ischaemic stroke.
Most existing studies focus on short-term SO2 exposure, and the associations of long-term exposure with cardio-respiratory health remain poorly understood [21]. Several previous cohort studies in China have reported positive associations between SO2 exposure and cardio-respiratory mortality, but effect sizes tended to be smaller than that observed in the present study [9,10,11]. This may be explained by relatively crude exposure assessment methods (e.g. fixed-site monitoring data assigned to residential zip codes) and lack of detailed adjustment for confounders in previous studies. We found some evidence of a threshold effect such that the harm of SO2 may exceed individuals’ ability to cope at certain levels, but this needs to be verified in other large cohort studies.
SO2 is often hypothesized as a proxy for other combustion-based pollutants [22] such as PM2.5, instead of having direct long-term health impact on major cardio-respiratory outcomes. We observed a lower correlation between PM2.5 and SO2 than seen in other studies, both in China [10] and elsewhere [23]. This could be due to our model of exposure assessment accounting for a greater degree of small-scale spatial and temporal variation, potentially reducing correlations between pollutants seen when using averages from fixed-site monitors [24]. It may also reflect increasingly stringent SO2 emission control (e.g., flue-gas desulphurisation) and the rapidly expanding car fleet in China in recent years, shifting relative PM contribution of older coal-fired power plants and industries to less sulphur-intense sources [25]. We found SO2 associated with respiratory disease after adjustment for other pollutants, suggesting some amount of an independent effect rather than SO2 being solely representative of exposure to other pollutants as previously hypothesised [22]. However, adjustment for some other pollutants did attenuate the association between SO2 exposure and cardiovascular disease.
To our knowledge, no study has examined the long-term cardio-respiratory effects of O3 exposure in China, and findings are mixed from the few studies performed in high-income countries. While some have found O3 exposure associated with cardio-respiratory mortality [26, 27] even after adjustment for PM2.5 [28, 29], others have found no clear association [30, 31]. We found modest associations between O3 exposure and cardiovascular disease (primarily ischemic stroke), including when adjusting for exposure to other pollutants. This provides new prospective study evidence, supporting previously observed associations in several large cohort studies [32]. The discrepancy between the present and some previous studies may be attributed to the significantly higher O3 exposure recorded in this study (mean 86.9 µg/m3 versus ~ 50–55 µg/m3) [30] and more extensive adjustment for individual-level confounders than in previous studies. Further studies to investigate the apparent non-linear dose–response relationship between O3 and respiratory disease is warranted.
The exact mechanisms linking O3 and SO2 with cardio-respiratory diseases remain to be confirmed. For example, experimental studies have shown O3 to be associated with numerous major pathways of cardiovascular disease development including oxidative stress, and inflammatory pathways [33, 34]. Similarly, experimental and toxicological studies have shown SO2 exposure capable of inducing oxidative damage and mitochondrial dysfunction in the heart and lungs of mice [35, 36] and associated with reduced cardiac vagal control in humans [37].
While this study found moderate inverse associations between long-term particulate matter exposure and cardio-respiratory disease, most previous studies observed significant positive associations, including the few existing cohort studies in China [9, 17, 38]. A large nationwide cohort study in China reported overall positive associations between PM2.5 and cardiovascular disease incidence and mortality, but found no clear association below 70 µg/m3 or in urban areas [17]. These results may reflect substantial regional variation in emission sources, and thus chemical composition, of particulate matter, with solid fuels and less efficient coal-fired power plants dominating rural AAP [39], which may be more harmful than particulate matter from mobile vehicles or industries [40], two major contributors of urban AAP. Declining PM levels may also have resulted in observable benefits on cardio-respiratory disease, as previously documented for mortality [41] and subclinical markers of inflammation [42] in China. It may also be due to residual confounding, especially by other pollutants, or the time delay between exposure and development of disease.
An important strength of this study is the inclusion of both non-fatal and fatal incident disease. A considerable proportion of the long-term burden relating to AAP exposure is likely captured by morbidity only, possibly more sensitive to AAP exposure than mortality [43, 44]. In addition, we used a spatio–temporal model to assign air pollution exposure instead of averages from fixed-site monitors, to attempt to account for small-scale variability in exposure and give a more robust exposure assessment [43]. Further, we included adjustment for a ranged of individual-level covariates and analysed all six criteria ambient air pollutants.
This study is limited by the short follow-up time coupled with latency in the development of numerous cardio-respiratory diseases. It is unlikely to capture all participants’ historic AAP exposure that may be associated with disease development. We attempted to alleviate this by examining disease endpoints representative of potentially shorter latency. For disease incidence not captured within the 3-year exposure period, extrapolation of AAP concentrations to additional years of follow-up was possible, as seen in other studies [16, 44]. However, given the substantial long-term trends seen in AAP, extrapolation would be unlikely to capture the exposure accurately [45]. We were unable to assign exposure based on residential addresses (though the CKB sampling strategy ensures that the vast majority of participants lived within 1 km of their assessment centre) and there is inherent measurement error in exposures predicted from a modelling strategy. Despite our adjustment for potential confounders at the individual level, residual confounding from factors such as socioeconomic status and health-seeking behaviour remain plausible.
Conclusion
The findings of this study provide new evidence that long-term exposure to O3 and SO2 is associated with increased risk of both cardiovascular and respiratory diseases. In contrast to the continuous declining trend of PM2.5 in China, anthropogenic O3 has been increasing rapidly in the past decade, prompting growing concerns of its potentially increasing proportional public health impact [46]. Our findings support more stringent regulatory policy to control other key criteria pollutants, in addition to PM2.5. Further cohort studies with greater geographic coverage, larger sample size, and longer follow-up will help to clarify the magnitude of associations between AAP exposures and disease.
Availability of data and materials
The China Kadoorie Biobank (CKB) is a global resource for the investigation of lifestyle, environmental, blood biochemical and genetic factors as determinants of common diseases. The CKB study group is committed to making the cohort data available to the scientific community in China, the UK and worldwide to advance knowledge about the causes, prevention and treatment of disease. For detailed information on what data is currently available to open access users and how to apply for it, visit: http://www.ckbiobank.org/site/Data+Access.
Researchers who are interested in obtaining the raw data from the China Kadoorie Biobank study that underlines this paper should contact ckbaccess@ndph.ox.ac.uk. A research proposal will be requested to ensure that any analysis is performed by bona fide researchers and—where data is not currently available to open access researchers—is restricted to the topic covered in this paper.
Abbreviations
- AAP:
-
Ambient air pollution
- CKB:
-
China Kadoorie Biobank
- INLA:
-
Integrated nested Laplace approximation
- SPDE:
-
Stochastic partial differential equation
- ICD-10:
-
International Classification of Diseases, 10th Revision
- IHD:
-
Ischemic heart disease
- COPD:
-
Chronic obstructive pulmonary disease
- TIA:
-
Transient ischemic attack
- HR:
-
Hazard ratio
- CI:
-
Confidence intervals
- SBP:
-
Systolic blood pressure
References
Health Effect Institute, State of global air 2020, in Special report. Boston: Health Effects Institute; 2020. ISSN 2578-6873.
Chan KH, Newell K, Lam KBH. The Effects of Air Pollution upon Public Health. In: Environmental Pollutant Exposures and Public Health. Royal Soc Chem; 2021. p. 115–50.
Organisation WH, Air quality guidelines. Global update 2005. Particulate matter, ozone, nitrogen dioxide and sulfur dioxide 2006, Editor.
China, M.o.E.a.E.f.t.P.s.R.o., Ambient air quality standard (GB 3095–2012). 2012.
Wong CM, et al. Public Health and Air Pollution in Asia (PAPA): a multicity study of short-term effects of air pollution on mortality. Environ Health Perspect. 2008;116(9):1195–202.
Tong L, Li K, Zhou Q. Promoted relationship of cardiovascular morbidity with air pollutants in a typical Chinese urban area. PLoS ONE. 2014;9(9): e108076.
Chen R, et al. Fine Particulate Air Pollution and Daily Mortality. A Nationwide Analysis in 272 Chinese Cities. Am J Respir Crit Care Med. 2017;196(1): 73–81.
Liu C, et al. Ambient carbon monoxide and cardiovascular mortality: a nationwide time-series analysis in 272 cities in China. Lancet Planet Health. 2018;2(1):e12–8.
Cao J, et al. Association between long-term exposure to outdoor air pollution and mortality in China: a cohort study. J Hazard Mater. 2011;186(2–3):1594–600.
Dong GH, et al. Long-term exposure to ambient air pollution and respiratory disease mortality in Shenyang, China: a 12-year population-based retrospective cohort study. Respiration. 2012;84(5):360–8.
Zhang P, et al. Long-term exposure to ambient air pollution and mortality due to cardiovascular disease and cerebrovascular disease in Shenyang, China. PLoS ONE. 2011;6(6): e20827.
Li G, et al. The impact of ambient particle pollution during extreme-temperature days in Guangzhou City. China Asia Pac J Public Health. 2014;26(6):614–21.
Chen X, et al. Long-term exposure to urban air pollution and lung cancer mortality: A 12-year cohort study in Northern China. Sci Total Environ. 2016;571:855–61.
Zhou M, et al. Particulate air pollution and mortality in a cohort of Chinese men. Environ Pollut. 2014;186:1–6.
Peng Z, et al. Long-term exposure to ambient air pollution and mortality in a Chinese tuberculosis cohort. Sci Total Environ. 2017;580:1483–8.
Yin P, et al. Long-term Fine Particulate Matter Exposure and Nonaccidental and Cause-specific Mortality in a Large National Cohort of Chinese Men. Environ Health Perspect. 2017;125(11): 117002.
Liang F, et al. Long-Term Exposure to Fine Particulate Matter and Cardiovascular Disease in China. J Am Coll Cardiol. 2020;75(7):707–17.
Chen Z, et al. China Kadoorie Biobank of 0.5 million people: survey methods, baseline characteristics and long-term follow-up. Int J Epidemiol. 2011;40(6):1652–66.
Wright N, et al. Estimating ambient air pollutant levels in Suzhou through the SPDE approach with R-INLA. Int J Hyg Environ Health. 2021;235: 113766.
Rue H, Martino S, Chopin N. Approximate Bayesian inference for latent Gaussian models by using integrated nested Laplace approximations. Journal of the royal statistical society: Series b (statistical methodology). 2009;71(2):319–92.
Newell K, et al. Cardiorespiratory health effects of gaseous ambient air pollution exposure in low and middle income countries: a systematic review and meta-analysis. Environ Health. 2018;17(1):41.
COMEAP, Cardiovascular Disease and Air Pollution A report by the Committee on the Medical Effects of Air Pollutants. 2006.
Beelen R, et al. Effects of long-term exposure to air pollution on natural-cause mortality: an analysis of 22 European cohorts within the multicentre ESCAPE project. The Lancet. 2014;383(9919):785–95.
Abbey DE, et al. Long-term inhalable particles and other air pollutants related to mortality in nonsmokers. Am J Respir Crit Care Med. 1999;159(2):373–82.
Li C, et al. India Is Overtaking China as the World’s Largest Emitter of Anthropogenic Sulfur Dioxide. Sci Rep. 2017;7(1):14304.
Zanobetti A, Schwartz J. Ozone and survival in four cohorts with potentially predisposing diseases. Am J Respir Crit Care Med. 2011;184(7):836–41.
Krewski D, et al. Extended follow-up and spatial analysis of the American Cancer Society study linking particulate air pollution and mortality. Res Rep Health Eff Inst. 2009;(140):5–114. discussion 115–36.
Turner MC, et al. Long-Term Ozone Exposure and Mortality in a Large Prospective Study. Am J Respir Crit Care Med. 2016;193(10):1134–42.
Jerrett M, et al. Spatial analysis of air pollution and mortality in California. Am J Respir Crit Care Med. 2013;188(5):593–9.
Atkinson RW, et al. Long-term exposure to outdoor air pollution and incidence of cardiovascular diseases. Epidemiology. 2013;24(1):44–53.
Raaschou-Nielsen O, et al. Long-term exposure to air pollution and mortality in the Danish population a nationwide study. EClinicalMedicine. 2020;28.
Lim CC, et al. Long-Term Exposure to Ozone and Cause-Specific Mortality Risk in the United States. Am J Respir Crit Care Med. 2019;200(8):1022–31.
Mirowsky JE, et al. Ozone exposure is associated with acute changes in inflammation, fibrinolysis, and endothelial cell function in coronary artery disease patients. Environ Health. 2017;16(1):126.
Devlin RB, et al. Controlled exposure of healthy young volunteers to ozone causes cardiovascular effects. Circulation. 2012;126(1):104–11.
Qin G, et al. Sulfur Dioxide Contributes to the Cardiac and Mitochondrial Dysfunction in Rats. Toxicol Sci. 2016;151(2):334–46.
Meng Z, et al. Oxidative damage of sulfur dioxide inhalation on lungs and hearts of mice. Environ Res. 2003;93(3):285–92.
Tunnicliffe WS, et al. The effect of sulphur dioxide exposure on indices of heart rate variability in normal and asthmatic adults. Eur Respir J. 2001;17(4):604–8.
Huang K, et al. Long term exposure to ambient fine particulate matter and incidence of stroke: prospective cohort study from the China-PAR project. BMJ. 2019;367: l6720.
Zhou Y, et al. A comprehensive biomass burning emission inventory with high spatial and temporal resolution in China. Atmos Chem Phys. 2017;17(4):2839–64.
Yu K, et al. Association of solid fuel use with risk of cardiovascular and all-cause mortality in rural China. JAMA. 2018;319(13):1351–61.
Su C, et al. Assessing responses of cardiovascular mortality to particulate matter air pollution for pre-, during- and post-2008 Olympics periods. Environ Res. 2015;142:112–22.
Rich DQ, et al. Association Between Changes in Air Pollution Levels During the Beijing Olympics and Biomarkers of Inflammation and Thrombosis in Healthy Young Adults. JAMA. 2012;307(19):2068–78.
Zhang LW, et al. Long-term exposure to high particulate matter pollution and cardiovascular mortality: a 12-year cohort study in four cities in northern China. Environ Int. 2014;62:41–7.
Pope CA 3rd, et al. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. JAMA. 2002;287(9):1132–41.
Liu Y, Shaddick G, Zidek JV. Incorporating high-dimensional exposure modelling into studies of air pollution and health. Stat Biosci. 2017;9(2):559–81.
Li K, et al. Increases in surface ozone pollution in China from 2013 to 2019: anthropogenic and meteorological influences. Atmos Chem Phys. 2020;20(19):11423–33.
Acknowledgements
The chief acknowledgment is to the participants, the project staff, and the China National Centre for Disease Control and Prevention (CDC) and its regional offices for assisting with the fieldwork. We thank Judith Mackay in Hong Kong; Yu Wang, Gonghuan Yang, Zhengfu Qiang, Lin Feng, Maigeng Zhou, Wenhua Zhao, Yan Zhang and Zheng Bian in China CDC; Lingzhi Kong, Xiucheng Yu, and Kun Li in the Chinese Ministry of Health; and Garry Lancaster, Sarah Clark, Martin Radley, Mike Hill, Hongchao Pan, and Jill Boreham in the CTSU, Oxford, for assisting with the design, planning, organization, and conduct of the study.
Professor Haidong Kan, School of Public Health Fudan University, Shanghai, for providing the fixed site monitoring data.
Dr Steve Hung Lam Yim, Chinese University of Hong Kong, for providing all meteorological variables.
Members of the China Kadoorie Biobank collaborative group:
International Steering Committee: Junshi Chen, Zhengming Chen (PI), Robert Clarke, Rory Collins, Yu Guo, Liming Li (PI), Chen Wang, Jun Lv, Richard Peto, Robin Walters.
International Co-ordinating Centre, Oxford: Daniel Avery, Maxim Barnard, Derrick Bennett, Ruth Boxall, Sushila Burgess, Ka Hung Chan, Yiping Chen, Zhengming Chen, Johnathan Clarke; Robert Clarke, Huaidong Du, Ahmed Edris Mohamed, Hannah Fry, Simon Gilbert, Pek Kei Im, Andri Iona, Maria Kakkoura, Christiana Kartsonaki, Hubert Lam, Kuang Lin, James Liu, Mohsen Mazidi, Iona Millwood, Sam Morris, Qunhua Nie, Alfred Pozarickij, Paul Ryder, Saredo Said, Dan Schmidt, Becky Stevens, Iain Turnbull, Robin Walters, Baihan Wang, Lin Wang, Neil Wright, Ling Yang, Xiaoming Yang, Pang Yao.
National Co-ordinating Centre, Beijing: Xiao Han, Can Hou, Chun Li, Chao Liu, Jun Lv, Pei Pei, Dianjianyi Sun, Canqing Yu.
10 Regional Co-ordinating Centres:
Guangxi Provincial CDC: Naying Chen, Duo Liu, Zhenzhu Tang. Liuzhou CDC: Ningyu Chen, Qilian Jiang, Jian Lan, Mingqiang Li, Yun Liu, Fanwen Meng, Jinhuai Meng, Rong Pan, Yulu Qin, Ping Wang, Sisi Wang, Liuping Wei, Liyuan Zhou. Gansu Provincial CDC: Caixia Dong, Pengfei Ge, Xiaolan Ren. Maiji CDC: Zhongxiao Li, Enke Mao, Tao Wang, Hui Zhang, Xi Zhang. Hainan Provincial CDC: Jinyan Chen, Ximin Hu, Xiaohuan Wang. Meilan CDC: Zhendong Guo, Huimei Li, Yilei Li, Min Weng, Shukuan Wu. Heilongjiang Provincial CDC: Shichun Yan, Mingyuan Zou, Xue Zhou. Nangang CDC: Ziyan Guo, Quan Kang, Yanjie Li, Bo Yu, Qinai Xu. Henan Provincial CDC: Liang Chang, Lei Fan, Shixian Feng, Ding Zhang, Gang Zhou. Huixian CDC: Yulian Gao, Tianyou He, Pan He, Chen Hu, Huarong Sun, Xukui Zhang. Hunan Provincial CDC: Biyun Chen, Zhongxi Fu, Yuelong Huang, Huilin Liu, Qiaohua Xu, Li Yin. Liuyang CDC: Huajun Long, Xin Xu, Hao Zhang, Libo Zhang. Jiangsu Provincial CDC: Jian Su, Ran Tao, Ming Wu, Jie Yang, Jinyi Zhou, Yonglin Zhou. Suzhou CDC: Yihe Hu, Yujie Hua, Jianrong Jin Fang Liu, Jingchao Liu, Yan Lu, Liangcai Ma, Aiyu Tang, Jun Zhang. Qingdao Qingdao CDC: Liang Cheng, Ranran Du, Ruqin Gao, Feifei Li, Shanpeng Li, Yongmei Liu, Feng Ning, Zengchang Pang, Xiaohui Sun, Xiaocao Tian, Shaojie Wang, Yaoming Zhai, Hua Zhang, Licang CDC: Wei Hou, Silu Lv, Junzheng Wang. Sichuan Provincial CDC: Xiaofang Chen, Xianping Wu, Ningmei Zhang, Weiwei Zhou. Pengzhou CDC: Xiaofang Chen, Jianguo Li, Jiaqiu Liu, Guojin Luo, Qiang Sun, Xunfu Zhong. Zhejiang Provincial CDC: Weiwei Gong, Ruying Hu, Hao Wang,Meng Wan, Min Yu. Tongxiang CDC: Lingli Chen, Qijun Gu, Dongxia Pan, Chunmei Wang, Kaixu Xie, Xiaoyi Zhang.
Funding
The CKB baseline survey and the first re-survey were supported by the Kadoorie Charitable Foundation in Hong Kong. The long-term follow-up has been supported by Wellcome grants to Oxford University (212946/Z/18/Z, 202922/Z/16/Z, 104085/Z/14/Z, 088158/Z/09/Z) and grants from the National Natural Science Foundation of China (82192901, 82192904, 82192900, 91846303) and the National Key Research and Development Program of China (2016YFC0900500).
The UK Medical Research Council (MC_UU_00017/1, MC_UU_12026/2, MC_U137686851), Cancer Research UK (C16077/A29186, C500/A16896) and the British Heart Foundation (CH/1996001/9454), provide core funding to the Clinical Trial Service Unit and Epidemiological Studies Unit at Oxford University for the project.
Ka Hung Chan acknowledges the support from the BHF Centre of Research Excellence, University of Oxford (RE/18/3/34214).
Katherine Newell acknowledges the support from the Oxford-MRC Doctoral Training Partnership.
UK Medical Research Council Global Challenges Research Fund (Foundation Award MR/P025080/1).
This research was funded in whole, or in part, by the Wellcome Trust (212,946/Z/18/Z, 202,922/Z/16/Z, 104,085/Z/14/Z, 088,158/Z/09/Z). For the purpose of open access, the author has applied a CC BY public copyright licence to any Author Accepted Manuscript version arising from this submission.
Author information
Authors and Affiliations
Contributions
NW and KN analysed the data. CK, KBHL, and OK conceived and designed the present study and supervised the analyses. KN and NW drafted the initial manuscript. ZC, JC, and LL conceived and designed the China Kadoorie Biobank. YG, PP, CY, JL were involved in the acquisition of data. SG and AH prepared and managed the data. All authors reviewed and revised the manuscript. The author(s) read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The Chinese Center for Disease Control and Prevention and the University of Oxford gave ethics clearance for the CKB study. Written informed consent was obtained from all participants.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wright, N., Newell, K., Chan, K.H. et al. Long-term ambient air pollution exposure and cardio-respiratory disease in China: findings from a prospective cohort study. Environ Health 22, 30 (2023). https://doi.org/10.1186/s12940-023-00978-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12940-023-00978-9