Abstract
Background
Prior epidemiological and animal work has linked in utero exposure to ambient air pollutants (AAP) with accelerated postnatal weight gain, which is predictive of increased cardiometabolic risk factors in childhood and adolescence. However, few studies have assessed changes in infant body composition or multiple pollutant exposures. Therefore, the objective of this study was to examine relationships between prenatal residential AAP exposure with infant growth and adiposity.
Methods
Residential exposure to AAP (particulate matter < 2.5 and 10 microns in aerodynamic diameter [PM2.5, PM10]; nitrogen dioxide [NO2]; ozone [O3]; oxidative capacity [Oxwt: redox-weighted oxidative potential of O3 and NO2]) was modeled by spatial interpolation of monitoring stations via an inverse distance-squared weighting (IDW2) algorithm for 123 participants from the longitudinal Mother’s Milk Study, an ongoing cohort of Hispanic mother-infant dyads from Southern California. Outcomes included changes in infant growth (weight, length), total subcutaneous fat (TSF; calculated via infant skinfold thickness measures) and fat distribution (umbilical circumference, central to total subcutaneous fat [CTSF]) and were calculated by subtracting 1-month measures from 6-month measures. Multivariable linear regression was performed to examine relationships between prenatal AAP exposure and infant outcomes. Models adjusted for maternal age, pre-pregnancy body mass index, socioeconomic status, infant age, sex, and breastfeeding frequency. Sex interactions were tested, and effects are reported for each standard deviation increase in exposure.
Results
NO2 was associated with greater infant weight gain (β = 0.14, p = 0.02) and TSF (β = 1.69, p = 0.02). PM10 and PM2.5 were associated with change in umbilical circumference (β = 0.73, p = 0.003) and TSF (β = 1.53, p = 0.04), respectively. Associations of Oxwt (pinteractions < 0.10) with infant length change, umbilical circumference, and CTSF were modified by infant sex. Oxwt was associated with attenuated infant length change among males (β = -0.60, p = 0.01), but not females (β = 0.16, p = 0.49); umbilical circumference among females (β = 0.92, p = 0.009), but not males (β = -0.00, p = 0.99); and CTSF among males (β = 0.01, p = 0.03), but not females (β = 0.00, p = 0.51).
Conclusion
Prenatal AAP exposure was associated with increased weight gain and anthropometric measures from 1-to-6 months of life among Hispanic infants. Sex-specific associations suggest differential consequences of in utero oxidative stress. These results indicate that prenatal AAP exposure may alter infant growth, which has potential to increase childhood obesity risk.
Similar content being viewed by others
Background
In the United States, obesity disproportionately impacts Hispanic youth as nearly 26% of those aged 2–19 have obesity, in contrast to non-Hispanic white (14%) and non-Hispanic Asian (11%) youth [1]. Importantly, obesity in childhood increases the risk for adverse cardiometabolic outcomes in adulthood and premature mortality [2, 3]. Accelerated weight trajectory during the first six months of life has been linked to obesity at three years of age [4] and greater than expected weight gain during any period through four years of age has been found to be associated with increased body mass index (BMI) in adolescence [5]. Recent work supports the hypothesis that developmental programming during the pre-, peri-, and early postnatal periods is sensitive to certain exposures (e.g., maternal nutrition/obesity) that appear to play a critical role in future obesity risk [6]. Exposure to air pollutants during these periods is also suggested as a risk factor for low birth weight, accelerated growth in early life, and/or childhood obesity [7,8,9,10,11]. This may partially explain the aforementioned disparities in childhood obesity, as Hispanics are exposed to higher levels of ambient (AAP) and traffic-related air pollutants [12, 13].
To date, studies examining the link between exposure to air pollutants and weight status have primarily focused on prenatal exposures and birth outcomes (e.g., low/high birth weight, intrauterine growth restriction [IUGR]) [14] or early life/childhood exposures and outcomes in childhood/adolescence [15, 16]. Few studies have examined the influence of prenatal exposure to air pollutants on early infant growth patterns. Greater prenatal exposure to traffic-related air pollution has been shown to result in more rapid infant weight-for-length gain over the first six months of life, particularly among those in the lowest quartile of fetal growth [9] and increased ozone exposure during the 3rd trimester was associated with greater rate of fat mass and adiposity accrual at 5 months old [17]. In other birth cohorts, prenatal exposure to PM10 (particulate matter < 10 microns in aerodynamic diameter) was inversely associated with weight-for-age z-score at six months of age [10] while prenatal NO2 (nitrogen dioxide) exposure during the 1st trimester was inversely associated both with infant length at 6 months and infant weight at 12 months, which were partially mediated by birth length and birth weight, respectively [8]. These associations between prenatal exposures and postnatal growth patterns may be metabolically programmed via epigenetic changes induced by elevated inflammation and/or oxidative/nitrosative stress [18].
Another important consideration with respect to in utero exposures and both fetal and infant growth patterns is the existence of sex-specific effects [19,20,21]. For instance, greater prenatal PM2.5 (particulate matter < 2.5 microns in aerodynamic diameter) exposure has been linked to lower weight at 2 years of age and beyond in males and an increased weight trajectory in females across the first 6 years of life [11] and greater waist-to-hip ratio among girls and increased fat mass and BMI z-score among boys at 4 years [22]. Two studies examining 1st trimester PM2.5 exposure found decreased adiposity among 5-month-old girls [17] and attenuated weight z-scores among pre-school males [23], respectively. Prenatal exposure models in rodents suggest that the potential mechanisms underlying these sexually dimorphic effects may involve differential microglial priming [24], methylation of the promoter region of the leptin gene [25], or altered expression of NPY [26].
As previous studies have been restricted to one pollutant and/or cross-sectional measures of infant growth, there is a need to examine multiple pollutants as well as changes in infant growth parameters over time in order to enable greater insight into the physiological response mechanisms to exposures. In addition, beyond weight and BMI trajectories, there is also a need to examine effects on infant body composition and relative adipose distribution, which are predictive of abdominal adiposity in childhood [27]. Therefore, in the present study, we examined the associations between prenatal residential exposure to ambient air pollutants – PM2.5, PM10, NO2, 24-h ozone (O3), and oxidative capacity (Oxwt: a redox-weighted proxy for oxidative potential utilizing NO2 and O3) – and changes in infant measures of growth and adiposity from 1 to 6 months of life, among Hispanic mother-infant dyads from the Southern California Mother’s Milk Study. We hypothesized that residential exposure to ambient air pollutants during the pregnancy period would be associated with greater change in measures of infant growth and adiposity.
Methods
Study population
The longitudinal Mother’s Milk Study is an ongoing cohort of Hispanic mother-infant dyads from Southern California examining growth and development in early life. Recruitment of study participants began in 2016 at Los Angeles County maternity clinics affiliated with the University of Southern California. Maternal inclusion criteria include ≥ 18 years of age at delivery; healthy, term, singleton birth; enrollment by one month postpartum; self-identified Hispanic ethnicity; intention to breastfeed for at least three months postpartum; and ability to read at the 5th grade level in either English or Spanish in order to understand study procedures [28]. Maternal exclusion criteria include medical conditions and/or medications known to potentially affect physical/mental health, nutritional status, or metabolism; tobacco use (defined as > 1 cigarette in the past week); recreational drug use; pre-term/low birth weight; and clinically diagnosed fetal abnormalities. Mothers were recruited to represent an even split of pre-pregnancy BMI class (normal weight [BMI: 18–24.9 kg/m2]; overweight [BMI: 25–29.9 kg/m2]; obese [BMI: > 30 kg/m2]). The Institutional Review Boards of the University of Southern California, Children’s Hospital of Los Angeles, and the University of Colorado Boulder approved study procedures. Written informed consent was obtained from participants prior to enrollment and all analyses in this study.
Study design
As of July 2020, 212 mother-infant dyads had been enrolled in the study. Maternal medical and family histories were documented at 1 month and infant measures of growth and adiposity were performed at both time points. Information pertinent to relevant covariates were collected, including maternal age, socioeconomic status, infant sex, pre-pregnancy BMI, breastfeeding frequency, and infant age in days. Pre-pregnancy BMI (kg/m2) was calculated via weight and height recalled prior to pregnancy. Breastfeeding frequency was ascertained from questionnaires at both time points and was dichotomized to ≥ 7 or < 7 feedings per day at the 6-month timepoint to capture differences over the first 6 months of life since the data was semi-quantitative and this provided a relatively balanced contrast in frequency in this cohort. The Hollingshead Index was utilized to capture socioeconomic status in a standardized fashion [29]. Briefly, this index incorporates four weighted scores consisting of both maternal and paternal educational attainment and occupational prestige, and accounts for marital status and parental living arrangements. Lower scores are indicative of lower SES, while higher scores are indicative of higher SES. Since students, stay-at-home parents, and unemployed persons have no assigned employment categories in the Hollingshead Index, modifications were made to assign these individuals a score of zero (given that these participants were likely to have very little or no income) so as to retain them in the analyses [30]. Alternative adjustment for a poverty index variable (a component of the CalEnviroScreen 3.0 score), representing area-level SES, did not yield appreciable differences in our models (Supplemental Table 3). For the current study, 123 of the 212 mother-infant dyads were included in final our analysis (Supplemental Figure 1). On average, the 123 mothers and infants included did not significantly differ on any important baseline characteristics from those excluded from the current analysis (Supplemental Table 1). Reasons for exclusion consisted of: 1-month clinical visit not completed (n = 3), 6-month clinical visit not yet completed (n = 20), missing air pollution exposure data (n = 24), missing skinfold measures at 6-month visit (n = 1), did not reside at the same address for at least 8 months of the pregnancy period (n = 26), or missing pregnancy address information (n = 4). Additionally, 9 were deemed outliers based on change in infant age (time between study visits) (n = 1), pollutant exposure levels (n = 3), or infant growth change variables (n = 5) as determined by > 3 standard deviations from the mean. Finally, two influential points were removed upon examination of Cook’s distance and residual diagnostics used to meet the assumptions of linear regression analysis.
Outcome assessment
All outcome measurements were performed by trained clinical research staff. At each visit, infant length was measured in duplicate to the nearest 0.1 centimeter (cm) using an infantometer; infant weight was twice calculated to the nearest 0.1 kilogram (kg) by subtraction of the weight of the mother holding the infant from the weight of the mother alone on a Tanita scale; and infant umbilical circumference was measured to the nearest 0.1 cm in duplicate using a tape measure resistant to stretching. In addition, infant skinfold thickness was measured at both study visits using a commercial caliper at the tricep, subscapular, suprailiac, and midthigh regions. Consistency of the caliper calibration was confirmed with a manufacturer-supplied metal step phantom immediately before its use on each infant. In these measurements, the operator’s thumb and index finger were used to elevate a double fold of skin and subcutaneous tissue in the natural cleavage lines of the skin approximately 1 cm from the site at which the skinfold was measured. For the tricep and midthigh skinfold measures, the corresponding limb was gently extended while the infant was laying down on one side and in the supine position, respectively. The subscapular skinfold was measured with the infant laying on one side at the lower angle of the scapula, in the axis of the skin crease. The suprailiac skinfold was taken immediately above the iliac crest, along the axis of anterior axillary line, with the infant in the supine position. Dynamic skinfold thickness was examined to adjust for tissue waste content by recording each skinfold measurement at exactly 15 s and 60 s with a stopwatch. The sums of the four skinfold measures were calculated to create a proxy for total subcutaneous fat (TSF) at each time point. Further, the sum of the suprailiac and subscapular skinfold thicknesses (representing truncal fat) were calculated and divided by the sum of all four regions to generate a proxy for the ratio of central-to-total subcutaneous fat (CTSF) [27]. For sensitivity analyses, weight-for-age z scores (WAZ) and weight-for-length z scores (WLZ) were also calculated at 1 and 6 months postpartum according to World Health Organization (WHO) standards [31]. Outcome variables representing early infant growth and adiposity were constructed by subtracting 1-month measures from 6-month measures.
Exposure Assessment
Residential exposure to ambient air pollutants (PM2.5, PM10, NO2, and O3) during the pregnancy period was modeled for all 123 mother-infant pairs. PM2.5 and PM10 were measured in micrograms per cubic meter (μg/m3) and NO2 and O3 were assessed as parts per billion (ppb). Oxwt was calculated from NO2 and O3 according to their respective weighted redox potentials [32], as such: [(1.07*NO2) + (2.075*O3)]/3.145.
Analyses were restricted to participants who had resided at the same address for at least eight months of the pregnancy period to minimize potential for exposure misclassification. Residential address histories were obtained via questionnaire during the baseline study visit and geocoded at the street level utilizing the Texas A&M Geocoder (http://geoservices.tamu.edu/Services/Geocode/). Monthly averages of ambient pollutant exposures were estimated from the U.S. Environmental Protection Agency’s Air Quality System (AQS, http://www.epa.gov/ttn/airs/airsaqs), which records hourly air quality data from ambient monitoring stations. Spatial interpolation of up to four monitoring stations within 50 km of participants’ homes was performed via an inverse distance-squared weighting (IDW2) algorithm. This method has been demonstrated to be robust to leave-one-out validation for the same data source in California [33], with R2 values of 0.76, 0.73, 0.53, and 0.46 for O3, NO2, PM2.5, and PM10, respectively. Mother-infant pairs were evenly disbursed across Southern California with participants largely clustered in urban centers of Los Angeles, including south-central Los Angeles. Prenatal exposure was modeled based upon the cumulative nine-month average prior to birth. Monthly average exposure estimates for each pollutant were available dating back 12 months from the baseline (1 month postpartum) study visit. Thus, the exposure window spanning from 10 months to 1 month prior to the baseline visit was utilized to capture in utero exposure.
Statistical analyses
Descriptive statistics are displayed as mean ± standard deviation (SD) for continuous variables and as frequency (%) for categorical variables. Correlations among pollutants were examined using Spearman’s rank tests. Multivariable linear regression analysis was performed to examine the relationship between exposure to residential ambient air pollutants during the pregnancy period and early life changes in infant growth and adiposity. Linear models were adjusted for infant sex, change in infant age (time between visits), pre-pregnancy BMI, breastfeeding frequency at 6 months (dichotomized to ≥ 7 or < 7 times per day), maternal age, and socioeconomic status based on the Hollingshead Index. Two participants were missing information needed for calculating a Hollingshead Index score and were assigned the median value. Levels of ambient air pollutants and infant growth change measures did not differ across tertiles of the Hollingshead Index (Supplemental Table 2). These covariates were chosen based on known associations with infant growth parameters and/or AAP exposure levels [34]. Based upon previous literature [11], infant sex was also examined as a potential effect modifier of the relationship between ambient air pollution and early infant growth. Sensitivity analyses were also performed, which additionally adjusted for birth weight and the baseline variable of each change outcome. However, these results should be interpreted with caution as birth weight may be on the causal pathway [8, 35] and inclusion of baseline values may be an over-adjustment [36]. Also, we did not have detailed information on gestational age (i.e., last menstrual period or physician’s assessment), which adds important context to birth weight [37]. Effect estimates in our single-pollutant models were scaled to one SD of exposure (PM2.5 [SD = 1.16 μg/m3], PM10 [SD = 3.58 μg/m3], NO2 [SD = 2.39 ppb], O3 [SD = 2.48 ppb], Oxwt [SD = 1.08]). Due to the high correlation among pollutants (Table 2), multipollutant models were not examined. Generalized additive models (GAM) with penalized spline smooth terms were fitted and we visually assessed plotted splines to determine linearity of exposure-outcome associations. Overall, we found some evidence for non-linear associations between prenatal PM10 exposure and change in umbilical circumference (pGAM = 0.02) as well as PM2.5 and change in total subcutaneous fat (pGAM = 0.04). For these models, we fitted cubic splines to investigate the non-linear associations and subsequently created tertiles to examine the non-linear dose-response relationships. All analyses were conducted with RStudio (Version 1.2.1335; RStudio, Inc.) and figures were made in Prism (Version 8.41; GraphPad Software, Inc.). Statistical significance was defined as a two-sided p-value less than 0.05. For interaction terms, statistical significance was defined as a two-sided p-value less than 0.1 by virtue of the quantified gain in power given our sample size and variables of interest [38]. Effect estimates are reported with 95% confidence intervals (CIs).
Results
Maternal and infant characteristics at 1 and 6 months are shown in Table 1. On average, mothers were 29.2 years old at baseline, and prior to pregnancy, 31 (25.2%) mothers were classified as normal weight, 48 (39.0%) as overweight, and 44 (35.8%) as obese. The proportion of women breastfeeding decreased from 99.2% at baseline to 76.4% at six months (p < 0.001). Approximately 42.3% of infants were male and the average birth weight was 3.4 kg (range: 2.3 to 4.5 kg). As expected, all infant growth parameters increased from 1 to 6 months of age (pall < 0.01). During the pregnancy period, mean residential exposure to PM2.5 and PM10 ranged between 10.6 and 14.6 μg/m3 and 25.3 and 38.3 μg/m3, respectively. For NO2, O3, and Oxwt, exposure levels ranged between 12.6 and 22.9 ppb, 22.1 and 31.2 ppb, and 21.3 and 25.8, respectively. Further, exposures to ambient air pollutants were significantly correlated during the pregnancy period (Table 2).
PM2.5 and PM10 were statistically significantly associated with increased measures of infant body composition (Table 3). For example, PM2.5 was associated with a larger increase in TSF (β = 1.53; CI: 0.11, 2.94; p = 0.04); however, there was some evidence for non-linearity (pGAM = 0.04). Therefore, we examined this association across tertiles of exposure and observed a positive association in the lowest tertile of exposure (β = 15.2; p = 0.03) and smaller inverse associations in the second (β = -2.2; p = 0.84) and third tertiles (β = -1.6; p = 0.43), which did not reach statistical significance. PM10 exposure was positively associated with change in TSF (β = 1.40; CI: -0.03, 2.82; p = 0.05). Additionally, PM10 (β = 0.73; CI: 0.25, 1.21; p = 0.003) was positively associated with greater changes in umbilical circumference from 1 to 6 months of age, which also showed evidence for non-linearity (pGAM = 0.02). Across tertiles of PM10 exposure, this association was positive in the lowest tertile of exposure (β = 2.37; p = 0.004) and modestly inversely associated in the second (B = -0.26; p = 0.37) and third tertiles (β = -0.10; p = 0.73) but these associations did not reach statistical significance. Overall, results were largely consistent with additional adjustment for infant birth weight or baseline measures of each outcome (Supplemental Figure 2). Lastly, the associations between PM exposure and measures of infant growth and body composition were not modified by infant sex (Pinteractions > 0.10) (Table 4).
Higher exposure to NO2 during the pregnancy period was associated with a greater increase in infant weight (β = 0.14; CI: 0.02, 0.25; p = 0.02) and TSF (β = 1.69; CI: 0.25, 3.12; p = 0.02) from 1 to 6 months of life. Oxwt was associated with change in umbilical circumference (β = 0.59; CI: 0.09, 1.09; p = 0.02) and an increased CTSF (β = 0.01; CI: 0.00, 0.02; p = 0.04) from 1 to 6 months of age, and infant sex was found to modify the effect of these relationships (pinteractions < 0.1) (Table 4). Specifically, Oxwt was associated with change in umbilical circumference among females (β = 0.92; CI: 0.24, 1.61; p = 0.009), but not males (β = -0.00; CI: -0.83, 0.82; p = 0.99), and change in CTSF among males (β = 0.01; CI: 0.00, 0.03; p = 0.03), but not females (β = 0.00; CI: -0.01, 0.01; p = 0.51). Oxwt and O3 were not associated with change in infant length (β = -0.13; CI: -0.46, 0.20; p = 0.45 and β = -0.20; CI: -0.53, 0.13; p = 0.23, respectively), but infant sex was found to modify the effect of these relationships (Pinteractions = 0.06). Specifically, Oxwt and O3 were each inversely associated with infant length change (β = -0.60; CI: -1.07, -0.12; p = 0.01 and β = -0.60; CI: -1.07, -0.13; p = 0.01, respectively) among males, but not females (β = 0.16; CI: -0.31, 0.63; p = 0.49 and β = 0.04; CI: -0.43, 0.51; p = 0.87, respectively). These associations were largely robust to further adjustments for birth weight and baseline values, respectively (Supplemental Figure 2). Lastly, we conducted a sensitivity analysis by examining changes in infant z-scores from 1 to 6 months and similarly found that prenatal AAP was associated with greater increases in some standardized measures of infant body composition (Supplemental Table 4).
Discussion
In this study of Hispanic mother-infant dyads from the Mother’s Milk Study cohort, prenatal ambient air pollution exposure was associated with increased postnatal growth and adiposity among infants from 1 to 6 months of life. Higher in utero exposure to NO2 was associated with greater increases in infant weight and total subcutaneous fat, while PM10 and PM2.5 were associated with larger increases in umbilical circumference and total subcutaneous fat, respectively. Upon examination of non-linearity, positive associations of particulate matter with infant body composition appeared to plateau beyond the first tertile of exposure. Consistent with previous studies [11, 22], we observed sex-specific findings. Oxwt was associated with change in umbilical circumference and a modestly larger increase in CTSF, with these associations observed only among females and males, respectively, following stratification by sex. Likewise, Oxwt and O3 exposure were inversely associated with change in infant length only among males. Overall, greater AAP exposure was associated with increased adiposity (i.e., TSF, umbilical circumference), but there was only one modest main effect for increased CTSF, which suggests that AAP may influence total adiposity while not favoring central adiposity to other fat depots at this life stage.
The potential molecular mechanisms underlying the associations between prenatal exposure to air pollutants [39] and altered postnatal phenotypes may involve formation of DNA adducts [40], mitochondrial oxidative stress [41, 42], telomere shortening [43, 44], polymorphisms of detoxification enzymes [45, 46], global hypomethylation [47], placental microRNA expression [48], and/or aryl hydrocarbon hydroxylase activity [49]. Upon exposure to particulate matter, several of these mechanisms may converge, directly and indirectly, to induce oxidative/nitrosative stress [50, 51] and affect growth patterns through epigenetic changes [18] and increased inflammation [52]. For example, greater exposure to PM2.5 during the second trimester of pregnancy has been shown to be associated with reduced methylation of the placental leptin promoter [53] and PM10 exposure during the first and second trimesters has been associated with methylation of genes regulating fetal growth and glucocorticoid metabolism [54]. Furthermore, exposure to air pollutants could influence early infant growth via alterations to the developing gut microbiome [55, 56]. It remains to be determined if air pollutants can physically traverse the placenta in humans, but recent work has identified black carbon particles at the fetal side of the human placenta [57] and a rabbit model found aggregates of nanoparticles in trophoblastic cells and fetal blood upon maternal exposure to diesel exhaust [58].
Findings from the INMA (Infancia y Medio Ambiente [Environment and Childhood]) cohort included an inverse association (trend) between prenatal NO2 exposure and length z-score at 6 months of age, particularly during the first trimester [8]. Although we did not find an association with NO2 and change in length, we observed attenuated growth in length from 1 to 6 months of age among males exposed to greater prenatal Oxwt, which incorporates both NO2 and O3. Oxwt acts as a proxy for the potential to induce oxidative stress. Indeed, previous work in newborn twins has shown that males have higher levels of 15-F2t-isoprostane, a marker for oxidative stress, in both like-sex and unlike sex pairs [59], indicating males may be particularly susceptible to in utero oxidative stress. This finding also coheres with the INMA study which found that placental mitochondrial DNA content, which is notably susceptible to oxidative stress, partially mediated the inverse relationship between prenatal NO2 exposure and length at 6 months [8]. Of relevance, restricted height attainment appears to account for the association of greater childhood BMI in those exposed in utero to maternal smoking [60].
Many, but not all [14], prenatal exposure studies have observed intrauterine growth restriction or low birth weight in response to higher exposure to air pollutants, and this effect has been hypothesized to be due to compromised transfer of nutrients from the mother to the developing fetus [61]. This is in line with the thrifty phenotype hypothesis, which postulates that cues from the environment during development can potentiate fetal reprogramming and alter growth trajectories, including “catch-up/rapid growth”, and increase future risk for metabolic diseases [62,63,64]. Findings from the Project Viva cohort supported the catch-up growth hypothesis, wherein those with the highest exposure to traffic density and black carbon had higher odds for the phenotype of lower fetal growth (birth weight) and more rapid 0 to 6 month weight-for-length gain [9]. In the current study, we did not observe an association between prenatal exposure and birth weight; however, we are limited to drawing any firm conclusions due to incomplete information on gestational age (i.e., we did not have date of last menstrual period or physician’s assessment of gestational age), which adds important context to birth weight and risk for future disease [37]. We did, however, find a similar pattern of increased rate of growth and adiposity measures from 1 to 6 months of life among those exposed to higher levels of NO2 and PM. Longitudinal examination of weight trajectory in response to prenatal PM2.5 in the Children’s HealthWatch cohort found consistently higher weight across the first 6 years of life among females with above median exposure [11]. This is in line with our finding of prenatal particulate matter exposure and increased rate of change in umbilical circumference and total subcutaneous fat, both measures of adiposity, and similarly, greater change in umbilical circumference among females in response to Oxwt exposure. Our findings specific to prenatal PM10 exposure were similar to those with PM2.5 and each showed evidence of non-linear dose–response effects, which could be attributable to a saturation point [65, 66] beyond the first tertile of PM exposure for these specific relationships. This could be due to a confluence of molecular mechanisms that impart epigenetic programming with an upper threshold of exposure [18]. In contrast, the Mothers and Children’s Environmental Health cohort found an inverse association with prenatal PM10 and weight-for-age z score at 6 months of age [10]. However, this effect did not persist at any other intervals up to age 5 years [10].
Whether early growth patterns linked to prenatal air pollutant exposure persist into adolescence and young adulthood remains to be determined. While prenatal exposure has been linked to slower fetal growth and lower birth weight, both low and high birth weight have been observed to predict a ‘stable obesity’ growth trajectory through childhood [6]. In addition, a similarly sized ‘later-onset obesity’ group has been identified that showed a high and earlier likelihood of obesity by 2 years of age and exhibited similar developmental programming risk factors [6], which may be reflective of subtle epigenetic changes that occur during the gestational period that increase susceptibility to exposures later in life [67]. The ACCESS (Asthma Coalition on Community, Environment and Social Stress) cohort found early and mid-pregnancy exposure to PM2.5 to be associated with fat mass and BMI z-score among boys and mid-pregnancy exposure with increased waist-to-hip ratio among girls, respectively, at 4 years old [22]. Additionally, the Mothers and Children Study found greater prenatal exposure to polycyclic aromatic hydrocarbons to be associated with higher BMI z-scores at ages 5 and 7 years [68]. Another cohort from Wuhan, China found that prenatal NO2 exposure, particularly in the 3rd trimester, was associated with childhood obesity at 2 years old and a faster growth in childhood BMI-for-age z-score [7]. In contrast, Project Viva found no association between prenatal exposure to traffic-related air pollution and BMI trajectory, including the timing and magnitude of BMI peak in infancy and adiposity rebound [69], both important predictors of obesity later in life [70]. This discrepancy may have been attributable to differences in race/ethnicity, maternal obesity, and breastfeeding practices from other cohorts [69].
This study should be interpreted with the context of several strengths and limitations. In contrast to previous relevant work, we examined the effects of multiple pollutant exposures (PM2.5, PM10, NO2, O3, Oxwt). This aids both in identifying the unique effects of different pollutants and distinguishing the exposures of greatest concern for a given outcome, which can help direct effective policy interventions. However, the high degree of correlation between pollutants, particularly NO2 and O3, which are known to be intimately related via their atmospheric photochemistry [32], may limit interpretability of their individual effects in this study. Also, despite being strongly inversely correlated with one another, O3 and NO2 were both positively associated with umbilical circumference (although neither model was statistically significant and both confidence intervals also included inverse associations). This may have been due to our relatively small exposure contrasts for these pollutants. Despite this, our additional use of Oxwt, the redox-weighted oxidative variable utilizing NO2 and O3, alternatively enabled investigation into their joint effects. The longitudinal study design allowed for examination of temporal exposure-outcome relationships and persistence of effects over time that cannot be captured cross-sectionally. All participants were of Hispanic ethnicity; while this may limit generalizability of study findings, this group is often exposed to higher levels of air pollutants and at greater risk for childhood obesity. Misclassification of exposure could have occurred due to either time spent away from residences or discordant indoor exposures; however, any misclassification likely would have biased effects towards the null [71]. The IDW2 interpolation exposure assessment method in this analysis is limited by spatial resolution relative to other exposure assessment methods like land-use-regression or hybrid models but is justified by the focus on associations with regional air pollutant levels in the present analysis. Furthermore, prior work by our group has shown that the IDW2 method in California has little bias on average (0.1 ppb, 0.7 ppb, 0.4 µg/m3, and 0.5 µg/m3 for O3, NO2, PM2.5, and PM10), and acceptable error (± 15%, 34%, 31%, and 35% for O3, NO2, PM2.5, and PM10) [33]. While our study had a relatively small sample size, the availability of information regarding important covariates and detailed phenotyping of mothers and infants allowed for adjustment of known confounders in several model iterations. In addition to traditional measures of growth, we included several measures of infant body composition enabling greater insight into infant growth. The use of a skinfold caliper to measure body composition (in place of DEXA) could be considered a limitation, but skinfold thickness may be a reasonable proxy for DEXA in this age group [72] and is positively associated with total and abdominal fat at six years of age [27]. Incomplete information with respect to date of conception and gestational age prevent us from adjusting for this in our statistical models, yet models with and without birth weight did not appear to appreciably influence the results. Future investigations in the Mother’s Milk Study will include both pregnancy and early life exposures to air pollutants through 36 months of age, which will be used to further investigate the effects of exposure to air pollutants on rapid infant growth and risk for childhood obesity.
Conclusion
Prenatal ambient air pollution exposure was associated with increased weight gain and anthropometric measures from 1 to 6 months of life in 123 Hispanic infants from Southern California. Sex-specific associations suggest differential consequences of in utero oxidative stress. These results indicate that prenatal ambient air pollution exposure may alter infant growth, which has potential to increase childhood obesity risk. Interventions to address the obesity and non-communicable disease epidemics should consider personal and policy-based strategies for reducing exposure during critical periods in early life.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- PM2.5 :
-
Fine particulate matter
- PM10 :
-
Coarse particulate matter
- NO2 :
-
Nitrogen dioxide
- O3 :
-
Ozone
- Ox wt :
-
Redox-weighted oxidative capacity
- TSF:
-
Total subcutaneous fat
- CTSF:
-
Central:total subcutaneous fat
- AAP:
-
Ambient air pollutants
- BMI:
-
Body mass index
References
Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of Obesity Among Adults and Youth: United States, 2015–2016. NCHS Data Brief. 2017;288:1–8.
Ayer J, Charakida M, Deanfield JE, Celermajer DS. Lifetime risk: childhood obesity and cardiovascular risk. Eur Heart J. 2015;36:1371–6.
Lindberg L, Danielsson P, Persson M, Marcus C, Hagman E. Association of childhood obesity with risk of early all-cause and cause-specific mortality: A Swedish prospective cohort study. PLoS Med. 2020;17:e1003078.
Taveras EM, Rifas-Shiman SL, Belfort MB, Kleinman KP, Oken E, Gillman MW. Weight Status in the First 6 Months of Life and Obesity at 3 Years of Age. Pediatrics. 2009;123:1177–83.
Stock K, Nagrani R, Gande N, Bernar B, Staudt A, Willeit P, et al. Birth Weight and Weight Changes from Infancy to Early Childhood as Predictors of Body Mass Index in Adolescence. J Pediatr. 2020;222:120-126.e3.
O’Connor TG, Williams J, Blair C, Gatzke-Kopp LM, Francis L, Willoughby MT. Predictors of Developmental Patterns of Obesity in Young Children. Front Pediatr. 2020;8:109.
Chen X, Liao J, Xu S, Zhang B, Wang W, Cao Z, et al. Associations of exposure to nitrogen dioxide and major roadways with growth trajectories and obesity at 2 years old: A prospective cohort study. Atmos Environ. 2020;232:117574.
Clemente DBP, Casas M, Janssen BG, Lertxundi A, Santa-Marina L, Iñiguez C, et al. Prenatal ambient air pollution exposure, infant growth and placental mitochondrial DNA content in the INMA birth cohort. Environ Res. 2017;157:96–102.
Fleisch AF, Rifas-Shiman SL, Koutrakis P, Schwartz JD, Kloog I, Melly S, et al. Prenatal Exposure to Traffic Pollution. Epidemiology. 2015;26:43–50.
Kim E, Park H, Park EA, Hong Y-C, Ha M, Kim H-C, et al. Particulate matter and early childhood body weight. Environ Int. 2016;94:591–9.
Rosofsky AS, Fabian MP, de Cuba SE, Sandel M, Coleman S, Levy JI, et al. Prenatal Ambient Particulate Matter Exposure and Longitudinal Weight Growth Trajectories in Early Childhood. Int J Environ Res Pu. 2020;17:1444.
Tessum CW, Apte JS, Goodkind AL, Muller NZ, Mullins KA, Paolella DA, et al. Inequity in consumption of goods and services adds to racial–ethnic disparities in air pollution exposure. Proc National Acad Sci. 2019;116:201818859.
Woodruff TJ, Parker JD, Kyle AD, Schoendorf KC. Disparities in exposure to air pollution during pregnancy. Environ Health Persp. 2003;111:942–6.
Stieb DM, Chen L, Eshoul M, Judek S. Ambient air pollution, birth weight and preterm birth: A systematic review and meta-analysis. Environ Res. 2012;117:100–11.
An R, Ji M, Yan H, Guan C. Impact of ambient air pollution on obesity: a systematic review. Int J Obes. 2018;42:1112–26.
Seo MY, Kim S-H, Park MJ. Air pollution and childhood obesity. Clin Exp Pediatr. 2020;10:382–8.
Starling AP, Moore BF, Thomas DSK, Peel JL, Zhang W, Adgate JL, et al. Prenatal exposure to traffic and ambient air pollution and infant weight and adiposity: The Healthy Start study. Environ Res. 2020;182:109130.
Saenen ND, Martens DS, Neven KY, Alfano R, Bové H, Janssen BG, et al. Air pollution-induced placental alterations: an interplay of oxidative stress, epigenetics, and the aging phenotype? Clin Epigenetics. 2019;11:124.
Clougherty JE. A Growing Role for Gender Analysis in Air Pollution Epidemiology. Environ Health Persp. 2010;118:167–76.
Ghosh R, Rankin J, Pless-Mulloli T, Glinianaia S. Does the effect of air pollution on pregnancy outcomes differ by gender? A systematic review. Environ Res. 2007;105:400–8.
Rosenfeld CS. Sex-Specific Placental Responses in Fetal Development. Endocrinology. 2015;156:3422–34.
Chiu Y-HM, Hsu H-HL, Wilson A, Coull BA, Pendo MP, Baccarelli A, et al. Prenatal particulate air pollution exposure and body composition in urban preschool children: Examining sensitive windows and sex-specific associations. Environ Res. 2017;158:798–805.
Fossati S, Valvi D, Martinez D, Cirach M, Estarlich M, Fernández-Somoano A, et al. Prenatal air pollution exposure and growth and cardio-metabolic risk in preschoolers. Environ Int. 2020;138:105619.
Bolton JL, Smith SH, Huff NC, Gilmour MI, Foster WM, Auten RL, et al. Prenatal air pollution exposure induces neuroinflammation and predisposes offspring to weight gain in adulthood in a sex-specific manner. Faseb J. 2012;26:4743–54.
Chen M, Wang X, Hu Z, Zhou H, Xu Y, Qiu L, et al. Programming of mouse obesity by maternal exposure to concentrated ambient fine particles. Part Fibre Toxicol. 2017;14:20.
Chen M, Liang S, Zhou H, Xu Y, Qin X, Hu Z, et al. Prenatal and postnatal mothering by diesel exhaust PM2.5-exposed dams differentially program mouse energy metabolism. Part Fibre Toxicol. 2017;14:3.
Santos S, Gaillard R, Oliveira A, Barros H, Hofman A, Franco OH, et al. Subcutaneous fat mass in infancy and cardiovascular risk factors at school-age: The generation R study. Obesity. 2016;24:424–9.
Berger PK, Plows JF, Jones RB, Alderete TL, Yonemitsu C, Poulsen M, et al. Human milk oligosaccharide 2’-fucosyllactose links feedings at 1 month to cognitive development at 24 months in infants of normal and overweight mothers. PLoS One. 2020;15:e0228323.
Hollingshead A. Four-factor index of social status. New Haven: Yale University. 1975.
Alderete TL, Chen Z, Toledo-Corral CM, Contreras ZA, Kim JS, Habre R, et al. Ambient and Traffic-Related Air Pollution Exposures as Novel Risk Factors for Metabolic Dysfunction and Type 2 Diabetes. Curr Epidemiol Rep. 2018;5:79–91.
Ebrahim GJ. WHO Child Growth Standards. Growth Velocity Based on Weight, Length and Head Circumference. * Methods and Development. J Trop Pediatr. 2009;56:136–136.
Williams ML, Atkinson RW, Anderson HR, Kelly FJ. Associations between daily mortality in London and combined oxidant capacity, ozone and nitrogen dioxide. Air Qual Atmosphere Heal. 2014;7:407–14.
Eckel SP, Cockburn M, Shu Y-H, Deng H, Lurmann FW, Liu L, et al. Air pollution affects lung cancer survival. Thorax. 2016;71:891.
VanderWeele TJ, Shpitser I. A New Criterion for Confounder Selection. Biometrics. 2011;67:1406–13.
Ananth CV, Schisterman EF. Confounding, causality, and confusion: the role of intermediate variables in interpreting observational studies in obstetrics. Am J Obstet Gynecol. 2017;217:167–75.
Glymour MM, Weuve J, Berkman LF, Kawachi I, Robins JM. When Is Baseline Adjustment Useful in Analyses of Change? An Example with Education and Cognitive Change. Am J Epidemiol. 2005;162:267–78.
Oken E, Kleinman KP, Rich-Edwards J, Gillman MW. A nearly continuous measure of birth weight for gestational age using a United States national reference. Bmc Pediatr. 2003;3:6.
Durand CP. Does raising type 1 error rate improve power to detect interactions in linear regression models? A simulation study. PLoS One. 2013;8:e71079.
Luyten LJ, Saenen ND, Janssen BG, Vrijens K, Plusquin M, Roels HA, et al. Air pollution and the fetal origin of disease: A systematic review of the molecular signatures of air pollution exposure in human placenta. Environ Res. 2018;166:310–23.
Pedersen M, Wichmann J, Autrup H, Dang DA, Decordier I, Hvidberg M, et al. Increased micronuclei and bulky DNA adducts in cord blood after maternal exposures to traffic-related air pollution. Environ Res. 2009;109:1012–20.
Clemente DBP, Casas M, Vilahur N, Begiristain H, Bustamante M, Carsin A-E, et al. Prenatal Ambient Air Pollution, Placental Mitochondrial DNA Content, and Birth Weight in the INMA (Spain) and ENVIR ON AGE (Belgium) Birth Cohorts. Environ Health Persp. 2016;124:659–65.
Janssen BG, Munters E, Pieters N, Smeets K, Cox B, Cuypers A, et al. Placental Mitochondrial DNA Content and Particulate Air Pollution during in Utero Life. Environ Health Persp. 2012;120:1346–52.
Bijnens E, Zeegers MP, Gielen M, Kicinski M, Hageman GJ, Pachen D, et al. Lower placental telomere length may be attributed to maternal residential traffic exposure; a twin study. Environ Int. 2015;79:1–7.
Martens DS, Nawrot TS. Air Pollution Stress and the Aging Phenotype: The Telomere Connection. Curr Environ Heal Rep. 2016;3:258–69.
Obolenskaya MYU, Teplyuk NM, Divi RL, Poirier MC, Filimonova NB, Zadrozna M, et al. Human placental glutathione S-transferase activity and polycyclic aromatic hydrocarbon DNA adducts as biomarkers for environmental oxidative stress in placentas from pregnant women living in radioactivity- and chemically-polluted regions. Toxicol Lett. 2010;196:80–6.
Topinka J, Binková B, Mračková G, Stávková Z, Peterka V, Beneš I, et al. Influence of GSTM1 and NAT2 genotypes on placental DNA adducts in an environmentally exposed population. Environ Mol Mutagen. 1997;30:184–95.
Janssen BG, Godderis L, Pieters N, Poels K, Kiciński M, Cuypers A, et al. Placental DNA hypomethylation in association with particulate air pollution in early life. Part Fibre Toxicol. 2013;10:22.
Tsamou M, Vrijens K, Madhloum N, Lefebvre W, Vanpoucke C, Nawrot TS. Air pollution-induced placental epigenetic alterations in early life: a candidate miRNA approach. Epigenetics. 2018;13:135–46.
Hincal F. Effects of Exposure to Air Pollution and Smoking on the Placental Aryl Hydrocarbon Hydroxylase (AHH) Activity. Arch Environ Heal Int J. 1986;41:377–83.
Grevendonk L, Janssen BG, Vanpoucke C, Lefebvre W, Hoxha M, Bollati V, et al. Mitochondrial oxidative DNA damage and exposure to particulate air pollution in mother-newborn pairs. Environ Health-uk. 2016;15:10.
Rossner P, Rossnerova A, Sram RJ. Oxidative stress and chromosomal aberrations in an environmentally exposed population. Mutat Res Fundam Mol Mech Mutagen. 2011;707:34–41.
de Melo JO, Soto SF, Katayama IA, Wenceslau CF, Pires AG, Veras MM, et al. Inhalation of fine particulate matter during pregnancy increased IL-4 cytokine levels in the fetal portion of the placenta. Toxicol Lett. 2015;232:475–80.
Saenen ND, Vrijens K, Janssen BG, Roels HA, Neven KY, Berghe WV, et al. Lower Placental Leptin Promoter Methylation in Association with Fine Particulate Matter Air Pollution during Pregnancy and Placental Nitrosative Stress at Birth in the ENVIR ON AGE Cohort. Environ Health Persp. 2017;125:262–8.
Cai J, Zhao Y, Liu P, Xia B, Zhu Q, Wang X, et al. Exposure to particulate air pollution during early pregnancy is associated with placental DNA methylation. Sci Total Environ. 2017;607:1103–8.
Fouladi F, Bailey MJ, Patterson WB, Sioda M, Blakley IC, Fodor AA, et al. Air pollution exposure is associated with the gut microbiome as revealed by shotgun metagenomic sequencing. Environ Int. 2020;138:105604.
Robertson RC, Manges AR, Finlay BB, Prendergast AJ. The Human Microbiome and Child Growth – First 1000 Days and Beyond. Trends Microbiol. 2018;27:131–47.
Bové H, Bongaerts E, Slenders E, Bijnens EM, Saenen ND, Gyselaers W, et al. Ambient black carbon particles reach the fetal side of human placenta. Nat Commun. 2019;10:3866.
Valentino SA, Tarrade A, Aioun J, Mourier E, Richard C, Dahirel M, et al. Maternal exposure to diluted diesel engine exhaust alters placental function and induces intergenerational effects in rabbits. Part Fibre Toxicol. 2016;13:39.
Minghetti L, Greco A, Zanardo V, Suppiej A. Early-life sex-dependent vulnerability to oxidative stress: the natural twining model. J Maternal-fetal Neonatal Med. 2012;26:259–62.
Howe LD, Matijasevich A, Tilling K, Brion M-J, Leary SD, Smith GD, et al. Maternal smoking during pregnancy and offspring trajectories of height and adiposity: comparing maternal and paternal associations. Int J Epidemiol. 2012;41:722–32.
Kannan S, Misra DP, Dvonch JT, Krishnakumar A. Exposures to Airborne Particulate Matter and Adverse Perinatal Outcomes: A Biologically Plausible Mechanistic Framework for Exploring Potential Effect Modification by Nutrition. Environ Health Persp. 2006;114:1636–42.
Barker DJP, Osmond C, Forsén TJ, Kajantie E, Eriksson JG. Trajectories of Growth among Children Who Have Coronary Events as Adults. N Engl J Med. 2005;353:1802–9.
Bhargava SK, Sachdev HS, Fall CHD, Osmond C, Lakshmy R, Barker DJP, et al. Relation of Serial Changes in Childhood Body-Mass Index to Impaired Glucose Tolerance in Young Adulthood. New Engl J Med. 2004;350:865–75.
Taveras EM, Rifas-Shiman SL, Sherry B, Oken E, Haines J, Kleinman K, et al. Crossing Growth Percentiles in Infancy and Risk of Obesity in Childhood. Arch Pediat Adol Med. 2011;165:993–8.
de Bont J, Casas M, Barrera-Gómez J, Cirach M, Rivas I, Valvi D, et al. Ambient air pollution and overweight and obesity in school-aged children in Barcelona Spain. Environ Int. 2019;125:58–64.
Pope CA, Burnett RT, Turner MC, Cohen A, Krewski D, Jerrett M, et al. Lung Cancer and Cardiovascular Disease Mortality Associated with Ambient Air Pollution and Cigarette Smoke: Shape of the Exposure-Response Relationships. Environ Health Persp. 2011;119:1616–21.
Heindel JJ, Vandenberg LN. Developmental origins of health and disease. Curr Opin Pediatr. 2015;27:248–53.
Rundle A, Hoepner L, Hassoun A, Oberfield S, Freyer G, Holmes D, et al. Association of Childhood Obesity With Maternal Exposure to Ambient Air Polycyclic Aromatic Hydrocarbons During Pregnancy. Am J Epidemiol. 2012;175:1163–72.
Fleisch AF, Aris IM, Rifas-Shiman SL, Coull BA, Luttmann-Gibson H, Koutrakis P, et al. Prenatal Exposure to Traffic Pollution and Childhood Body Mass Index Trajectory. Front Endocrinol. 2019;9:771.
Geserick M, Vogel M, Gausche R, Lipek T, Spielau U, Keller E, et al. Acceleration of BMI in Early Childhood and Risk of Sustained Obesity. N Engl J Med. 2018;379:1303–12.
Crouse DL, Peters PA, van Donkelaar A, Goldberg MS, Villeneuve PJ, Brion O, et al. Risk of Nonaccidental and Cardiovascular Mortality in Relation to Long-term Exposure to Low Concentrations of Fine Particulate Matter: A Canadian National-Level Cohort Study. Environ Health Persp. 2012;120:708–14.
Schmelzle HR, Fusch C. Body fat in neonates and young infants: validation of skinfold thickness versus dual-energy X-ray absorptiometry. Am J Clin Nutr. 2002;76:1096–100.
Acknowledgements
Research described in this article was conducted under contract to the Health Effects Institute (HEI), an organization jointly funded by the United States Environmental Protection Agency (EPA) (Assistance Award No. CR 83998101) and certain motor vehicle and engine manufacturers. The contents of this article do not necessarily reflect the views of HEI, or its sponsors, nor do they necessarily reflect the views and policies of the EPA or motor vehicle and engine manufacturers.
Funding
This work was supported by the NIH NIDDK (R01 DK11079), The Gerber Foundation (15PN-013), NIEHS (R00 ES027853), and the Health Effects Institute Rosenblith Award. Design of the study; collection, analysis, and interpretation of data; and writing the manuscript was strictly the responsibility of the authors.
Author information
Authors and Affiliations
Contributions
WP conducted the formal analysis and prepared the original draft. JG and NN helped prepare datasets for covariates. RJ, PB, and JP reviewed and revised the manuscript. HM and FL modeled air pollutant exposure estimates and reviewed and revised the manuscript. MG supervised all data collection, acquired funding, and reviewed and revised the manuscript. TA conceived the hypothesis and data analysis plan, supervised the formal analysis, acquired funding, and reviewed and revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The Institutional Review Boards of the University of Southern California, Children’s Hospital of Los Angeles, and the University of Colorado Boulder approved study procedures. Written informed consent was obtained from participants prior to enrollment and all analyses in this study.
Consent for publication
Not applicable.
Competing interests
MIG is a scientific advisor for Yumi and receives book royalties. The other authors declare they have no actual or potential competing financial interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Supplemental Table 1.
Mother-Infant Dyads were Similar to those Excluded from the Current Analysis. 1-month characteristics of Hispanic mother-infant dyads from the Southern California Mother’s Milk Study are shown for both those included and those excluded from the current study. Data are reported mean and standard deviation (SD) unless otherwise noted. Group means of continuous variables were compared via two-sample t-tests and binary variables were compared via Chi-square test. Breastfeeding frequency at 6 months of age is shown dichotomized as ≥ 7 or < 7 times per day. Total subcutaneous fat is the sum of the four infant skinfold thickness measures in millimeters. The Central:Total subcutaneous fat variable is the sum of the suprailiac and subscapular skinfold thicknesses divided by all four skinfold measures.
Additional file 2: Supplemental Table 2.
Levels of Ambient Air Pollutant Exposures and Infant Growth Outcomes Across Tertiles of Socioeconomic Status. Means and standard deviations of ambient air pollutant exposure levels and infant growth change variables are presented across tertiles of socioeconomic status (Hollingshead Index). Tertile means of continuous variables were compared via one-way ANOVA and p-values are shown. TSF represents the change in infant total subcutaneous fat, which is the sum of the four infant skinfold thickness measures in millimeters. CTSF represents the change in infant central:total subcutaneous, which is the sum of the suprailiac and subscapular skinfold thicknesses divided by all four skinfold measures. Oxwt is the redox-weight oxidative potential of NO2 and O3.
Additional file 3: Supplemental Table 3.
Sensitivity Analysis: Prenatal Exposures and Infant Changes from 1- to 6-Months Postpartum with Adjustment for Area-Level Socioeconomic Status. Multivariable linear regression was performed to examine the relationships between prenatal exposure to ambient air pollutants and changes in infant growth from 1 to 6 months of age with adjustment for a poverty index variable in place of the individual-level socioeconomic status variable in the main model. Beta coefficients and 95% confidence intervals are shown for a one standard deviation increase in exposure (PM2.5 [SD = 1.15 μg/m3], PM10 [SD = 3.54 μg/m3], NO2 [SD = 2.34 ppb], O3 [SD = 2.38 ppb], Oxwt [SD = 1.04 ppb]). Multivariable linear models adjust for infant sex, infant age, pre-pregnancy BMI, breastfeeding frequency, maternal age, and poverty index (a component of the CalEnviroScreen 3.0 score). TSF and CTSF represent changes in total subcutaneous fat and the ratio of central to total subcutaneous fat, respectively.
Additional file 4: Supplemental Table 4.
Sensitivity Analysis: Prenatal Exposures and Infant Z-Score Changes from 1- to 6-Months Postpartum. Table displays the results for multivariable linear regression models examining prenatal ambient air pollutant exposure and changes in infant z-score measures (weight-for-age and weight-for-length). Beta coefficients are shown for a one standard deviation increase in exposure (PM2.5 [SD = 1.15 μg/m3], PM10 [SD = 3.54 μg/m3], NO2 [SD = 2.34 ppb], O3 [SD = 2.38 ppb], Oxwt [SD = 1.04]). Models adjusted for pre-pregnancy BMI, breastfeeding frequency, maternal age, and socioeconomic status.
Additional file 5: Supplemental Figure 1.
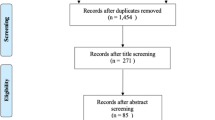
Flowchart Detailing Participants Retained for the Present Analysis. At the time of our analyses, 212 participants had been enrolled in the Mother’s Milk Study. There were 24 participants who had completed both clinical study visits but did not yet have ambient pollutant exposure estimates available. Among the 5 change in infant growth variable outliers, 2 were outliers from infant length change, 1 was an outlier from infant weight change, 1 was an outlier from umbilical circumference change, and 1 was from change in CTSF.
Additional file 6: Supplemental Figure 2.
Sensitivity Analysis: Multivariable Linear Models Additionally Adjusted for Birth Weight and Baseline of Change Variables. Fully adjusted models controlled for infant sex, infant age, pre-pregnancy BMI, breastfeeding frequency, maternal age, and socioeconomic status (effect estimates denoted by circles). Sensitivity analyses examining multivariable linear regression models additionally adjusting for birth weight (effect estimates denoted by squares) or the baseline value of the change variable (effect estimates denoted by black triangles), respectively, were performed to further evaluate the relationships between prenatal exposure to ambient air pollutants and changes in infant growth from 1 to 6 months of age. Beta coefficients and 95% confidence intervals are shown for a one standard deviation increase in exposure (PM2.5 [SD = 1.15 μg/m3], PM10 [SD = 3.54 μg/m3], NO2 [SD = 2.34 ppb], O3 [SD = 2.38 ppb], Oxwt [SD = 1.04]).
Additional file 7: Supplemental Figure 3.
Prenatal NO2 and O3 were Associated with a Greater Change in Umbilical Circumference. Scatterplots display infant change in umbilical circumference in relation to prenatal NO2 and O3, respectively. Plots were made to visualize the distribution of these data due to a strong inverse correlation between NO2 and O3, yet similar direction of effect.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Patterson, W.B., Glasson, J., Naik, N. et al. Prenatal exposure to ambient air pollutants and early infant growth and adiposity in the Southern California Mother’s Milk Study. Environ Health 20, 67 (2021). https://doi.org/10.1186/s12940-021-00753-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12940-021-00753-8