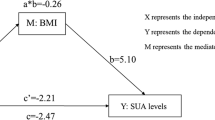
Abstract
Background
Certain foods and food groups could positively or negatively impact serum uric acid (SUA) levels. However, evidence on the holistic dietary strategy to prevent and control hyperuricemia (HUA) development remains limited.
Objective
The aim of this research work was to explore the association of dietary approaches to stop hypertension (DASH) diet with SUA levels and odds of HUA among Chinese adults.
Methods
This research premise included 66,427 Chinese adults aged 18 and above who were part of the China Adult Chronic Disease and Nutrition Surveillance in 2015. Dietary consumptions were assessed via the household condiment weighing approach and a three-day, 24-hour dietary recall. Total fat, saturated fat, calcium, protein, potassium, cholesterol, magnesium, fiber, and sodium were all adopted to calculate the DASH score (score range, 0–9). The associations of DASH score with SUA levels and odds of HUA were evaluated using multiple linear and logistic regression models, respectively.
Results
We established that a higher DASH score was linked with a lower SUA levels (β = − 0.11; 95% CI: − 0.12, − 0.1; p < 0.001) and odds of HUA (OR = 0.85; 95% CI: 0.83, 0.87; p < 0.001) after adjustment for age, sex, ethnicity, education status, marital status, health behaviours and health factors. The association of the DASH diet with odds of HUA was stronger among men (p-interaction = 0.009), non-Han Chinese (p-interaction< 0.001) as well as rural residents (p-interaction< 0.001).
Conclusions
Our results illustrate that the DASH diet was remarkably negatively with SUA levels and odds of HUA in the Chinese adult population.
Similar content being viewed by others
Introduction
Hyperuricemia (HUA) is a metabolic disease caused by the metabolism disorder and/or impaired renal uric acid excretion [1]. HUA is the main pathogenic factor for gout [1], and might participate in the onset and progress of hypertension, type 2 diabetes, chronic kidney disease, metabolic syndrome, as well as cardiovascular disease [2,3,4,5]. Over the last few decades, the occurrence of HUA has risen in developed along with developing countries [6]. In China, HUA has been increasing in prevalence from 8.4 to 14% in adults during 2009 to 2019, and has become a public health problem to be addressed [7].
Diet, as an important determinant of serum uric acid (SUA) levels [8], could have a positive or negative impact on the prevention of HUA [9]. Intake of alcohol, sugary sweetened beverages (SSB) and some purine-rich foods for instance red meat and shellfish triggers the increase of SUA levels [10]. In contrast, consumption of some specific elements of diet including low fat milk [10], vitamin C [11] and dietary fiber [12] may reduce SUA levels. However, focusing on single nutrient or food may not comprehensively reflect the impact of overall dietary quality on health outcomes due to the diversity and complexity of food intake [13]. Previous studies indicated that the conventional low-purine (i.e., low-protein) dietary approach to lowing SUA levels may worsen insulin resistance and elevate levels of fasting blood glucose, triglycerides and LDL cholesterol as a result of compensatory higher intake of refined carbohydrates or fats [14, 15]. Therefore, a holistic dietary strategy is needed to better prevent, as well as control the development of HUA.
The Dietary Approaches to Stop Hypertension (DASH) diet, which underscores foods rich in protein, calcium, fiber, potassium along with magnesium, and consisting of fruits, low-fat dairy products, vegetables, nuts, beans, as well as whole grains, whereas limiting foods harboring high saturated fat and sugar, was originally developed for hypertension management [16]. The DASH diet has also been found to reduce the odds of some HUA related comorbidities for instance cardiovascular disease, chronic kidney disease, metabolic syndrome, as well as type 2 diabetes [17]. HUA is causally related with insulin resistance [18], and thus might also be improved by DASH diet, which has the potential to enhance insulin sensitivity [19]. Two intervention studies conducted in the U.S. have observed significant reductions in SUA levels among participants with hypertension or gout following DASH diet [20, 21]. However, in a randomised controlled trial (RCT), the benefit effects of DASH diet on SUA levels was only found among African Americans hypertensive patients who had a a higher baseline SUA levels [22]. So far, the existing epidemiological research evidence on this issue in the general population is very limited. A previous cross-sectional study conducted in Tangshan city, China observed that adherence to DASH diet has been documented to be linked with a lower odds of HUA among Chinese general adult population [23]. But, in this study, all participants were residents of the same community, who are mainly Northern Han Chinese. Thus, the focus of our research was to explore link between a DASH diet with SUA levels and the odds of HUA in 66,427 Chinese adults using the most recent nationally representative data.
Materials and methods
Study population
Data were derived from the China Adult Chronic Disease and Nutrition Surveillance (CACDNS) 2015. The CNCDNS is a national representative cross-sectional research work which was designed to assess food intake, health status and lifestyle behaviors of Chinese adults aged 18 and above. Participants were enrolled from 31 provinces in China (except Hong Kong, Macao, and Taiwan) via a multistage stratified cluster random sampling approach along with a probability proportionate to population size sampling technique, which has been documented previously in details [24]. A total of 69,909 individuals with available data of dietary intake, lifestyle behaviors, medical history, laboratory results along with physical examination, were included in this study. Participants with implausible total energy intakes (< 800 kcal/day (kcal/day) or > 4500 kcal/day)(n = 2169), pregnant women (n = 141), cancer patients(n = 1019), as well as nursing mothers(n = 153) were further excluded from further analysis. The excluded participants did not show significant differences regarding socio-demographics, BMI, or serum uric acid levels. Finally, 66,427 participants were involved in the analysis.
At the start of the survey, all participants granted a written informed consent form. The Chinese Center for Disease Control and Prevention’s Ethical Committee authorized the research work protocols (No. 201519-B).
Assessment of dietary scores
The 3-day (two weekdays coupled with one weekend day) food record approach was used to determine dietary intake of each individual. The amounts of edible oil and each condiments (such as salt, sugar and other ingredients) used at the household were assessed by the three-day household condiments weighing method, using a uniformly calibrated electronic dietary scale with a precision of 2 g [25]. The interviewers consisted of public health physicians drawn from local community health centers as well as disease control and prevention centers who had been trained on dietary data recording. In the course of the household interviews, staff utilized standard forms of dietary recalls harboring picture aids, as well as food models. Throughout the survey, participants were required not to modify their eating or lifestyle habits. Dietary data along with the Chinese Food Composition Table were utilized to estimate nutrient and energy intake [26, 27].
The DASH diet score for each participant were calculated based on the Mellen et al. formula in this study [28]. Briefly, nine targeted nutrients including total fat, fiber, saturated fat, calcium, protein, potassium, cholesterol, magnesium, and sodium were identified for DASH goals. Micronutrient goals were expressed per 1000 kcal. We assigned a value of 1 if the participant met the DASH target of a nutrient, a value of 0.5 reflected meeting a nutrient’s intermediate target, whilst 0 reflected no target met. By adding all nutrient targets met, the total DASH score is then calculated, which varies from 0 to 9. The detailed target for each nutrient is illustrated in Supplementary Table 1.
Ascertainment of outcome
This study focused on serum urate concentration as the primary outcome. SUA contents of ≥7.0 mg/dL in men or ≥ 6.0 mg/dL in women defined HUA [29]. We collected blood samples from consenting participants following an overnight fasting of 10–12 hours, and spun and fractionated into plasma, as well as serum within 0.5–1.0 hours post collection. Serum specimens were stored at − 80 °C for downstream analyses. SUA contents were further measured with the Hitachi Automatic Platform 7600 (Hitachi Co., Tokyo, Japan). Each of the measures listed above was performed in a laboratory by specialists under stringent quality control.
Measurement of other covariates
Information was obtained from each participant on demographic characteristics (date of birth, region, gender, education along with marital status), health behaviors (smoking status, level of physical activity, and drinking status) coupled with medical histories with the use of a questionnaire completed by an interviewer.
In previous studies, these factors, in addition to dietary factors, have been found to be associated with the odds of hyperuricemia in Chinese adults [7]. Attainment of education was stratified into three classes; less than high school, high school and higher education. Marital status consisted of either living single (widowed, separated, or divorced) and not single (living with partner or married). Physical activity was summarized and expressed in metabolic equivalents (METs) minutes/week, and then classified into three categories: vigorous (MET> 3000), moderate (600 ≤ MET≤3000), or sedentary (MET< 600) [30]. Status of smoking along with drinking were classified as yes (current or former) or no (never).
Body height and weight were assessed via calibrated electronic digital scales with a precision of 0.1 cm and 0.01 kg, respectively. Body mass index (BMI) was then computed as kg/m2. Systolic and diastolic blood pressures were assessed for three times with a 1-minute interval between each measurement with an automatic measurement device (Omron HBP-1300; OMRON Healthcare, the Netherlands). In the analysis, the average of three measurements was utilized.
The contents of total cholesterol, fasting blood glucose, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, as well as low-density lipoprotein cholesterol were assessed on the Hitachi Automatic Platform 7600 (Hitachi Co., Tokyo, Japan). Diabetes was defined by having a self-reported history of diabetes mellitus or having a fasting glucose level of more than 7.0 mmol/L. The criteria of defining hypertension consisted of a self-reported hypertension history, or an average SBP or an average SBP ≥ 140 mmHg or DBP ≥90 mmHg. Dyslipidemia was defined as total cholesterol (TC) level ≥ 5.7 mmol/L, or high-density lipoprotein cholesterol (HDL-C) level < 1.04 mmol/L, or low-density lipoprotein cholesterol (LDL-C) level ≥ 3.64 mmol/L, or current use of antihyperlipidemic drugs. Participants with any of the above diseases were defined as having a chronic disease noncommunicable diseases (NCDs). The medication use was included in the medical history referred to whether the participants were taking anti-hypertensive drugs, anti-hyperglycemic drugs, and lipid-lowering drugs. Urologic disease, for instance kidney stones, chronic nephritis prostatitis, was assessed via self-report.
Statistical analysis
Participants were stratified into quartiles on the basis of the DASH score beginning with the lowest (the 1st quartile) to the highest (the 4th quartile). Basic features and dietary intake of the study participants were reported with the use of median (interquartile range [IQR]) or means (standard deviation [SD]) for continuous variables and number (percentage) for categorical variables. As the proportion of participants without dietary intake of dairy products (85.9%), sugar-sweetened beverages (SSB) (96.4%) is relatively large, we used number (percentage) instead of median (interquartile range [IQR]) to express the intake status of these food types. Differences between groups were analyzed via Kruskal-Wallis test for continuous variables and chi-square test for categorical variables. We further used generalized linear regression and logistic regression models to explore the the association of DASH diet scores with SUA levels and odds of HUA. Multivariable regression models were adjusted for these covariates: Model 1: adjusted for age, sex; Model 2: further adjusted for body mass index, region (city, rural), ethic (han, others), marital status (married or living with partner, living alone), education (less than high school, high school, higher than higer school), physical activity (sedentary, moderate, vigorous), smoking status (yes, no) and alcohol consumption (never, former, current), NCDs status (yes, no), medication use (yes, no), urologic disease status (yes, no). P values for trend (P-trend) were computed via the median within each group to assess the ordered relation across groups of the DASH score for both the continuous (SUA) and binary (HUA) outcomes. We also tested interaction between the DASH diet and age (< 45y, > = 45 y), sex, ethic group (Han, others), region (urban, rural), body mass index (< 24, > = 24) and health status (with NCDs, without NCDs) in the fully adjusted model. Statistically significance was defined as P < 0.05. The statistical analyses were conducted using the R software version 3.6.3.
Results
This study enrolled 66,427 adults, including 31,920 males and 34,507 females. The overall prevalence of HUA in the entire sample was 14% (18% in men; 10.3% in women). Table 1 shows the characteristics of the study participants across the DASH score quartiles. Participants with higher DASH scores were more likely female, greater than 60 years old, rural residents, non-Han Chinese, with less than a high school education, not single, vigorous physical activity, not smoke or drink alcohol, with NCDs and use concomitant medications, without the urological disease. Relative to participants in the lowest quartile, those with DASH score in the highest quartile had a lower level of BMI and SUA levels.
The dietary intake information including nutrients and food categories of the study participants according to DASH score quartiles is presented in Table 2. Participants with DASH score in the highest quartile showed a dietary trend consisting of a higher intake of protein, fiber, protein, fiber, magnesium accompanied by a higher ingestion of vegetables, nuts and legumes and whole grains. In contrast, those participants with lowest DASH score showed a higher consumption of saturated fat, total fat, cholesterol, sodium accompanied by a higher ingestion of red along with processed meat and fruit. The fraction of participants with dietary intake of dairy products was lower among participants with DASH score in the highest quartile whereas the reverse was true with sugar-sweetened beverages (SSB).
Table 3 provides the multiple linear regression data, exhibiting the association of DASH score with alteration in SUA contents. Following correcting for prospective various confounders (Model 2), an inverse association of the DASH score with SUA contents was reported (β = − 0.11; 95% CI − 0.12, − 0.1). In contrast to the first quartile, the β along with the 95% CI of Q2, Q3, and Q4 were − 0.08 (− 0.1, − 0.05), − 0.23 (− 0.25, − 0.2), and − 0.34 (− 0.37, − 0.31) (P-trend < 0.001) in Model 2.
Table 4 provides the multivariable-adjusted ORs along with the 95% CI for HUA on the basis of the DASH score. Following correcting for prospective confounders (Model 2), more adherence to the DASH dietary pattern exhibited beneficial effects on HUA (OR = 0.85; 95% CI 0.83, 0.87). Relative to the lowest quartile of DASH score, the ORs along with 95% CI for HUA were 0.88 (0.83, 0.94), 0.72 (0.68, 0.76) and 0.65 (0.61, 0.69) (P-trend < 0.001) in Model 2.
Table 5 provides the data of stratified assessment for the relationship of DASH score with HUA risk. The association between DASH score and odds of HUA were similar in sub-groups stratified by age group (p-interaction = 0.723), body mass index (p-interaction = 0.372) and health status (p-interaction = 0.762), and was stronger among male (p-interaction = 0.009; OR = 1.05; 95% CI = 1.01–1.1), non-Han Chinese (p-interaction < 0.001; OR = 0.83; 95% CI = 0.76–0.91) as well as rural residents(p-interaction < 0.001; OR = 0.89; 95% CI = 0.84–0.95).
Discussion
In this nationally representative cross-sectional research work, we established that the DASH diet was inversely linked to SUA contents and corresponding odds of HUA among 66,427 Chinese adults. These associations remained following adjusting for prospective confounders consisting of age, gender, body mass index, region, race, marital status, education background, physical activity, smoking status, drinking status, NCDs status and urological disease status.
As far as we know, this is the first research premise to reveal the beneficial impacts of DASH diet on SUA levels and odds of HUA among Chinese adults using a nationally representative sample. Our results were consistent with findings from several intervention studies conducted in the United States. In a 30-day randomized, crossover feeding trial, the DASH diet reduced SUA levels in adults with prehypertension or stage I hypertension, and the effect was stronger among those who had a baseline SUA level ≥ 7 mg/dL with an average decrease of 1.29 mg/dL in SUA levels which almost achieve the effect of pharmacologic urate-lowering therapy [20]. In another randomized, controlled, cross-over pilot investigation, the dietitian-directed, DASH-patterned groceries was found to reduce SUA levels modestly among gout participants not on urate-lowering drugs in 4 weeks [21]. Results from a parallel arm, controlled-randomized trial demonstrated that the DASH diet lowered SUA in the course of 30 days in prehypertensive or hypertensive adults, with a sustained effect at 90 days [31]. In addition, by partially replacing typical diet with DASH foods, a randomized trial lowered SUA contents in hypertensive African American with higher baseline contents of serum UA [22]. A similar observation was reported by two observational studies (refs). The first was a prospective cohort investigation of with 26 years of follow-up established that higher adherence to DASH diet in American males result in lower gout risk, illustrating that its influence of decreasing uric acid contents in participants with HUA corresponds a lower risk of gout [32]. The second was a cross-sectional investigation conducted in residents of the Kailuan community of Tangshan City, China, who are mainly Northern Han Chinese, found the DASH diet was linked to a low likelihood of developing HUA in adults [23]. Our study reconfirmed this beneficial effect in a sample of Chinese adults from different ethnic groups and regions throughout China, and thus could be generalizable to the entire Chinese population.
The mechanism underlying our findings are not known explicitly but could be related to the following factors. It has been suggested that insulin resistance alters glycolysis and renal handling of uric acid, thereby increasing the diversion of glycolysis inter-mediates to uric acid and decreasing urine uric acid clearance [33, 34]. The DASH diet has been proved to improve insulin resistance and increase insulin sensitivity significantly, and thus may have favourable effects on uric acid excretion [19, 35]. Substituting plant proteins of legumes and nuts for animal protein sources in the DASH diet may also be a reason for our results. High meat consumption, particularly red meat and seafood, have long been documented as a risk factor for HUA [10]. In contrast, higher soy food intake was found to be linked to lower serum uric acid levels in adults by a recent cross-sectional research conducted in Henan Province, China [36]. Previous studies have shown that although legumes are considered a purine-rich food, some special constituents such as phytic acid and polyphenols may have a beneficial influence on uric acid levels [37, 38]. In addition, actual purine intake may be lower due to the purines loss of soy foods during processing and cooking [39, 40]. At present, studies on the relation between nuts consumption and HUA risk are limited, but peanuts, one of the nuts commonly found in China, has been documented to have an positive impact on SUA levels [10]. Whole grain, as a important component of the DASH diet, may also contribute to the decrease in SUA levels. The consumption of whole grains improves glucose tolerance and peripheral insulin sensitivity, which may lead to a decrease in SUA levels [41]. Cereal fiber rich in whole grains has also been found to be negatively linked with blood uric acid contents and the odds of HUA in previous national representative studies in China and the United States [42, 43].
In subgroup analysis, we established that the effect of DASH diet were more strongly associated with HUA among men than women. This may be because men generally have higher levels of SUA than women, while the DASH diet may have a greater effect on those with higher levels of SUA [20]. Our study also explored the differences in the association between DASH diet and HUA among residents of different ethnic groups in China, and the results showed that the association was stronger among non-Han Chinese. Genetic factors may play an indispensable role in the risk association [10]. In addition, we established that the effect of DASH diet on HUA was significantly stronger in rural residents relative to urban residents. Higher levels of residential greenness and better sleeping patterns were documented to be related with lower risk of HUA in previous Chinese studies [44,45,46]. Therefore, we speculate that differences in living environment and lifestyle between urban and rural areas may be attributable to the effect modification.
The DASH diet is based on the Western dietary pattern and was initially developed for the American population. Over the past three decades, the dietary patterns of Chinese adults have shown a trend toward Western, mainly in the form of reduced intakes of vegetables and whole grains and increased intakes of red meat, processed meat, sugary drinks, saturated fats, and alcohol [47]. In this situation, the DASH dietary pattern as a healthy nutritional strategy may also contribute to the quality of the diet of the Chinese population. However, given the cultural and dietary differences, we still need to develop a modified DASH diet more suitable for our population, as Japan and Korea have done [17]. For example, since the Chinese diet has a low dairy product intake, we can increase calcium intake by increasing traditional foods such as beans and tofu. The development of the DASH diet for the Chinese population considering traditional staples, dishes, and other dietary habits will focus on our subsequent studies.
There are several strengths to our study. First, this is the first research premise to assess the relationship of the DASH diet with SUA levels and the odds of HUA among Chinese adults using a nationally representative sample. Thus, our results can be generalizable to the whole Chinese adults population. Second, the inverse association of the DASH diet with SUA contents and HUA was sustained following adjustment for numerous prospective confounding variables. Thirdly, Standardized methods as well as data collecting processes, and also training along with stringent quality control for all participants, assured the credibility of this research work’s findings.
Several limitations are also present in this research premise. Due to the cross-sectional aspect of this premise, we were unable to confirm a causal relationship of the DASH diet with SUA levels and HUA. Those conclusions would need to be confirmed by longitudinal studies. In addition, we could not exclude patients with gout who were finally enrolled in our study due to lack of information. According to the estimated prevalence of gout among Chinese adults in 2017 [48], there might be nearly 285 potential (0.429%) gout patients in our research. Therefore, the use of urate-lowering therapies and diuretics of these patients may still have an impact on our results although the number of these patients was small. At last, since we estimated levels of dietary intake using the 24-hour dietary recall method, long-term dietary intake may not accurately be reflected in our findings. Nevertheless, relative to the questionnaire of food frequency, the 24-hour recall yielded greater data on the kind and amount of food consumed, potentially reducing the possibility of under- or over-estimating nutrient intake levels [49].
Conclusions
Herein, a negative relationship of DASH diet with SUA contents and the odds of HUA among the Chinese adult population was reported. This association was stronger among man, non-Han Chinese and rural residents. These findings may provide new dietary strategies into the prevention and management of HUA.
Availability of data and materials
The data are not allowed to be disclosed according to the National Institute for Nutrition and Health, Chinese Center for Disease Control and Prevention.
Abbreviations
- SUA:
-
Serum uric acid
- HUA:
-
Hyperuricemia
- DASH:
-
Dietary Approaches to Stop Hypertension
- BMI:
-
Body mass index
- NCDs:
-
Noncommunicable diseases
References
Benn CL, Dua P, Gurrell R, et al. Physiology of Hyperuricemia and Urate-Lowering Treatments. Front Med (Lausanne). 2018;5:160. Published 2018 May 31. https://doi.org/10.3389/fmed.2018.00160.
Mortada I. Hyperuricemia, type 2 diabetes mellitus, and hypertension: an emerging association. Curr Hypertens Rep. 2017;19(9):69. https://doi.org/10.1007/s11906-017-0770-x.
Ponticelli C, Podestà MA, Moroni G. Hyperuricemia as a trigger of immune response in hypertension and chronic kidney disease. Kidney Int. 2020;98(5):1149–59. https://doi.org/10.1016/j.kint.2020.05.056.
Yanai H, Adachi H, Hakoshima M, Katsuyama H. Molecular biological and clinical understanding of the pathophysiology and treatments of hyperuricemia and its association with metabolic syndrome, cardiovascular diseases and chronic kidney disease. Int J Mol Sci. 2021;22(17):9221. https://doi.org/10.3390/ijms22179221.
Nishizawa H, Maeda N, Shimomura I. Impact of hyperuricemia on chronic kidney disease and atherosclerotic cardiovascular disease. Hypertens Res. 2022;45(4):635–40. https://doi.org/10.1038/s41440-021-00840-w.
Kuo C-F, Grainge MJ, Zhang W, Doherty M. Global epidemiology of gout: prevalence, incidence and risk factors. Nat Rev Rheumatol. 2015;11:649–62. https://doi.org/10.1038/nrrheum.2015.91.
Zhang M, Zhu X, Wu J, et al. Prevalence of hyperuricemia among Chinese adults: findings from two nationally representative cross-sectional surveys in 2015-16 and 2018-19. Front Immunol. 2022;12:791983. Published 2022 Feb 7. https://doi.org/10.3389/fimmu.2021.791983.
Nuki G, Simkin PA. A concise history of gout and hyperuricemia and their treatment. Arthritis Res Ther. 2006;8 Suppl 1(Suppl 1):S1. https://doi.org/10.1186/ar1906.
Choi HK, Liu S, Curhan G. Intake of purine-rich foods, protein, and dairy products and relationship to serum levels of uric acid: the third National Health and nutrition examination survey. Arthritis Rheum. 2005;52(1):283–9. https://doi.org/10.1002/art.20761.
Major TJ, Topless RK, Dalbeth N, Merriman TR. Evaluation of the diet wide contribution to serum urate levels: meta-analysis of population based cohorts. BMJ. 2018;363:k3951. Published 2018 Oct 10. https://doi.org/10.1136/bmj.k3951.
Cox CL, Stanhope KL, Schwarz JM, et al. Consumption of fructose- but not glucose-sweetened beverages for 10 weeks increases circulating concentrations of uric acid, retinol binding protein-4, and gamma-glutamyl transferase activity in overweight/obese humans. Nutr Metab (Lond). 2012;9(1):68. Published 2012 Jul 24. https://doi.org/10.1186/1743-7075-9-68.
Carabin IG, Lyon MR, Wood S, Pelletier X, Donazzolo Y, Burdock GA. Supplementation of the diet with the functional fiber PolyGlycoplex®is well tolerated by healthy subjects in a clinical trial. Nutr J. 2009;8:9. https://doi.org/10.1186/1475-2891-8-9.
Jacobs DR, Tapsell LC. Food synergy: the key to a healthy diet. Proc Nutr Soc. 2013;72(2):200–6. https://doi.org/10.1017/S0029665112003011.
Fam AG. Gout, diet, and the insulin resistance syndrome. J Rheumatol. 2002;29(7):1350–5.
Dessein PH, Shipton EA, Stanwix AE, Joffe BI, Ramokgadi J. Beneficial effects of weight loss associated with moderate calorie/carbohydrate restriction, and increased proportional intake of protein and unsaturated fat on serum urate and lipoprotein levels in gout: a pilot study. Ann Rheum Dis. 2000;59(7):539–43. https://doi.org/10.1136/ard.59.7.539.
Appel LJ, Moore TJ, Obarzanek E, et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH collaborative research group. N Engl J Med. 1997;336(16):1117–24. https://doi.org/10.1056/NEJM199704173361601.
Song Y, Lobene AJ, Wang Y, Hill Gallant KM. The DASH diet and Cardiometabolic health and chronic kidney disease: a narrative review of the evidence in east Asian countries. Nutrients. 2021;13(3):984. Published 2021 Mar 18. https://doi.org/10.3390/nu13030984.
McCormick N, O'Connor MJ, Yokose C, et al. Assessing the causal relationships between insulin resistance and hyperuricemia and gout using bidirectional Mendelian randomization. Arthritis Rheum. 2021;73(11):2096–104. https://doi.org/10.1002/art.41779.
Asemi Z, Tabassi Z, Samimi M, Fahiminejad T, Esmaillzadeh A. Favourable effects of the dietary approaches to stop hypertension diet on glucose tolerance and lipid profiles in gestational diabetes: a randomised clinical trial. Br J Nutr. 2013;109(11):2024–30. https://doi.org/10.1017/S0007114512004242.
Juraschek SP, Gelber AC, Choi HK, Appel LJ, Miller ER 3rd. Effects of the dietary approaches to stop hypertension (DASH) diet and sodium intake on serum uric acid. Arthritis Rheum. 2016;68(12):3002–9. https://doi.org/10.1002/art.39813.
Juraschek SP, Miller ER 3rd, Wu B, et al. A randomized pilot study of DASH patterned groceries on serum urate in individuals with gout. Nutrients. 2021;13(2):538. Published 2021 Feb 7. https://doi.org/10.3390/nu13020538.
Juraschek SP, White K, Tang O, Yeh HC, Cooper LA, Miller ER 3rd. Effects of a dietary approach to stop hypertension (DASH) diet intervention on serum uric acid in African Americans with hypertension. Arthritis Care Res. 2018;70(10):1509–16. https://doi.org/10.1002/acr.23515.
Gao Y, Cui LF, Sun YY, et al. Adherence to the dietary approaches to stop hypertension diet and hyperuricemia: a cross-sectional study. Arthritis Care Res. 2021;73(4):603–11. https://doi.org/10.1002/acr.24150.
Song PK, Man QQ, Li H, Pang SJ, Jia SS, Li YQ, et al. Trends in lipids level and dyslipidemia among Chinese adults, 2002-2015. Biomed Environ Sci. 2019;32:559–70. https://doi.org/10.3967/bes2019.074.
Liu Z, Zhao L, Man Q, Wang J, Zhao W, Zhang J. Dietary micronutrients intake status among Chinese elderly people living at home: data from CNNHS 2010-2012. Nutrients. 2019;11(8):1787. https://doi.org/10.3390/nu11081787.
Yang Y, Wang G, Pan X. Chinese food composition table, 2nd ed. Beijing: Peking University Medical Press; 2009.
Yang YX. China food composition tables, Standard Edition. Beijing: Peking University Medical Press; 2018.
Mellen PB, Gao SK, Vitolins MZ, Goff DC Jr. Deteriorating dietary habits among adults with hypertension: DASH dietary accordance, NHANES 1988–1994 and 1999–2004. Arch Intern Med. 2008;168:308–14. https://doi.org/10.1001/archinternmed.2007.119.
Chen J, Chen H, Feng J, Zhang L, Li J, Li R, et al. Association between hyperuricemia and metabolic syndrome in patients suffering from bipolar disorder. BMC Psychiatry. 2018;18:390. https://doi.org/10.1186/s12888-018-1952-z.
Li C, Kang B, Zhang T, Gu H, Song P, Chen J, et al. Dietary pattern and dietary energy from fat associated with sarcopenia in community-dwelling older Chinese people: a cross-sectional study in three regions of China. Nutrients. 2020;12:3689. https://doi.org/10.3390/nu12123689.
Tang O, Miller ER 3rd, Gelber AC, Choi HK, Appel LJ, Juraschek SP. DASH diet and change in serum uric acid over time. Clin Rheumatol. 2017;36(6):1413–7. https://doi.org/10.1007/s10067-017-3613-x.
Rai SK, Fung TT, Lu N, Keller SF, Curhan GC, Choi HK. The dietary approaches to stop hypertension (DASH) diet, Western diet, and risk of gout in men: prospective cohort study. BMJ. 2017;357:j1794. Published 2017 May 9. https://doi.org/10.1136/bmj.j1794.
Leyva F, Wingrove CS, Godsland IF, Stevenson JC. The glycolytic pathway to coronary heart disease: a hypothesis. Metabolism. 1998;47(6):657–62. https://doi.org/10.1016/s0026-0495(98)90026-9.
Facchini F, Chen YD, Hollenbeck CB, Reaven GM. Relationship between resistance to insulin-mediated glucose uptake, urinary uric acid clearance, and plasma uric acid concentration. JAMA. 1991;266(21):3008–11.
Rahimi H, Yuzbashian E, Zareie R, et al. Dietary approaches to stop hypertension (DASH) score and obesity phenotypes in children and adolescents. Nutr J. 2020;19(1):112. Published 2020 Oct 4. https://doi.org/10.1186/s12937-020-00631-y.
Cui N, Dong X, Xue Y, et al. Gender-specific inverse associations between beans intake, serum urate levels, and hyperuricemia: a cross-sectional analysis based on the Henan rural cohort study. Front Nutr. 2021;7:593599. Published 2021 Jan 21. https://doi.org/10.3389/fnut.2020.593599.
Ikenaga T, Noguchi H, Kakumoto K, et al. Effect of phytic acid on postprandial serum uric acid level in healthy volunteers: a randomized, double-blind, crossover study. Nucleosides Nucleotides Nucleic Acids. 2020;39(4):504–17. https://doi.org/10.1080/15257770.2019.1656337.
Mehmood A, Zhao L, Wang C, et al. Management of hyperuricemia through dietary polyphenols as a natural medicament: a comprehensive review. Crit Rev Food Sci Nutr. 2019;59(9):1433–55. https://doi.org/10.1080/10408398.2017.1412939.
Yamakita J, Yamamoto T, Moriwaki Y, Takahashi S, Tsutsumi Z, Higashino K. Effect of tofu (bean curd) ingestion and on uric acid metabolism in healthy and gouty subjects. Adv Exp Med Biol. 1998;431:839–42. https://doi.org/10.1007/978-1-4615-5381-6_161.
Wolfram G. Ernährungstherapie bei Gicht [Diet therapy in gout]. Ther Umsch. 1995;52(8):524–7.
McKeown NM. Whole grain intake and insulin sensitivity: evidence from observational studies. Nutr Rev. 2004;62(7 Pt 1):286–91. https://doi.org/10.1111/j.1753-4887.2004.tb00054.x.
Sun Y, Sun J, Zhang P, Zhong F, Cai J, Ma A. Association of dietary fiber intake with hyperuricemia in U.S. adults. Food Funct. 2019;10(8):4932–40. https://doi.org/10.1039/c8fo01917g.
Zhu Q, Yu L, Li Y, et al. Association between Dietary Fiber Intake and Hyperuricemia among Chinese Adults: Analysis of the China Adult Chronic Disease and Nutrition Surveillance (2015). Nutrients. 2022;14(7):1433. Published 2022 Mar 30. https://doi.org/10.3390/nu14071433.
Dong X, Liu X, Zhang L, et al. Residential greenness associated with lower serum uric acid levels and hyperuricemia prevalence in a large Chinese rural population. Sci Total Environ. 2021;770:145300. https://doi.org/10.1016/j.scitotenv.2021.145300.
Wang Y, Zeng Y, Zhang X, et al. Daytime napping duration is positively associated with risk of hyperuricemia in a Chinese population. J Clin Endocrinol Metab. 2021;106(5):e2096–105. https://doi.org/10.1210/clinem/dgab043.
Yu X, Gong S, Chen J, et al. Short sleep duration increases the risk of hyperuricemia among Chinese adults: findings from the China health and nutrition survey. Sleep Med. 2021;84:40–5. https://doi.org/10.1016/j.sleep.2021.05.014.
He Y, Li Y, Yang X, et al. The dietary transition and its association with cardiometabolic mortality among Chinese adults, 1982-2012: a cross-sectional population-based study. Lancet Diabetes Endocrinol. 2019;7(7):540–8. https://doi.org/10.1016/S2213-8587(19)30152-4.
Tang YM, Zhang L, Zhu SZ, et al. Gout in China, 1990-2017: the global burden of disease study 2017. Public Health. 2021;191:33–8. https://doi.org/10.1016/j.puhe.2020.06.029.
Bingham SA, Gill C, Welch A, et al. Comparison of dietary assessment methods in nutritional epidemiology: weighed records v. 24 h recalls, food-frequency questionnaires and estimated-diet records. Br J Nutr. 1994;72(4):619–43. https://doi.org/10.1079/bjn19940064.
Acknowledgments
We are grateful to all participants and staff members involved in the China Adult Chronic Disease and Nutrition Surveillance (2015). Q.Z. thanks the China Scholarship Council for a scholarship.
Funding
This study was supported by the National Health Commission of the People’s Republic of China Medical Reform Major Program: China National Chronic Diseases and Nutrition Surveillance of Adults (2015–2017). The study was also financed by the National Institute for Nutrition and Health, China CDC project: Research on Dietary and Nutritional Status of Chinese Elderly (No. 150052). Q.Z. is funded by a China Scholarship Council PhD Scholarship (No. 202109110099). There was no influence of the funders on the design of the investigation, data collection, or analysis, or on the decision to publish or the preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
J.Z.: contributed to the study concept, design and critical revision of the manuscript for important intellectual content; L.Y., Y.L.,Q.M.,S.J.,B.L.: acquired the data; Q.Z., Y.Z.,H.Z.,W.Z.: analyzed the data; Q.Z. wrote the manuscript; all authors: reviewed and approved the final manuscript. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Nation Institute for Nutrition and Health, Chinese Center for Disease Control and Prevention (protocol code: 201519-B; date of approval: June 2015). Informed consent was obtained from all participants involved in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
Nutrient targets for DASH score.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhu, Q., Yu, L., Li, Y. et al. Association between dietary approaches to stop hypertension (DASH) diet and hyperuricemia among Chinese adults: findings from a nationwide representative study. Nutr J 22, 21 (2023). https://doi.org/10.1186/s12937-023-00845-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12937-023-00845-w