Abstract
Objectives
Epidemiologic studies have reported that diet is associated with diabetes and its complications through different pathways. We sought to examine the associations between the Dietary Approaches to Stop Hypertension (DASH) diet and the odds of diabetic nephropathy (DN) developing in Iranian women with existing type 2 diabetes.
Methods
In this case–control study, 105 women with DN and 105 controls, matched for age and diabetes duration, were selected from the Kowsar Diabetes Clinic in Semnan, Iran. DASH, estimated using dietary intake, was assessed using a validated and reliable food frequency questionnaire with 147 items. Anthropometric measurements were assessed for all subjects. Logistic regression was performed to examine the association between DASH and the odds of developing DN.
Results
After controlling for potential confounders, subjects in the highest intake of DASH diet adherence have 84% lower odds of DN, compared to those with the lowest intake (OR = 0.16, 95% CI = 0.07–0.34, P < 0.001). Among DASH diet subcategories, intakes of vegetables (80%), fruits (88%), nuts and legumes (87%), and low-fat dairy (73%) decreased the risk of DN after adjustment for confounders (P < 0.001).
Conclusions
This study showed that the DASH diet is associated with lower odds of DN development in women with type 2 diabetes.
Similar content being viewed by others
Introduction
Diabetic nephropathy (DN) is one the most important of diabetes complications that can lead to renal dysfunction and end-stage renal disease (ESRD) in diabetic patients [1, 2]. In DN, proteins excrete from kidney [3], and high blood pressure (BP), decreasing kidney function, and excretion of more than 300 mg/day of protein are criteria for DN [2]. Many previous studies have indicated that family history, ethnicity, gestational diabetes, dyslipidemia, obesity, hypertension, and insulin resistance are major risk factors for DN [4]. About 50% of global ESRD is due to diabetes [5], and predictions indicate that 7.7% of the global population will have diabetes by 2030 [6,7,8]. Additional reports have demonstrated that the rate of diabetes will increase by about 70% between 2010 and 2030 [6,7,8], whilst according to a study in Iran, the prevalence of DN was reported to be about 31% [2]. DN, as one of the most serious microvascular complications of diabetes, accounts for 4 million mortalities per year in the world [8,9,10]. As the incidence of type 2 diabetes (T2D) increases, efforts to stop the progression of diabetes to diabetic kidney disease and ESRD are essential. Modification of risk factors, such as precise control of blood glucose, and management of hypertension and hyperlipidemia may contribute to delayed progression of diabetic kidney disease [6]. Diet can be used to control hypertension and blood glucose [11, 12], where it appears that greater adherence to plant-based diets and fewer processed foods can have positive affects diabetic nephropathy [13].
One of the diets that can help with DN is the dietary approaches to stop hypertension diet (DASH). In the DASH diet, individuals are encouraged to consume whole grains, fruits, vegetables, low-fat dairy products, legumes, seeds, fish, and poultry (lean meats), but, on the other hand, consumption of saturated fat, red meats, sweets, and sugar-sweetened beverages (SSBs) is limited [14, 15]. Higher amounts of protein, fiber, magnesium, calcium, potassium, antioxidant components, and unsaturated fatty acids in this diet, can help to reduce the risk of diabetes [16]. Studies have demonstrated that adherence to the DASH diet can prevent diabetes [11]. However, some clinical trials reported inconsistent results about the relationship between the DASH diet and diabetes [12, 17,18,19]. In one study, it was noted that adherence to the DASH diet, concomitant to an exercise program, can effect diabetes in persons with hypertension and overweight [20]. Further, blood pressure, as a risk factor for kidney diseases and diabetic nephropathy, is shown to be reduced by adherence to the DASH diet [21].
Given the high prevalence of diabetes and its progression to complications, such as DN and eventually ESRD, as well as the economic burden on health systems, it is important to discern preventative measures for DN progression. Since the DASH diet is effective in controlling both diabetes and BP, which are serious factors in the progression of DN, we sought to examine the relationship between DASH diet and odds of DN.
Methods
Study population
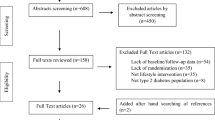
In the current case–control study, 210 women (105 cases and 105 controls) were recruited from the Kowsar Diabetes Clinic by convenience sampling in Semnan, Iran. 105 diabetic women without DN were recruited as the control group by a 1:1 matching to the DN cases, by age at 1-year intervals and by the duration of diabetes in 6-months intervals, from the same center. The inclusion criteria were women with T2D, aged between 30 and 65 years, and with a history of 3–10 years of T2D. The definition of diabetes used in this study is based on the American Diabetes Association (ADA) criteria: 2-h post-load blood glucose (2hrBG) ≥ 200 mg/dl or fasting blood glucose (FBG) ≥ 126 mg/dl; glycosylated hemoglobin (HbA1c) ≥ 6.5% [22]. The exclusion criteria were having autoimmune disorders or previous history of cancer, coronary angiography, hepatic disease, myocardial infarction, or stroke. Total energy intake of < 500 or > 3500 kcal/day and/or poor response to the food-frequency questionnaire (FFQ) were also considered as exclusion criteria. DN was defined as urinary albumin-to-creatinine ratio (ACR) ≥ 30 mg/g in a random spot urine sample in the present study [23]. This work was approved by the Ethics Committee of Tehran University of Medical Sciences (Ethics Number: IR.TUMS.REC.1395.2644) and the Ethics Committee of Semnan University of Medical Sciences (Ethics Number: IR. SEMUMS. REC.1395.66) and was conducted in line with the guidelines of the Declaration of Helsinki.
Dietary intake assessment and DASH diet score calculation
A validated FFQ was used to assess dietary intake of participants [24]. Then, participants reported their intake of food items daily, weekly, monthly, or yearly. Portion sizes were finally converted into g/day using household measurements. After that, these amounts were adjusted for energy intake using the residual method [25]. For estimating the energy and nutrient intakes, dietary intakes were analyzed using NUTRITIONIST 4 (First Data Bank, San Bruno, CA) software. For computing the DASH diet score, the components were classified into groups, based on their intake ranking. The component score for nuts and legumes, fruits, vegetables, low-fat dairy products, and whole grains were ranked by decile. For example, decile 1 was assigned 1 point and decile 10, 10 points. For sodium, sweetened beverages, red and processed meats, lower intakes were desired. Hence, the lowest decile was given a score of 10 points and the highest 0 points. The component scores were finally summed to obtain an overall DASH score, ranging from 0 to 80 [26].
Assessment of other variables
Age, diabetes duration, medical history, and current drug usage were recorded by trained interviewers. Weight (kg) was measured while subjects were wearing light clothing, without shoes. Body mass index (BMI, kg/m2) was computed as weight (kg) divided by the square of height (m). After a resting period ≥ 5 min, BP was measured on the left arm using a manual sphygmomanometer. A validated physical activity questionnaire (IPAQ) [27] was utilized to evaluate individuals’ physical activity (PA).
Blood biomarkers assessment
Participant’s past 3 months medical records were used to obtain their fasting blood sugar (FBS), 2hrBG, HbA1c, total cholesterol (TC), triglycerides (TG), low-density lipoprotein (LDL), high-density lipoprotein (HDL), total serum creatinine (Cr), and blood urea nitrogen (BUN) levels.
Statistical analysis
The distribution of the quantitative variables was assessed using the Kolmogorov–Smirnov test. Independent samples T tests and chi-square tests were used to compare quantitative and qualitative variables between cases and controls, and they were presented as mean ± SD and frequency (%), respectively. Energy-adjusted dietary intakes, across DASH diet scores, were compared using independent samples T tests. To investigate the relationship between DASH diet and DN, logistic regression was used to determine the odds ratio (OR) of DN and its 95% confidence interval (CI).
Linear regression analysis was used to determine the relationship between DASH score and its subcategories with biochemical markers, in crude and adjusted models. In adjusted models, energy intake, age, PA, BMI, cardiovascular disease history, and type of drug used (angiotensin receptor blockers, angiotensin-converting enzyme inhibitors, beta-blockers, metformin, sulphonylurea, and insulin) were controlled. Data analysis was performed using SPSS software (Version 25, SPSS Inc., Chicago, IL, USA) and P < 0.05 was, a priori, considered statistically significant.
Results
Participants and study characteristics across case and control group
Overall, 210 subjects were enrolled in the study, including 105 cases and 105 control with DM. The basic characteristics of the participants, according to case and control status, are presented in Table 1. There was a significant difference between serum albumin, ACR, Hb1Ac, LDL, creatinine, Angiotensin receptor blockers (ARBs), and Angiotensin-converting enzyme (ACEIs) usage (P < 0.001). The frequency of cases and controls across following DASH diet are shown in Fig. 1. Counts of patients above vs below the median of DASH diet scores were significantly different (P < 0.001). 64.5% of subjects who were in the lower adherence group had DN, whilst 35.5% of patients with higher adherence had DN. On other hand, mean ± SD of age, BMI, and diabetes duration of cases was 55.33 ± 7.04 (years), 28.686 ± 4.74 (kg/m2), and 7.60 ± 2.21 (years), respectively (P > 0.05). Finally, mean ± SD of FBS (167.10 ± 50.62) and cholesterol level (185.15 ± 38.12) (mg/dl) were marginally higher among the cases group (P < 0.05).
Dietary intakes of cases and controls across lower and higher adherence of DASH diet
The DASH diet subgroups, macronutrients, fat and water soluble vitamins, minerals, and other parts of dietary intakes were reported in Table 2. Overall, the adherence to DASH diet was divided to two groups of above and below the median. Among DASH diet subcategories, consumption of fruits, vegetables, nuts and legumes, and dairy products were increased in both case and control groups (P < 0.05). Meat intake was decreased among case and control groups, whilst sweetened beverages and whole grains intake were significantly different between lower and higher adherence of DASH diet in the case group with DN (P < 0.05).
The DN cases consumed significantly more magnesium and zinc (P < 0.05). In addition, energy, protein, carbohydrate, and fat intake were decreased with increasing DASH diet adherence in the control group (P < 0.05). Among simple sugars, consumption of sucrose was significantly reduced in higher DASH diet adherence in both cases and controls (P < 0.05).
Baseline characteristics of cases and controls groups among lower and higher adherence of median of DASH diet
The general characteristics and dietary intakes of the participants in increasing adherence of the DASH diet were shown in Table 3. The participants in the highest column of the DASH diet had lower albumin serum level (6.11 ± 5.097), height (159.93 ± 6.177), BUN (0.87 ± 0.18), and higher creatinine level (0.87 ± 0.18), after adjusting for confounders including age, energy intake, and physical activity, across the control group (P < 0.05). Compared to the control group, diastolic blood pressure (DBP), FBS, and HbA1c levels were decreased in case groups after adjusting for mentioned confounders, aligned with higher adherence to the DASH diet(P < 0.05).
Association between adherence to a DASH-style diet and the odds of DN
The ORs for DN across the lowest and highest median of the DASH diet subcategories were detailed in Table 4. In the crude model of total DASH diet, participants in the high adherence group of the DASH diet were 74% less likely to have DN (ORs: 0.26; 95% CI 0.14–0.47; P < 0.001). After controlling for energy intake, age, physical activity, and BMI, adherence to the DASH diet remained negatively associated with the odds of DN (ORs: 0.20; 95% CI 0.10–0.38; P < 0.001) (Model 1). In model 2, which was adjusted for model 1 + diabetes duration, cardiovascular diseases history, and drug usage (ARBs, ACEIs, beta-blockers, metformin, sulphonyl urea, and insulin), the inverse association remained (OR: 0.16; 95% CI 0.07–0.34; P < 0.001). Among DASH diet subcategories, intakes of vegetables (80%), fruits (88%), nuts and legumes (87%), and low-fat dairy (73%) decreased the risk of DN in model 2 (P < 0.001). In addition, intake of whole grains was inversely associated with high risk of DN among women (OR: 0.51; 95% CI 0.27–0.95; P = 0.03) in model 2. Moreover, logistic regression showed that red meat consumption increased the risk of DN in the model 2 (OR: 1.20; 95% CI 0.10–0.41; P < 0.001).
Association between DASH-style diet and biochemical markers of participants
The association between DASH score and subcategories with biochemical markers, are presented in Table 5. There was no significant association between FBS, TG, HDL, total cholesterol and BUN with DASH score and subcategories (P > 0.05). However, there was an inverse relationship between DASH score and both creatinine (β = − 0.08, 95% CI = − 0.14, − 0.01, P value = 0.02) and DBP (β = − 6.50, 95% CI = − 11.91, − 1.08, P value = 0.01), after adjusting for potential confounders the results remained significant. Among DASH diet subcategories, low fat dairy was inversely related to LDL (β = − 16.28, 95% CI = − 29.7, − 2.77, P value = 0.01) (in model 2), DBP (β = − 6.41, 95% CI = − 11.63, − 1.20, P value = 0.01), and Alb (β = − 6.85, 95% CI = − 2.11, − 11.59, P value = 0.005) (in crude and adjusted model). Additionally, vegetables maintained negative association with ACR (β = − 45.05, 95% CI = − 90.95, 0.84, P value = 0.04), and SBP (β = − 9.09, 95% CI = − 15.94, − 2.25, P value = 0.01) after adjusting in model 1 and 2. Fruits also displayed a negative association with creatinine in model 2 (β = − 0.06, 95% CI = − 0.13, − 0.001, P value = 0.04). Moreover, nuts and legumes were inversely associated with ACR (β = − 76.73, 95% CI = − 130.05, − 23.42, P value = 0.005) and Alb (β = − 7.04, 95% CI = − 12.82, − 1.27, P value = 0.01), after adjusting for confounders, the results remained significant. Whole grains had a negative relationship with both creatinine after adjustment (β = − 0.07, 95% CI = − 0.14, 0.01, P value = 0.02), and DBP (β = − 6.47, 95% CI = − 11.72, − 1.22, P value = 0.01). Besides, sodium demonstrated a positive association with DBP (β = − 6.58, 95% CI = − 13.19, − 0.51, P value = 0.03), which remained significant after adjusting. Red meat was also positively related to creatinine (β = − 0.096, 95% CI = − 0.16, 0.02, P value = 0.009) after adjusting in model 2.
Discussion
The present study demonstrated that higher adherence to the DASH diet yielded a 74% reduction in the odds of DN. These findings support our hypothesis that people who adhere to the DASH diet decrease the risk of DN among the adult population. After adjusting for confounders, such as energy intake, age, physical activity, and BMI, the DASH diet remained negatively associated with the odds of DN. Thus, the DASH diet appears to be effective in controlling the risk of DN among adults. To our knowledge, the present study is the first to examine the effects of the DASH eating pattern and subcategories on DN and other biochemical markers among type 2 diabetes.
As the incidence of T2D increases, efforts to stop the progression of diabetes to diabetic kidney disease and ESRD are essential [28]. There are several main risk factors for DN, including, family history, ethnicity, gestational diabetes, dyslipidemia, hyperglycemia, obesity, being a smoker, high blood cholesterol, hypertension, and insulin resistance. The DASH diet, which promotes the consumption of fruits, vegetables, low-fat dairy products, and sodium restriction, was created for people with hypertension [29]. This diet is rich in antioxidants, unsaturated fatty acids, fiber, and low-fat dairy, all of which may be crucial for reducing insulin resistance, inflammatory levels, and metabolic disruption [30].
In the present study we found that, there was an inverse relationship between DASH score and serum Cr. In line with our study, a cross-sectional study of participants with T2D in Taiwan demonstrated that greater adherence to a healthy diet and higher intake of fish and vegetables was negatively correlated with serum Cr, and positively associated with eGFR [31]. Given our results, nuts and legumes were inversely associated with ACR and Alb. The DASH diet plan’s positive benefits on metabolic parameters could possibly be attributed to a larger consumption of legumes. Additionally, the DASH diet includes more soy products, which may be linked to better cardio-metabolic health and lower plasma levels of CRP [32]. The DASH study shown that despite an increase in calories from protein, the DASH diet did not raise urinary albumin excretion rate [33].
In our study, we indicated an inverse association between DASH score and DBP. Previous investigations indicated that this diet can be used to control hypertension and blood glucose, according to recent studies [11, 12]. It appears that greater adherence to plant-based diets and less consumption of processed foods can have a positive impact on DN [13]. In the DASH diet, individuals are encouraged to consume whole grains, fruits, vegetables, low-fat dairy products, legumes, seeds, fish, and poultry (lean meats), whilst consumption of saturated fat, red meats, sweets, and SSBs is restricted [14, 15]. Higher amounts of protein, fiber, magnesium, calcium, potassium, antioxidant components, and unsaturated fatty acids in this diet, can help to reduce the risk of diabetes [34]. Several studies have demonstrated the association between the DASH diet and the risk of diseases; for instance, the insulin resistance atherosclerosis study demonstrated that adherence to the DASH dietary pattern may have the potential to prevent T2D [11].
In the present study, vegetables maintained negative association with ACR and SBP after adjusting for confounding variables. Additionally, in an adjusted model, Fruits also displayed a negative association with Cr.
A prospective cohort study among south Korean found that intake of a diet rich in fruits, and vegetables were associated with a decreased risk of CKD [35]. The DASH trial demonstrated that dietary patterns rich in vegetables, fruit, and low-fat dairy products can reduce systolic and diastolic blood pressure [36]. Hypertension is a risk factor for kidney diseases and also DN, which is reduced by adherence to the DASH diet, according to recent studies [21]. Concomitantly intervention studies have revealed that the DASH diet has beneficial effects on total and LDL cholesterol, insulin sensitivity, and weight management [37, 38]. Moreover, in postmenopausal women without diabetes, better adherence to the DASH diet was associated with a lower prevalence of metabolic syndrome, according to a cross-sectional study using Korean National Health and Nutrition Examination Survey (KNHANES) [39]. Additionally, the DASH diet can significantly protect against cardiovascular disease (CVD) 20%, Coronary heart disease (CHD) 21%, stroke 19%, and Heart failure (HF) 29%, according to a systematic review and meta-analysis of observational prospective studies [38]. In addition, elderly adults with a high intake of the DASH-style diet were reported to have lower odds of having Chronic kidney disease (CKD), according to a cross-sectional study using the KNHANES data [40]. Furthermore, the DASH diet has been associated with a lower risk of kidney damage, particularly the decline in glomerular filtration rate [35, 41]. Additionally, the relationship between the DASH diet and CKD progression or CKD-related complications has not been reported in East Asia [42]. Some clinical trial studies have demonstrated inconsistent results regarding the relationship between the DASH diet and diabetes [43, 44]. In addition, adherence to the DASH diet with a routine exercise program can affect diabetes in persons with hypertension and overweight [38]. The National Health and Nutrition Survey, Japan, found that better adherence to the DASH diet was inversely associated with metabolic risk factors, including waist circumference, TC, LDL cholesterol, and BMI [45]. Generally, the potential direct relationship between greater adherence to the DASH and kidney function has been largely explained by positive impacts of this diets on levels of cardiometabolic risk factors including blood pressure [46, 47], glycemic control [11], and lipid profile [48].
Furthermore, concordant with our hypothesis, there is an association between the DASH diet and the risk of DN. Evidence regarding the association between diets and DN patients is scarce; however, a case–control study among 105 women demonstrated that adherence to the DASH was inversely and dose-dependently associated with risk of DN [49]. Moreover, a prospective cohort study revealed that a DASH-style diet was associated with a lower risk for CKD and DN [50]. In addition, a prospective study using data from the Singapore Chinese Health Study found that greater adherence to a DASH-style diet was associated with a 29% lower risk of developing T2D [51]. The DASH dietary pattern may be beneficial both in the prevention and management of diabetes mellitus nephropathy [41].
The strengths of our study include that, to best our knowledge, this is the first study to have examined the association of DASH diet and risk of DN among Iranian adults, as well as considering a wide range of confounders. On the other hand, our study has several limitations that should be noted. The case–control design of the study precludes casual inferences. The second limitation is the use of FFQ for dietary assessment, which might result in misclassification of participants. Although we controlled for several confounding variables, the existence of residual confounding cannot be excluded. Furthermore, as in all epidemiologic studies, random errors might affect our results because diet and lifestyle information might be collected with some degree of error.
Conclusion
We found that DASH diet adherence may be associated with lower odds of DN. Further studies, with large sample sizes, are needed to confirm the veracity of this association.
Availability of data and materials
The authors confirm that the data supporting the findings of this study are available within the manuscript and in the included tables.
Abbreviations
- ACR:
-
Albumin-to-creatinine ratio
- ADA:
-
American Diabetes Association
- ARBs:
-
Angiotensin receptor blockers
- ACEIs:
-
Angiotensin-converting enzyme
- ANCOVA:
-
Analysis of covariance
- BMI:
-
Body mass index
- BUN:
-
Blood urea nitrogen
- BP:
-
Blood pressure
- CI:
-
Confidence interval
- CKD:
-
Chronic kidney disease
- Cr:
-
Creatinine
- CVD:
-
Cardiovascular disease
- CHD:
-
Coronary heart disease
- DASH:
-
Dietary approaches to stop hypertension diet
- DBP:
-
Diastolic blood pressure
- DN:
-
Diabetic nephropathy
- ESRD:
-
End-stage renal disease
- FBG:
-
Fasting blood glucose
- FBS:
-
Fasting blood sugar
- FFQ:
-
Fod-frequency questionnaire
- 2hrBG:
-
2-Hour post-load blood glucose
- HF:
-
Heart failure
- HbA1c:
-
Glycosylated hemoglobin
- HDL:
-
High-density lipoprotein
- KNHANES:
-
Korean National Health and Nutrition Examination Survey
- LDL:
-
Low-density lipoprotein
- IPAQ:
-
International Physical Activity Questionnaire
- OR:
-
Odds ratio
- PA:
-
Physical activity
- SSBs:
-
Sugar-sweetened beverages
- SDs:
-
Standard deviations
- T2D:
-
Type 2 diabetes
- TC:
-
Total cholesterol
- TG:
-
Triglycerides
- TUMS:
-
Tehran University of Medical Sciences
References
System USRD. 2015 USRDS annual data report: epidemiology of kidney disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive; 2015.
Rabieenia E, Jalali R, Mohammadi M. Prevalence of nephropathy in patients with type 2 diabetes in Iran: a systematic review and meta-analysis based on geographic information system (GIS). Diabetes Metab Syndr. 2020;14(5):1543–50.
Ansar MM, ShahrokhiRad R, Lebady MK. Risk factors of microalbuminuria and macroalbuminuria in type-2 diabetic patients in North of Iran-Rasht. Nephrourol Mon. 2016.
Raile K, Galler A, Hofer S, Herbst A, Dunstheimer D, Busch P, et al. Diabetic nephropathy in 27,805 children, adolescents, and adults with type 1 diabetes: effect of diabetes duration, A1C, hypertension, dyslipidemia, diabetes onset, and sex. Diabetes Care. 2007;30(10):2523–8.
Tuttle KR, Bakris GL, Bilous RW, Chiang JL, De Boer IH, Goldstein-Fuchs J, et al. Diabetic kidney disease: a report from an ADA Consensus Conference. Diabetes Care. 2014;37(10):2864–83.
Sawaf H, Thomas G, Taliercio JJ, Nakhoul G, Vachharajani TJ, Mehdi A. Therapeutic advances in diabetic nephropathy. J Clin Med. 2022;11(2):378.
Wang N, Zhu F, Chen L, Chen K. Proteomics, metabolomics and metagenomics for type 2 diabetes and its complications. Life Sci. 2018;212:194–202.
CE ML, San Martín Ojeda CA, JJ RP, CJ FZ. Pathophysiology of diabetic nephropathy: a literature review. Medwave. 2017;17(1):e6839-e.
Papadopoulou-Marketou N, Chrousos GP, Kanaka-Gantenbein C. Diabetic nephropathy in type 1 diabetes: a review of early natural history, pathogenesis, and diagnosis. Diabetes Metab Res Rev. 2017;33(2):e2841.
Tesch GH. Diabetic nephropathy—Is this an immune disorder? Clin Sci. 2017;131(16):2183–99.
Liese AD, Nichols M, Sun X, D’Agostino RB Jr, Haffner SM. Adherence to the DASH Diet is inversely associated with incidence of type 2 diabetes: The insulin resistance atherosclerosis study. Diabetes Care. 2009;32(8):1434–6.
Lopes HF, Martin KL, Nashar K, Morrow JD, Goodfriend TL, Egan BM. DASH diet lowers blood pressure and lipid-induced oxidative stress in obesity. Hypertension. 2003;41(3):422–30.
Saneei P, Hashemipour M, Kelishadi R, Esmaillzadeh A. The Dietary Approaches to Stop Hypertension (DASH) diet affects inflammation in childhood metabolic syndrome: a randomized cross-over clinical trial. Ann Nutr Metab. 2014;64(1):20–7.
Sacks FM, Moore TJ, Appel LJ, Obarzanek E, Cutler JA, Vollmer WM, et al. A dietary approach to prevent hypertension: a review of the Dietary Approaches to Stop Hypertension (DASH) Study. Clin Cardiol. 1999;22(S3):6–10.
Vogt TM, Appel LJ, Obarzanek E, Moore TJ, Vollmer WM, Svetkey LP, et al. Dietary approaches to stop hypertension: rationale, design, and methods. J Am Diet Assoc. 1999;99(8):S12–8.
Blumenthal JA, Babyak MA, Sherwood A, Craighead L, Lin P-H, Johnson J, et al. Effects of the dietary approaches to stop hypertension diet alone and in combination with exercise and caloric restriction on insulin sensitivity and lipids. Hypertension. 2010;55(5):1199–205.
Lien LF, Brown AJ, Ard JD, Loria C, Erlinger TP, Feldstein AC, et al. Effects of PREMIER lifestyle modifications on participants with and without the metabolic syndrome. Hypertension. 2007;50(4):609–16.
Al-Solaiman Y, Jesri A, Mountford WK, Lackland DT, Zhao Y, Egan BM. DASH lowers blood pressure in obese hypertensives beyond potassium, magnesium and fibre. J Hum Hypertens. 2010;24(4):237–46.
Al-Solaiman Y, Jesri A, Zhao Y, Morrow JD, Egan BM. Low-Sodium DASH reduces oxidative stress and improves vascular function in salt-sensitive humans. J Hum Hypertens. 2009;23(12):826–35.
Azizi F, Rahmani M, Emami H, Mirmiran P, Hajipour R, Madjid M, et al. Cardiovascular risk factors in an Iranian urban population: Tehran lipid and glucose study (phase 1). Sozial-und präventivmedizin. 2002;47(6):408–26.
Liese AD, Roach AK, Sparks KC, Marquart L, D’Agostino RB Jr, Mayer-Davis EJ. Whole-grain intake and insulin sensitivity: the insulin resistance atherosclerosis study. Am J Clin Nutr. 2003;78(5):965–71.
Targets G. Standards of medical care in diabetes-2019. Diabetes Care. 2019;42(Suppl 1):S61-s70.
Molitch ME, DeFronzo RA, Franz MJ, Keane WF, Mogensen CE, Parving HH, et al. Nephropathy in diabetes. Diabetes Care. 2004;27(Suppl 1):S79-83.
Esfahani FH, Asghari G, Mirmiran P, Azizi F. Reproducibility and relative validity of food group intake in a food frequency questionnaire developed for the Tehran Lipid and Glucose Study. J Epidemiol. 2010;20(2):150–8.
Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr. 1997;65(4 Suppl):1220S-1228S (discussion 9S–31S).
Mirzababaei A, Khorsha F, Togha M, Yekaninejad MS, Okhovat AA, Mirzaei K. Associations between adherence to dietary approaches to stop hypertension (DASH) diet and migraine headache severity and duration among women. Nutr Neurosci. 2020;23(5):335–42.
Craig CL, Marshall AL, Sjöström M, Bauman AE, Booth ML, Ainsworth BE, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35(8):1381–95.
Lea JP, Nicholas SB. Diabetes mellitus and hypertension: key risk factors for kidney disease. J Natl Med Assoc. 2002;94(8 Suppl):7S.
Vollmer WM, Sacks FM, Ard J, Appel LJ, Bray GA, Simons-Morton DG, et al. Effects of diet and sodium intake on blood pressure: subgroup analysis of the DASH-sodium trial. Ann Intern Med. 2001;135(12):1019–28.
Azadbakht L, Mirmiran P, Esmaillzadeh A, Azizi T, Azizi F. Beneficial effects of a Dietary Approaches to Stop Hypertension eating plan on features of the metabolic syndrome. Diabetes Care. 2005;28(12):2823–31.
Hsu C-C, Jhang H-R, Chang W-T, Lin C-H, Shin S-J, Hwang S-J, et al. Associations between dietary patterns and kidney function indicators in type 2 diabetes. Clin Nutr. 2014;33(1):98–105.
Azadbakht L, Atabak S, Esmaillzadeh A. Soy protein intake, cardiorenal indices, and C-reactive protein in type 2 diabetes with nephropathy: a longitudinal randomized clinical trial. Diabetes Care. 2008;31(4):648–54.
Jacobs DR Jr, Gross MD, Steffen L, Steffes MW, Yu X, Svetkey LP, et al. The effects of dietary patterns on urinary albumin excretion: results of the Dietary Approaches to Stop Hypertension (DASH) Trial. Am J Kidney Dis. 2009;53(4):638–46.
Smith PJ, Blumenthal JA, Babyak MA, Craighead L, Welsh-Bohmer KA, Browndyke JN, et al. Effects of the dietary approaches to stop hypertension diet, exercise, and caloric restriction on neurocognition in overweight adults with high blood pressure. Hypertension. 2010;55(6):1331–8.
Banerjee T, Crews DC, Tuot DS, Pavkov ME, Burrows NR, Stack AG, et al. Poor accordance to a DASH dietary pattern is associated with higher risk of ESRD among adults with moderate chronic kidney disease and hypertension. Kidney Int. 2019;95(6):1433–42.
Conlin PR, Chow D, Miller ER, Svetkey LP, Lin P-H, Harsha DW, et al. The effect of dietary patterns on blood pressure control in hypertensive patients: results from the Dietary Approaches to Stop Hypertension (DASH) trial. Am J Hypertens. 2000;13(9):949–55.
Razavi Zade M, Telkabadi MH, Bahmani F, Salehi B, Farshbaf S, Asemi Z. The effects of DASH diet on weight loss and metabolic status in adults with non-alcoholic fatty liver disease: a randomized clinical trial. Liver Int. 2016;36(4):563–71.
Shirani F, Salehi-Abargouei A, Azadbakht L. Effects of Dietary Approaches to Stop Hypertension (DASH) diet on some risk for developing type 2 diabetes: a systematic review and meta-analysis on controlled clinical trials. Nutrition. 2013;29(7–8):939–47.
Kim HY, Lee J, Kim J. Association between dietary inflammatory index and metabolic syndrome in the general Korean Population. Nutrients. 2018;10(5):648.
Jo YS, Choi SM, Lee J, Park YS, Lee S-M, Yim J-J, et al. The relationship between chronic obstructive pulmonary disease and comorbidities: a cross-sectional study using data from KNHANES 2010–2012. Respir Med. 2015;109(1):96–104.
Rebholz CM, Crews DC, Grams ME, Steffen LM, Levey AS, Miller ER III, et al. DASH (Dietary Approaches to Stop Hypertension) diet and risk of subsequent kidney disease. Am J Kidney Dis. 2016;68(6):853–61.
Song Y, Lobene AJ, Wang Y, Hill Gallant KM. The DASH diet and cardiometabolic health and chronic kidney disease: a narrative review of the evidence in East Asian Countries. Nutrients. 2021;13(3):984.
Paula TP, Viana LV, Neto AT, Leitão CB, Gross JL, Azevedo MJ. Effects of the DASH diet and walking on blood pressure in patients with type 2 diabetes and uncontrolled hypertension: a randomized controlled trial. J Clin Hypertens (Greenwich). 2015;17(11):895–901.
Hosseinpour-Niazi S, Mirmiran P, Hadaegh F, Mahdavi M, Khalili D, Daneshpour MS, et al. Improvement of glycemic indices by a hypocaloric legume-based DASH diet in adults with type 2 diabetes: a randomized controlled trial. Eur J Nutr. 2022.
Murakami K, Livingstone MBE, Sasaki S. Diet quality scores in relation to metabolic risk factors in Japanese adults: a cross-sectional analysis from the 2012 National Health and Nutrition Survey. Japan Eur J Nutr. 2019;58(5):2037–50.
Forman JP, Stampfer MJ, Curhan GC. Diet and lifestyle risk factors associated with incident hypertension in women. JAMA. 2009;302(4):401–11.
Toledo E, Carmona-Torre FDA, Alonso A, Puchau B, Zulet MA, Martinez JA, et al. Hypothesis-oriented food patterns and incidence of hypertension: 6-year follow-up of the SUN (Seguimiento Universidad de Navarra) prospective cohort. Public Health Nutr. 2010;13(3):338–49.
Ajala O, English P, Pinkney J. Systematic review and meta-analysis of different dietary approaches to the management of type 2 diabetes. Am J Clin Nutr. 2013;97(3):505–16.
Jayedi A, Mirzaei K, Rashidy-Pour A, Yekaninejad MS, Zargar MS, Akbari Eidgahi MR. Dietary approaches to stop hypertension, mediterranean dietary pattern, and diabetic nephropathy in women with type 2 diabetes: a case-control study. Clin Nutr ESPEN. 2019;33:164–70.
Yuzbashian E, Asghari G, Mirmiran P, Amouzegar-Bahambari P, Azizi F. Adherence to low-sodium Dietary Approaches to Stop Hypertension-style diet may decrease the risk of incident chronic kidney disease among high-risk patients: a secondary prevention in prospective cohort study. Nephrol Dial Transplant. 2018;33(7):1159–68.
Chen GC, Koh WP, Neelakantan N, Yuan JM, Qin LQ, van Dam RM. Diet quality indices and risk of type 2 diabetes mellitus: the Singapore Chinese health study. Am J Epidemiol. 2018;187(12):2651–61.
Acknowledgements
We thank the school of Nutritional and Dietetics at Tehran University of medical sciences and participants in this investigation.
Funding
This work was supported by the Tehran University of Medical Sciences (Grant Number: 94-04-161-31155).
Author information
Authors and Affiliations
Contributions
KM designed the study. AM, FA, SH, NB, SN, AMB, and DH contributed to the literature searches, data collection, and measurements. AM drafted the manuscript. KM, SH, CC, and AM critically revised the manuscript; and agree to be fully accountable for ensuring the integrity and accuracy of the work. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study protocol was approved by the Ethics Commission of Tehran University of Medical Sciences (Ethics Number: IR.TUMS.REC.1395.2644) and the Ethics Committee of Semnan University of Medical Sciences (Ethics Number: IR. SEMUMS. REC.1395.66). Then, written informed consent was obtained from all patients. All methods were performed in accordance with the declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Mirzababaei, A., Abaj, F., Hajishizari, S. et al. The association of dietary approaches to stop hypertension (DASH) with the odds of diabetic nephropathy and metabolic markers in women: a case–control study. BMC Women's Health 23, 63 (2023). https://doi.org/10.1186/s12905-022-02140-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12905-022-02140-y