Abstract
Background
Endothelial dysfunction serves as an early marker for the risk of cardiovascular disease (CVD); therefore, it is an attractive site of therapeutic interventions to reduce the risk of CVD. This study was conducted to investigate the effect of folic acid supplementation on endothelial function markers in randomized controlled trials (RCTs).
Methods
PubMed, ISI web of science, and Scopus databases were searched up to July 2022 for detecting eligible studies. A random-effects model was used for meta-analysis, and linear Meta-regression and non-linear dose-response analysis were performed to assess whether the effect of folic acid supplementation was affected by the dose and duration of intervention. Cochrane tools were also used to assess the risk of bias in the included studies.
Results
Twenty-one studies, including 2025 participants (1010 cases and 1015 controls), were included in the present meta-analysis. Folic acid supplementation significantly affected the percentage of flow-mediated dilation (FMD%) (WMD: 2.59%; 95% CI: 1.51, 3.67; P < 0.001) and flow-mediated dilation (FMD) (WMD: 24.38 μm; 95% CI: 3.08, 45.68; P = 0.025), but not end-diastolic diameter (EDD) (WMD: 0.21 mm; 95% CI: − 0.09, 0.52; P = 0.176), and intercellular adhesion molecule (ICAM) (WMD: 0.18 ng/ml; 95% CI: − 10.02, 13.81; P = 0.755).
Conclusions
These findings suggest that folic acid supplementation may improve endothelial function by increasing FMD and FMD% levels.
Trial registration
PROSPERO registration cod: CRD42021289744.
Similar content being viewed by others
Background
The importance of healthy endothelium has been increasingly recognized in the maintenance of normal vascular function [1]. Endothelial cells have a notable role in the preservation of vascular integrity, preventing platelet aggregation, regulating thrombosis, and angiogenesis through releasing of different signaling molecules [2, 3]. However, vascular problems resulting from conditions like angioplasty, stenting, diabetes, hypertension, and imbalances in the production of vasodilator molecules (nitric oxide (NO)) and vasoconstrictor substances (endothelin), can lead to endothelial dysfunction [4,5,6]. Loss of normal endothelial functions (endothelial dysfunction) plays a pivotal role in the progression of atherosclerosis and coronary artery disease (CAD) and it can be accompanied by various cardiovascular risk factors [7,8,9]. Consequently, maintaining a healthy endothelium could represent a promising therapeutic approach for the prevention of these pathological conditions [10]. Studies have suggested that supplements with endothelium-protective properties might be beneficial in the management of cardiovascular diseases [11].
Endothelial dysfunction is a systemic disorder and a key variable in the pathogenesis of atherosclerosis and its complications [12]. Several biological markers, including intercellular adhesion molecule (ICAM), flow-mediated dilation (FMD), and end-diastolic diameter (EDD), have been used as indicators of endothelial dysfunction. A systemic increase in the expression of adhesion molecules (e.g., ICAM) on the surface of endothelial cells can increase the risk of endothelium dysfunction [12]. Soluble forms of these molecules in the blood increase, which can be assessed by laboratory tests using blood serum. FMD of the brachial artery, an index of endothelium-dependent vasodilation, reflects NO production in the endothelium and correlates with coronary artery endothelial function [13]. EDD is commonly used to determine endothelium dysfunction in vascular tone modulation [14], which is dependent on a multitude of factors, ranging from NO production, prostanoids, endothelin-1, and other endothelium-derived hyperpolarizing factors [15].
Epidemiological studies have demonstrated a reduction in cardiovascular risk within societies with folic acid-fortified foods [16, 17]. Folic acid (an oxidized form of folate with high bioavailability) insufficiency has been associated with endothelial dysfunction and increased incidence of cardiovascular diseases (CVD)s [18]. Several promising findings suggest that folic acid effectively reduces atherogenesis by improving oxidative stress, inflammation, blood pressure, lipid profile, and glycemic control [19,20,21,22,23]. Homocysteine may be responsible for vascular endothelial cell dysfunction that occurs at the onset CVD pathology [24]. However, the underlying mechanism of action of folic acid has not been fully established. Decreased folate concentrations and/or homocysteine in high doses can increase inflammation [25]. In endothelial cells, inflammatory cytokines result in increased circulating levels of intercellular adhesion molecule (ICAM) and vascular cell adhesion molecule (VCAM); these are transmembrane proteins that promote endothelial dysfunction [8]. Folic acid reduces homocysteine levels through its anti-inflammatory characteristics with a decline in the expression of ICAM-1 [25, 26]. In addition, another important indicator of endothelial dysfunction that can be addressed is flow-mediated dilation (FMD) [27, 28]. A substantial body of evidence suggests that significant improvement in FMD is evident after folic acid supplementation [29,30,31,32], while others do not support these findings [33,34,35]. Furthermore, there is conflicting and uncertain evidence regarding the effectiveness of folic acid on ICAM levels [31, 36].
Based on the above considerations, and also to address the inconsistency in the literature, we conducted this comprehensive systematic review, dose-response, meta-regression, and meta-analysis study to investigate the effectiveness of folic acid supplementation on biomarkers of endothelial function in adults.
Methods
This study was performed in line with the preferred reporting items for systematic reviews and meta-analyses (PRISMA) protocol for reporting systematic reviews and meta-analyses [37]. Moreover, we designed this meta-analysis based on PICOS criteria (Population: adults, Intervention: folic acid, Comparison: control group, Outcome: endothelial function parameters, Study: clinical trials).
Search strategy
We systematically reviewed electronic databases including PubMed/Medline, Scopus, and ISI Web of Science to find relevant RCTs up to July 2022. The MESH and non-MESH terms were used as follows: ((“folate” OR “folic acid” OR “Vitamin M” OR “Vitamin B9” OR “Folacin” OR “Folvite” OR “Pteroylglutamic Acid” OR “folates” OR “tetrahydrofolates” OR “Formyltetrahydrofolates”) AND (“Endothelium function” OR “FMD” OR “endothelin” OR “end-diastolic diameter” OR “EDD” OR “intercellular adhesion molecule” OR “ICAM”) AND (intervention OR “controlled trial” OR randomized OR random OR randomly OR placebo OR “clinical trial” OR trial OR “randomized clinical trial” OR RCT OR trial OR trials “Cross-Over Studies” OR “Cross-Over” OR “Cross-Over Study” OR parallel OR “parallel study” OR “parallel trial”) (Supplementary Table 1). No restriction was made on the year of publication or language of the identified papers. We conducted a manual search in google scholar and the reference lists of the related publications to avoid the possibility of missing any eligible studies. Unpublished records were also not considered.
Study selection and eligibility criteria
All recorded articles found by electronic or manual searches were exported into EndNote software for screening (EndNote X8, Thomson Reuters, New York). The title and abstract of all publications found in the initial search were evaluated independently by two investigators (D.A.L. and B.N.). To select eligible articles, the following criteria were considered: a) the population (adults aged ≥18 years); b) RCTs with either parallel or crossover design investigating the effects of folic acid supplementation on endothelial function; c) studies that reported means and standard deviations (SDs) for indicators of endothelial function (EDD, FMD%, FMD, and ICAM) or any other effect sizes, by which the calculation of means±SDs is possible; d) studies that were placebo-controlled; e) intervention was tried for 1 week or longer durations. For multiple papers from the same dataset, the most complete ones are selected. Clinical trials with an additional arm were considered two separate studies. Articles were excluded if they: a) employed children, adolescent, or pregnant women; b) were letters, comments, short communications, reviews, meta-analyses, ecologic studies, and experimental studies; c) had no control group; d) examined the impact of acid folic in combination with other ingredients where the independent effect of folic acid could not be determined.
Data extraction
Two independent reviewers (M.R.K. and S.S.) performed the study selection, and a chief researcher (O.A) was responsible for resolving any conflicts. The subsequent information was extracted from the eligible trials: first author’s name, year of publication, study location, study design (parallel or cross-over), gender, the health status of participants, study sample size, the duration of interventions, supplementation dosage, the mean age of participants, and the mean ± SD of the EDD, FMD%, FMD and ICAM levels throughout the trial for the intervention and control groups. We converted the data reported in different units for endothelial function measures to the most frequently used ones. All studies reported FMD in percentage and micrometers (μm), and also reported EDD in millimeters (mm). For ICAM we converted all units to nanograms per milliliter (ng/ml).
Risk of bias assessment
The Cochrane scoring system was used to evaluate the quality of the included studies [38]. This tool contained seven domains including: 1) random sequence generation, 2) allocation concealment, 3) blinding of participants and personnel, 4) blinding of outcomes assessors, 5) incomplete outcome data reporting, 6) selective reporting, and 7) other sources of bias. Each domain was given a “high risk” score if RCT comprised methodological defects that may have affected the results, a “low risk” score if the defect was considered ineffectual and an “unclear risk” score if the information was not sufficient to determine the impact. If the trial had “low risk” for all domains, it was labeled as a high-quality study with a totally low risk of bias.
Statistical analysis
For data analysis, we utilized Stata software version 14 (StataCorp, College Station, Texas). Furthermore, we used mean change and standard deviation (SD) of the EDD, FMD%, FMD, and ICAM levels to assess the pooled effect size. Effect sizes for all variables were listed as weighted mean differences (WMDs) and 95% confidence interval (CI) [39]. When the SD of the mean difference was not reported, we calculated it using the following formula: SD change = square root ([SD baseline] 2 + [SD final] 2 – [2R × SD baseline × SD final]) [40]. For studies that only reported standard error of the mean (SEM), SD was obtained using the following formula: SD = SEM × √n, where “n” is the number of subjects in each group. Cochrane’s Q test (significance accepted at P < 0.05) and I2 index were used to determine heterogeneity between studies. We assessed the presence of potential sources of between-study heterogeneity through subgroup analysis based on trial duration, intervention dose, and health status. Subgroup analyses were conducted based on, the duration of intervention (8 ≥ vs. 8 < weeks), the dosage of folic acid supplement (≥5 vs. < 5 mg/day), and health status (CVD vs. no-CVD). The potential non-linear dose-response relationship between dosage and duration of folic acid supplementation was examined by fractional polynomial modeling. Meta-regression analysis was executed to evaluate the association between pooled effect size and folic acid dosage (mg/day) and duration of intervention). A sensitivity analysis was also performed to determine the effect of each trial on the pooled effect size [41]. Publication bias was assessed by the funnel plot inspection as well as Egger’s test.
Certainty assessment
The overall certainty of evidence across the studies was graded according to the guidelines of the GRADE (Grading of Recommendations Assessment, Development, and Evaluation) Working Group. The quality of evidence was classified into four categories, according to the corresponding evaluation criteria: high, moderate, low, and very low [42].
Results
Study selection
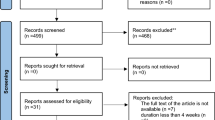
The flowchart of the screening and study selection process is shown in Fig. 1. When we initiated the search, we identified a total of 628 records and recognized and removed 233 duplicates in the resultant set. Furthermore, through the title and abstract screening of 395 articles, 367 were removed. After reviewing the full texts of the remaining 28 articles, 7 trials were excluded due to not reporting the required information. Finally, 21 RCTs were included in the final meta-analysis [29,30,31,32,33,34,35,36, 43,44,45,46,47,48,49,50,51,52,53,54,55].
Characteristics of the included studies
The general features of included studies are outlined in Table 1. The 21 eligible studies were published between 1999 and 2016 and had 1–52 weeks of follow-up. A total of 2025 participants were included (1010 cases and 1015 controls). These studies were carried out in the United Kingdom [33, 34, 43,44,45,46,47,48, 52], Australia [35], USA [50], China [32, 53], Iran [55], Italy [54], Greece [29], Netherland [36, 50], Canada [31] and Belgium [51]. Daily supplemental dosage of folic acid varied between 0.4 and 10 mg/day across the studies. Twelve studies had a parallel design [29, 30, 33, 34, 36, 46, 47, 49, 50, 52,53,54,55], and the rest were cross-over [31, 32, 35, 43,44,45, 48, 51]. All studies included both sexes [29,30,31,32,33,34,35,36, 43,44,45,46,47,48,49,50,51,52,53], except 2 studies that enrolled just female subjects [54, 55]. The sample size in the included trials ranged from 17 [32] to 530 [49] participants. The mean age of the individuals ranged from 26 [54] to 66 [36] years old and the mean baseline BMI varied from 24 [55] to 29 [50] kg/m2. Participants in these studies were patients with coronary artery disease (CAD) [30, 34, 46,47,48, 50, 52], type 2 diabetes mellitus (T2DM) [31, 36], acute myocardial infarction [51], hyperhomocysteinaemia [43], hypercholesterolemia [29], predialysis renal failure [33], polycystic ovary syndrome [54], preeclampsia [55], and healthy adults [32, 35, 45, 49, 53].
Quality assessment
Based on the Cochrane risk assessment, most of the included studies had a high risk of bias [29,30,31,32,33,34,35, 43, 44, 46,47,48,49,50,51,52,53,54], however, 3 RCTs had a moderate risk of bias [36, 45, 55] (Table 2).
The effects of folic acid supplementation on EDD
Findings from analysis of 9 effect sizes, including 542 subjects (269 cases and 273 controls), demonstrated that folic acid supplementation did not significantly affect the level of EDD compared to placebo (WMD: 0.21 mm; 95% CI: − 0.10, 0.53; P = 0.176), with significant heterogeneity among the studies (I2 = 88.8%, P < 0.001) (Fig. 2a). Moreover, subgroup analyses did not reveal significant effects (Table 3).
The effects of folic acid supplementation on FMD%
A total of 7 effect sizes, including 360 subjects (186 cases and 174 controls), evaluated the effects of folic acid supplementation on FMD%. The pooled analysis using a random-effects model indicated a significant elevation in FMD% levels following folic acid supplementation compared to placebo (WMD: 2.59%; 95% CI: 1.51, 3.67; P < 0.001), with a significant degree of between-study heterogeneity (I2 = 90.0%, P < 0.001) (Fig. 2b). Folic acid supplementation increased FMD% in all subgroups (Table 3).
The effects of folic acid supplementation on FMD
Overall, 10 effect sizes, including 836 subjects (408 cases and 428 controls), evaluated the effects of folic acid supplementation on FMD levels. The pooled analysis using a random-effects model indicated a significant elevation in FMD levels following folic acid supplementation compared to placebo (WMD: 24.38 μm; 95% CI: 3.09, 45.68; P = 0.025), with a significant degree of between-study heterogeneity (I2 = 98.6%, P < 0.001) (Fig. 2c). Based on subgroup analysis, we found that folic acid supplementation increased FMD levels when the intervention dose was ≥5 mg/day and the intervention conducted in the CVD group (Table 3).
The effects of folic acid supplementation on ICAM
The meta-analysis of three effect sizes involving 609 individuals (306 cases and 303 controls) revealed no significant change in ICAM levels after folic acid supplementation (WMD: 1.90 ng/ml; 95% CI: − 10.02, 13.82; P = 0.755), with a high heterogeneity between studies (I2 = 82.1%, P = 0.004) (Fig. 2d). Due to the limited number of studies, subgroup analysis was not possible.
Sensitivity analysis and publication bias
The sensitivity analysis suggested no significant changes following the sequential removal of each study performed for FMD%, FMD, and ICAM. However, by excluding Thambyrajah et al. study, the overall effect of folic acid supplementation on EDD was significantly changed (WMD: 0.32 mm; 95% CI: 0.00, 0.63). Furthermore, visual inspection of the funnel plot (Fig. 3a-d) and Egger’s test revealed no evidence of publication bias for studies evaluating the effects of folic acid supplementation on EDD (P = 0.263), FMD% (P = 0.100), FMD (P = 0.174), and ICAM (P = 0.642).
Linear Meta-regression analyses between dose and duration of folic acid supplementation and endothelial function measures
Meta-regression analysis did not show a linear relationship between dose and changes in EDD (Coefficient = 1.83, P = 0.15), FMD% levels (Coefficient = − 0.42, P = 0.13), and FMD levels (Coefficient = − 0.01, P = 0.68) (Fig. 4a-c). In addition, no significant relationship was found between the duration of intervention and changes in EDD (Coefficient = 0.33, P = 0.78) and FMD (Coefficient = 0.02, P = 0.66) levels (Fig. 5a, b). However, there is a significant relationship between the duration of intervention and changes in FMD% (Coefficient = − 3.40, P = 0.03) (Fig. 5c).
Non-linear dose–response analyses between dose and duration of folic acid supplementation and endothelial function measures
Non-linear dose-response analysis demonstrated that there is a significant relationship between dose of intervention and changes in FMD (Coefficient = 1058.98, P = 0.035) (Fig. 6a), but not for EDD (Coefficient = − 15.24, P = 0.119) and FMD% (Coefficient = 0.61, P = 0.145) (Fig. 6b, c). Moreover, a significant relationship was shown between the duration of the intervention and changes in EDD (Coefficient = − 1989.69, P = 0.005) (Fig. 7a), but not for FMD% (Coefficient = 0.35, P = 0.051) and FMD (Coefficient = − 790.68, P = 0.272) (Fig. 7b, c).
GRADE assessment
Based on the GRADE assessment, the quality of evidence for EDD and ICAM was very low due to very serious limitations in inconsistency and serious limitations in imprecision. There was also a low quality of evidence for FMD% and FMD because of very serious limitation in inconsistency (Table 4).
Discussion
The present meta-analysis of RCTs showed that supplementation with folic acid increases FMD% compared to placebo group. In addition, high doses of folic acid (≥ 5 mg/day) and intervention in CVD patients increases FMD. However, there was no significant difference between folic acid supplementation and placebo groups regarding the levels of EDD, and ICAM.
Previous observational studies have identified an inverse link between both folic acid intake and blood folate concentration and cardiovascular health [56, 57]. Indeed, a meta-analysis involving 82,334 participants showed a 10% lower risk of stroke and a 4% lower risk of overall CVD with folic acid supplementation, especially among participants with lower plasma folate levels [58].
Folic acid exerts its protective effects against endothelial dysfunction through several mechanisms.
Folic acid improves NO bioavailability via 1) increased endothelial nitric oxide (NO) synthase (eNOS) dimerization [59] and enhancing the effectiveness of BH4 on eNOS uncoupling [60] and 2) serving as a direct scavenger of reactive oxygen species, which preserves bioavailable NO, both of which are mediated independent of its homocysteine-lowering effect [61].
Endothelial dysfunction, mechanistically induced by the loss of NO bioavailability [62], plays an initial role in the pathogenesis of atherosclerosis [63, 64]. Further, folate has been identified to improve endothelial dysfunction via homocysteine lowering pathways [65]. The link between elevated plasma homocysteine and endothelial cell damage has been well established and is mediated through attenuating the amount of available NO [66, 67].
Although the abovementioned mechanisms indicate both homocysteine-dependent and independent impacts of folate on endothelial function, the evidence suggests that simply lowering plasma homocysteine does not seem to improve CVD outcomes. Indeed, some studies have shown that folate supplementation with dosages ≥5 mg/day is required for its endothelial benefits, even without further decrease in homocysteine levels [31, 46, 68].
The FMD is a frequently used method to test endothelial dysfunction which indicates the bioavailability of endothelium-derived NO [62]. Our results are consistent with the findings of previous studies that demonstrated an improvement in FMD with high doses of folic acid (≥5 mg) in unhealthy subjects [46, 50]. However; some studies have reported that despite a significant reduction in total serum homocysteine, low-dose folic acid (400 μg/day) did not affect endothelial function in healthy adults [45, 49]. Doshi et al. demonstrated a significant improvement in FMD with folic acid supplementation, even before the reduction in plasma homocysteine concentration occurred, indicating that the enhancement was independent of the changes in homocysteine levels [46].
Inflammation is pivotally involved in all stages of atherosclerosis [69]. Endothelial cell dysfunction (ECD) is the earliest detectable manifestation of atherosclerotic lesions. The injured endothelium begins to increase the expression of adhesion molecules, such as E-selectin, vascular cell adhesion molecule-1 (VCAM-1), and ICAM-1, which recruits monocytes and T lymphocytes to the endothelium and leads to the inflammatory response propagation [70, 71]. Our findings from three RCTs demonstrated that supplementation with folic acid was not associated with any significant change in plasma concentrations of ICAM. However, the lack of improvement in ICAM concentrations in this study does not preclude a direct or indirect impact of folate on ICAM levels, as it might be influenced by other mediatory factors, such as blood homocysteine levels which we did not include in our data analysis. Further future large-scale studies need to be carried out on healthy populations and in subjects with reversible vascular dysfunction to better discern the effect of FA supplementation.
The present study possesses notable strengths. First, to our knowledge, this is the first systematic review and meta-analysis to investigate the effects of folic acid supplementation on a range of biomarkers of endothelial dysfunction. Another strength of this meta-analysis relates to the inclusion of several long-term studies, which has the advantage of documenting the long-term effects of folic acid supplementation on endothelial markers and allowing comparisons to shorter-duration designs. However, the existence of publication bias and heterogeneity in the analysis should be interpreted as limitations to this study.
Conclusion
In conclusion, our data suggest that pharmacological doses of folic acid supplementation, especially in higher doses (≥ 5 mg/day), were associated with a significant improvement in endothelial function as measured with FMD and FMD%. Further in vivo mechanistic research in humans will elucidate the protective and therapeutic roles of folate in the development and progression of endothelial dysfunction in both healthy and unhealthy populations. Finally, more research is required to establish the safety of long-term intake before recommending it as a routine medication.
Availability of data and materials
Not applicable.
References
Vanhoutte PM, Shimokawa H, Tang EH, Feletou M. Endothelial dysfunction and vascular disease. Acta Physiol. 2009;196(2):193–222.
Godo S, Shimokawa H. Endothelial functions. Arterioscler Thromb Vasc Biol. 2017;37(9):e108–e14.
Lara J, Ashor AW, Oggioni C, Ahluwalia A, Mathers JC, Siervo M. Effects of inorganic nitrate and beetroot supplementation on endothelial function: a systematic review and meta-analysis. Eur J Nutr. 2016;55(2):451–9.
Gallo G, Pierelli G, Forte M, Coluccia R, Volpe M, Rubattu S. Role of oxidative stress in the process of vascular remodeling following coronary revascularization. Int J Cardiol. 2018;268:27–33.
Sena CM, Carrilho F, Seiça RM. Endothelial dysfunction in type 2 diabetes: targeting inflammation. Endothelial Dysfunct. 2018;24:23110.
Strohbach A, Pennewitz M, Glaubitz M, Palankar R, Groß S, Lorenz F, et al. The apelin receptor influences biomechanical and morphological properties of endothelial cells. J Cell Physiol. 2018;233(8):6250–61.
Gori T, Münzel T. Oxidative stress and endothelial dysfunction: therapeutic implications. Ann Med. 2011;43(4):259–72.
Medina-Leyte DJ, Zepeda-García O, Domínguez-Pérez M, González-Garrido A, Villarreal-Molina T, Jacobo-Albavera L. Endothelial dysfunction, inflammation and coronary artery disease: potential biomarkers and promising Therapeutical approaches. Int J Mol Sci. 2021;22(8):3850.
Sun HJ, Wu ZY, Nie XW, Bian JS. Role of endothelial dysfunction in cardiovascular diseases: the link between inflammation and hydrogen sulfide. Front Pharmacol. 2019;10:1568.
Shafabakhsh R, Milajerdi A, Reiner Ž, Kolahdooz F, Amirani E, Mirzaei H, et al. The effects of catechin on endothelial function: a systematic review and meta-analysis of randomized controlled trials. Crit Rev Food Sci Nutr. 2020;60(14):2369–78.
Konukoglu D, Uzun H. Endothelial dysfunction and hypertension. Hypertension. 2016;956:511–40.
Bonetti PO, Lerman LO, Lerman A. Endothelial dysfunction: a marker of atherosclerotic risk. Arterioscler Thromb Vasc Biol. 2003;23(2):168–75.
Maruhashi T, Kajikawa M, Kishimoto S, Hashimoto H, Takaeko Y, Yamaji T, et al. Diagnostic criteria of flow-mediated vasodilation for Normal endothelial function and nitroglycerin-induced vasodilation for Normal vascular smooth muscle function of the brachial artery. J Am Heart Assoc. 2020;9(2):e013915.
Chia PY, Teo A, Yeo TW. Overview of the assessment of endothelial function in humans. Front Med (Lausanne). 2020;7:542567.
Vanhoutte PM, Shimokawa H, Feletou M, Tang EH. Endothelial dysfunction and vascular disease - a 30th anniversary update. Acta Physiol (Oxf). 2017;219(1):22–96.
Bentley TG, Weinstein MC, Willett WC, Kuntz KM. A cost-effectiveness analysis of folic acid fortification policy in the United States. Public Health Nutr. 2009;12(4):455–67.
Yang Q, Botto LD, Erickson JD, Berry RJ, Sambell C, Johansen H, et al. Improvement in stroke mortality in Canada and the United States, 1990 to 2002. Circulation. 2006;113(10):1335–43.
Wang L, Li H, Zhou Y, Jin L, Liu J. Low-dose B vitamins supplementation ameliorates cardiovascular risk: a double-blind randomized controlled trial in healthy Chinese elderly. Eur J Nutr. 2015;54(3):455–64.
Asbaghi O, Salehpour S, Rezaei Kelishadi M, Bagheri R, Ashtary-Larky D, Nazarian B, et al. Folic acid supplementation and blood pressure: a GRADE-assessed systematic review and dose-response meta-analysis of 41,633 participants. Crit Rev Food Sci Nutr. 2021:1–16.
Asbaghi O, Ghanavati M, Ashtary-Larky D, Bagheri R, Rezaei Kelishadi M, Nazarian B, et al. Effects of folic acid supplementation on oxidative stress markers: a systematic review and Meta-analysis of randomized controlled trials. Antioxidants (Basel). 2021;10(6):871.
Asbaghi O, Ashtary-Larky D, Bagheri R, Nazarian B, Pourmirzaei Olyaei H, Rezaei Kelishadi M, et al. Beneficial effects of folic acid supplementation on lipid markers in adults: a GRADE-assessed systematic review and dose-response meta-analysis of data from 21,787 participants in 34 randomized controlled trials. Crit Rev Food Sci Nutr. 2021:1–19.
Asbaghi O, Ashtary-Larky D, Bagheri R, Moosavian SP, Olyaei HP, Nazarian B, et al. Folic acid supplementation improves glycemic control for diabetes prevention and management: a systematic review and dose-response Meta-analysis of randomized controlled trials. Nutrients. 2021;13(7):2355.
Asbaghi O, Ashtary-Larky D, Bagheri R, Moosavian SP, Nazarian B, Afrisham R, et al. Effects of folic acid supplementation on inflammatory markers: a Grade-assessed systematic review and dose-response Meta-analysis of randomized controlled trials. Nutrients. 2021;13(7):2327.
Cui S, Li W, Wang P, Lv X, Gao Y, Huang G. Folic acid inhibits homocysteine-induced cell apoptosis in human umbilical vein endothelial cells. Mol Cell Biochem. 2018;444(1–2):77–86.
Bajic Z, Sobot T, Skrbic R, Stojiljkovic MP, Ponorac N, Matavulj A, et al. Homocysteine, vitamins B6 and folic acid in experimental models of myocardial infarction and heart failure-how strong is that link? Biomolecules. 2022;12(4):536.
Li M, Chen J, Li YS, Feng YB, Gu X, Shi CZ. Folic acid reduces adhesion molecules VCAM-1 expession in aortic of rats with hyperhomocysteinemia. Int J Cardiol. 2006;106(2):285–8.
Moens AL, Goovaerts I, Claeys MJ, Vrints CJ. Flow-mediated vasodilation: a diagnostic instrument, or an experimental tool? Chest. 2005;127(6):2254–63.
Yoshida T, Kawano H, Miyamoto S, Motoyama T, Fukushima H, Hirai N, et al. Prognostic value of flow-mediated dilation of the brachial artery in patients with cardiovascular disease. Intern Med. 2006;45(9):575–9.
Lekakis JP, Papamichael CM, Papaioannou TG, Dagre AG, Stamatelopoulos KS, Tryfonopoulos D, et al. Oral folic acid enhances endothelial function in patients with hypercholesterolaemia receiving statins. Eur J Cardiovasc Prev Rehabil. 2004;11(5):416–20.
Title LM, Cummings PM, Giddens K, Genest JJ Jr, Nassar BA. Effect of folic acid and antioxidant vitamins on endothelial dysfunction in patients with coronary artery disease. J Am Coll Cardiol. 2000;36(3):758–65.
Title LM, Ur E, Giddens K, McQueen MJ, Nassar BA. Folic acid improves endothelial dysfunction in type 2 diabetes--an effect independent of homocysteine-lowering. Vasc Med. 2006;11(2):101–9.
Woo KS, Chook P, Lolin YI, Sanderson JE, Metreweli C, Celermajer DS. Folic acid improves arterial endothelial function in adults with hyperhomocystinemia. J Am Coll Cardiol. 1999;34(7):2002–6.
Thambyrajah J, Landray MJ, McGlynn FJ, Jones HJ, Wheeler DC, Townend JN. Does folic acid decrease plasma homocysteine and improve endothelial function in patients with predialysis renal failure? Circulation. 2000;102(8):871–5.
Thambyrajah J, Landray MJ, Jones HJ, McGlynn FJ, Wheeler DC, Townend JN. A randomized double-blind placebo-controlled trial of the effect of homocysteine-lowering therapy with folic acid on endothelial function in patients with coronary artery disease. J Am Coll Cardiol. 2001;37(7):1858–63.
Woodman RJ, Celermajer DE, Thompson PL, Hung J. Folic acid does not improve endothelial function in healthy hyperhomocysteinaemic subjects. Clin Sci (Lond). 2004;106(4):353–8.
Spoelstra-de MA, Brouwer CB, Terheggen F, Bollen JM, Stehouwer CD, Smulders YM. No effect of folic acid on markers of endothelial dysfunction or inflammation in patients with type 2 diabetes mellitus and mild hyperhomocysteinaemia. Neth J Med. 2004;62(7):246–53.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535.
Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928.
DerSimonian R, Kacker R. Random-effects model for meta-analysis of clinical trials: an update. Contemp Clin Trials. 2007;28(2):105–14.
Borenstein M, Hedges LV, Higgins JP, Rothstein HR. Introduction to meta-analysis: John Wiley & Sons; 2011.
Sahebkar A. Are curcuminoids effective C-reactive protein-lowering agents in clinical practice? Evidence from a meta-analysis. Phytother Res. 2014;28(5):633–42.
Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–6.
Bellamy MF, McDowell IF, Ramsey MW, Brownlee M, Newcombe RG, Lewis MJ. Oral folate enhances endothelial function in hyperhomocysteinaemic subjects. Eur J Clin Investig. 1999;29(8):659–62.
Doshi SN, McDowell IF, Moat SJ, Lang D, Newcombe RG, Kredan MB, et al. Folate improves endothelial function in coronary artery disease: an effect mediated by reduction of intracellular superoxide? Arterioscler Thromb Vasc Biol. 2001;21(7):1196–202.
Pullin CH, Ashfield-Watt PA, Burr ML, Clark ZE, Lewis MJ, Moat SJ, et al. Optimization of dietary folate or low-dose folic acid supplements lower homocysteine but do not enhance endothelial function in healthy adults, irrespective of the methylenetetrahydrofolate reductase (C677T) genotype. J Am Coll Cardiol. 2001;38(7):1799–805.
Doshi SN, McDowell IF, Moat SJ, Payne N, Durrant HJ, Lewis MJ, et al. Folic acid improves endothelial function in coronary artery disease via mechanisms largely independent of homocysteine lowering. Circulation. 2002;105(1):22–6.
Doshi S, McDowell I, Moat S, Lewis M, Goodfellow J. Folate improves endothelial function in patients with coronary heart disease. Clin Chem Lab Med. 2003;41(11):1505–12.
Doshi SN, Moat SJ, Lewis MJ, McDowell IF, Giddings JC, Goodfellow J. Short-term high-dose folic acid does not alter markers of endothelial cell damage in patients with coronary heart disease. Int J Cardiol. 2004;94(2–3):203–7.
Durga J, van Tits LJ, Schouten EG, Kok FJ, Verhoef P. Effect of lowering of homocysteine levels on inflammatory markers: a randomized controlled trial. Arch Intern Med. 2005;165(12):1388–94.
Moat SJ, Madhavan A, Taylor SY, Payne N, Allen RH, Stabler SP, et al. High- but not low-dose folic acid improves endothelial function in coronary artery disease. Eur J Clin Investig. 2006;36(12):850–9.
Moens AL, Claeys MJ, Wuyts FL, Goovaerts I, Van Hertbruggen E, Wendelen LC, et al. Effect of folic acid on endothelial function following acute myocardial infarction. Am J Cardiol. 2007;99(4):476–81.
Shirodaria C, Antoniades C, Lee J, Jackson CE, Robson MD, Francis JM, et al. Global improvement of vascular function and redox state with low-dose folic acid: implications for folate therapy in patients with coronary artery disease. Circulation. 2007;115(17):2262–70.
Woo K, Chook P, Yip T, Kwong S, Hu Y, Huang X, et al. Folic acid and vitamin B12 supplementation improves arterial function and structure in subjects with subnormal intake. Heart Lung Circ. 2008;17:S201–S2.
Palomba S, Falbo A, Giallauria F, Russo T, Tolino A, Zullo F, et al. Effects of metformin with or without supplementation with folate on homocysteine levels and vascular endothelium of women with polycystic ovary syndrome. Diabetes Care. 2010;33(2):246–51.
Hashemi M, Heshmat-Ghahdarijani K, Zarean E, Baktash F, Mortazavi ZS. Evaluation of the effect of high-dose folic acid on endothelial dysfunction in pre-eclamptic patients: a randomized clinical trial. J Res Med Sci. 2016;21:114.
Connor SL, Ojeda LS, Sexton G, Weidner G, Connor WE. Diets lower in folic acid and carotenoids are associated with the coronary disease epidemic in central and Eastern Europe. J Am Diet Assoc. 2004;104(12):1793–9.
Eichholzer M, Lüthy J, Gutzwiller F, Stähelin HB. The role of folate, antioxidant vitamins and other constituents in fruit and vegetables in the prevention of cardiovascular disease: the epidemiological evidence. Int J Vitam Nutr Res. 2001;71(1):5–17.
Li Y, Huang T, Zheng Y, Muka T, Troup J, Hu FB. Folic acid supplementation and the risk of cardiovascular diseases: a Meta-analysis of randomized controlled trials. J Am Heart Assoc. 2016;5(8):e003768.
Taylor SY, Dixon HM, Yoganayagam S, Price N, Lang D. Folic acid modulates eNOS activity via effects on posttranslational modifications and protein-protein interactions. Eur J Pharmacol. 2013;714(1–3):193–201.
Chalupsky K, Kračun D, Kanchev I, Bertram K, Görlach A. Folic acid promotes recycling of tetrahydrobiopterin and protects against hypoxia-induced pulmonary hypertension by recoupling endothelial nitric oxide synthase. Antioxid Redox Signal. 2015;23(14):1076–91.
Stanhewicz AE, Kenney WL. Role of folic acid in nitric oxide bioavailability and vascular endothelial function. Nutr Rev. 2017;75(1):61–70.
Zhong Q, Nong Q, Mao B, Pan X, Meng L. Association of Impaired Vascular Endothelial Function with increased cardiovascular risk in asymptomatic adults. Biomed Res Int. 2018;2018:3104945.
Sitia S, Tomasoni L, Atzeni F, Ambrosio G, Cordiano C, Catapano A, et al. From endothelial dysfunction to atherosclerosis. Autoimmun Rev. 2010;9(12):830–4.
Davignon J, Ganz P. Role of endothelial dysfunction in atherosclerosis. Circulation. 2004;109(23 Suppl 1):Iii27–32.
Zhang M, Wen J, Wang X, Xiao C. High-dose folic acid improves endothelial function by increasing tetrahydrobiopterin and decreasing homocysteine levels. Mol Med Rep. 2014;10(3):1609–13.
Tyagi N, Sedoris KC, Steed M, Ovechkin AV, Moshal KS, Tyagi SC. Mechanisms of homocysteine-induced oxidative stress. Am J Physiol Heart Circ Physiol. 2005;289(6):H2649–56.
Chernyavskiy I, Veeranki S, Sen U, Tyagi SC. Atherogenesis: hyperhomocysteinemia interactions with LDL, macrophage function, paraoxonase 1, and exercise. Ann N Y Acad Sci. 2016;1363(1):138–54.
de Bree A, van Mierlo LA, Draijer R. Folic acid improves vascular reactivity in humans: a meta-analysis of randomized controlled trials. Am J Clin Nutr. 2007;86(3):610–7.
Raggi P, Genest J, Giles JT, Rayner KJ, Dwivedi G, Beanlands RS, et al. Role of inflammation in the pathogenesis of atherosclerosis and therapeutic interventions. Atherosclerosis. 2018;276:98–108.
Thayse K, Kindt N, Laurent S, Carlier S. VCAM-1 target in non-invasive imaging for the detection of atherosclerotic plaques. Biology (Basel). 2020;9(11):368.
Manduteanu I, Simionescu M. Inflammation in atherosclerosis: a cause or a result of vascular disorders? J Cell Mol Med. 2012;16(9):1978–90.
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
O.A contributed to the conception and design of the study; D.A.L and M.Z contributed to data extraction; S.S and K.N screened articles for inclusion criteria; O.A contributed to data analysis, C.C.T.C, SR and F.R contributed in manuscript drafting; O.A and S R supervised the study. All authors approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zamani, M., Rezaiian, F., Saadati, S. et al. The effects of folic acid supplementation on endothelial function in adults: a systematic review and dose-response meta-analysis of randomized controlled trials. Nutr J 22, 12 (2023). https://doi.org/10.1186/s12937-023-00843-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12937-023-00843-y