Abstract
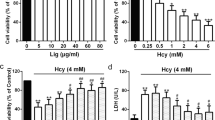
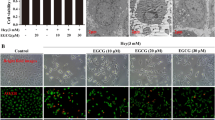
Homocysteine may be responsible for vascular endothelial cell injury, which occurs early in the pathology of cardiovascular disease. Homocysteine metabolism requires enzymatic interaction with vitamins such as folic acid, vitamin B12, and vitamin B6. We hypothesized that folic acid alleviated homocysteine-induced vascular injury by regulating the metabolic pathway of apoptosis. Human umbilical vein endothelial cells were incubated for 48 h with folic acid at the concentrations of 0–1000 nmol/L, in combination with either 1000 μmol/L homocysteine or vehicle for the first 24 h. We then assessed cell viability and apoptosis by methyl thiazolyl tetrazolium assay and flow cytometry, respectively. To further investigate how folic acid influenced cell apoptosis, we also analyzed the activities of caspase-3/7 and the mRNA and protein expressions of BCL2, BAX, TP53, CASP3, and CASP8 in human umbilical vein endothelial cells. We showed that folic acid increased cell viability and decreased apoptosis in a dose-dependent manner, and that this effect was mediated by decreased caspase-3/7 activity, upregulated BCL2/BAX ratio, and downregulated TP53, CASP3, and CASP8 expressions. Thus, we conclude that folic acid inhibits cell apoptosis and ameliorates homocysteine toxicity by regulating the expression of apoptosis-related genes in human umbilical vein endothelial cells.







Similar content being viewed by others
Abbreviations
- AMV:
-
Avian myeloblastosis virus
- ANOVA:
-
Analysis of variance
- BCL2:
-
B-cell lymphoma 2
- BAX:
-
Bcl-2-associated protein X
- Caspase:
-
Cysteinyl aspartate-specific proteinase
- Hcy:
-
Homocysteine
- HUVEC:
-
Human umbilical vein endothelial cells
- HRP:
-
Horseradish peroxidase
- MTT:
-
Methyl thiazolyl tetrazolium
- PCR:
-
Polymerase chain reaction
- SDS-PAGE:
-
Sodium dodecyl sulfate polyacrylamide gel
- TP53:
-
Tumor suppressor p53
References
McCully KS (1969) Vascular pathology of homocysteinemia: implications for the pathogenesis of arteriosclerosis. Am J Pathol 56(1):111–128
Han S, Wu H, Li W, Gao P (2015) Protective effects of genistein in homocysteine-induced endothelial cell inflammatory injury. Mol Cell Biochem 403(1–2):43–49. https://doi.org/10.1007/s11010-015-2335-0
Yang XH, Li P, Yin YL, Tu JH, Dai W, Liu LY, Wang SX (2015) Rosiglitazone via PPARgamma-dependent suppression of oxidative stress attenuates endothelial dysfunction in rats fed homocysteine thiolactone. J Cell Mol Med 19(4):826–835. https://doi.org/10.1111/jcmm.12510
Chernyavskiy I, Veeranki S, Sen U, Tyagi SC (2016) Atherogenesis: hyperhomocysteinemia interactions with LDL, macrophage function, paraoxonase 1, and exercise. Ann N Y Acad Sci 1363:138–154. https://doi.org/10.1111/nyas.13009
Wu S, Gao X, Yang S, Meng M, Yang X, Ge B (2015) The role of endoplasmic reticulum stress in endothelial dysfunction induced by homocysteine thiolactone. Fundam Clin Pharmacol 29(3):252–259. https://doi.org/10.1111/fcp.12101
Jia F, Wu C, Chen Z, Lu G, Sun J (2016) Atorvastatin attenuates atherosclerotic plaque destabilization by inhibiting endoplasmic reticulum stress in hyperhomocysteinemic mice. Mol Med Rep 13(4):3574–3580. https://doi.org/10.3892/mmr.2016.4975
Duthie SJ, Beattie JH, Gordon MJ, Pirie LP, Nicol F, Reid MD, Duncan GJ, Cantlay L, Horgan G, McNeil CJ (2015) Nutritional B vitamin deficiency alters the expression of key proteins associated with vascular smooth muscle cell proliferation and migration in the aorta of atherosclerotic apolipoprotein E null mice. Genes Nutr 10(1):446. https://doi.org/10.1007/s12263-014-0446-y
Gurda D, Handschuh L, Kotkowiak W, Jakubowski H (2015) Homocysteine thiolactone and N-homocysteinylated protein induce pro-atherogenic changes in gene expression in human vascular endothelial cells. Amino Acids 47(7):1319–1339. https://doi.org/10.1007/s00726-015-1956-7
Zhou YH, Tang JY, Wu MJ, Lu J, Wei X, Qin YY, Wang C, Xu JF, He J (2011) Effect of folic acid supplementation on cardiovascular outcomes: a systematic review and meta-analysis. PLoS ONE 6(9):e25142. https://doi.org/10.1371/journal.pone.0025142
Lee M, Hong KS, Chang SC, Saver JL (2010) Efficacy of homocysteine-lowering therapy with folic acid in stroke prevention: a meta-analysis. Stroke 41(6):1205–1212. https://doi.org/10.1161/STROKEAHA.109.573410
Steegers-Theunissen RP, Wathen NC, Eskes TK, van Raaij-Selten B, Chard T (1997) Maternal and fetal levels of methionine and homocysteine in early human pregnancy. Br J Obstet Gynaecol 104(1):20–24
Tian X, Shi Y, Liu N, Yan Y, Li T, Hua P, Liu B (2016) Upregulation of DAPK contributes to homocysteine-induced endothelial apoptosis via the modulation of Bcl2/Bax and activation of caspase 3. Mol Med Rep 14(5):4173–4179. https://doi.org/10.3892/mmr.2016.5733
Vermes I, Haanen C, Steffens-Nakken H, Reutelingsperger C (1995) A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J Immunol Methods 184(1):39–51
Schmittgen TD, Livak KJ (2008) Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 3(6):1101–1108
Brunelle JK, Letai A (2009) Control of mitochondrial apoptosis by the Bcl-2 family. J Cell Sci 122(Pt 4):437–441. https://doi.org/10.1242/jcs.031682
Clarke R, Daly L, Robinson K, Naughten E, Cahalane S, Fowler B, Graham I (1991) Hyperhomocysteinemia: an independent risk factor for vascular disease. New Engl J Med 324(17):1149–1155. https://doi.org/10.1056/NEJM199104253241701
Liu LH, Guo Z, Feng M, Wu ZZ, He ZM, Xiong Y (2012) Protection of DDAH2 overexpression against homocysteine-induced impairments of DDAH/ADMA/NOS/NO pathway in endothelial cells. Cell Physiol Biochem 30(6):1413–1422. https://doi.org/10.1159/000343329
Li F, Chen Q, Song X, Zhou L, Zhang J (2015) MiR-30b Is involved in the homocysteine-induced apoptosis in human coronary artery endothelial cells by regulating the expression of Caspase 3. Int J Mol Sci 16(8):17682–17695. https://doi.org/10.3390/ijms160817682
Li J, Luo M, Xie N, Wang J, Chen L (2016) Curcumin protects endothelial cells against homocysteine induced injury through inhibiting inflammation. Am J Transl Res 8(11):4598–4604
Mierzecki A, Kloda K, Bukowska H, Chelstowski K, Makarewicz-Wujec M, Kozlowska-Wojciechowska M (2013) Association between low-dose folic acid supplementation and blood lipids concentrations in male and female subjects with atherosclerosis risk factors. Med Sci Monit 19:733–739. https://doi.org/10.12659/MSM.889087
Hodis HN, Mack WJ, Dustin L, Mahrer PR, Azen SP, Detrano R, Selhub J, Alaupovic P, Liu CR, Liu CH, Hwang J, Wilcox AG, Selzer RH, Group BR (2009) High-dose B vitamin supplementation and progression of subclinical atherosclerosis: a randomized controlled trial. Stroke 40(3):730–736. https://doi.org/10.1161/STROKEAHA.108.526798
Wang L, Li H, Zhou Y, Jin L, Liu J (2015) Low-dose B vitamins supplementation ameliorates cardiovascular risk: a double-blind randomized controlled trial in healthy Chinese elderly. Eur J Nutr 54(3):455–464. https://doi.org/10.1007/s00394-014-0729-5
Zhang Y, Bao YL, Wu Y, Yu CL, Huang YX, Sun Y, Zheng LH, Li YX (2013) Alantolactone induces apoptosis in RKO cells through the generation of reactive oxygen species and the mitochondrial pathway. Mol Med Rep 8(4):967–972. https://doi.org/10.3892/mmr.2013.1640
Ren H, Mu J, Ma J, Gong J, Li J, Wang J, Gao T, Zhu P, Zheng S, Xie J, Yuan B (2016) Selenium inhibits homocysteine-induced endothelial dysfunction and apoptosis via activation of AKT. Cell Physiol Biochem 38(3):871–882. https://doi.org/10.1159/000443041
Catena C, Colussi G, Url-Michitsch M, Nait F, Sechi LA (2015) Subclinical carotid artery disease and plasma homocysteine levels in patients with hypertension. J Am Soc Hypertens 9(3):167–175. https://doi.org/10.1016/j.jash.2014.12.020
Yang B, Fan S, Zhi X, Wang Y, Wang Y, Zheng Q, Sun G (2014) Prevalence of hyperhomocysteinemia in China: a systematic review and meta-analysis. Nutrients 7(1):74–90. https://doi.org/10.3390/nu7010074
Chen H, Liu S, Ji L, Wu T, Ma F, Ji Y, Zhou Y, Zheng M, Zhang M, Huang G (2015) Associations between Alzheimer’s disease and blood homocysteine, vitamin B12, and folate: a case-control study. Curr Alzheimer Res 12(1):88–94
Boot MJ, Steegers-Theunissen RP, Poelmann RE, Van Iperen L, Lindemans J, Gittenberger-de Groot AC (2003) Folic acid and homocysteine affect neural crest and neuroepithelial cell outgrowth and differentiation in vitro. Dev Dyn 227(2):301–308. https://doi.org/10.1002/dvdy.10303
Buemi M, Marino D, Di Pasquale G, Floccari F, Ruello A, Aloisi C, Corica F, Senatore M, Romeo A, Frisina N (2001) Effects of homocysteine on proliferation, necrosis, and apoptosis of vascular smooth muscle cells in culture and influence of folic acid. Thromb Res 104(3):207–213
Huang RF, Yaong HC, Chen SC, Lu YF (2004) In vitro folate supplementation alleviates oxidative stress, mitochondria-associated death signalling and apoptosis induced by 7-ketocholesterol. Br J Nutr 92(6):887–894
Craciunescu CN, Brown EC, Mar MH, Albright CD, Nadeau MR, Zeisel SH (2004) Folic acid deficiency during late gestation decreases progenitor cell proliferation and increases apoptosis in fetal mouse brain. J Nutr 134(1):162–166
Wang ZB, Liu YQ, Cui YF (2005) Pathways to caspase activation. Cell Biol Int 29(7):489–496. https://doi.org/10.1016/j.cellbi.2005.04.001
Mercer J, Bennett M (2006) The role of p53 in atherosclerosis. Cell Cycle 5(17):1907–1909. https://doi.org/10.4161/cc.5.17.3166
Yamaguchi H, Chen J, Bhalla K, Wang HG (2004) Regulation of Bax activation and apoptotic response to microtubule-damaging agents by p53 transcription-dependent and -independent pathways. J Biol Chem 279(38):39431–39437. https://doi.org/10.1074/jbc.M401530200
Borghetti G, Yamaguchi AA, Aikawa J, Yamazaki RK, de Brito GA, Fernandes LC (2015) Fish oil administration mediates apoptosis of Walker 256 tumor cells by modulation of p53, Bcl-2, caspase-7 and caspase-3 protein expression. Lipids Health Dis 14:94. https://doi.org/10.1186/s12944-015-0098-y
Wu R, Tang S, Wang M, Xu X, Yao C, Wang S (2016) MicroRNA-497 induces apoptosis and suppresses proliferation via the Bcl-2/Bax-caspase9-caspase3 pathway and cyclin D2 protein in HUVECs. PLoS ONE 11(12):e0167052. https://doi.org/10.1371/journal.pone.0167052
Berens HM, Tyler KL (2011) The proapoptotic Bcl-2 protein Bax plays an important role in the pathogenesis of reovirus encephalitis. J Virol 85(8):3858–3871. https://doi.org/10.1128/JVI.01958-10
McIlwain DR, Berger T, Mak TW (2013) Caspase functions in cell death and disease. Cold Spring Harb Perspect Biol 5(4):a008656. https://doi.org/10.1101/cshperspect.a008656
Chipuk JE, Kuwana T, Bouchier-Hayes L, Droin NM, Newmeyer DD, Schuler M, Green DR (2004) Direct activation of Bax by p53 mediates mitochondrial membrane permeabilization and apoptosis. Science 303(5660):1010–1014. https://doi.org/10.1126/science.1092734
Zhu M, Li B, Ma X, Huang C, Wu R, Zhu W, Li X, Liang Z, Deng F, Zhu J, Xie W, Yang X, Jiang Y, Wang S, Wu J, Geng S, Xie C, Zhong C, Liu H (2016) Folic acid protected neural cells against aluminum-maltolate-induced apoptosis by preventing miR-19 downregulation. Neurochem Res 41(8):2110–2118. https://doi.org/10.1007/s11064-016-1926-9
Sanz AB, Santamaria B, Ruiz-Ortega M, Egido J, Ortiz A (2008) Mechanisms of renal apoptosis in health and disease. J Am Soc Nephrol 19(9):1634–1642. https://doi.org/10.1681/ASN.2007121336
Xi H, Zhang Y, Xu Y, Yang WY, Jiang X, Sha X, Cheng X, Wang J, Qin X, Yu J, Ji Y, Yang X, Wang H (2016) Caspase-1 inflammasome activation mediates homocysteine-induced pyrop-apoptosis in endothelial cells. Circ Res 118(10):1525–1539. https://doi.org/10.1161/CIRCRESAHA.116.308501
Lavrik IN, Golks A, Krammer PH (2005) Caspases: pharmacological manipulation of cell death. J Clin Invest 115(10):2665–2672. https://doi.org/10.1172/JCI26252
Chen CS, Tseng YT, Hsu YY, Lo YC (2013) Nrf2-Keap1 antioxidant defense and cell survival signaling are upregulated by 17beta-estradiol in homocysteine-treated dopaminergic SH-SY5Y cells. Neuroendocrinology 97(3):232–241. https://doi.org/10.1159/000342692
Sun Z, Lan X, Ahsan A, Xi Y, Liu S, Zhang Z, Chu P, Song Y, Piao F, Peng J, Lin Y, Han G, Tang Z (2016) Phosphocreatine protects against LPS-induced human umbilical vein endothelial cell apoptosis by regulating mitochondrial oxidative phosphorylation. Apoptosis 21(3):283–297. https://doi.org/10.1007/s10495-015-1210-5
Xu Z, Lu G, Wu F (2009) Simvastatin suppresses homocysteine-induced apoptosis in endothelial cells: roles of caspase-3, cIAP-1 and cIAP-2. Hypertens Res 32(5):375–380. https://doi.org/10.1038/hr.2009.24
Acknowledgements
This research was supported by a grant from the National Natural Science Foundation of China (No. 81373002).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Rights and permissions
About this article
Cite this article
Cui, S., Li, W., Wang, P. et al. Folic acid inhibits homocysteine-induced cell apoptosis in human umbilical vein endothelial cells. Mol Cell Biochem 444, 77–86 (2018). https://doi.org/10.1007/s11010-017-3232-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-017-3232-5