Abstract
Background
Most patients attending cancer clinics have hypovitaminosis D. Correcting or preventing this abnormal condition could mitigate the emotional and physical complications of their disease, but clinical trials of vitamin D therapy in this setting are hindered by the unavailability of safe, effective and practical loading dose regimens.
Methods
In this single arm open-label pharmacokinetic trial, outpatients with advanced lung cancer consumed 20,000 IU vitamin D daily with the largest meal of the day for 14 days followed by 10,000 IU per day for a further 7 days. Plasma concentrations of 25-hydroxyvitamin D [25(OH)D], parathyroid hormone, calcium, vitamin C and C-reactive protein were measured on protocol days 0, 14 and 21, and serum vitamin D binding protein (VDBP) concentrations on days 0 and 21. As a secondary objective, preliminary information was obtained regarding clinical effects of rapid vitamin D loading on mood and symptoms by administering appropriate questionnaires two times at baseline and after 14 and 21 days of vitamin D therapy.
Results
Of the 91 patients enrolled in the study, 85 % had hypovitaminosis D and 41 % had hypovitaminosis C. Plasma VDBP concentrations were in the normal range. The vitamin D load increased the average plasma 25(OH)D concentration to 116 ± 34 nmol/L (mean ± SD); the median concentration was 122 nmol/L (interquartile range 103–134); VDBP concentrations did not change. Final plasma 25(OH)D concentrations were subnormal (<75 nmol/L) for 13 % of the patients and sub-target (<120 nmol/L) for 44 % of them. In most cases, subnormal and sub-target 25(OH)D concentrations were attributable to obesity and/or a low baseline 25(OH)D concentration. Mood and symptom scores did not change significantly throughout the 3-week protocol.
Conclusion
Hypovitaminosis D and C are very common in outpatients with advanced lung cancer. A vitamin D load of 20,000 IU per day for 14 days failed to achieve the target concentration in 44 % of the participants in this trial. These results suggest that a loading dose of 30,000 IU per day for 14 days would be safe and effective for patients who are obese or at risk of severe hypovitaminosis D. The preliminary nature of the study design, and the failure to achieve target 25(OH)D concentrations for a large proportion of the patients, do not allow any firm conclusion about the clinical effects of correcting hypovitaminosis D in this patient population. Nevertheless, no evidence was obtained that partial correction of hypovitaminosis D greatly improved mood, reduced distress or relieved cancer-related symptoms. This trial was registered at clinicaltrials.gov as NCT01631526.
Similar content being viewed by others
Background
Hypovitaminosis D–a plasma 25-hydroxyvitamin D [25(OH)D] concentration < 75 nmol/L [1]–is present in the great majority of patients attending cancer clinics [2–4], but its clinical consequences are unknown. There is general agreement that biochemical vitamin D deficiency [plasma 25(OH)D < 25 nmol/L] requires prevention and treatment, but the benefits of correcting vitamin D insufficiency [plasma 25(OH)D between 25 and 75 nmol/L] are uncertain and a topic of active clinical investigation [1, 5–7].
Correcting subclinical vitamin D deficiency in patients with advanced cancer, including lung cancer, could mitigate some of the emotional and physical complications of their disease, including pain [8, 9] and certain side effects of cytotoxic chemotherapy [10], since vitamin D has important functions in relation to brain metabolism [11–13], muscle function [14–16] and immunity [16–21].
Clinical trials of vitamin D therapy in actively progressing diseases like lung cancer are hindered by the unavailability of safe, effective and practical loading dose regimens. The primary objective of this study was to test the ability of a simple vitamin D loading regimen to rapidly attain a target 25(OH)D concentration in every patient. The resulting plasma 25(OH)D concentrations were interpreted in the context of a systematic review of the available data pertaining to rapid vitamin D loading in a variety of patient populations. As a secondary objective, we tested the ability of three questionnaires to measure mood, well-being, and cancer-related symptoms in the context of vitamin D loading. More than one subclinical micronutrient deficiency can occur in a patient with cancer [22]. To explore this possibility, we measured plasma vitamin C concentrations in the study participants.
Methods
Justification of the loading dose protocol
A loading dose is necessary
Exploratory clinical trials in patients with actively progressing disease have to be implemented promptly and be of relatively short duration, a time constraint that rules out simple maintenance therapy which requires months to attain a steady-state target plasma 25(OH)D concentration [23, 24].
Target 25(OH)D concentration
A variety of biological and observational data suggest that 25(OH)D concentrations in the range of 100–130 nmol/L are safe and potentially optimal for human health [6, 7, 25], hence a promising target in clinical trials with non-bone-related endpoints. A suitable loading dose regimen will bring the plasma 25(OH)D concentration of every patient–not merely the group average–into the target range. The target plasma 25(OH)D concentration in this study was 120 nmol/L for every patient.
Avoid very large individual doses
Vitamin D3 (cholecalciferol) is the precursor of the active form of the vitamin, 1,25-dihydroxyvitamin D, but cholecalciferol itself has some biological activity and when administered in unphysiologically large individual doses could engender adverse effects consequent to variable storage and release of the unmetabolized vitamin [26–30]. We reasoned that a safe and reliable loading regimen should avoid daily doses much greater than the maximum rate of sunlight-induced endogenous vitamin D synthesis, approximately 10,000–20,000 IU per day [30–32].
Bioavailability
Fat-soluble vitamins like vitamin D are better absorbed when consumed with fat-containing meals [33–35].
Practicality
High quality pharmaceutical vitamin D formulations in a dose of 10,000 IU per tablet are widely available.
Safety
The loading dose had to be conservative enough to mitigate concerns about potential toxicity among treating oncologists and patients considering participation in a clinical trial.
Details of the loading dose protocol
Study participants took 20,000 IU vitamin D (two tablets) daily for 14 days (total dose 280,000 IU) followed by 10,000 IU per day for a further 7 days (for a total dose of 350,000 IU over the entire 3-week study period). Study participants were instructed to take their daily vitamin D dose with the largest meal of the day rather than on an empty stomach. The daily maintenance dose of 10,000 IU was chosen because of evidence that it is safe to use in a clinical trial [32, 36] and can sustain plasma 25(OH)D concentrations in the vicinity of 120 nmol/L for almost every patient [6].
Vitamin D binding protein
Approximately 90 % of the 25(OH)D measured in a serum or plasma sample is bound to the vitamin D binding protein (VDBP) [30, 37]. It is unclear how much changes in plasma VDBP concentrations affect measured plasma 25(OH)D concentrations or biological effects in people who are acutely sick, since systemic inflammation expands the extravascular space and redistributes large proteins into the extracellular space, potentially confounding the analysis of kinetic studies of vitamin D concentration and effect [30, 38–40]. To address this possibility we measured plasma VDBP concentrations at baseline and after 3 weeks of vitamin D therapy.
Outcome measures
The primary pharmacokinetic outcome was plasma 25(OH)D concentrations measured at baseline, after 14 days of vitamin D loading (20,000 IU per day), and after a further 7 days of the maintenance dose of 10,000 IU per day. Secondary outcome measures were mood, distress, and cancer-related symptoms evaluated at the same time points. The prevalence of hypovitaminosis D and C in this patient population were documented as well as their relationship to certain biologically relevant factors, including systemic inflammation. In addition to parathyroid hormone concentrations, standard information obtained for clinical purposes was also analyzed, specifically including serum calcium and C-reactive protein.
Participants
Mentally competent ambulatory outpatients of any age > 18 years with advanced lung cancer were eligible to participate whether or not they were receiving specific anti-cancer therapy, with the following exceptions: current use of any vitamin D supplement equivalent to > 1000 IU per day, current therapy with any dose of calcitriol, a recent history of extensive sunlight exposure (>30 min of summer sunlight exposure more than 5 days per week), non-fluency in French or English and no capable neutral translator available, current diagnosis of primary hyperparathyroidism, existing nephrocalcinosis, hypercalcemia, current or suspected tuberculosis, histoplasmosis, sarcoidosis or other granulomatous disease, pregnancy, or anticipated death within 2 months.
Study site and details
The Pulmonary Oncology Peter Brojde Lung Cancer Centre accepts approximately 180 new referrals each year; approximately 375 patients are being followed at any time. Approximately 80 % of newly referred patients have advanced lung cancer treated with palliative chemotherapy, radiotherapy and supportive care. The vitamin D used in the trial was provided to patients by the hospital’s research pharmacy in a labeled bottle containing 35 tablets of 10,000 IU (Laboratoire Riva Inc, Blainville QC, Canada; DIN 00821772). The patients were followed by the dietitian (NS) who administered the questionnaires and made pill counts at each visit to verify compliance. The accrual goal of the study was completion of the 3-week protocol by 80 patients (“completed study group”). The baseline data for patients who enrolled in the study but did not complete the entire protocol (“initial study group”) were used to determine the frequency and clinical correlates of hypovitaminosis C and D. The study protocol was approved by the Research Ethics Committee of the Jewish General Hospital and registered at clinicaltrials.gov (NCT 01631526).
Research analytical measurements
Plasma 25(OH)D, VDBP, vitamin C and other concentrations
Total 25-hydroxyvitamin D was measured by competitive electrochemiluminescence binding (Roche, Laval, QC) on a Cobas e 602 module (Roche, Laval, QC). The assay uses a vitamin D binding protein (VDBP), which binds both 25-hydroxyvitamin D2 and 25-hydroxyvitamin D3, as a capture protein. At a concentration of 21 nmol/L the assay has intra-assay and inter-assay coefficients of variation of 6.8 and 13.1 % respectively; and at a concentration of 174 nmol/L, 2.2 and 3.4 % respectively. VDBP was measured in plasma by means of a solid phase enzyme immunoassay using the Quantikine Human Vitamin D Binding Protein kit (R&D Systems, Minneapolis, MN). Patient samples were stored at < −20 ° C prior to analysis and analyzed in batches using kits of the same lot number. The coefficients of variation of intra-assay and inter-assay concentrations were 4.6 and 9.1 % respectively. For internal validation, we measured plasma VDBP in 22 healthy volunteers not receiving any medication and taking either no vitamin D supplements or < 1000 IU vitamin D daily. The values obtained for these volunteers (average 196 μg/mL, SD 79, range 74–318) fell within the reference interval provided by the manufacturer. Intact parathyroid hormone (reference interval 10–70 ng/L) was measured in plasma by electrochemiluminescence immunoassay on a Cobas e 602 module (Roche, Laval, QC). Plasma C-reactive protein (reference interval 1–10 mg/L) was measured by latex particle enhanced immunoturbidimetry on a Cobas e 502 module (Roche, Laval, QC). Plasma vitamin C was measured as described previously [41].
Mood and symptom assessment
POMS-B
The Profile of Mood States™ (POMS™) is a widely used 65 item questionnaire that measures mood in healthy, physically ill and psychiatric populations; the instrument generates a total mood disturbance score [42, 43]. Briefer versions of the POMS™ have been developed to accommodate the limited reserve of physically ill patients and found to be practical and valid [42, 44, 45]. The 30-item POMS™-B was chosen as the most appropriate instrument to assess the mood of cancer patients because it is a validated, extensively used, broad spectrum tool that can be conveniently and quickly administered even to sick, hospitalized patients [46].
Distress thermometer
This simple, validated one-item measure of psychological distress asks the patient to indicate their level of distress alongside an image of a thermometer [47–49]. The DT is formally recommended by several clinical organizations as a valid, easy to use and simple tool for detecting distress in people with cancer [47].
Revised Edmonton Symptom Assessment System (ESAS-r)
This very widely used 9-question self-report symptom intensity tool is used to assess the intensity of nine common symptoms experienced by cancer patients. The items scored by this questionnaire are: pain, tiredness, drowsiness, nausea, lack of appetite, shortness of breath, anxiety, depression and well-being [50].
Systematic review
In order to interpret our observations and explore whether people with advanced lung cancer differ importantly in their response to vitamin D loading from other people, we carried out a systematic review of the literature pertaining to the effects of vitamin D loading regimens on plasma or serum 25(OH)D concentrations in the 3 to 21-day time period in adults. Pertinent articles were identified by studying and up-dating three published systematic reviews of the kinetics of high-dose vitamin D conversion to 25(OH)D [51–53] that included data in the 3 to 21-day period. This process identified six articles [54–59]. Ten more pertinent articles were identified by studying the references cited in these reviews and updating the searches [60–69]. One otherwise pertinent study [70] was excluded because it provided incremental, but no baseline data. The search process identified 16 articles that reported 24 different kinetic studies that included plasma 25(OH)D measurements at early time points after large oral doses of vitamin D.
Statistical methods
Statistical analyses were performed using GraphPad Prism version 5.01 (GraphPad Software Inc., San Diego, Ca). Non-parametric tests were used in all cases. Correlation analyses between baseline metabolites were done using Spearman coefficients. Wilcoxon matched pairs tests were used to verify differences between pre-baseline and baseline results. The Friedman test and Dunn’s post test were performed to detect differences between baseline and post-treatment results. The significant level of P < 0.05 was adopted in all analyses.
Results
The first participant was enrolled on June 14, 2012 and the final, eightieth one completed the protocol on November 3, 2015. The study flow diagram is shown in Fig. 1. During the approximately 3.5-year duration of the study, 747 patients were screened for eligibility, of whom 525 were ineligible (reasons indicated in Table 1). Of the 222 eligible patients, 125 were uninterested in participating or lost in the rapid flux of patients in and out of the clinic. Six patients who agreed to participate withdrew or had to be withdrawn before the protocol commenced, leaving 91 people who began the study (initial study group). Eleven participants were withdrawn before the protocol could be completed (reasons indicated in the study flow diagram, Fig. 1), leaving 80 patients who completed the protocol. The baseline characteristics of the 91 patients who commenced the study (initial study group, n = 91) and the 80 who completed it (study completed group, n = 80) are shown in Table 2. The characteristics of the study-completed group were closely similar to those in the initial study group. Although we cannot rule the possibility out, in almost every case the reasons for ineligibility do not suggest that the nonparticipants were clinically or metabolically very different from the people who enrolled in and completed the study. No patient was prescribed calcitriol or suffered from renal insufficiency.
Baseline data
As shown in Table 2, 85 % of the patients in the initial study group (n = 91) had hypovitaminosis D; 26 % of them had previously undiagnosed biochemical vitamin D deficiency [plasma 25(OH)D concentration < 25 nmol/L]. Plasma VDBP concentrations were in the normal range. Plasma 25(OH)D was strongly correlated with plasma VDBP (P = 0.0015), although the predictive value of the association was low (Spearman r = 0.33). The usual negative correlation between plasma 25(OH)D and parathyroid hormone concentrations was observed (P = 0.002, r = −0.32). Hypovitaminosis C was present in 41 % of the patients. There was a strong negative correlation between plasma vitamin C and C-reactive protein, an indicator of systemic inflammation (P = 0.0068, r = −0.28), but no correlation between plasma VDBP and C-reactive protein nor between 25(OH)D and C-reactive protein.
Effects of the loading dose on plasma 25(OH)D and VDBP concentrations
As shown in Table 3 and Fig. 2, the vitamin D loading regimen increased plasma 25(OH)D concentrations dramatically within 14 days, with a further small increase by day 21. The average final plasma 25(OH)D concentration was 116 ± 34 nmol/L (mean ± SD); the median concentration was 122 nmol/L (interquartile range 103–134). Plasma parathyroid hormone levels decreased after vitamin D therapy (Table 3), the expected biological response to correcting vitamin D deficiency. Plasma VDBP concentrations remained constant and normal during the protocol (Table 3 and Fig. 3).
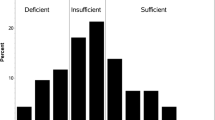
Plasma 25(OH)D concentrations on day 21 were strongly associated with body mass index (BMI) and baseline 25(OH)D concentration, as shown in Fig. 4. Final plasma 25(OH)D concentrations were < 120 nmol/L for 44 % of the participants (35 patients, 26 of whom had a BMI > 30 kg/m2 or baseline 25(OH)D < 30 nmol/L. Ten participants had a final 25(OH)D concentration < 75 nmol/L; nine of them had a very low baseline 25(OH)D concentration, and two had a BMI > 30 kg/m2.
The results of the systematic review of articles reporting plasma 25(OH)D concentrations shortly after high-dose vitamin D administration are displayed in Table 4.
Toxicity
Serum calcium levels remained constant and normal for every patient. As shown in Fig. 2, one patient whose 25(OH)D concentration was unusually high at baseline experienced an increase to 258 nmol/L, marginally higher than the upper value of the reference interval, 250 nmol/L [5].
Effects of the loading dose on mood, distress and symptoms
The POMS-B, DT and ESAS-r were administered twice, a week apart, before commencing vitamin D therapy, and repeated on protocol days 14 and 21. As shown in Table 3, average mood and symptom scores did not change upon repeat measurement nor did they change significantly throughout the 3-week protocol, despite an indication that cancer symptoms were slightly reduced by week 3 of vitamin D therapy (P = 0.012). There was no strong indication of an important effect of vitamin D therapy on any sub-scale of the questionnaires nor of benefit in those patients whose 25(OH)D concentration was very low at baseline and normalized during the 21-day duration of the study.
Discussion
This study corroborates previous reports that hypovitaminosis D is highly prevalent in outpatients being treated for advanced cancer [2–4] and further demonstrates a high prevalence of hypovitaminosis C. One-quarter of the patients in this study had previously unsuspected biochemical vitamin D deficiency, a diagnosis that calls for immediate therapy. Neither 25(OH)D nor VDBP levels were related to the level of systemic inflammation as indicated by plasma C-reactive protein concentrations. These data indicate that, unlike vitamin C [41], 25(OH)D and VDBP do not behave as negative acute-phase reactants in this patient population.
The vitamin D loading protocol was safe in that no patient experienced a 25(OH)D level significantly greater than normal, even in the small minority of patients whose 25(OH)D level was normal at baseline. No patient experienced hypercalcemia or any toxicity attributable to vitamin D administration. However, the protocol failed to increase plasma 25(OH)D concentrations into the normal range (75 nmol/L or greater) in 13 % of the participants, and it failed to achieve the target of 120 nmol/L for 44 % of the participants. Obesity [24, 71, 72] and a very low baseline plasma 25(OH)D concentration [23] are known to predict a muted 25(OH)D response to vitamin D therapy. Of the ten patients whose final 25(OH)D concentration was < 75 nmol/L at the end of the protocol, nine had a very low baseline 25(OH)D concentration, and two had a BMI > 30 kg/m2. Of the 35 patients whose final 25(OH)D concentration was < 120 nmol/L, 74 % of them either had a BMI > 30 kg/m2 or baseline 25(OH)D < 30 nmol/L.
Our observations regarding the short-term effect of this vitamin D loading regimen on 25(OH)D concentrations were interpreted by reference to the systemic review of published data displayed in Table 4. The wide heterogeneity of dose regimens and patient types precludes a statistical analysis, but a qualitative pattern is readily discernable and the present data are consistent with it. It appears that people with advanced lung cancer do not differ from other people in their response to vitamin D loading. Obesity and markedly subnormal baseline 25(OH)D concentrations predict a muted 25(OH)D response to vitamin D loading, whereas active tuberculosis (a disease in which vitamin D metabolism can be altered) is associated with very large increases [55, 69], perhaps partly attributable to the diminished body fat store [73] that so often accompanies active tuberculosis [55].
The present data suggest a convenient way to choose an appropriate vitamin D loading dose using readily available formulations. Even while acknowledging the non-linearity of vitamin D kinetics [74] it would be reasonable to routinely prescribe a loading dose 50 % higher than used in this study, namely, 30,000 IU per day for 14 days for every patient or, alternatively, prescribe 20,000 IU per day for 14 days for nonobese patients but 30,000 IU for patients who are obese [71] or predicted to have severe hypovitaminosis D on the basis of no prior use of a vitamin D supplement, limited sunlight exposure, dark skin coloration, gastrointestinal disease, or use of certain medications [24].
As a secondary objective, we tested the reproducibility of the mood and symptom questionnaires to determine whether they are robust instruments in this patient population, and to gain some preliminary notion of whether any large or striking effect on mood and symptoms could be detected. The mood, emotional well-being and symptom scales used in this study proved to be practical and reproducible; they could be used in future clinical trials in this population. It is emphasized that this study was not designed to, nor could it, answer specific questions about the effects of short-term vitamin D replacement on mood and symptoms. For one thing, the dosing protocol fell short of the target 25-hydroxyvitamin D concentration. For another, the study was not blinded. That having been said, rapid partial correction of vitamin D status as the sole nutritional had no obvious major effect on mood, distress or cancer-related symptoms in this study.
Malnutrition is common in patients with advanced cancer despite their commonly normal body mass index [75]. Such patients are at risk of subclinical deficiencies of other micronutrients [22], as was demonstrated in this study with regard to vitamin C. It is reasonable to presume that normal status with regard to all vitamins and essential minerals, not just certain selected ones, will provide the best outcomes. Future controlled clinical trials testing the benefits of correcting micronutrient deficiencies in people with advanced cancer should use a combination of micronutrient supplements that target documented subclinical deficiencies, in addition to optimal standard symptom management.
Conclusion
Hypovitaminosis D and C are highly prevalent among outpatients with advanced lung cancer. Although the consumption of 20,000 IU vitamin D per day for 14 days failed to achieve the target 25(OH)D concentration of 120 nmol/L for 44 % of the participants, the data suggest that a loading dose of 30,000 IU per day for 14 days would be safe and effective for patients who are obese or have severe hypovitaminosis D at baseline.
Abbreviations
- 25(OH)D:
-
25-hydroxyvitamin D3
- BMI:
-
Body mass index
- DT:
-
Distress thermometer
- ESAS-r:
-
Revised Edmonton Symptom Assessment System
- IU:
-
International units
- POMS-B:
-
Profile of Mood States-B™
- VDBP:
-
Vitamin D binding protein
References
Rosen CJ. Vitamin D, insufficiency. N Engl J Med. 2011;364:248–54.
Anthony HM, Schorah CJ. Severe hypovitaminosis C in lung-cancer patients: the utilization of vitamin C in surgical repair and lymphocyte-related host resistance. Br J Cancer. 1982;46:354–67.
Vashi PG, Trukova K, Lammersfeld CA, Braun DP, Gupta D. Impact of oral vitamin D supplementation on serum 25-hydroxyvitamin D levels in oncology. Nutr J. 2010;9:60.
Harvie M. Nutritional supplements and cancer: potential benefits and proven harms. Am Soc Clin Oncol Educ Book. 2014;e478–e486.
Souberbielle JC, Body JJ, Lappe JM, Plebani M, Shoenfeld Y, Wang TJ, et al. Vitamin D and musculoskeletal health, cardiovascular disease, autoimmunity and cancer: Recommendations for clinical practice. Autoimmun Rev. 2010;9:709–15.
Garland CF, French CB, Baggerly LL, Heaney RP. Vitamin D supplement doses and serum 25-hydroxyvitamin D in the range associated with cancer prevention. Anticancer Res. 2011;31:607–11.
Heaney RP. Health is better at serum 25(OH)D above 30 ng/mL. J Steroid Biochem Mol Biol. 2013;136:224–8.
Turner MK, Hooten WM, Schmidt JE, Kerkvliet JL, Townsend CO, Bruce BK. Prevalence and clinical correlates of vitamin D inadequacy among patients with chronic pain. Pain Med. 2008;9:979–84.
Straube S, Andrew MR, Derry S, McQuay HJ. Vitamin D and chronic pain. Pain. 2009;141:10–3.
Fink M. Vitamin D, deficiency is a cofactor of chemotherapy-induced mucocutaneous toxicity and dysgeusia. J Clin Oncol. 2011;29:e81–2.
McCann JC, Ames BN. Is there convincing biological or behavioral evidence linking vitamin D deficiency to brain dysfunction? FASEB J. 2008;22:982–1001.
Anglin RES, Samaan Z, Walter SD, McDonald SD. Vitamin D deficiency and depression in adults: systematic review and meta-analysis. Br J Psychiatry. 2013;202:100–7.
Spedding S. Vitamin D, and depression: a systematic review and meta-analysis comparing studies with and without biological flaws. Nutrients. 2014;6:1501–18.
Stockton KA, Mengersen K, Paratz JD, Kandiah D, Bennell KL. Effect of vitamin D supplementation on muscle strength: a systematic review and meta-analysis. Osteoporos Int. 2011;22:859–71.
Dawson-Hughes B. Serum 25-hydroxyvitamin D and muscle atrophy in the elderly. Proc Nutr Soc. 2012;71:46–9.
Bischoff-Ferrari HA, Dawson-Hughes B, Stocklin E, Sidelnikov E, Willett WC, Edel JO, et al. Oral supplementation with 25(OH)D3 versus vitamin D3: effects on 25(OH)D levels, lower extremity function, blood pressure, and markers of innate immunity. J Bone Miner Res. 2012;27:160–9.
Chadha MK, Fakih M, Muindi J, Tian L, Mashtare T, Johnson CS, et al. Effect of 25-hydroxyvitamin D status on serological response to influenza vaccine in prostate cancer patients. Prostate. 2011;71:368–72.
Youssef DA, Ranasinghe T, Grant WB, Peiris AN. Vitamin D’s potential to reduce the risk of hospital-acquired infections. Dermatoendocrinol. 2012;4:167–75.
Vuolo L, Di SC, Faggiano A, Colao A. Vitamin D and cancer. Front Endocrinol. 2012;3:58.
Jolliffe DA, Griffiths CJ, Martineau AR. Vitamin D in the prevention of acute respiratory infection: systematic review of clinical studies. J Steroid Biochem Mol Biol. 2013;136:321–9.
Korf H, Decallonne B, Mathieu C. Vitamin D for infections. Curr Opin Endocrinol Diabetes Obes. 2014;21:431–6.
Tastekin D, Erturk K, Bozbey HU, Olmuscelik O, Kiziltan H, Tuna S, et al. Plasma homocysteine, folate and vitamin B12 levels in patients with lung cancer. Exp Oncol. 2015;37:218–22.
van Groningen L, Opdenoordt S, van Sorge A, Telting D, Giesen A, de Boer H. Cholecalciferol loading dose guideline for vitamin D-deficient adults. Eur J Endocrinol. 2010;162:805–11.
Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911–30.
Heaney RP. Toward a physiological referent for the vitamin D requirement. J Endocrinol Invest. 2014;37:1127–30.
Heaney RP, Armas LA, Shary JR, Bell NH, Binkley N, Hollis BW. 25-Hydroxylation of vitamin D3: relation to circulating vitamin D3 under various input conditions. Am J Clin Nutr. 2008;87:1738–42.
Dawson-Hughes B, Harris SS. High-dose vitamin D supplementation: too much of a good thing? JAMA. 2010;303:1861–2.
Hollis BW, Wagner CL. Clinical review: The role of the parent compound vitamin D with respect to metabolism and function: Why clinical dose intervals can affect clinical outcomes. J Clin Endocrinol Metab. 2013;98:4619–28.
Zheng YT, Cui QQ, Hong YM, Yao WG. A meta-analysis of high dose, intermittent vitamin D supplementation among older adults. PLoS ONE. 2015;10:e0115850.
Heaney RP, Armas LA. Quantifying the vitamin D economy. Nutr Rev. 2015;73:51–67.
Vieth R. Vitamin D, supplementation, 25-hydroxyvitamin D concentrations, and safety. Am J Clin Nutr. 1999;69:842–56.
Hathcock JN, Shao A, Vieth R, Heaney R. Risk assessment for vitamin D. Am J Clin Nutr. 2007;85:6–18.
Mulligan GB, Licata A. Taking vitamin D with the largest meal improves absorption and results in higher serum levels of 25-hydroxyvitamin D. J Bone Miner Res. 2010;25:928–30.
Dawson-Hughes B, Harris SS, Lichtenstein AH, Dolnikowski G, Palermo NJ, Rasmussen H. Dietary fat increases vitamin D-3 absorption. J Acad Nutr Diet. 2015;115:225–30.
Borel P, Caillaud D, Cano NJ. Vitamin D bioavailability: state of the art. Crit Rev Food Sci Nutr. 2015;55:1193–205.
Amir E, Simmons CE, Freedman OC, Dranitsaris G, Cole DE, Vieth R, et al. A phase 2 trial exploring the effects of high-dose (10,000 IU/day) vitamin D(3) in breast cancer patients with bone metastases. Cancer. 2010;116:284–91.
Alpers DH. Vitamins as drugs: the importance of pharmacokinetics in oral dosing. Curr Opin Gastroenterol. 2011;27:146–51.
Reid D, Toole BJ, Knox S, Talwar D, Harten J, O’Reilly DS, et al. The relation between acute changes in the systemic inflammatory response and plasma 25-hydroxyvitamin D concentrations after elective knee arthroplasty. Am J Clin Nutr. 2011;93:1006–11.
Waldron JL, Ashby HL, Cornes MP, Bechervaise J, Razavi C, Thomas OL, et al. Vitamin D: a negative acute phase reactant. J Clin Pathol. 2013;66:620–2.
Silva MC, Furlanetto TW. Does serum 25-hydroxyvitamin D decrease during acute-phase response? A systematic review. Nutr Res. 2015;35:91–6.
Wang Y, Liu XJ, Robitaille L, Eintracht S, Macnamara E, Hoffer LJ. Effects of vitamin C and vitamin D administration on mood and distress in acutely hospitalized patients. Am J Clin Nutr. 2013;98:705–11.
McNair DM, Heuchert JW. Profile of Mood States Technical Update. Toronto: MHS; 2005.
Nyenhuis DL, Yamamoto C, Luchetta T, Terrien A, Parmentier A. Adult and geriatric normative data and validation of the profile of mood states. J Clin Psychol. 1999;55:79–86.
Baker F, Denniston M, Zabora J, Polland A, Dudley WN. A POMS short form for cancer patients: psychometric and structural evaluation. Psychooncology. 2002;11:273–81.
Yeun EJ, Shin-Park KK. Verification of the Profile of Mood States-Brief: cross-cultural analysis. J Clin Psychol. 2006;62:1173–80.
Lam RW, Michalak EE, Swinson RP. Assessment Scales in Depression, Mania and Anxiety. London: Taylor & Francis; 2005.
Holland JC, Bultz BD, National comprehensive Cancer Network (NCCN). The NCCN guideline for distress management: a case for making distress the sixth vital sign. J Natl Compr Cancer Netw. 2007;5:3–7.
Mitchell AJ. Short screening tools for cancer-related distress: a review and diagnostic validity meta-analysis. J Natl Compr Cancer Netw. 2010;8:487–94.
Carlson LE, Groff SL, Maciejewski O, Bultz BD. Screening for distress in lung and breast cancer outpatients: a randomized controlled trial. J Clin Oncol. 2010;28:4884–91.
Watanabe SM, Nekolaichuk C, Beaumont C, Johnson L, Myers J, Strasser F. A multicenter study comparing two numerical versions of the Edmonton Symptom Assessment System in palliative care patients. J Pain Symptom Manage. 2011;41:456–68.
Autier P, Gandini S, Mullie P. A systematic review: influence of vitamin D supplementation on serum 25-hydroxyvitamin D concentration. J Clin Endocrinol Metab. 2012;97:2606–13.
Kennedy DA, Cooley K, Skidmore B, Fritz H, Campbell T, Seely D. Vitamin D: pharmacokinetics and safety when used in conjunction with the pharmaceutical drugs used in cancer patients: a systematic review. Cancers. 2013;5:255–80.
Kearns MD, Alvarez JA, Tangpricha V. Large, single-dose, oral vitamin D supplementation in adult populations: a systematic review. Endocr Pract. 2014;20:341–51.
Ilahi M, Armas LA, Heaney RP. Pharmacokinetics of a single, large dose of cholecalciferol. Am J Clin Nutr. 2008;87:688–91.
Martineau AR, Nanzer AM, Satkunam KR, Packe GE, Rainbow SJ, Maunsell ZJ, et al. Influence of a single oral dose of vitamin D(2) on serum 25-hydroxyvitamin D concentrations in tuberculosis patients. Int J Tuberc Lung Dis. 2009;13:119–25.
Romagnoli E, Mascia ML, Cipriani C, Fassino V, Mazzei F, D’Erasmo E, et al. Short and long-term variations in serum calciotropic hormones after a single very large dose of ergocalciferol (vitamin D2) or cholecalciferol (vitamin D3) in the elderly. J Clin Endocrinol Metab. 2008;93:3015–20.
Amrein K, Sourij H, Wagner G, Holl A, Pieber TR, Smolle KH, et al. Short-term effects of high-dose oral vitamin D3 in critically ill vitamin D deficient patients: a randomized, double-blind, placebo-controlled pilot study. Crit Care. 2011;15:R104.
Rossini M, Adami S, Viapiana O, Fracassi E, Idolazzi L, Povino MR, et al. Dose-dependent short-term effects of single high doses of oral vitamin D(3) on bone turnover markers. Calcif Tissue Int. 2012;91:365–9.
Grossmann RE, Zughaier SM, Kumari M, Seydafkan S, Lyles RH, Liu S, et al. Pilot study of vitamin D supplementation in adults with cystic fibrosis pulmonary exacerbation: A randomized, controlled trial. Dermatoendocrinol. 2012;4:191–7.
Weisman Y, Schen RJ, Eisenberg Z, Amarilio N, Graff E, Edelstein-Singer M, et al. Single oral high-dose vitamin D3 prophylaxis in the elderly. J Am Geriatr Soc. 1986;34:515–8.
Cipriani C, Romagnoli E, Scillitani A, Chiodini I, Clerico R, Carnevale V, et al. Effect of a single oral dose of 600,000 IU of cholecalciferol on serum calciotropic hormones in young subjects with vitamin D deficiency: a prospective intervention study. J Clin Endocrinol Metab. 2010;95:4771–7.
Roth DE, Al MA, Raqib R, Black RE, Baqui AH. Pharmacokinetics of a single oral dose of vitamin D3 (70,000 IU) in pregnant and non-pregnant women. Nutr J. 2012;11:114.
De Jong A, Woods K, Van Gestel L, Suresh M, Porteous M. Vitamin D insufficiency in osteoporotic hip fracture patients: rapid substitution therapy with high dose oral cholecalciferol (vitamin D3). Acta Orthop Belg. 2013;79:578–86.
Drincic A, Fuller E, Heaney RP, Armas LA. 25-Hydroxyvitamin D response to graded vitamin D3 supplementation among obese adults. J Clin Endocrinol Metab. 2013;98:4845–51.
Cantor I. A clinical protocol demonstrating rapid, safe, and effective treatment of vitamin D deficiency: a potential role in oncology alongside conventional treatment. Integr Cancer Ther. 2014;13:411–6.
Kearns MD, Binongo JN, Watson D, Alvarez JA, Lodin D, Ziegler TR, et al. The effect of a single, large bolus of vitamin D in healthy adults over the winter and following year: a randomized, double-blind, placebo-controlled trial. Eur J Clin Nutr. 2015;69:193–7.
Oliveri B, Mastaglia SR, Brito GM, Seijo M, Keller GA, Somoza J, et al. Vitamin D3 seems more appropriate than D2 to sustain adequate levels of 25OHD: a pharmacokinetic approach. Eur J Clin Nutr. 2015;69:697–702.
Rousseau AF, Damas P, Ledoux D, Lukas P, Carlisi A, Le GC, et al. Vitamin D status after a high dose of cholecalciferol in healthy and burn subjects. Burns. 2015;41:1028–34.
Tukvadze N, Sanikidze E, Kipiani M, Hebbar G, Easley KA, Shenvi N, et al. High-dose vitamin D3 in adults with pulmonary tuberculosis: a double-blind randomized controlled trial. Am J Clin Nutr. 2015;102:1059–69.
Armas LA, Hollis BW, Heaney RP. Vitamin D2 is much less effective than vitamin D3 in humans. J Clin Endocrinol Metab. 2004;89:5387–91.
Dhaliwal R, Mikhail M, Feuerman M, Aloia JF. The vitamin D dose response in obesity. Endocr Pract. 2014;20:1258–64.
Didriksen A, Grimnes G, Hutchinson MS, Kjaergaard M, Svartberg J, Joakimsen RM, et al. The serum 25-hydroxyvitamin D response to vitamin D supplementation is related to genetic factors, BMI, and baseline levels. Eur J Endocrinol. 2013;169:559–67.
Gallagher JC, Yalamanchili V, Smith LM. The effect of vitamin D supplementation on serum 25(OH)D in thin and obese women. J Steroid Biochem Mol Biol. 2013;136:195–200.
Boullata JI. Vitamin D, supplementation: a pharmacologic perspective. Curr Opin Clin Nutr Metab Care. 2010;13:677–84.
Shaw C, Fleuret C, Pickard JM, Mohammed K, Black G, Wedlake L. Comparison of a novel, simple nutrition screening tool for adult oncology inpatients and the Malnutrition Screening Tool (MST) against the Patient-Generated Subjective Global Assessment (PG-SGA). Support Care Cancer. 2015;23:47–54.
Acknowledgements
None.
Funding
This trial was funded by a grant from the Lotte and John Hecht Memorial Foundation and further supported by the Pulmonary Oncology Peter Brojde Lung Cancer Centre and by the Faculty of Medicine, McGill University.
Availability of data and materials
The raw individual data points generated during and analysed in this study are not publicly available for reasons of simplicity. The authors will provide them to anyone requesting them.
Authors’ contributions
LJH originated the study concept, which was refined by all the authors, and was responsible for seeking funding, institutional review approval and registration. Prospective patients were identified and screened by NS and LR. LR was responsible for blood sample handling, storage and analysis and data entry and analysis. NS followed the patients and administered the questionnaires. JA, VC, DS and CP treated the patients and wrote the vitamin D prescriptions. SE organized, arranged and supervised the 25(OH)D, parathyroid hormone and VDBP analyses. The manuscript was drafted by LJH and LR; all authors were involved in revision and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
As indicated in the Methods section, the study protocol was approved by the Research Ethics Committee of the Jewish General Hospital and registered at clinicaltrials.gov (NCT 01631526).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Hoffer, L.J., Robitaille, L., Swinton, N. et al. Appropriate vitamin D loading regimen for patients with advanced lung cancer. Nutr J 15, 84 (2015). https://doi.org/10.1186/s12937-016-0203-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12937-016-0203-8