Abstract
Background
Water resource development projects are essential for increasing agricultural productivity and ensuring food security. However, these activities require the modification of pre-existing environmental settings, which may alter mosquito larval habitat availability and seasonality. The intensive utilization of current adult vector control tools results in insecticide resistance among the main vectors. When coupled with behavioural resistances, a shift in malaria vector feeding and resting behaviours could compromise the effectiveness of the current adult vector control strategies. Thus, it is important to look for new or alternative vector control interventions for immatures to complement adult control by focusing on different larval habitats and their seasonal availability. Thus, this study investigated larval habitat seasonality and seasonal larval abundance and distribution in irrigated sugar cane plantation settings in Ethiopia.
Methods
Anopheles mosquito larval habitats were surveyed and visited twice a month for a period of 14 months. Anopheline larvae and pupae were collected, reared, and fed finely ground fish food. Adults were provided with sucrose solution and kept under standard conditions. Female Anopheles mosquitoes were identified morphologically and using a species-specific PCR assay. Environmental parameters, which include habitats’ physico-chemical characteristics, were assessed. Larval habitat diversity and larval abundance and distribution were determined across different seasons.
Results
The study revealed that Anopheles gambiae sensu lato (s.l.) was the most predominant 4197(57%) vector species, followed by Anopheles coustani complex 2388 (32.8%). Molecular analysis of sub-samples of An. gambiae s.l. resulted in Anopheles arabiensis (77.9%) and Anopheles amharicus (21.5%), and the remaining 1.1% (n = 7) sub-samples were not amplified. Physico-chemical parameters such as temperature (t = 2.22, p = 0.028), conductivity (t = 3.21, p = 0.002), dissolved oxygen (t = 7.96, p = 0.001), nitrate ion (t = 2.51, p = 0.013), and ammonium ion (t = 2.26, p = 0.025) showed a significant and direct association with mosquito larval abundance. Furthermore, mosquito larval abundance was correlated with distance to the nearest houses (r = − 0.42, p = 0.001), exposure to sunlight (r = 0.34, p = 0.001), during long and short rainy season animal hoof prints, truck tires/road puddles and rain pools were negatively correlated (r = − 0.22, p = 0.01) and types of habitat (r = − 0.20, p = 0.01). Significant habitat type productivity were observed in man-made pools (t = 3.881, P = 0.01163), rain pools, animal hoof prints, (t = − 4.332, P = 0.00749 in both short and long rainy season, whereas, during dry seasons habitat type productivity almost similar and have no significance difference.
Conclusion
The study found that different larval habitats had variable productivity in different seasons, and that physical and physicochemical features like ammonium and nitrate, as well as the distance between larval habitats and households, are related to larval production. As a result, vector control should take into account the seasonality of Anopheles larval habitat as well as the impact of pesticide application on larval source management.
Similar content being viewed by others
Background
A rapidly growing human population needs to increase agricultural productivity to ensure food security. This requires environmental modifications to get additional land to promote economic growth and alleviate poverty in the developing world [1]. Environmental modification, such as irrigation projects, may alter the existing ecological setting, increase diversity and number of mosquito-breeding habitats [2, 3], and expose larval habitats to sunlight, which in turn increases aquatic temperature and shortens the developmental cycle of larval stages of malaria vectors [4]. It also changes the microclimate, which affects the survival of both immature stages and adult mosquitoes [5,6,7]. However, past experience indicates less attention is being given to environmental modification problems related to vector-borne disease prevalence, distribution, and public health challenges [8].
In recent years, the prevalence and incidence of malaria have shown significant reductions as a result of the scaling up of vector control interventions and active case management [9, 10]. Though the current vector control interventions focus on adult vectors, mainly the application of indoor residual insecticide spraying (IRS) and insecticide-treated nets (ITNs) [11]. The major malaria vectors have developed resistance to most of the insecticide classes used and potentially to be used for public health [12, 13], which makes the existing vector control approach insufficient for malaria elimination. Hence, the implementation of integrated vector management interventions that focus on the immature stages might become important.
The abundance of immature stages of malaria vectors has been found to increase after rainy seasons and throughout the year in irrigated areas in different parts of Africa [14, 15]. Studies from different parts of the world show that larval control reduces on malaria cases and reduces human vector contact [16,17,18,19]. A similar study conducted in western Kenya indicates that the combination of LSM and ITNs results in enhanced protection [20].
Even though larval source management (LSM) has been shown to be effective in different settings in malaria-endemic regions when habitats are few, fixed, and findable [21], numerous aquatic habitats and mosquito larvae species can be found all year in environmentally modified areas [22]. Understanding habitat seasonality and Anopheles larvae abundance has a significant impact on designing and implementing possible larval stage control timing and approaches [5, 14, 23]. However, larval abundance may not imply vector productivity in particular habitats; rather, it may be a good predictor of vector availability in the area [24].
Various chemical properties of the larval habitat, such as pH, optimum temperature, and ammonia, nitrate, and sulfate concentrations, have been discovered to affect larval development and survival [25, 26]. Moreover, the physicochemical parameters of the irrigation site are critical for mosquito breeding. In the process of increasing crop production, herbicides, insecticides, fungicides, nematicides, and fertilizers were used in higher quantities [27,28,29]. Studies indicate that one of the causes of insecticide resistance in malaria vector populations is the extensive use of agrochemicals [30]. Furthermore, the irrigation site may result in rapid larval development due to high proportions of sulfate, nitrate, and phosphate and dissolved solids from the fertilizer, and most of the irrigation system uses river water [31]. River waters contain chemicals such as calcium, magnesium, sulfate, nitrate, phosphate, and dissolved solids in high proportions as nutrients. This might result in the larvae’s exposure to important nutrients for their rapid development and exposure to different chemicals that have similar insecticide groups, which might lead to the development of insecticide resistance in a population through time in generations.
Recent widespread irrigation projects in Ethiopia have increased agricultural productivity and promote economic growth. Such large-scale water resource development projects may increase the burden of malaria by increasing vector density. The risk of such types of activities has not been well studied in Ethiopia. It is important to determine the physical and psycho-chemical properties of breeding habitats, as well as the seasonal abundance of Anopheles, in each eco-epidemiological context. In order to develop and implement larval source management methods together with the current intervention strategies, it is essential to have an understanding of the physical and psycho-chemical properties of larval habitats. Despite the fact that several larval ecology studies have been conducted in Ethiopia, there is limited data on the seasonality, succession, and physiochemical properties of larval habitats in relation to larval abundance. Although adult vector control plays a greater role in malaria prevalence and incidence reduction, interventions often focus on adult vectors only or neglect the larval/immature stages.
Thus, this study aimed at understanding the abundance of immature stages of Anopheline mosquitoes in irrigated sugar cane development plantations in different seasons and determining the psycho-chemical characteristics of mosquito larval habitats.
Methods
Study area
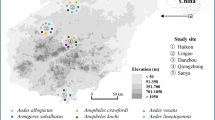
The study was conducted at the Arjo-Didessa sugarcane plantation irrigation scheme, which is located between East Wollega and Buno Bedele Zone, a low malaria transmission setting in south-west Ethiopia (8° 41′ 60″ N, 36° 23′ 60″ E) (Fig. 1). The average altitude in the area is 1350 m above sea level. On average, it receives 1400 mm of rain per year [32]. The area mostly has black soil and occasionally has red and brown soil, which has a slow rate of percolation. As result, rainwater can accumulate in the top layers of the soil and form swamps in the area. The area has seasonal malaria transmission, with Plasmodium falciparum and Plasmodium vivax being the principal malaria parasite species responsible for the majority of the infections: The predominant species in the study area are Anopheles arabiensis, Anopheles amharicus, Anopheles pharoensis, Anopheles coustani complex, Anopheles funestus group, and Anopheles squamosus [15, 33].
Habitat selection and study site mapping
Identification and follow-up of Anopheles larval habitats positive for aquatic mosquito immature stages was conducted from September 2020 to October 2021. First, an exhaustive survey for potential Anopheline larvae habitats was done in the study area. A larval habitat was selected for inclusion in the study if it was positive for Anopheline larvae during the first survey. Each selected habitat was given a permanent identification number using a wooden frame or stand and was geo-referenced using a handheld Global Positioning System (GPS) unit.
Larval sampling and identification
Mosquito larval habitats positive for Anopheles larvae were surveyed and visited twice a month for a period of 14 months (29 visits). Larval sampling was done using a standard dipper (350 ml, Bio Quip Products, Inc., California, USA). Mosquito larvae habitat physical characteristics like distance to the nearest house, exposure to sunlight, and habitat permanence were measured with visual inspection. Mosquito larvae were immediately filtered using mesh in the field so as to avoid larvae competitors, predators, and unwanted organic debris, and then transported to the field insectary along with water samples taken from the mosquito’s natural breeding sites and reared to adult stages with fish food. The species were sorted by genus and sex during the adult stage following identification keys by Gillies and Coetzee [34, 35] and kept separately in separate cages with a 10% sucrose solution.
Molecular identification of An. gambiae sensu lato
Sub-samples of Anopheles gambiae s.l. that emerged from larval collections from the three seasons were randomly selected and identified as species using a species-specific polymerase chain reaction (PCR) assay. In brief, genomic DNA was extracted using a DNA extraction kit (Qiagen, Sigma Aldrich, USA) from whole mosquitoes. PCR amplification was carried out according to the methods of Scott et al. [36] using species-specific primers for An. arabiensis (AR: 5′-AAGTGTCCTTCTCCATCCTA-3′) and An. amharicus, formerly Anopheles quadriannulatus B (QD: 5′-CAGACCAAGAGAGATGGTTAGTAT-3′). Anopheles gambiae (GA: 5′-CTGGTTTGGTCGGCACGTTT-3′) and a universal primer (UN: 5′-GTGTGCCCCTTCCTC GATGT-3′). Then the amplicon was loaded on a 2% agarose gel stained with ethidium bromide and run for gel electrophoresis. Anopheles arabiensis from the Sekoru insectary colony and previously confirmed An. amharicus [33] were used as positive controls.
Physico-chemical characterization of breeding habitats
Physico-chemical variables such as pH, total dissolved solids, temperature, conductivity, dissolved oxygen, nitrate, ammonium, and orthophosphate ion content of water were measured for all habitats. pH, TDS, temperature, electrical conductivity, and dissolved oxygen were measured using a portable multi-meter (pH Tester 10, Oaklon, USA). The rest, ammonium (NH4–N), soluble reactive phosphorous (SRP), and nitrate (NO3–N), were analysed according to the standard methods described by APHA [37].
Statistical analysis
Data analysis was done using the R statistical software package, version 4.2.0. The differences in larval abundance across seasons, habitat types, habitat permanence, distance to the nearest house, and exposure to sunlight were compared using multiple regression analysis. Correlation analysis was used to investigate the relationship between physical and physico-chemical characteristics and Anopheles larval abundance. The ratio of the total number of Anopheles larvae to the total number of dips taken from each larval habitat was used to calculate the mean Anopheles larval density [38].
A mathematical formula was employed to assess the species diversity index, species evenness, and abundance in a community. The species diversity index (SDI) was calculated using Simpson’s Diversity Index Equation [39,40,41] for measuring species heterogeneity or homogeneity for all weeks in different habitat types. Values near zero correspond to a highly diverse or heterogeneous community, and values near one correspond to a more homogeneous community.
where Pi is the fraction of a species that belongs to the nth species, that is, 0 ≤ D ≤ 1 where p is the proportion of individuals in each species, N is the number of species.
Pielou’s evenness index expresses how evenly the individuals in a community are distributed among the different species. Values near one represent a community with near perfect evenness, and they decrease to zero as the relative abundances of the species diverge from evenness [41, 42].
where J′ is Evenness index, H′ is Shannon winner index and used the formula one and S is species richness.
Results
Seasonal larval habitat abundance
A total of 78 larval habitats were positive for Anopheles during the habitat survey and categorized into eleven types of breeding habitats. In total, 11,031 larvae of Anopheles mosquitoes were collected from gate valve leakages, earth-bottom irrigation canals, man-made pools, animal hoof prints, truck tires/road puddles, rain pools, river edges, hippo trenches, spring seepage, farm ditches, and swamps. Of the total collected larvae, 6781 (61.5%) emerged female Anopheles species and 2878 (26%) as males, while the remaining 1372 (12.4%) were dead before emerging adults.
Overall, animal hoof prints, rain pools, tire truks/road puddles, and man-made pools yielded a relatively higher larval density, with mean densities of 7.13, 7.05, 6.15, and 5.4 larvae per dip, respectively. The remaining seven breeding habitats, gate valve leakage, earth bottom irrigation canals, river edges, hippo trenches and swamps, farm ditches, and spring seepage, had a mean larval density of 3.13, 3.09, 1.34, 2.25, 1.55, 2.4, and 2.9 larvae/dip, respectively Additional file 1).
Anopheles larvae were more abundant from September to November and declined in December and January. In the long rainy season (June–August), there was high rain overflow/runoff, which reduced mosquito larval abundance. However, during the short rainy season, the larval abundance was relatively similar in different larval breeding habitats. In general, different larvae habitats have different contribution in different seasons, and this differs significantly between seasons (F = 8.2687 p = 0.005) (Fig. 2).
Figure 2 shows that Anopheles mosquito abundance was different by season and types of larval habitats. During the long rainy season, rain pools, animal hoof prints, tire tracks/road puddles, and man-made pools contributed in higher proportions, while river edges, hippo trenches, and spring seepage had no contribution. Whereas during the dry season, man-made pools, rain pools, animal hoof prints, gate valve leakage, earth-bottom irrigation canals, hippo trenches, and swamps contributed more, whereas, spring seepage, and river edges contributed a small proportion. During the short rainy season, the contributions of the different larval breeding habitats were relatively similar.
Over the study period, the Simpson model showed that there were variations in species diversity over the sampling weeks (Fig. 3a) and seasons: the dry season had a Diversity Index (DI) of 0.95, the short rain season (DI = 0.3), and the long rain season (DI = 1.0). Whereas low species diversity was found between larval habitats; DI was between 0.963 and 0.67. The pielous evenness model (J) indicates that species were distributed unevenly throughout the season in different habitats (Fig. 3b).
Anopheles species abundance and species composition
As indicated in Table 1 below, An. gambiae s.l. had a higher abundance across all three seasons, while An. coustani complex and An. pharoensis had lower abundances. In this finding, there was a significant number of Anopheles species emerging from man-made pools, rain pools, animal hoof prints and earth-bottom irrigation canals throughout the season (F = 4.9632, p = 0.027) (Fig. 4).
Molecular identification of the An. gambiae complex
Out of the 629 An. gambiae s.l. sub-samples that were tested for species identification using PCR, 77.9% (n = 490) were found to be An. arabiensis, 21.5% (n = 132) were An. amharicus (formerly called An. quadriannulatus B), and 1.1% (n = 7) could not be amplified (Fig. 5).
Association between larval abundance and habitat variables
In this study, the regression model depicted that parameters such as season (t = − 2.876, p = 0.00463), distance to the nearest house (t = − 3.847, p = 0.000177), and exposure to sunlight (t = 2.803, P = 005748) were the main predictors of Anopheles larval abundance in the study area, whereas habitat type (t = 0.071, p = 0.943466) and habitat permanence were not significantly associated with larval abundance (t = 0.477, p = 0.633859) (Table 2).
Parameters such as temperature (t = − 2.224, p = 0.027), conductivity (t = 3.210, p = 0.001), dissolved oxygen (t = 7.964, p = 4.44e−13), nitrate ion (t = 2.513, p = 0.01305), and ammonium ion (t = 2.257, p = 0.025) were significantly associated with larval abundance. In contrast, pH (0.022, p = 0.98246), TDS (t = − 1.33, p = 0.185), and orthophosphate ion (mg/l) (t = − 1.627, p = 0.105) were not significantly associated with mosquito larval abundance.
Anopheles larvae abundance was positively correlated with exposure to sunlight (r = 0.34, p = 0.001), conductivity (r = 0.28, p = 0.001), TDS (r = 0.27, p = 0.001), dissolved oxygen (r = 0.48, p = 0.0010), and nitrate ion (mg/l) (r = 0.37, p = 0.001). Whereas season (r = − 0.22, p = 0.01), types of habitat (r = − 0.20, p = 0.01), and distance to the nearest house (r = − 0.42, p = 0.001) were negatively correlated with Anopheles larval abundance (Table 3).
Table 4 indicates that season (t = − 2.228, p = 0.027), distance to the nearest house (t = − 3.812, p = 0.000), exposure to sunlight (t = 2.738, p = 0.006942), and dissolved oxygen (t = − 3.177, p = 0.002) had a significant associated with adult mosquito abundance. However, types of habitats (t = − 0.071, p = 0.943), habitat permanence (t = − 0.477, p = 0.633), and the rest of the physico-chemical variables were not significantly associated with adult abundance (Table 4).
Adult Anopheles species abundance was positively correlated with habitat permanence (r = 0.28, p = 0.00), exposure to sunlight (r = 0.32, p = 0.00), conductivity (r = 0.28, p = 0.00), TDS (r = 0.27, p = 0.00), and nitrate ion (mg/l) (r = 0.35, p = 0.00). Whereas season (r = − 0.16, p = 0.05), types of habitats (r = − 0.19, p = 0.02), distance to nearest house (r = − 0.41, p = 0.00), and dissolved oxygen (r = − 0.28, p = 0.00) were negatively correlated with adult Anopheles mosquito abundance.
Discussion
This study revealed that larval abundance varies across seasons in different larval habitats. Early dry seasons had a higher larval abundance, whereas long rainy seasons had lower larval productivity. This result was consistent with studies conducted in different parts of sub-Saharan Africa [5, 43,44,45], which showed that larval abundance and adult productivity decreased in long rainy seasons while increasing in early dry seasons. The reduced larval abundance during long rainy seasons might be the result of an over-flooding effect, i.e., washing of eggs, larvae, and pupa from larval habitats, which was similar to studies conducted in western Kenya and elsewhere in Ethiopia [14, 43, 46].
The high larval abundance in this study correlates with the country’s high malaria transmission season. The main transmission season lasts from October to December in most parts of the country, following the main rainy season from June to September, and there is also a short transmission season from April to May, following the short rainy season [9].
Anopheles larvae breed in various types of habitats, ranging from large, permanent collections to small, temporary ones. Irrespective of land setup and types of study, numerous habitat types were identified as breeding sites in this and other studies in Ethiopia [15, 46, 47].
In the long rainy season, in this study, rain pools, animal hoof prints, tire tracks/road puddles, and man-made pools all contributed to a higher proportion of larval abundance. A similar cross-sectional study conducted in the same country indicates that rain pools, animal hoof prints, tire tracks/road puddles were the major contributors during the long rainy season [15, 47]. In contrast, during the long rainy season, river edges, hippo trenches, and spring seepage make no or little contribution. During the dry season, man-made pools, gate valve leakages, earth-bottom irrigation canals, river edges, hippo trenches, spring seepage, and swamps play a greater role in this study. Studies conducted in western Kenya found that larval source management is effective where mosquito larval habitats are accessible and distinct [16]. This would give an opportunity to utilize the seasonality of habitats and larval abundance for the effective suppression of larvae and adult mosquito abundance by targeting larval habitats based on seasonal occurrence.
Studies indicated that there were more than 47 documented species and subspecies of Anopheles mosquitoes in Ethiopia [48, 49]. In the current study area, the Anopheles mosquito species occurring in the area were An. arabiensis, An. amharicus, An. coustani complex, An. pharoensis, and An. squamosus [13, 20, 50]. Different findings indicate that in East Africa, the abundance of An. gambiae sensu stricto (s.s.) and An. arabiensis increased in the early dry season [43,44,45]. Similarly, in the current study, An. arabiensis is highly abundant during the dry season. The rest of the secondary and suspected vectors have a relatively equal proportion. In particular, An. coustani complex, regardless of habitat preference, shows a similar pattern of occurrence to An. gambiae s.l. in all seasons.
Over the study period, the Simpson model demonstrated that there was species dominance in early, dry, and long rainy seasons by An. gambiae s.l. over the other species. Whereas there was species heterogeneity in the late dry season and short rainy season. A similar study conducted elsewhere in East Africa indicates that An. gambiae s.l. is the predominant species [43]. In this study, low species diversity was found between larval habitat types. Similarly, studies conducted elsewhere in Africa indicate habitat types have low species diversity [51, 52]. In the current study, species evenness was found to be unequal across seasons and habitats. This contrasts with a study done elsewhere in Ethiopia, which shows that the species were equally distributed with equitability values which indicating the absence of any dominant species [51]. This might suggest that land use practices have significant influence on mosquito species diversity and abundance.
The distance between larval habitat and houses showed an influence on larval abundance in this study, which might indicate the potential for increased indoor vector density [15, 43, 53]. As an evolutionary strategy for energy conservation, it might be suggested that gravid mosquitoes prefer to lay eggs in habitats near human dwellings to conserve energy lost while flying long distances in search of oviposition sites [54].
In this study, water temperature was associated with larval abundance. Similar findings show that larval habitat water temperature is the most important water quality parameter; it affects the quantity of oxygen that can be found in water as a result of photosynthesis by algae and other aquatic plants. Studies reported that moderately high temperatures were necessary for the optimum growth of Anopheles larvae [55]. Further, studies indicate elevated water temperatures are also permissible for more microorganisms to grow and serve as a food source for mosquito larvae [56, 57].
The quantity of sunlight significantly affects Anopheles larval density. In this study, there was a strong correlation between sunlit exposure and the number of larvae. This finding is similar to previous studies conducted in different sub-Saharan African countries [58,59,60]. The average daily water temperature is higher in habitats that are directly exposed to sunlight than in habitats that are shaded. The larval growth stage can be prolonged in shaded habitats, which increasing the chance of stunted larvae and the risk of predation [4, 60, 61].
Furthermore, in this study, various physicochemical characteristics, such as conductivity, dissolving oxygen, ammoniums, and nitrate, were found to significantly influence Anopheles larval abundances. Recent studies in Ethiopia and neighboring Kenya have found that the above physicochemical characteristics influence Anopheles larval abundance [25, 46, 47, 62, 63]. Application of nitrogenous fertilizers in different agro-ecosystems has been demonstrated to lower water turbidity and consequently significantly influence mosquito larval abundance [6, 62,63,64], since Anopheles mosquitoes prefer to oviposit in areas with lower turbidity [65]. In this study, TDS and orthophosphate ions did not show any significant influence on larval abundance. Similarly, phosphate and TDS appear to have no effect on Anopheles larval density in a study conducted in western Kenya and Nigeria [21, 66].
Physicochemical studies at different irrigation sites show that agrochemicals, such as herbicides, pesticides, and fertilizers, improve productivity, increase yield, which alter the physicochemical characteristics of larval habitats. This could expose immature stages to different chemicals with similar insecticide groups [27,28,29]. Selection pressure can lead to the development of insecticide resistance [28, 29, 67]. This could eventually lead to the development of insecticide resistance in malaria vector populations [30, 67]. In different parts of Africa, evidence suggests that agrochemical use results in increases in insecticide resistance in An. gambiae s.l. in Burkina Faso and northern Benin [27, 29]. Resistance to An. arabiensis in Khartoum State, Sudan, and northern Tanzania [68].
Conclusions
The study revealed that different larval habitats have different productivity in different seasons. Physical and physicochemical properties such as ammonium and nitrate, as well as the distance between larval habitats and houses, are associated with larval productivity. Therefore, vector control should consider Anopheles larval habitat seasonality as well as the impact of agrochemical application on LSM. Furthermore, the current vector control should take into account LSM in the dry season based on seasonal habitat availability in malaria-endemic settings, which may allow for addressing dry-season refugal-larval habitats.
Availability of data and materials
The corresponding author can provide the datasets used in this study upon reasonable request.
Abbreviations
- LSM:
-
Larval source management
- SDI:
-
Species diversity index
- TDS:
-
Total dissolved solids
References
Lipton M, Litchfield J, Faurès JM. The effects of irrigation on poverty: a framework for analysis. Water Policy. 2003;5:13–27.
Keiser J, De Castro MC, Maltese MF, Bos R, Tanner M, Singer BH, Utzinger J. Effect of irrigation and large dams on the burden of malaria on a global and regional scale. Am J Trop Med Hyg. 2005;72:392–406.
Awulachew SB, Loulseged M, Yilma AD. Impact of irrigation on poverty and environment in Ethiopia. In: Proceeding of the symposium and exhibition held at Ghion Hotel, Addis Ababa; 2008. https://core.ac.uk/download/pdf/6506866.pdf.
Munga S, Minakawa N, Zhou G, Githeko AK, Yan G. Survivorship of immature stages of Anopheles gambiae s.l (Diptera: Culicidae) in natural habitats in western Kenya highlands. J Med Entomol. 2007;44:758–64.
Munga S, Minakawa Nl, Zhou G, Mushinzimana E, Barrack OOJ, Githeko AK, et al. Association between land cover and habitat productivity of malaria vectors in western Kenyan highlands. Am J Trop Med Hyg. 2006;74:69–75.
Mwangangi JM, Muturi EJ, Shililu J, Muriu SM, Jacob B, Kabiru EW, et al. Survival of immature Anopheles arabiensis (Diptera: Culicidae) in aquatic habitats in Mwea rice irrigation scheme, central Kenya. Malar J. 2006;5:114.
Hawaria D, Kibret S, Demissew A, Tsegaye A, Bitew D, Yan G, Yewhalaw D. Survivorship of Anopheles gambiae sensu lato in irrigated sugarcane plantation scheme in Ethiopia. Parasit Vectors. 2021;14:142.
Yasuoka J. Impact of deforestation and agricultural development on Anopheline ecology and malaria epidemiology. Am J Trop Med Hyg. 2007;76:450–60.
Federa Ministry of Health (FMOH). National malaria guidelines. 3rd ed. Addis Ababa: Federa Ministry of Health (FMOH); 2012.
Sougoufara S, Ottih EC, Tripet F. The need for new vector control approaches targeting outdoor biting Anopheline malaria vector communities. Parasit Vectors. 2020;13:295.
WHO. World malaria report 2015. Geneva: World Health Organization; 2016.
Yeshiwondim AK, Gopal S, Hailemariam AT, Dengela DO, Patel HP. Spatial analysis of malaria incidence at the village level in areas with unstable transmission in Ethiopia. Int J Health Geogr. 2009;8:5.
Balkew M, Ibrahim M, Koekemoer LL, Brooke BD, Engers H, Aseffa A, et al. Insecticide resistance in Anopheles arabiensis (Diptera: Culicidae) from villages in central, northern and south west Ethiopia and detection of kdr mutation. Parasit Vectors. 2010;3:40.
Kweka EJ, Zhou G, Lee MC, Gilbreath TM, Mosha F, Munga S, et al. Evaluation of two methods of estimating larval habitat productivity in western Kenya highlands. Parasit Vectors. 2011;4:110.
Hawaria D, Demissew A, Kibret S, Lee MC, Yewhalaw D, Yan G. Effects of environmental modification on the diversity and positivity of Anopheline mosquito aquatic habitats at Arjo-Dedessa irrigation development site, Southwest Ethiopia. Infect Dis Poverty. 2020;9:9.
Fillinger U, Sonye G, Killeen GF, Knols BGJ, Becker N. The practical importance of permanent and semipermanent habitats for controlling aquatic stages of Anopheles gambiae sensu lato mosquitoes: operational observations from a rural town in western Kenya. Trop Med Int Health. 2004;9:1274–89.
Yapabandara AM, Curtis CF, Wickramasinghe MB, Fernando WP. Control of malaria vectors with the insect growth regulator pyriproxyfen in a gem-mining area in Sri Lanka. Acta Trop. 2001;80:265–76.
Geissbühler Y, Kannady K, Chaki PP, Emidi B, Govella NJ, Mayagaya V, Kiama M, Mtasiwa D, Mshinda H, Lindsay SW, Tanner M. Microbial larvicide application by a large-scale, community-based program reduces malaria infection prevalence in urban Dar es Salaam, Tanzania. PLoS ONE. 2009;4:e5107.
Yapabandara AM, Curtis CF. Control of vectors and incidence of malaria in an irrigated settlement scheme in Sri Lanka when using the insect growth regulator pyriproxyfen. J Am Mosq Control Assoc. 2004;20:395–400.
Fillinger U, Ndenga B, Githeko A, Lindsay SW. Integrated malaria vector control with microbial larvicides and insecticide-treated nets in western Kenya: a controlled trial. Bull World Health Organ. 2009;87:655–65.
Afrane YA, Zhou G, Lawson BW, Githeko AK, Yan G. Effects of microclimatic changes caused by deforestation on the survivorship and reproductive fitness of Anopheles gambiae in western Kenya highlands. Am J Trop Med Hyg. 2005;42:974–80.
Afrane YA, Zhou G, Lawson BW, Githeko AK, Yan G. Effects of microclimatic changes caused by deforestation on the survivorship and reproductive fitness of Anopheles gambiae in western Kenya highlands. Am J Trop Med Hyg. 2006;74:772–8.
Ndenga BA, Simbauni JA, Mbugi JP, Githeko AK, Fillinger U. Productivity of malaria vectors from different habitat types in the western Kenya highlands. PLoS ONE. 2011;6:e19473.
Suryanarayana Murty U, Srinivasa Rao M, Arunachalam N. The effects of climatic factors on the distribution and abundance of Japanese encephalitis vectors in Kurnool district of Andhra Pradesh, India. J Vector Borne Dis. 2002;39:833–41.
Mutero CM, Wekoyela P, Githure J, Konradsen F. Ammonium sulphate fertiliser increases larval populations of Anopheles arabiensis and culicine mosquitoes in rice fields. Acta Trop. 2004;89:187–92.
Okogun GR, Anosike JC, Okere A, Nwoke B, Esekhegbe A. Epidemiological implications of preferences of breeding sites of mosquito speciesin midwestern Nigeria. Ann Agric Environ Med. 2003;10:217–22.
Yadouleton A, Martin T, Padonou G, Chandre F, Asidi A, Djogbenou L, et al. Cotton pest management practices and the selection of pyrethroid resistance in Anopheles gambiae population in northern Benin. Parasit Vectors. 2011;4:60.
Chouaïbou M, Etang J, Brevault T, Nwane P, Hinzoumbé CK, Mimpfoundi R, Simard F. Dynamics of insecticide resistance in the malaria vector Anopheles gambiae s.l. from an area of extensive cotton cultivation in northern Cameroon. Trop Med Int Health. 2008;13:476–86.
Diabate A, Baldet T, Chandre F, Akogbeto M, Guiguemde TR, Guillet P, et al. The role of agricultural use of insecticides in resistance to pyrethroids in Anopheles gambiae s.l. in Burkina Faso. Am J Trop Med Hyg. 2002;67:617–22.
Mouhamadou CS, de Souza SS, Fodjo BK, Zoh MG, Bli NK, Koudou BG. Evidence of insecticide resistance selection in wild Anopheles coluzzii mosquitoes due to agricultural pesticide use. Infect Dis Poverty. 2019;8:64.
Grillet ME. Factors associated with distribution of Anopheles aquasalis and Anopheles oswaldoi (Diptera: Culicidae) in a malarious area, northeastern Venezuela. J Med Entomol. 2000;37:231–8.
Ethiopian Sugar Industry Group. Arjo Dediessa sugar factory. https://etsugar.com/esig/2023/01/30/arjo-dediessa-sugar-factory/2023. Accessed 26 Feb 2023.
Demissew A, Hawaria D, Kibret S, Animut A, Tsegaye A, Lee MC, et al. Impact of sugarcane irrigation on malaria vector Anopheles mosquito fauna, abundance and seasonality in Arjo-Didessa, Ethiopia. Malar J. 2020;19:344.
Gillies MT, Coetzee M. A supplement to the Anophelinae of Africa South of the Sahara. Publ S Afr Inst Med Res. 1987;55:1–43.
Coetzee M. Key to the females of afrotropical Anopheles mosquitoes (Diptera: Culicidae). Malar J. 2020;19:70.
Scott JA, Brogdon WG, Collins FH. Identification of single specimens of the Anopheles gambiae complex by the polymerase chain reaction. Am J Trop Med Hyg. 1993;49:520–9.
Baird RB, Eaton AD, Federation WE. Standard methods for the examination of water and wastewater. 23rd ed. New York: Pharmabooks.
Williams J, Pinto J. Training manual on malaria entomology for entomology and vector control technicians (basic level). Washington, DC: USAID; 2012. p. 78.
Alatalo R, Alatalo R. Components of diversity: multivariate analysis with interaction. Ecology. 1977;58:900–6.
Norris JL, Pollock KH. Non-parametric MLE for Poisson species abundance models allowing for heterogeneity between species. Environ Ecol Stat. 1998;5:391–402.
Diversity indices. https://bio.libretexts.org/Courses/Gettysburg_College/01%3A_Ecology_for_All/22%3A_Biodiversity/22.02%3A_Diversity_Indices. Accessed 26 Feb 2023.
Southwood TR, Henderson PA. Ecological methods. 3rd ed. Oxford: Blackwell Science Ltd; 2000. p. 575.
Kweka EJ, Zhou G, Munga S, Lee MC, Atieli HE, Nyindo M, et al. Anopheline larval habitats seasonality and species distribution: a prerequisite for effective targeted larval habitats control programmes. PLoS ONE. 2012;7:e52084.
Minakawa N, Githure JI, Beier JC, Yan G. Anopheline mosquito survival strategies during the dry period in western Kenya. J Med Entomol. 2001;38:388–92.
Mala AO, Irungu LW, Shililu JI, Muturi EJ, Mbogo CC, Njagi JK, et al. Dry season ecology of Anopheles gambiae complex mosquitoes at larval habitats in two traditionally semi-arid villages in Baringo, Kenya. Parasit Vectors. 2011;4:25.
Getachew D, Balkew M, Tekie H. Anopheles larval species composition and characterization of breeding habitats in two localities in the Ghibe River Basin, southwestern Ethiopia. Malar J. 2020;19:65.
Keno H, Ejeta D, Negisho T, Wakjira M, Muleta G, Natea G, et al. Characterization of Anopheles mosquito larval habitats and species composition in Bambasi District, Northwestern Ethiopia. Int J Trop Insect Sci. 2022;42:2325–36.
Gaffigan TV, Wilkerson RC, Pecor JE, Stoffer JA, Anderson T. Systematic catalog of Culicidae. Walter Reed Biosystematics Unit, Walter Reed Army Inst Res. 2013.
Irish SR, Kyalo D, Snow RW, Coetzee M. Updated list of Anopheles species (Diptera: Culicidae) by country in the afrotropical region and associated islands. Zootaxa. 2020;4747:401–49.
Jaleta KT, Hill SR, Seyoum E, Balkew M, Gebre-Michael T, Ignell R, et al. Agro-ecosystems impact malaria prevalence: large-scale irrigation drives vector population in western Ethiopia. Malar J. 2013;12:350.
Asmare Y, Wale M, Adem S. Larval Anopheles species composition and diversity at different habitats and seasons of Gondar Zuria District, Ethiopia. J Trop Med. 2022;2022:9767155.
Dida GO, Anyona DN, Abuom PO, Akoko D, Adoka SO, Matano AS, et al. Spatial distribution and habitat characterization of mosquito species during the dry season along the Mara River and its tributaries, in Kenya and Tanzania. Infect Dis Poverty. 2018;7:2.
Minakawa N, Seda P, Yan G. Influence of host and larval habitat distribution on the abundance of African malaria vectors in western Kenya. Am J Trop Med Hyg. 2002;67:32–8.
Minakawa N, Mutero CM, Githure JI, Beier JC, Yan G. Spatial distribution and habitat characterization of Anopheline mosquito larvae in western Kenya. Am J Trop Med Hyg. 1999;61:1010–6.
Kipyab PC, Khaemba BM, Mwangangi JM, Mbogo CM. The physicochemical and environmental factors affecting the distribution of Anopheles merus along the Kenyan coast. Parasit Vectors. 2015;8:221.
Paaijmans KP, Takken W, Githeko AK, Jacobs AFG. The effect of water turbidity on the near-surface water temperature of larval habitats of the malaria mosquito Anopheles gambiae. Int J Biometeorol. 2008;52:747–53.
Gimmg JE, Ombok M, Kamau L, Hawley WA. Characteristics of larval anopheline (Diptera: Culicidae) habitats in western Kenya. J Med Entomol. 2001;38:282–8.
Aklilu E, Kindu M, Gebresilassie A, Yared S, Tekie H, Balkew M. Environmental factors associated with larval habitats of Anopheline mosquitoes (Diptera: Culicidae) in Metema District, Northwestern Ethiopia. J Arthropod Borne Dis. 2020;14:153–61.
Kenea O, Balkew M, Gebre-Michael T. Environmental factors associated with larval habitats of Anopheline mosquitoes (Diptera: Culicidae) in irrigation and major drainage areas in the middle course of the Rift Valley, central Ethiopia. J Vector Borne Dis. 2011;48:85–92.
Wang X, Zhou G, Zhong D, Wang X, Wang Y, Yang Z, et al. Life-table studies revealed significant effects of deforestation on the development and survivorship of Anopheles minimus larvae. Parasit Vectors. 2016;9:323.
Emidi B, Kisinza WN, Mmbando BP, Malima R, Mosha FW. Effect of physicochemical parameters on Anopheles and Culex mosquito larvae abundance in different breeding sites in a rural setting of Muheza, Tanzania. Parasit Vectors. 2017;10:304.
Sanford MR, Chan K, Walton WE. Effects of inorganic nitrogen enrichment on mosquitoes (Diptera: Culicidae) and the associated aquatic community in constructed treatment wetlands. J Med Entomol. 2005;42:766–76.
Muturi EJ, Mwangangi J, Shililu J, Muriu S, Jacob B, Kabiru E, et al. Mosquito species succession and physicochemical factors affecting their abundance in rice fields in Mwea, Kenya. J Med Entomol. 2007;44:336–44.
Mwangangi J, Shililu J, Muturi E, Gu W, Mbogo C, Kabiru E, Jacob B, Githure J, Novak R. Dynamics of immature stages of Anopheles arabiensis and other mosquito species (Diptera: Culicidae) in relation to rice cropping in a rice agro-ecosystem in Kenya. J Vector Ecol. 2006;31:245–51.
Blaustein L, Chase JM. Interactions between mosquito larvae and species that share the same trophic level. Annu Rev Entomol. 2007;52:489–507.
Imam A, Deeni Y. Larval productivity and detoxification enzymes profile in response to physico-chemical environmental factors of Anopheles gambiae breeding ecologies in Nigeria. Br J Appl Sci Technol. 2015;5:595–612.
Reid MC, McKenzie FE. The contribution of agricultural insecticide use to increasing insecticide resistance in African malaria vectors. Malar J. 2016;15:107.
Abuelmaali SA, Elaagip AH, Basheer MA, Frah EA, Ahmed FT, Elhaj HF, et al. Impacts of agricultural practices on insecticide resistance in the malaria vector Anopheles arabiensis in Khartoum State, Sudan. PLoS ONE. 2013;8:e80549.
Acknowledgements
We would like to thank the Arjo-Didessa sugar factory and the surrounding community for their cooperation in carrying out this research. We are grateful to the ICEMR field Entomology data collectors for their assistance in mosquito collection and rearing. We also acknowledge the Tropical and Infectious Diseases Research Center (TIDRC) Laboratory Staff at Jimma University.
Funding
National Institutes of Health (NIH) provided financial support for this research (Grant Nos. D43TW001505, R01A1050243 and U19AI129326). The funders played no role in the study design, data collection and analysis, publication decision, or manuscript writing.
Author information
Authors and Affiliations
Contributions
AT, DY, and GY conceived the study, and AT drafted the manuscript; AT, XW, and GZ analyzed and interpreted the data; and AT, AD, AA, DH, KH, and HG were involved in field data collection; AT and AD performed morphological and molecular species identification. Study sites were mapped by ML. The manuscript was critically examined by DY, GY, and TD for significant intellectual content. All authors read and approved the final draft of the paper.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The Ethiopian National Research Ethics Review Committee (NRERC) examined and approved the protocol (Ref. no.: 10/31/2018). In addition, permission was given by the Buno Bedele and East Wollega Zone Health Offices, as well as the Arjo-Didessa Sugar Factory in Oromia Regional State.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Tsegaye, A., Demissew, A., Hawaria, D. et al. Anopheles larval habitats seasonality and environmental factors affecting larval abundance and distribution in Arjo-Didessa sugar cane plantation, Ethiopia. Malar J 22, 350 (2023). https://doi.org/10.1186/s12936-023-04782-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12936-023-04782-1