Abstract
Background
Over the last decades, enormous successes have been achieved in reducing malaria burden globally. In Latin America, South East Asia, and the Western Pacific, many countries now pursue the goal of malaria elimination by 2030. It is widely acknowledged that Plasmodium spp. infections cluster spatially so that interventions need to be spatially informed, e.g. spatially targeted reactive case detection strategies. Here, the spatial signature method is introduced as a tool to quantify the distance around an index infection within which other infections significantly cluster.
Methods
Data were considered from cross-sectional surveys from Brazil, Thailand, Cambodia, and Solomon Islands, conducted between 2012 and 2018. Household locations were recorded by GPS and finger-prick blood samples from participants were tested for Plasmodium infection by PCR. Cohort studies from Brazil and Thailand with monthly sampling over a year from 2013 until 2014 were also included. The prevalence of PCR-confirmed infections was calculated at increasing distance around index infections (and growing time intervals in the cohort studies). Statistical significance was defined as prevalence outside of a 95%-quantile interval of a bootstrap null distribution after random re-allocation of locations of infections.
Results
Prevalence of Plasmodium vivax and Plasmodium falciparum infections was elevated in close proximity around index infections and decreased with distance in most study sites, e.g. from 21.3% at 0 km to the global study prevalence of 6.4% for P. vivax in the Cambodian survey. In the cohort studies, the clustering decreased with longer time windows. The distance from index infections to a 50% reduction of prevalence ranged from 25 m to 3175 m, tending to shorter distances at lower global study prevalence.
Conclusions
The spatial signatures of P. vivax and P. falciparum infections demonstrate spatial clustering across a diverse set of study sites, quantifying the distance within which the clustering occurs. The method offers a novel tool in malaria epidemiology, potentially informing reactive intervention strategies regarding radius choices of operations around detected infections and thus strengthening malaria elimination endeavours.
Similar content being viewed by others
Background
Globally, enormous successes have been achieved over the last decades in reducing the malaria burden [1]. The main instruments were the distribution of long-lasting insecticidal nets and clinical case management after passive case detection. The strategies deployed so far have proven particularly successful in battling Plasmodium falciparum transmission. However, Plasmodium vivax causes relapses [2] and to a higher degree asymptomatic low-parasitaemia infections that escape passive case detection but contribute to transmission [3, 4]. The effectiveness against P. vivax malaria has thus been less pronounced so that P. vivax has predominance over P. falciparum in many areas [1].
In Latin America, South East Asia, and the Western Pacific, the overall reduction of malaria burden in many countries has paved the way for the ambitious goal of malaria elimination by 2030 [1, 5]. The necessity to incorporate active finding of infections in order to tackle the silent reservoir of on-going transmission is increasingly acknowledged [3, 4, 6,7,8,9]. One strategy considered as part of current recommendations for malaria elimination are reactive interventions, i.e. active finding, testing, and treatment of other infections in a delimited area around passively detected malaria cases [10]. However, the radius of the area in which to optimally implement interventions remains unclear.
Infection with human Plasmodium spp. through an Anopheles mosquito bite depends on a complex interplay of factors of the pathogen, host, vector, and environment. Each of these factors, such as proximity to the vector’s breeding grounds, bed net usage, Anopheles species composition, and host immunity, can vary geographically [11, 12]. Geographical heterogeneity in malaria infections appears across multiple spatial scales varying from between households [13,14,15,16] to within and between villages [9, 11]. At low incidence, the possibility to identify single malaria transmission clusters has long been reported [17].
Reducing malaria transmission country-wide usually leads to a geographical fragmentation of areas where malaria risk remains high, further increasing spatial heterogeneity [18]. Tailoring interventions to high-risk areas within these countries is at the core of recommended strategies for malaria elimination [10, 19]. Obtaining a good understanding of the spatial dependence between malaria infections will help underpin spatially targeted control efforts.
To date, most efforts to characterise the spatial clustering of malaria have relied on comparing infection prevalence at community or household scales [11, 13]. In addition, some studies have used space–time clustering statistics such as the k-nearest neighbour method for binary testing whether spatiotemporal clustering of cases occurred [17]. The introduction of spatial scanning [20, 21] to malaria epidemiology further stimulated studies on spatial heterogeneity in disease or infection risk. This method scans the study area for (size-variable) circular areas with statistically significant clustering of infections (‘hotspots’). Such hotspots were identified in diverse study areas in South America [22,23,24,25,26], Africa [27,28,29,30,31,32,33,34,35,36,37,38,39], and South East Asia [9, 40,41,42,43,44], for P. falciparum [25, 32, 40, 41], P. vivax [25, 26, 32, 40, 41], and Plasmodium malariae [40, 41]. However, the effectiveness of the method in forecasting hotspots remains unclear and it does not generalize spatial patterns between infections.
In this study, a global clustering statistic is introduced to malaria epidemiology, borrowing strongly from earlier development in the epidemiology of dengue and other pathogens [45, 46]. The spatial signature quantifies both the magnitude of clustering of infections and the radius within which it is statistically significant, potentially informing reactive interventions as part of malaria elimination endeavours. Spatial signatures of PCR-confirmed P. vivax and P. falciparum infections were revealed by assessing the pooled prevalence of infections around confirmed index infections in widening spatial and temporal windows. Spatial and temporal clustering of infections was demonstrated in both cross-sectional surveys and cohort studies from a diverse spectrum of study sites in Brazil [6, 47], Thailand [7, 8], Cambodia [9], and Solomon Islands [48].
Methods
Cross-sectional malaria prevalence surveys
Data from 5 cross-sectional surveys from 4 countries, i.e. Brazil, Thailand, Cambodia, and Solomon Islands were used. The study design and basic descriptions are summarized in Table 1. A cross-sectional survey in 17 villages in the high-incidence, rural province Mondulkiri, in North-Eastern Cambodia, in the dry season in December 2017 until April 2018 was included (as described in [9]). The 4200 study participants were 2–79 years old and from randomly sampled households based on a census prior to the survey. Villages of residence were classified into categories of proximity to the forest based on a forest cover remote sensing analysis [49]. In this and all following included studies, finger-prick blood samples were tested for P. vivax and P. falciparum infections by real-time PCR and GPS locations of households were collected.
From Kanchanaburi and Ratchaburi provinces in western Thailand, data from a cross-sectional survey in September–October 2012 were considered [7]. In this survey, 4309 study participants aged 0–92 years were enrolled.
Data were added from two cross-sectional surveys in a peri-urban part of Manaus, Amazonas State, Brazil from November 2012 until January 2013 (rainy season) and from August until September 2013 (dry season), respectively [6]. After all inhabitants had been invited to the study, 2010 and 2073 participated in the two surveys, respectively (963 in both surveys).
From Solomon Islands, data were used from a cross-sectional survey in several villages across Ngella, Central Islands province from May until June 2012 [48]. From a representative household-based sample, 3501 individuals agreed to participate.
Longitudinal cohort studies
In addition to the cross-sectional surveys, data were used from two longitudinal cohort studies with similar design: Monthly active follow-up with finger-prick blood-sampling for P. vivax and P. falciparum real-time PCR testing and collection of household locations by GPS at baseline. One study was conducted in two villages in Kanchanaburi and Ratchaburi provinces, Thailand, respectively, in May 2013 until June 2014, enrolling 999 participants (Table 1) [8]. The other study took place in the peri-urban part of Manaus, Brazil, from April 2013 until March 2014 with 1274 enrolled participants [47].
Spatial signature of malaria infection
In order to characterise the spatial dependence between infections in both the cross-sectional surveys and for each follow-up period in the longitudinal studies, the average prevalence \(\pi (d)\) of PCR-positive infections was calculated within a distance \(d\) of PCR-positive “index” infections. To do this, the study participants that lived within distance \(d\) of each PCR-positive participant were identified, and the proportion that were also PCR-positive was calculated. \(d\) was varied in incremental steps by 1 km for \(\pi\) across the entire study region; and by 50 m for \(\pi\) within 1 km.
The observed prevalence was compared with that expected under a null distribution where no spatial dependence exists. A bootstrap null distribution of \(\pi\) was generated after 10,000 random re-allocations of infection locations and statistical significance was defined outside of the null’s 95th percentile interval.
For the cohort studies, the spatial signature for P. vivax infections was calculated as above, pooling across the monthly study visits, i.e. considering only index-neighbour pairs in the same study month. If multiple monthly PCR results were positive for an individual, only the first, “incident” positive result was considered.
The underlying distribution of pairwise distances between the study participants per study site as well as the underlying distribution of pairwise distances between infections and all study participants are provided in the Additional file 1: Figures S1.1a-S1.7.
Spatiotemporal signature of malaria infection
In the two longitudinal cohort studies, the extent of spatiotemporal dependence was also explored. The period prevalence \(\rho (d, t)\) of PCR-positive neighbouring infections was calculated within a distance \(d\) and within a time window \(t\) of all incident PCR-positive index infections for P. vivax as the ratio of the number of pairs of index and neighbouring incident infections within \(d\) and \(t\) and the number of pairs of study participants within \(d\) (steps by 1 km for \(\rho\) across the entire study region; by 50 m for \(\rho\) within 1 km). A study participant was considered an incident infection at the first study visit with a PCR-positive blood sample. A bootstrap null distribution of \(\rho\) was generated after 1000 random re-allocations of locations of infection time series.
From the cohort study in Thailand, PCR-positive blood samples for P. vivax were genotyped as described in [8]. Spatiotemporal signatures were calculated as described above but respecting only P. vivax infection pairs with a matching genotype.
Comparative analysis of distance to 50% reduction in prevalence
From each spatial signature across the cross-sectional surveys and cohort studies, the distance was drawn within which the prevalence around index infection has fallen by 50% (between the prevalence at 0 m and the study’s global prevalence). The distance was approximated as the mean of the pair of 50 m-increments of distance \(d\) within which the spatial signature falls below the 50% of prevalence.
Software
The data were described, analysed, and visualised in R 4.1.2 [50]. The maps were created with the ggmaps package in R and background Landsat-8 image courtesy of the U.S. Geological Survey.
Results
Spatial signatures from cross-sectional surveys
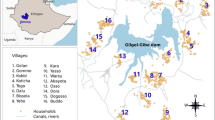
In the Cambodian cross-sectional survey, P. vivax and P. falciparum infections were found in all villages across the study region (Fig. 1A). Across all villages, the P. vivax prevalence was 6.4%, the P. falciparum prevalence was 3.0%, and the prevalence of co-infection was 1.1%. The prevalence of P. vivax around index infections decreased threefold from 21.3% at 0 km to the global study prevalence of 6.4% at maximum distance (Fig. 1B). The estimate at 0 km may include household members and inhabitants of the same building. For P. falciparum, prevalence decreased from 12.9 to 3.0%. Within 1 km, it decreased to 13.3% for P. vivax infections and to 7.9% for P. falciparum infections. Amongst the villages inside the forest, P. vivax prevalence decreased from 38.4% at 0 km to 28.2% within 1 km (Fig. 1C). For P. falciparum, prevalence fell from 23.9 to 17.3%. For both species, prevalence remains spatially clustered beyond the null distribution within 1 km amongst the villages inside and outside the forest but to a lesser degree, if at all, amongst the villages at the forest fringe.
Spatial signature of P. vivax or P. falciparum infections in the Cambodian cross-sectional survey. Prevalence of infections at increasing distance around index infections. Panel A: Household locations in shades of blue, purple, or green per village outside the forest, at the forest-fringe, or inside the forest, respectively. P. vivax and P. falciparum infections in red. B: The spatial signature across the entire study region (left) or within 1 km around infections (right). Ribbon: 95%-quantile interval of null distribution. Horizontal line: Global study prevalence. C: Stratified by the villages’ forest proximity within 1 km
In the cross-sectional survey across three sites in Western Thailand (Fig. 2A), prevalence of P. vivax infections around index infections decreased from 6.6% at 0 km to 3.6% at 1 km and for P. falciparum from 3.1 to 1.0% (Fig. 2B). Significant spatial clustering for P. vivax infections was found only within a radius of 75 m.
Spatial signatures of infections in the cross-sectional surveys in Thailand, Brazil, and Solomon Islands. Prevalence of P. vivax or P. falciparum infections at increasing distance around index infections in Thailand (upper panels A–B), Brazil (middle, C–D), and Solomon Islands (lower, E–F). Panels A, C, E: Maps with household locations in light gold. P. vivax (and for first Brazilian survey also P. falciparum) infections in red. Maps of P. falciparum infections in the Thailand survey, maps of the infections in the second Brazilian survey, and complete maps of P. vivax and P. falciparum infections in all surveyed villages in Solomon Islands are in the Supplementary Material. B, D, F: The spatial signatures per survey within 1 km around infections. Ribbon: 95%-quantile interval of null distribution. Horizontal line: Global study prevalence
In the peri-urban part of Manaus, P. vivax and P. falciparum infections were detected in the first of the two cross-sectional surveys (Fig. 2C) and P. vivax infections in the second survey (maps in the Additional file 1: Figure S3). Only one P. falciparum infection was found in the second survey, precluding any signature analysis. P. vivax prevalence dropped from 11.4% at 0 km around index infections to 5.3% within 1 km in the first survey and from 9.6 to 3.7% in the second survey (Fig. 2D). Prevalence around P. vivax index infections was significantly elevated around index cases within 1 km in the first survey and up to 70 m in the second survey. Prevalence of P. falciparum infections in the first survey did not follow a decreasing pattern.
In the cross-sectional survey in Solomon Islands, P. vivax prevalence was 23.7% at 0 km around index infections and hovered between 19.3 and 21.8% until a distance of 1 km (a decreasing pattern is more apparent from the signature across the entire study region in the Additional file 1: Figure S5). For P. falciparum, prevalence decreased from 11.1% at 0 km around index infections until 1.8% within 1 km. Prevalence was elevated from the null distribution for both species across the distance of 1 km.
Spatial and spatiotemporal signatures from cohort studies
Pooling across the study visits of the longitudinal cohort studies, P. vivax prevalence around index infections per study visit decreased from 9.5% at 0 km to 4.1% at 1 km in the study in Thailand (Fig. 3B) and from 13.1 to 6.4% in the Brazilian study (Fig. 3E). The signature remains above the null distribution until 250 m in the Thai study and entirely within 1 km in the study in Brazil.
Spatial and spatiotemporal signatures of infections in the cohort studies in Thailand and Brazil. Prevalence of P. vivax infections at increasing distance (and in widening time windows) around index infections in Thailand (left, panels A–C) and Brazil (right, D–F). Panels A, D: Maps with household locations in light gold. P. vivax infections in red. B, E: The spatial signatures within 1 km around infections, pooling spatial clustering across the study visits. Ribbon: 95%-quantile interval of null distribution. C, F: The spatiotemporal signatures of incident P. vivax infections within 1 km and widening time windows in months
In the cohort study in Thailand, P. vivax period prevalence decreased from 4.5% at 0 km around incident infections to 2.3% within 1 km and the same study month, from 4.3 to 2.3% within 2 months, and from 3.1 to 1.9% across the entire study period of 14 months (Fig. 3C). Period prevalence was above the null distribution until 150 m within the same study month and within 2 months, and 25 m for infections across the study period.
In the Brazilian cohort study, P. vivax period prevalence fell from 7.3% at 0 km to 3.5% within 1 km and the same study month, from 5.7 to 3.1% within 2 months, and from 4.7 to 2.9% across the entire study period of 13 months (Fig. 3F). Period prevalence was above the null distribution until 150 m within the same study month, until 250 m for infections within 2 months, and 100 m for infections across the study period.
Considering only infection pairs with a matching genotype in the cohort study from Thailand, P. vivax period prevalence decreased from 1.4% at 0 km to 0.4% within 1 km and the same study month, from 0.9 to 0.3% for infections up to 2 months apart, and from 0.7 to 0.2% across the entire study period of 14 months (Fig. 4). Period prevalence was above the null distribution until 200 m within the same study month, until 250 m for infections within 2 months, and 250 m for infections across the study period.
Stronger spatial clustering at lower global prevalence
Across the spatial signatures of the cross-sectional surveys and cohort studies, the distance within which prevalence is reduced by 50% varies from 3175 m in the Solomon Islands survey to 25 m in the Thailand survey for P. vivax and from 975 m in the Cambodian survey to 75 m in the Thailand and Solomon Islands surveys for P. falciparum (Table 2, Fig. 5). Overall, the distance to 50% reduction tends to be shorter with lower prevalence.
Discussion
Across multiple studies in diverse malaria-endemic regions, clustering of P. vivax and P. falciparum infections in close proximity around index infections was demonstrated, decreasing with distance. Similarly, co-infections with both species cluster spatially (Additional file 1: Figure S6). Comparing the signature to a null distribution after random re-allocation of infection locations shows statistically significant clustering in most settings. In a village in Senegal, spatial clustering of P. falciparum infections has previously been shown with this method [14]. The finding of spatial/spatiotemporal clustering is in line with previous studies close to the study sites [23, 24, 40, 41] or in other malaria-endemic regions [22, 25,26,27,28, 30,31,32,33,34,35,36,37,38,39, 42,43,44], deploying the SaTScan scanning method.
Clustering for P. falciparum was not found in the first Brazilian cross-sectional survey. Given very low global P. falciparum prevalence in both surveys [6], this could be due to a lack of statistical power. Considering the distribution of infection locations on the map, almost all infections surround a forested, sparsely inhabited area in which no samples were collected. The resulting spatial signature is possibly an artefact due to a peculiar distribution of household locations in an insufficiently sampled transmission hotspot.
The signature of P. falciparum infections in the cross-sectional survey in Thailand also lacks statistical significance, however follows a decreasing form. This lack of significance most likely stems from low prevalence and insufficient statistical power. Clustering of P. vivax infections in this and the second Brazilian survey was significant, however only within a short distance. Statistical power decreases with fewer cases and a tendency of clustering towards shorter distances was observed at lower global survey prevalence. Clustering might thus become increasingly difficult to demonstrate with statistical significance as prevalence decreases.
A significant spatial signature was also not observed in the Cambodian cross-sectional survey among the villages at the forest fringe. This is most likely because exposure of inhabitants of villages outside the forest is mainly driven by forest-going behaviour [9], and vectors in this region are mainly found in the forests [12].
Plasmodium vivax infection distribution in Solomon Islands’ cross-sectional survey showed significant clustering in a flat signature within 1 km distance around index infections, with clustering decreasing at greater distances. There was substantial variation in malaria prevalence between villages [48]. By calculating a spatial signature pooled across all villages that were sampled in the survey, significant within-village clustering was potentially missed in high transmission areas.
Spatial clustering was also shown in the cohort studies, particularly within shorter time windows. The amplitude of the signatures flattens with increasing time windows, demonstrating temporal clustering. Relapses could affect the spatiotemporal signature for P. vivax. Repeat infections in the same individual do not contribute towards the presented spatiotemporal signatures as only the first PCR-positive sample was considered. Relapses may still cause a higher degree of measured spatiotemporal clustering of P. vivax infections in a geographic region as relapsing neighbours of an index case also contribute to the detected clustering. From a programmatic perspective, this would still make the signature informative to guide the detection of both new and relapse infections. Considering only the incident infection does not take into account that subsequent infections in the same person can also be new infections and may reduce the ability to detect correlations over time. More detailed genetic analysis would be required to delineate the direction of the effect [51].
Considering only infection pairs of a matching genotype, the P. vivax spatiotemporal signature in the Thai cohort becomes even more pronounced. While the genotyping methods used here lack the resolution to define separate transmission chains with certainty, the finding that infections with a matching genotype cluster in space and time corroborates the principle of the spatial signature method.
The study is subject to certain limitations. First, all included studies were based on household locations. In an area with peri-domestic vector exposure, spatial clustering and a decrease with distance based on household locations can be readily interpreted, mainly by the vectors’ host-seeking. The interpretation in areas with mainly behavioural exposure is less straightforward, e.g. in villages outside the forest in the Cambodian site [9]. Significant clustering of infections based on household location could still occur though, e.g. resulting from forest-goers tending to live closer to each other in the villages or more general clustering of household locations based on socio-economic factors. The fact that even in these settings clustering might be found is useful from a programmatic perspective.
Another limitation is that the form of the signatures depends on the locations of the households and villages that were sampled, i.e. the underlying distribution of pairwise distances in the data. Pooling across villages attempts to generalize the prevalence dynamics. Studies that sampled across adjacent villages (e.g. the Cambodian survey) are easily accessible for this approach. However, if sampled villages are far apart, it can complicate the interpretation (e.g. the survey in Solomon Islands). This limitation was controlled for by ensuring that no gaps occur in the pairwise distance distributions. However, the P. falciparum signature in the first Brazilian survey may be an example where these distributions cause artefacts in the signature.
It is generally acknowledged that malaria elimination efforts need to be spatially tailored towards areas where (the highest) transmission risk persists [10, 19]. The scanning method has proven effective for detecting and locating clusters of infections. However, the method detects a single or multiple hotspots, ordered by their likelihood. The localization of hotspots with the strongest clustering can distract from spatial clustering that also exists outside the statistically most significant cluster. While the assessment of village-level prevalence and scanning methods are very valuable for identifying local spatial structure (e.g. identifying hotspots), these tools provide limited information on the more general properties of the spatial nature of malaria infection. Also, while some studies found ‘stable hotspots’, i.e. recurring hotspots in the same area from 1 year to another [36,37,38], others did not find that temporal predictive value [32]. Repeating resource-intensive observational studies in order to regularly update hotspot mapping is not feasible for malaria elimination programmes.
Reactive interventions are part of the recommended toolbox for malaria elimination [10]. Unless they choose levels such as households or villages, malaria control programmes need to be informed on the radius around index infections in which to operate. Scanning methods report the spatial extent of the detected hotspots which is very specific to the investigated area and only partially generalizable. The spatial signature method allows to detect clustering, to quantify its magnitude, and shows its dynamics across increasing distance. For that purpose, it generalizes the spatial clustering across the entire study area and data points. From a programmatic perspective, it can inform a cost–benefit approach for the optimal selection of a radius around detected index infections, balancing the trade-off between the total number of households included, and the expected proportion of infections found. That clustering on the basis of household locations was found even in areas with occupationally driven exposure is encouraging. From a reactive intervention perspective, such socio-economic clustering may still allow effective targeting, regardless of the underlying dynamics.
The distance to 50% reduction in prevalence was considered a suitable measure for comparing the signatures across study sites. For both P. vivax and P. falciparum, a trend towards stronger spatial clustering was observed at lower global study prevalence. This suggests that reactive infection detection strategies could become increasingly effective in low transmission settings approaching malaria elimination.
Conclusion
Spatial clustering of P. vivax and P. falciparum infections was demonstrated across a diverse set of study sites and transmission intensities. Introducing a novel method in spatiotemporal malaria epidemiology, the distance within which clustering occurs around index infections was quantified. These distances are often short, e.g. below 200 m, tending to lower values at lower global study prevalence. The spatial signature of Plasmodium spp. infections offers a new tool to extract insights on Plasmodium spp. epidemiology from observational epidemiological field studies. It also provides a method to inform reactive infection detection strategies regarding effective and feasible radius choices of interventions around detected infections.
Availability of data and materials
The observational studies from which data were used list their data availability statements in the original publications.
Abbreviations
- CSS:
-
Cross-sectional survey
- GPS:
-
Global positioning system
- PCR:
-
Polymerase chain reaction
References
WHO. World malaria report 2021. Geneva: World Health Organization; 2021.
Mueller I, Galinski MR, Baird JK, Carlton JM, Kochar DK, Alonso PL, et al. Key gaps in the knowledge of Plasmodium vivax, a neglected human malaria parasite. Lancet Infect Dis. 2009;9:555–66.
Angrisano F, Robinson LJ. Plasmodium vivax - How hidden reservoirs hinder global malaria elimination. Parasitol Int. 2022;87:102526.
Tadesse FG, Slater HC, Chali W, Teelen K, Lanke K, Belachew M, et al. The relative contribution of symptomatic and asymptomatic Plasmodium vivax and Plasmodium falciparum infections to the infectious reservoir in a low-endemic setting in Ethiopia. Clin Infect Dis. 2018;66:1883–91.
WHO. Zeroing in on malaria elimination. Geneva: World Health Organization; 2021.
Almeida ACG, Kuehn A, Castro AJM, Vitor-Silva S, Figueiredo EFG, Brasil LW, et al. High proportions of asymptomatic and submicroscopic Plasmodium vivax infections in a peri-urban area of low transmission in the Brazilian Amazon. Parasit Vectors. 2018;11:194.
Nguitragool W, Mueller I, Kumpitak C, Saeseu T, Bantuchai S, Yorsaeng R, et al. Very high carriage of gametocytes in asymptomatic low-density Plasmodium falciparum and P. vivax infections in western Thailand. Parasit Vectors. 2017;10:512.
Nguitragool W, Karl S, White M, Koepfli C, Felger I, Singhasivanon P, et al. Highly heterogeneous residual malaria risk in western Thailand. Int J Parasitol. 2019;49:455–62.
Sandfort M, Vantaux A, Kim S, Obadia T, Pepey A, Gardais S, et al. Forest malaria in Cambodia: the occupational and spatial clustering of Plasmodium vivax and Plasmodium falciparum infection risk in a cross-sectional survey in Mondulkiri province. Cambodia Malar J. 2020;19:413.
WHO. Guidelines for malaria. Geneva: World Health Organization; 2022.
Greenwood BM. The microepidemiology of malaria and its importance to malaria control. Trans R Soc Trop Med Hyg. 1989;83(Suppl):25–9.
Vantaux A, Riehle MM, Piv E, Farley EJ, Chy S, Kim S, et al. Anopheles ecology, genetics and malaria transmission in northern Cambodia. Sci Rep. 2021;11:6458.
Gamage-Mendis AC, Carter R, Mendis C, De Zoysa AP, Herath PR, Mendis KN. Clustering of malaria infections within an endemic population: risk of malaria associated with the type of housing construction. Am J Trop Med Hyg. 1991;45:77–85.
Niang M, Sandfort M, Mbodj AF, Diouf B, Talla C, Faye J, et al. Fine-scale spatiotemporal mapping of asymptomatic and clinical Plasmodium falciparum infections: epidemiological evidence for targeted malaria elimination interventions. Clin Infect Dis. 2021;73:2175–83.
Bannister-Tyrrell M, Srun S, Sluydts V, Gryseels C, Mean V, Kim S, et al. Importance of household-level risk factors in explaining micro-epidemiology of asymptomatic malaria infections in Ratanakiri Province. Cambodia Sci Rep. 2018;8:11643.
Gul D, Rodriguez-Rodriguez D, Nate E, Auwan A, Salib M, Lorry L, et al. Investigating differences in village-level heterogeneity of malaria infection and household risk factors in Papua New Guinea. Sci Rep. 2021;11:16540.
Chadee DD, Kitron U. Spatial and temporal patterns of imported malaria cases and local transmission in Trinidad. Am J Trop Med Hyg. 1999;61:513–7.
Lana R, Nekkab N, Siqueira AM, Peterka C, Marchesini P, Lacerda M, et al. The top 1%: quantifying the unequal distribution of malaria in Brazil. Malar J. 2021;20:87.
Carter R, Mendis KN, Roberts D. Spatial targeting of interventions against malaria. Bull World Health Organ. 2000;78:1401–11.
Kulldorff M, Nagarwalla N. Spatial disease clusters: detection and inference. Stat Med. 1995;14:799–810.
Kulldorff M. A spatial scan statistic. Commun. Stat. 1997;26:1481–96.
Hiwat H, Martinez-Lopez B, Cairo H, Hardjopawiro L, Boerleider A, Duarte EC, et al. Malaria epidemiology in Suriname from 2000 to 2016: trends, opportunities and challenges for elimination. Malar J. 2018;17:418.
Kohara Melchior LA, Chiaravalloti NF. Spatial and spatio-temporal analysis of malaria in the state of Acre, western Amazon. Brazil Geospat Health. 2016;11:443.
Ueno T, Lima L, Sardinha DM, Rodrigues YC, Souza HUS, Teixeira PR, et al. Socio-epidemiological features and spatial distribution of malaria in an area under mining activity in the Brazilian Amazon Region. Int J Environ Res Public Health. 2021;18:10384.
Rosas-Aguirre A, Guzman-Guzman M, Gamboa D, Chuquiyauri R, Ramirez R, Manrique P, et al. Micro-heterogeneity of malaria transmission in the Peruvian Amazon: a baseline assessment underlying a population-based cohort study. Malar J. 2017;16:312.
Rosas-Aguirre A, Ponce OJ, Carrasco-Escobar G, Speybroeck N, Contreras-Mancilla J, Gamboa D, et al. Plasmodium vivax malaria at households: spatial clustering and risk factors in a low endemicity urban area of the northwestern Peruvian coast. Malar J. 2015;14:176.
Brooker S, Clarke S, Njagi JK, Polack S, Mugo B, Estambale B, et al. Spatial clustering of malaria and associated risk factors during an epidemic in a highland area of western Kenya. Trop Med Int Health. 2004;9:757–66.
Alemu K, Worku A, Berhane Y. Malaria infection has spatial, temporal, and spatiotemporal heterogeneity in unstable malaria transmission areas in northwest Ethiopia. PLoS ONE. 2013;8:e79966.
Solomon T, Loha E, Deressa W, Gari T, Lindtjorn B. Spatiotemporal clustering of malaria in southern-central Ethiopia: a community-based cohort study. PLoS ONE. 2019;14:e0222986.
Gwitira I, Mukonoweshuro M, Mapako G, Shekede MD, Chirenda J, Mberikunashe J. Spatial and spatio-temporal analysis of malaria cases in Zimbabwe. Infect Dis Poverty. 2020;9:146.
Coleman M, Coleman M, Mabuza AM, Kok G, Coetzee M, Durrheim DN. Using the SaTScan method to detect local malaria clusters for guiding malaria control programmes. Malar J. 2009;8:68.
Seyoum D, Yewhalaw D, Duchateau L, Brandt P, Rosas-Aguirre A, Speybroeck N. Household level spatio-temporal analysis of Plasmodium falciparum and Plasmodium vivax malaria in Ethiopia. Parasit Vectors. 2017;10:196.
Ndiath M, Faye B, Cisse B, Ndiaye JL, Gomis JF, Dia AT, et al. Identifying malaria hotspots in Keur Soce health and demographic surveillance site in context of low transmission. Malar J. 2014;13:453.
Gomez-Barroso D, Garcia-Carrasco E, Herrador Z, Ncogo P, Romay-Barja M, Ondo Mangue ME, et al. Spatial clustering and risk factors of malaria infections in Bata district. Equatorial Guinea Malar J. 2017;16:146.
Coulibaly D, Rebaudet S, Travassos M, Tolo Y, Laurens M, Kone AK, et al. Spatio-temporal analysis of malaria within a transmission season in Bandiagara. Mali Malar J. 2013;12:82.
Mosha JF, Sturrock HJ, Greenwood B, Sutherland CJ, Gadalla NB, Atwal S, et al. Hot spot or not: a comparison of spatial statistical methods to predict prospective malaria infections. Malar J. 2014;13:53.
Nourein AB, Abass MA, Nugud AH, El Hassan I, Snow RW, Noor AM. Identifying residual foci of Plasmodium falciparum infections for malaria elimination: the urban context of Khartoum. Sudan PLoS One. 2011;6:e16948.
Mirghani SE, Nour BY, Bushra SM, Elhassan IM, Snow RW, Noor AM. The spatial-temporal clustering of Plasmodium falciparum infection over eleven years in Gezira State, The Sudan. Malar J. 2010;9:172.
Bejon P, Williams TN, Liljander A, Noor AM, Wambua J, Ogada E, et al. Stable and unstable malaria hotspots in longitudinal cohort studies in Kenya. PLoS Med. 2010;7:e1000304.
Durnez L, Pareyn M, Mean V, Kim S, Khim N, Menard D, et al. Identification and characterization of areas of high and low risk for asymptomatic malaria infections at sub-village level in Ratanakiri, Cambodia. Malar J. 2018;17:27.
Sluydts V, Heng S, Coosemans M, Van Roey K, Gryseels C, Canier L, et al. Spatial clustering and risk factors of malaria infections in Ratanakiri Province, Cambodia. Malar J. 2014;13:387.
Rejeki DSS, Fuad A, Widartono BS, Murhandarwati EEH, Kusnanto H. Spatiotemporal patterns of malaria at cross-boundaries area in Menoreh Hills, Java, Indonesia. Malar J. 2019;18:80.
Surendra H, Wijayanti MA, Murhandarwati EH, Irnawati, Yuniarti T, Mardiati, et al. Analysis of serological data to investigate heterogeneity of malaria transmission: a community-based cross-sectional study in an area conducting elimination in Indonesia. Malar J. 2019;18:227.
Murhandarwati EE, Fuad A, Nugraheni MD, Wijayanti MA, Sulistyawati, Widartono BS, et al. Early malaria resurgence in pre-elimination areas in Kokap Subdistrict, Kulon Progo, Indonesia. Malar J. 2014;13:130.
Salje H, Lessler J, Endy TP, Curriero FC, Gibbons RV, Nisalak A, et al. Revealing the microscale spatial signature of dengue transmission and immunity in an urban population. Proc Natl Acad Sci USA. 2012;109:9535–8.
Lessler J, Salje H, Grabowski MK, Cummings DA. Measuring spatial dependence for infectious disease epidemiology. PLoS ONE. 2016;11:e0155249.
Monteiro W, Karl S, Kuehn A, Almeida A, White M, Vitor-Silva S, et al. Prevalence and force of Plasmodium vivax blood-stage infection and associated clinical malaria burden in the Brazilian Amazon. Mem Inst Oswaldo Cruz. 2022;117:e210330.
Waltmann A, Darcy AW, Harris I, Koepfli C, Lodo J, Vahi V, et al. High rates of asymptomatic, sub-microscopic Plasmodium vivax infection and disappearing Plasmodium falciparum malaria in an area of low transmission in Solomon Islands. PLoS Negl Trop Dis. 2015;9:e0003758.
Pepey A, Souris M, Vantaux A, Morand S, Lek D, Mueller I, et al. Studying land cover changes in a malaria-endemic Cambodian district: considerations and constraints. Remote Sens. 2020;12:2972.
R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2021.
Taylor AR, Watson JA, Chu CS, Puaprasert K, Duanguppama J, Day NPJ, et al. Resolving the cause of recurrent Plasmodium vivax malaria probabilistically. Nat Commun. 2019;10:5595.
Acknowledgements
The authors express their gratitude to the participants and field teams of the original studies. We also thank the reviewers for their availability and agreement to review this manuscript.
Funding
This study is part of the International Centers of Excellence for Malaria Research program “Understanding, tracking and eliminating malaria transmission in the Asia–Pacific Region”, funded by the National Institutes of Health, MD, US (Grant 1U19AI129392-01) and received additional funding by NHMRC (Australia, GNT1092789). Some of the included studies were supported by the TransEPI consortium funded by the Bill & Melinda Gates Foundation, USA (www.gatesfoundation.org). IM is supported by an NHMRC Principal Research Fellowship (GNT1155075). MS is part of the PhD program of the doctoral school ED393 Pierre Louis de santé publique and was supported by the Sorbonne Université (contract n°2695/2017). MW is supported by the European Research Council (852373). HS is supported by the European Research Council (804744). LJR is supported by an NHMRC Fellowship (Australia, GNT1161627). The funding bodies had no role in the design of the study, the collection, analysis, and interpretation of data, and in writing the manuscript.
Author information
Authors and Affiliations
Contributions
MW, IM, and MS conceived and designed the study and methodology and were overall responsible for the study. WM, ML, WN, JS, AW, AV, BW, LJR, and IM contributed the data. MS implemented the method and conducted the analyses. MW and IM supervised the analysis. IM and MW contributed to data interpretation. MS wrote the original draft of the manuscript. AW, HS, LJR, IM, and MW contributed to the writing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The observational studies from which data were used list their ethics approval references in the original publications.
Consent for publication
The observational studies from which data were used state the consent for publication in the original manuscripts.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Figures S1–S5.
Description of data: Distributions of distance between pairs of study participants or between pairs of cases and all other study participants across the studies; maps of households and P. falciparum infections in the cross-sectional survey in Thailand; maps of households and P. vivax and P. falciparum infections in the second cross-sectional survey in Brazil; maps of households and P. vivax and P. falciparum infections in the villages of the cross-sectional survey in Solomon Islands; spatial signature of prevalence of P. vivax or P. falciparum infections in the cross-sectional survey in Solomon Islands across the full distance range. Figure S6. The spatial signature of prevalence of co-infections of P. vivax an P. falciparum infections in the cross-sectional survey in Cambodia across the full distance range (left) and within 1 km (right). Ribbon: 95%-quantile interval of null distribution. Horizontal line: Global survey prevalence.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Sandfort, M., Monteiro, W., Lacerda, M. et al. The spatial signature of Plasmodium vivax and Plasmodium falciparum infections: quantifying the clustering of infections in cross-sectional surveys and cohort studies. Malar J 22, 75 (2023). https://doi.org/10.1186/s12936-023-04515-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12936-023-04515-4