Abstract
Background
Mosquito larval source management (LSM) is a valuable additional tool for malaria vector control. Understanding the characteristics of mosquito larval habitats and its ecology in different land use types can give valuable insight for an effective larval control strategy. This study determined the stability and productivity of potential anopheline larval habitats in two different ecological sites: Anyakpor and Dodowa in southern Ghana.
Methods
A total of 59 aquatic habitats positive for anopheline larvae were identified, and sampled every two weeks for a period of 30 weeks using a standard dipping method. Larvae were collected using standard dippers and were raised in the insectary for identification. Sibling species of the Anopheles gambiae sensu lato (s.l.) were further identified by polymerase chain reaction. The presence of larval habitats, their stability and larvae positive habitats were compared between the two sites using Mann–Whitney U and the Kruskal–Wallis test. Factors affecting the presence of An. gambiae larvae and physicochemical properties at the sites were determined using multiple logistic regression analysis and Spearman’s correlation.
Results
Out of a total of 13,681 mosquito immatures collected, 22.6% (3095) were anophelines and 77.38% (10,586) were culicines. Out of the 3095 anophelines collected, An. gambiae s.l. was predominant (99.48%, n = 3079), followed by Anopheles rufipes (0.45%, n = 14), and Anopheles pharoensis (0.064%, n = 2). Sibling species of the An. gambiae consisted of Anopheles coluzzii (71%), followed by An. gambiae s.s. (23%), and Anopheles melas (6%). Anopheles mean larval density was highest in wells [6.44 (95% CI 5.0–8.31) larvae/dip], lowest in furrows [4.18 (95% CI 2.75–6.36) larvae/dip] and man-made ponds [1.20 (95% CI 0.671–2.131) larvae/dip].The results also revealed habitat stability was highly dependent on rainfall intensity, and Anopheles larval densities were also dependent on elevated levels of pH, conductivity and TDS.
Conclusion
The presence of larvae in the habitats was dependent on rainfall intensity and proximity to human settlements. To optimize the vector control measures of malaria interventions in southern Ghana, larval control should be focused on larval habitats that are fed by underground water, as these are more productive habitats.
Graphical Abstract

Similar content being viewed by others
Background
Anopheles mosquitoes are an important vector of malaria and lymphatic filariasis in sub-Saharan Africa [1, 2]. The distribution of long-lasting insecticide-treated nets (LLINs) and indoor residual spraying (IRS) has been profound in aiding the reduction of a malaria vector population [3, 4], and malaria transmission in sub-Saharan Africa [5,6,7]. However, these strategies are only effective for adult vectors that rest indoors. The adult populations that rest or feed outdoors, and the immature stages that develop in water bodies, are not covered by the current vector control measures. More emphasis needs to be placed on controlling the aquatic stages, particularly as protection offered by current tools seems to be hampered by the emergence and spread of insecticide resistance across Africa [3, 8,9,10,11,12]. Larval source management (LSM) could provide an additional effective tool for the control of malaria vectors [13,14,15,16]. However, the use of LSM requires adequate knowledge of the larval ecology of the vectors, as well as characterization of their larval habitats in different ecological settings [17].
In Ghana, Anopheles gambiae sensu lato (s.l.), (incl. An.gambiae sensu stricto (s.s.), Anopheles arabiensis, Anopheles coluzzii, and Anopheles melas), and Anopheles funestus s.s. are the major vectors of human malaria [18,19,20]. Anopheles mosquitoes breeds in varying habitats: An. gambiae breeds in clear, sunlit pools of water that can be natural or man-made, and permanent or temporary [21, 22], but, An. funestus prefer to breed in shady permanent or semi-permanent water bodies with floating or emergent vegetation. In order to make LSM feasible in Ghana, a better understanding of larval habitats and with their physicochemical parameters in endemic areas is crucial, especially as mosquito control is becoming increasingly difficult due to the spread of insecticide resistance [23, 24] and alteration in land use [25, 26].
Environmental alterations due to deforestation, vegetation clearance for agricultural activities, urbanization, and human population growth enhances the proliferation of larval habitats of malaria vectors [21, 27, 28]. In addition, created dams and irrigation systems in farming communities contribute immensely to the number of suitable larval habitats [29]. Changes in land use can increase exposure to sunlight, which in turn contributes to the availability of larval habitats with enabling conditions for mosquito larvae productivity [30,31,32]. However, the productivity and stability of larval habitats can be influenced by a myriad of factors: climatic (e.g., temperature and rainfall), environmental (e.g., vegetation cover, presence of predators and competitors, habitat size, and amount of sunlight) and the physicochemical properties of the water in the habitats [22, 29, 33].
The abundance of rainfall and vegetation cover in a breeding habitat can influence the distribution and density of larvae, which in turn can influence the abundance of adult Anopheles vectors [32, 34, 35]. Water temperature can also affect the development of eggs or allow the development of more microorganisms that are required by the larvae for food [32, 36]. Varying physicochemical properties of mosquito larval habitats can also have a direct and indirect effect on the biology, including oviposition, survival and spatial distribution of malaria vectors [37, 38].
Monitoring larval population dynamics in different land use settings over a period of time, and evaluating the ecology of larval mosquitoes has implications for vector control [13, 39, 40]. There is the need to understand the productivity and stability of larval habitats in order to model and predict if LSM is feasible. This study aimed to determine the stability and productivity of anopheline larval habitats in Dodowa (savannah-forest transition area) and Anyakpor (the coastal savannah area) in southern Ghana. The effect of environmental, physicochemical parameters, rainfall intensity on Anopheles larvae productivity and habitat stability was also studied in these two areas. The results will provide valuable information to help model and predict the suitability of larval control strategies in these malaria-endemic areas.
Methods
Study sites
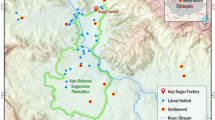
This study was undertaken in two rural areas: Anyakpor and Dodowa both located in the Greater Accra region of southern Ghana (Fig. 1). Ongoing studies in these sites have revealed diverse vector species composition and larval habitat types [41]. Malaria vectors reported in these sites include An. gambiae sensu lato (incl. An. gambiae s.s., An. arabiensis, An. coluzzii and An. melas), Anopheles pharoensis and An. funestus s.s. as dominant in both sites [42].
Anyakpor (5°45′59.99ʺ N 0°36′59.99ʺ E) is a coastal village in Ada Foah in southern Ghana, and it is about 110 km from the city of Accra. It has a dry equatorial climate with temperatures ranging from 23 to 28 °C throughout the year and maximum temperatures reaching 33 °C. Its rainfall pattern is bimodal, with a long rainy season from April to June and a short rainy season from October to November with an annual rainfall of 750 mm. Anyakpor has coastal savannah type vegetation. Main farming activity in this area includes irrigated vegetable farming in low-lying areas consisting of dug-out wells and furrows that connect the wells. The area has a high-water table, and as a result water seeps into these dug-out wells, which creates breeding sites for mosquitoes.
Dodowa (5° 52′ 58.3212ʺ N 0° 5′ 52.9548ʺ W) is a community located in the savannah-forest transition zone in the Shai Osudoku District and is about 39 km from the city of Accra. It has an average temperature of 27 ℃ with a bimodal rainfall pattern like Anyakpor. It has secondary forest type vegetation with little original virgin forest left as a result of deforestation. Due to intense human activities in Dodowa, water accumulates at construction sites, unpaved roads and low-lying areas creating suitable larval habitats for mosquitoes.
Measurement of habitat productivity and stability
In September 2020, a preliminary survey was conducted in the study sites, and most of the larval habitats were found to be located approximately 2 km from the centre of each community (Fig. 2). These areas were selected for detailed study. Thorough searches of Anopheles larval habitats (e.g., man-made ponds, wells, swamps, furrows, puddles) were conducted and their locations mapped using a global positioning system (GPS: Garmin etrex® 10) unit. A total of 59 larval habitats within the study sites, identified to consistently have immature mosquitoes, were chosen: 18 in Dodowa, and 42 in Anyakpor (Fig. 2). These 59 positive habitats were sampled for mosquito larvae once every two weeks from October 2020 to May 2021.
Individual habitats were numbered, and their surface area recorded along with the land use type and vegetation cover. The percentage of vegetation covering the surface of the habitats was visually estimated, and categorized as: 0 if vegetation was not present in the habitat, ≤ 24% surface coverage, and 25–49, 50–74 and 75–100% surface coverage [41]. Habitats were classified into land use types based on the activities taking place on the land where the larval habitat was found. During each survey, the physiochemical properties (temperature, pH, conductivity, dissolved oxygen, temperature, salinity, total dissolved solids (TDS) of the water in the habitats were measured on site using handheld multi-parameter tester (APERA Instrument PC60 Premium Multi-Parameter) based on guidelines provided by the manufacturer. The multi-parameter was calibrated and rinsed with distilled water before each use.
The larval habitats were grouped into temporary or permanent habitats. Temporary habitats were mainly rain-dependent and dried up when rain ceased for a while [43]. The permanent habitats was defined as habitats in which Anopheles larvae were found at least once, and contained water that was fed by natural underground sources throughout the sampling period [43].
Habitat stability was indicated by the availability of water in a habitat for 14 days, following previous reported studies that showed that egg-adult cycle of An. gambiae s.l. can be completed in this length of time [17, 37]. To determine productivity, the habitats were visited and examined once every two weeks for the presence of aquatic stages of anopheline and culicine mosquitoes. In addition, the area (length and width) of the water surface was measured and recorded in metres with a metal ruler and grouped as small (≤ 10 m2) or large (10–100 m2).
Mosquito larval surveys were also carried out to generate stage-specific estimates of larval densities. Water was dipped up to 20 times using a standard dipper (350 mL, BioQuip Products, Inc., CA, USA). When a habitat was too small to make 20 dips, water was dipped as many times as possible. Larval abundance was calculated as the number of larvae per number of dips made in each habitat. The number of larvae and pupae in each habitat was collected and recorded, with larvae classified as early instars (L1 and L2) or late instars (L3 and L4). Larval samples collected from each habitat were pooled into sterile plastic containers and transported to the insectary of the Department of Medical Microbiology, University of Ghana Medical School, where they were bred into adults. At the insectary, the larvae were fed on Tetramin® fish meal and maintained at 27 ± 2 °C.
Mosquito species identification
Emerged adult mosquitoes were morphologically identified using the taxonomic keys of Gillies and Coetzee [44]. Anopheles gambiae s.l. was further identified to sibling species and molecular forms using polymerase chain reaction (PCR) [45, 46].
Meteorological data
For the entire study period, precipitation data were obtained from Ghana Meteorological Service (https://www.meteo.gov.gh/). The weather station located in Anyakpor and Akropong measured the regional daily precipitation throughout the study period.
Data analysis
Data were entered in Microsoft Excel and analysed using STATA v15 (StataCorp. 2017). Descriptive statistical analysis was done to compare the presence of the various habitat types and larval densities in the different study sites and seasons. Larval densities were calculated by dividing the total number of larvae collected by the total number of dips taken.
The density of Anopheles mosquito larvae was compared among the various larval habitats and study sites. The Mann–Whitney U and the Kruskal–Wallis test were used to test the associations between larval densities in the two different sites. The Chi-square and Fisher's exact tests were first used to test the association between two categorical variables. Multiple logistic regression was then performed to assess the association between the habitat characteristics with categorical data and the presence/absence of Anopheles larvae. To find out whether rainfall intensity had any impact on the presence of Anopheles larval abundance, the average bi-weekly rainfall (mm) was calculated and then compared to the mean larval density using linear regression in both study sites, and Spearman’s correlation was used to determine the correlation between occurrences of mosquito larvae.
Results
Habitat characteristics and presence of mosquito larvae
Anyakpor (the coastal savannah zone) had more larval habitats (69.49%; 41/59) than Dodowa (30.5%; 18/59) in the savannah-forest transition zone. In the total number of times the larval habitats were sampled, 38.6% (168/435) of the habitats were inhabited by Anopheles larvae, while 61.38% (267/435) were not (Table 1). Anopheles larvae were mostly present in puddles (76.7%; 33/43) and furrows (58.3%; 7/12), followed by wells 40% (78/193) and man-made ponds 21.38% (31/145) (Table 1).
Five different habitat types (man-made pond, well, swamp, furrow, puddle) were encountered and recorded during the study. The likelihood of finding Anopheles larvae was higher in wells (OR = 2.085 (1.042–4.173); p = 0.038) than in man-made ponds (Table 2). Habitats with no vegetation had on average twice as many Anopheles larvae (B = 2.284 (0.792–3.779) than habitats with some vegetation cover (1 to 24%) (Additional file 1: Table S1). Anopheles larvae were more likely to be encountered in habitats which were 151–200 m from nearest human settlement (OR = 0.345; 95% CI 0.141–0.844: p = 0.020), compared to habitats that were more than 200 m from human settlement (Table 2). However, Anopheles larval density increased by a small margin for every metre a habitat was from human settlement (B = 0.023 (0.002–0.043); p < 0.05) (Additional file 1: Table S1). The presence of algae and land use type had no significant effect on the presence of Anopheles larvae).
Mosquito vector larval presence and species composition at the study sites
A total of 13,681 mosquito larvae belonging to two genera (Anopheles and Culex species) were collected from 59 different larval habitats in Anyakpor (the coastal savannah zone) and Dodowa (savannah-forest transition zone) over the 30 weeks. Among the total mosquito larvae collected from the larval habitats in Anyakpor and Dodowa, 22.6% (3095/13,681) were anophelines and 77.4% (10,586/13,681) were culicines (Table 3). In Anyakpor, of a total 11,403 mosquito larvae collected from the different habitats, 77.8% (8869/11,403; 95% CI 77.0–78.5) were Culex and 22.2% (2534/11,403, 95% CI 21.5–23.0) were Anopheles larvae (Table 3). Of a total 2,278 mosquito larvae collected from the different habitats in Dodowa, 75.4% (1717/2278, 95% CI 73.5–77.12) were Culex and 23.9% (545/2278, 95% CI 21.5–23.0) were Anopheles. Of the 3,095 Anopheles mosquitoes collected overall, 99.5% (3079/3095) were An. gambiae s.l., 0.5% (14/3095) were Anopheles rufipes and 0.1% (2/3095) were An. pharoensis. A sub-sample of 559 An. gambiae s.l. from the two study sites were analysed for the identification of their respective sibling species. Overall, An. coluzzii accounted for 71% (95% CI 67.4–75.1), followed by An. gambiae s.s. [23% (95% CI 19.2–26.3)], and An. melas [6% (95% CI 3.9–7.9)]. In Anyakpor, 30% (387/2534) of An. gambiae s.l. were discriminated into sibling species and of these, 70.7% (284/387, 95% CI 68.6–77.7) were An. coluzzii, 19.9% (70/387, 95% CI 14.5–22.4) were An. gambiae and 8.8% (31/387, 95% CI 5.6–11.3) were An. melas (Table 3). Anopheles melas was only found in the coastal town of Anyakpor. In Dodowa, 30% (172/561) of An. gambiae s.l. analysed for their respective sibling species revealed 66.9% (115/172, 95% CI 59.23–73.7) were An. coluzzii, and 32.6% (56/172, 95% CI 25.7–40.2) were An. gambiae.
The mean larval density in Anyakpor was higher (4.779 larvae/dip, 95% CI 3.67–6.22) than in Dodowa (0.996 (95% CI 0.663–1.495) larvae/dip) (p = 0.0128, χ2 = 6.12, df = 1). The types of habitats in this study affected both the presence (p < 0.000, χ2 = 36.53, df = 4) of Anopheles mosquitoes. Overall, wells were the most productive habitats (with 6.44 (95% CI 5.0–8.31) larvae/dip), followed by furrows (4.18 (95% CI 2.75–6.36) larvae/dip) and man-made ponds (1.20 (95% CI 0.671–2.131) larvae/dip). In Anyakpor, furrows (7.16 (95% CI 3.96–12.93) larvae/dip) and wells (6.0 (CI 4.72–7.62) larvae/dip) were the most productive larval habitats. While in Dodowa, furrows (4.14 (95% CI 2.08–8.24) larvae/dip) and swamps (1.30 (95% CI 0.25–6.88) larvae/dip) were the most productive larval habitats.
Effect of rainfall on habitat stability and Anopheles larval densities
Overall, rainfall influenced the availability of mosquito larval habitats at both sites (Fig. 3). However, the extent to which habitat frequencies fluctuated between rainy and dry season varied greatly between the two study sites. The mean length of weeks that a habitat contained water was significantly shorter in Dodowa site compared to Anyakpor (7.5 vs 10.7 weeks during rain and 2.9 vs 8.4 weeks dry season) (Table 4). The mean number of times that a habitat dried up in Dodowa was four times higher than that of the Anyakpor during the rainy season (4.0 vs 1.1), and two times more in the dry season (6.2 vs 3.9) (Table 4). Overall, the percentage of habitats that were aquatic on any given day in Dodowa and Anyakpor sites reduced during the rainy season from 95 vs 80.3% to 60.6 vs 55.0% in the dry season.
Anopheles gambiae s.l. larval densities exhibited temporal differences as shown in Fig. 4. Over all, high rainfall intensity led to an increase in mosquito larval densities (p = 0.001). The occurrence of An. gambiae larval densities was significantly associated (p < 0.05) with rainfall in Anyakpor (p = 0.0023) and in Dodowa (p = 0.047).
The effect of physicochemical parameters on Anopheles larval presence
The results of analysis using Spearman’s rho coefficient analysis revealed the overall presence of Anopheles larvae was significantly influenced by elevated levels of pH (rho = 0.119). Further, increasing level of conductivity (rho = 0.246) and total dissolved solids (TDS) (rho = 0.227) had a positive and significant correlation with Anopheles larval density. In Anyakpor, An. gambiae larval density was significantly associated with conductivity (p = 0.011), TDS (p = 0.010), salinity (p = 0.0075), and pH (p = 0.0407). However, in Dodowa, there was no significant difference between all the studied physicochemical parameters and An. gambiae larval density (p < 0.05).
Table 5 shows the mean standard deviations of physicochemical parameters in the different categories of Anopheles larval habitats in Anyakpor and Dodowa. Compared to Dodowa, swamps and furrows in Anyakpor had the highest conductivity (10.4 ± 2.5 vs 11.25 ± 1.97 µs), TDS (7.23 ± 1.69 vs 7.43 ± 1.73 ppm), salinity (5.21 ± 1.26 vs 5.56 ± 2.01 ppm), and pH (7.05 ± 0.08 vs 7.02 ± 0.02).
Discussion
The availability of anopheline larval habitats and their productivity have important implications for malaria transmission [17, 21, 47, 48]. This study investigated the productivity, stability and effect of physicochemical parameters on larval density of malaria vectors in two sites in southern Ghana: Dodowa (savannah-forest transition area) and Anyakpor (the coastal savannah area). This study revealed Anopheles larval productivity and habitat stability was highly dependent on rainfall intensity. The results also indicated that presence of some physicochemical parameters in mosquito larval habitats at various levels increased mosquito vector larval density. This can contribute significantly to adult mosquito populations, vector distribution and disease transmission.
In this study, productivity of larval habitats was found to be positively correlated with rainfall at both sites, implying that when it rained, more habitats that are suitable for oviposition and larval development are produced. This can translate to a high production of adult mosquitoes and increase malaria transmission. Similar observations have been reported in Kenya by Imbahale et al. [48] and Sang et al. [49], where mosquitoes larvae productivity was found to be correlated with high rainfall intensity. However, studies by Munga et al. [50] and Afrane et al. [51] reported that higher amounts of rainfall correlated negatively with larval abundance and productivity.
For cost-effective mosquito larval habitat monitoring and control, understanding the impact of rainfall on the stability of larval habitats is of great importance [58, 59]. In this study, the stability of larval habitats was positively correlated with rainfall. The larval habitats in Anyakpor were more stable than the Dodowa site. The increase in stability of larval habitats in Anyakpor compared to Dodowa was because the water table is high in the former, and water seeps from the ground into the different habitat types allowing for larval development [52, 53]. Not only was stability of habitats lower in Dodowa, but also the frequency of positive anopheline larval habitats was significantly lower. Compared to Dodowa, Anyakpor is an intensive farming community, and as such, man-made ponds and wells created for irrigation purposes enhances the creation of stable aquatic habitats for the completion of the mosquito life cycle. Similar observations have been reported in Kenya by Minakawa et al. [54] and Himeidan et al. [17] where habitat stability was positively correlated with rainfall.
It was interesting to note that in the Anyakpor site, conductivity, TDS and salinity of the water in larval habitats had a significant influence on the larval density of Anopheles larvae compared to Dodowa site. High levels of conductivity and pH may be due to the application of agricultural fertilizers (organic or inorganic) and pesticides in Anyakpor [55,56,57,58]. The findings concur with previous studies conducted in northern Ethiopia [59] and Tanzania [37] which reported that high pH and conductivity were significantly associated with Anopheles larval density. However, this study’s findings are contradictory to studies conducted in Nigeria which reported that conductivity and TDS had no influence on Anopheles larval density [60], and low pH had a significant association with Anopheles density [61].
Anopheles gambiae s.l. was found to be more abundant than other Anopheles species (An. rufipes and An. pharoensis) in the two study sites. Sibling species of An. gambiae s.l. revealed a dominance of An. coluzzii, followed by few An. gambiae s.s. and An. melas. Anopheles melas was detected only in Anyakpor which has habitats that are fed by salty underground water from the sea, which An. melas prefers to breed in, unlike in Dodowa where habitats are fed by rainwater. A previous study conducted in southern Ghana has shown similar findings across similar habitat types [41, 62].
Conclusion
The findings from this study reveal that the density of Anopheles larvae are affected by rainfall and physicochemical parameters present in their breeding sites. This calls for further studies to investigate the possible reasons for tolerance of high levels of physicochemical parameters among Anopheles mosquito populations. Additionally, this study revealed that human activity contributed to the majority of larval habitats at the study sites, and therefore, the involvement of the population would be highly beneficial in larval source management approaches.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CI:
-
Confidence interval
- GEE:
-
Generalized estimating equation
- OR:
-
Odds ratio
- SD:
-
Standard deviation
- Ppm:
-
Parts per million
- TDS:
-
Total dissolved solids
- µS:
-
Microsiemens per centimetre
- χ2 :
-
Chi square
- Df:
-
Degrees of freedom
References
WHO. Global Plan for insecticide resistance management in malaria vectors (GPIRM). Geneva: World Health Organization; 2012.
Hemingway J, Vontas J, Poupardin R, Raman J, Lines J, Schwabe C, et al. Country-level operational implementation of the Global Plan for Insecticide Resistance Management. Proc Natl Acad Sci USA. 2013;110:9397–402.
Bayoh MN, Mathias DK, Odiere MR, Mutuku FM, Kamau L, Gimnig JE, et al. Anopheles gambiae: historical population decline associated with regional distribution of insecticide-treated bed nets in western Nyanza Province, Kenya. Malar J. 2010;9:62.
Mutuku FM, King CH, Mungai P, Mbogo C, Mwangangi J, Muchiri EM, et al. Impact of insecticide-treated bed nets on malaria transmission indices on the south coast of Kenya. Malar J. 2011;10:356.
Kleinschmidt I, Schwabe C, Benavente L, Torrez M, Ridl FC, Segura JL, et al. Marked Increase in child survival after four years of intensive malaria control. Am J Trop Med Hyg. 2009;80:882–8.
WHO. World malaria report 2010. Geneva: World Health Organization; 2010.
Smithuis FM, Kyaw MK, Phe UO, van der Broek I, Katterman N, Rogers C, et al. The effect of insecticide-treated bed nets on the incidence and prevalence of malaria in children in an area of unstable seasonal transmission in western Myanmar. Malar J. 2013;12:363.
Reddy MR, Overgaard HJ, Abaga S. Outdoor host seeking behaviour of Anopheles gambiae mosquitoes following initiation of malaria vector control on Bioko Island, Equatorial Guinea. Malar J. 2011;10:184.
Russell TL, Govella NJ, Azizi S, Drakeley CJ, Kachur SP, Killeen GF. Increased proportions of outdoor feeding among residual malaria vector populations following increased use of insecticide-treated nets in rural Tanzania. Malar J. 2011;10:80.
Derua YA, Alifrangis M, Hosea KM, Meyrowitsch DW, Magesa SM, Pedersen EM, et al. Change in composition of the Anopheles gambiae complex and its possible implications for the transmission of malaria and lymphatic filariasis in north-eastern Tanzania. Malar J. 2012;11:188.
Bhatt S, Weiss DJ, Cameron E, Bisanzio D, Mappin B, Dalrymple U, et al. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature. 2015;526:207–11.
Cohen JM, Smith DL, Cotter C, Ward A, Yamey G, Sabot OJ, et al. Malaria resurgence: a systematic review and assessment of its causes. Malar J. 2012;11:122.
Fillinger U, Ndenga B, Githeko A, Lindsay SW. Integrated malaria vector control with microbial larvicides and insecticide-treated nets in western Kenya: a controlled trial. Bull World Health Organ. 2009;87:655–65.
Worrall E, Fillinger U. Large-scale use of mosquito larval source management for malaria control in Africa: a cost analysis. Malar J. 2011;10:338.
Tusting LS, Thwing J, Sinclair D, Fillinger U, Gimnig J, Bonner KE, et al. Mosquito larval source management for controlling malaria. Cochrane Database Syst Rev. 2013;2013:CD008923.
Mwangangi JM, Kahindi SC, Kibe LW, Nzovu JG, Luethy P, Githure JI, et al. Wide-scale application of Bti/Bs biolarvicide in different aquatic habitat types in urban and peri-urban Malindi. Kenya Parasitol Res. 2011;108:1355–63.
Himeidan YE, Zhou G, Yakob L, Afrane Y, Munga S, Atieli H, et al. Habitat stability and occurrences of malaria vector larvae in western Kenya highlands. Malar J. 2009;8:234.
Appawu M, Owusu-Agyei S, Dadzie S, Asoala V, Anto F, Koram K, et al. Malaria transmission dynamics at a site in northern Ghana proposed for testing malaria vaccines. Trop Med Int Health. 2004;9:164–70.
De Souza D, Kelly-Hope L, Lawson B, Wilson M, Boakye D. Environmental factors associated with the distribution of Anopheles gambiae s.s. in Ghana; an important vector of lymphatic filariasis and malaria. PLoS ONE. 2010;5:e9927.
Dadzie SK, Brenyah R, Appawu MA. Role of species composition in malaria transmission by the Anopheles funestus group (Diptera: Culicidae) in Ghana. J Vector Ecol. 2013;38:105–10.
Munga S, Yakob L, Mushinzimana E, Zhou G, Ouna T, Minakawa N, et al. Land use and land cover changes and spatiotemporal dynamics of anopheline larval habitats during a four-year period in a highland community of Africa. Am J Trop Med Hyg. 2009;81:1079–84.
Rejmánková E, Grieco J, Achee N, Roberts DR. Ecology of Larval Habitats. In: Manguin S, editor. Anopheles mosquitoes: new insights into malaria vectors. London: InTech Open; 2013. p. 397–446.
Coetzee M, van Wyk P, Booman M, Koekemoer LL, Hunt RH. Insecticide resistance in malaria vector mosquitoes in a gold mining town in Ghana and implications for malaria control. Bull Soc Pathol Exot. 2006;99:400–3.
Hunt RH, Fuseini G, Knowles S, Stiles-Ocran J, Verster R, Kaiser ML, et al. Insecticide resistance in malaria vector mosquitoes at four localities in Ghana, West Africa. Parasit Vectors. 2011;4:107.
Baeza A, Santos-Vega M, Dobson AP, Pascual M. The rise and fall of malaria under land-use change in frontier regions. Nat Ecol Evol. 2017;1:0108.
Fornace KM, Diaz AV, Lines J, Drakeley CJ. Achieving global malaria eradication in changing landscapes. Malar J. 2021;20:69.
Tuno N, Okeka W, Minakawa N, Takagi M, Yan G. Survivorship of Anopheles gambiae sensu stricto (Diptera: Culicidae) larvae in Western Kenya Highland forest. J Med Entomol. 2005;42:270–7.
Sovi A, Govoétchan R, Tokponnon F, Hounkonnou H, Aïkpon R, Agossa F, et al. Impact of land-use on malaria transmission in the Plateau region, southeastern Benin. Parasit Vectors. 2013;6:352.
Mereta ST, Yewhalaw D, Boets P, Ahmed A, Duchateau L, Speybroeck N, et al. Physico-chemical and biological characterization of anopheline mosquito larval habitats (Diptera: Culicidae): implications for malaria control. Parasit Vectors. 2013;6:320.
Wanjala CL, Kweka EJ. Impact of highland topography changes on exposure to malaria vectors and immunity in Western Kenya. Front Public Health. 2016;4:227.
Githeko AK, Ayisi JM, Odada PK, Atieli FK, Ndenga BA, Githure JI, et al. Topography and malaria transmission heterogeneity in western Kenya highlands: prospects for focal vector control. Malar J. 2006;5:107.
Minakawa N, Munga S, Atieli F, Mushinzimana E, Zhou G, Githeko AK, et al. Spatial distribution of anopheline larval habitats in Western Kenyan highlands: effects of land cover types and topography. Am J Trop Med Hyg. 2005;73:157–65.
Muturi EJ, Mwangangi J, Shililu J, Jacob BG, Mbogo C, Githure J, et al. Environmental factors associated with the distribution of Anopheles arabiensis and Culex quinquefasciatus in a rice agro-ecosystem in Mwea, Kenya. J Vector Ecol. 2008;33:56–63.
Ma M, Huang M, Leng P. Abundance and distribution of immature mosquitoes in urban rivers proximate to their larval habitats. Acta Trop. 2016;163:121–9.
Minakawa N, Sonye G, Mogi M, Yan G. Habitat characteristics of Anopheles gambiae s.s. larvae in a Kenyan highland. Med Vet Entomol. 2004;18:301–5.
Shililu J, Ghebremeskel T, Mengistu S, Fekadu H, Zerom M, Mbogo C, et al. Distribution of anopheline mosquitoes in Eritrea. Am J Trop Med Hyg. 2003;69:295–302.
Emidi B, Kisinza WN, Mmbando BP, Malima R, Mosha FW. Effect of physicochemical parameters on Anopheles and Culex mosquito larvae abundance in different breeding sites in a rural setting of Muheza, Tanzania. Parasit Vectors. 2017;10:304.
Chaiphongpachara T, Yusuk P, Laojun S, Kunphichayadecha C. Environmental factors associated with mosquito vector larvae in a malaria-endemic area in Ratchaburi, Province, Thailand. ScientificWorld J. 2018;2018:4519094.
Killeen GF, Tanner M, Mukabana WR, Kalongolela MS, Kannady K, Lindsay SW, et al. Habitat targeting for controlling aquatic stages of malaria vectors in Africa. Am J Trop Med Hyg. 2006;74:517–8.
Gu W, Novak RJ. Habitat-based modeling of impacts of mosquito larval interventions on entomological inoculation rates, incidence, and prevalence of malaria. Am J Trop Med Hyg. 2005;73:546–52.
Hinne IA, Attah SK, Mensah BA, Forson AO, Afrane YA. Larval habitat diversity and Anopheles mosquito species distribution in different ecological zones in Ghana. Parasit Vectors. 2021;14:193.
Appawu MA, Baffoe-Wilmot A, Afari EA, Dunyo S, Koram KA, Nkrumah FK. Malaria vector studies in two ecological zones in southern Ghana. Afr Entomol. 2001;9:59–65.
Mattah P, Futagbi G, Amekudzi LK, Mattah MM, de Souza DK, Kartey-Attipoe WD, et al. Diversity in breeding sites and distribution of Anopheles mosquitoes in selected urban areas of southern Ghana. Parasit Vectors. 2017;10:25.
Gillies MT, Coetzee M. A supplement to the Anophelinae of Africa South of the Sahara. Publ Sth Afr Inst Med Res. 1987;55:1–143.
Scott JA, Brogdon WG, Collins FH. Identification of single specimens of the Anopheles gambiae complex by the polymerase chain reaction. Am J Trop Med Hyg. 1993;49:520–9.
Fanello C, Santolamazza F, della Torre A. Simultaneous identification of species and molecular forms of the Anopheles gambiae complex by PCR-RFLP. Med Vet Entomol. 2002;16:461–4.
Kweka EJ, Zhou G, Munga S, Lee M-C, Atieli HE, Nyindo M, et al. Anopheline larval habitats seasonality and species distribution: a prerequisite for effective targeted larval habitats control programmes. PLoS ONE. 2012;7:e52084.
Imbahale SS, Paaijmans KP, Mukabana WR, van Lammeren R, Githeko AK, Takken W. A longitudinal study on Anopheles mosquito larval abundance in distinct geographical and environmental settings in western Kenya. Malar J. 2011;10:81.
Sang R, Lutomiah J, Said M, Makio A, Koka H, Koskei E, et al. Effects of irrigation and rainfall on the population dynamics of Rift Valley Fever and other arbovirus mosquito vectors in the epidemic-prone Tana River County, Kenya. J Med Entomol. 2016;54:460–70.
Munga S, Minakawa N, Zhou G, Mushinzimana E, Barrack OO, Githeko AK, et al. Association between land cover and habitat productivity of malaria vectors in western Kenyan highlands. Am J Trop Med Hyg. 2006;74:69–75.
Afrane YA, Lawson BW, Brenya R, Kruppa T, Yan G. The ecology of mosquitoes in an irrigated vegetable farm in Kumasi, Ghana: abundance, productivity and survivorship. Parasit Vectors. 2012;5:233.
Mwangangi JM, Muturi EJ, Shililu J, Muriu SM, Jacob B, Kabiru EW, et al. Survival of immature Anopheles arabiensis (Diptera: Culicidae) in aquatic habitats in Mwea rice irrigation scheme, central Kenya. Malar J. 2006;5:114.
Mwangangi JM, Shililu J, Muturi EJ, Muriu S, Jacob B, Kabiru EW, et al. Anopheles larval abundance and diversity in three rice agro-village complexes Mwea irrigation scheme, central Kenya. Malar J. 2010;9:228.
Minakawa N, Sonye G, Yan G. Relationships between occurrence of Anopheles gambiae s.l. (Diptera: Culicidae) and size and stability of larval habitats. J Med Entomol. 2005;42:295–300.
Nkya TE, Akhouayri I, Kisinza W, David JP. Impact of environment on mosquito response to pyrethroid insecticides: facts, evidences and prospects. Insect Biochem Mol Biol. 2013;43:407–16.
Sunish IP, Reuben R. Factors influencing the abundance of Japanese encephalitis vectors in ricefields in India–I. Abiotic. Med Vet Entomol. 2001;15:381–92.
Philbert AL, Lyantagaye SL, Nkwengulila G. A review of agricultural pesticides use and the selection for resistance to insecticides in malaria vectors. Adv Entomol. 2014;2:120–8.
Garba Y, Olayemi IK. Spartial variation in physicochemical characteristics of wetland rice fields mosquito larval habitats in Minna, North Central Nigeria. In: International Conference on Agricultural, Ecological and Medical Sciences. 2015. p. 53–6.
Dejenie T, Yohannes M, Assmelash T. Characterization of mosquito breeding sites in and in the vicinity of tigray microdams. Ethiop J Health Sci. 2011;21:57–66.
Imam AA, Deeni YJ. Common types of Anopheles gambiae breeding habitats in north western Nigeria. Innov Res Eng Sci. 2014;4:496–504.
Adebote DA, Oniye SJ, Muhammed YA. Studies on mosquitoes breeding in rock pools on inselbergs around Zaria, northern Nigeria. J Vector Borne Dis. 2008;45:21–8.
Orsborne J, Mohammed AR, Jeffries CL, Kristan M, Afrane YA, Walker T, et al. Evidence of extrinsic factors dominating intrinsic blood host preferences of major African malaria vectors. Sci Rep. 2020;10:741.
Acknowledgements
The authors are grateful to the people of Anyakpor and Dodowa for permitting us to sample mosquito larvae in their communities and farms. We greatly appreciate Sebastian Mensah and Abdul Rahim Mohammed for their technical assistance during field surveys. We also thank all the community field assistants for helping in the data collection in the various communities.
Funding
This study was supported by a grant from the American Association of University Women (AAUW) 2020–2021 Research Publication Grant in Engineering, Medicine and Science. This study was also supported by Grants from the National Institute of Health (NIH: R01 A1123074 and D43 TW 011513).
Author information
Authors and Affiliations
Contributions
AOF conceived, designed, performed the field and laboratory work, analysed data and drafted the manuscript. YAA conceived, supervised the study, analysed data and revised the manuscript. IAH and IKS performed field and laboratory experiments. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1
: Table S1. Generalized estimation equation regression of larval habitat characteristics and Anopheles larval densities from October 2020 to May 2021.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Forson, A.O., Hinne, I.A., Sraku, I.K. et al. Larval habitat stability and productivity in two sites in Southern Ghana. Malar J 22, 74 (2023). https://doi.org/10.1186/s12936-023-04498-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12936-023-04498-2