Abstract
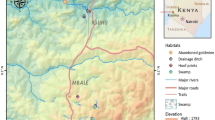
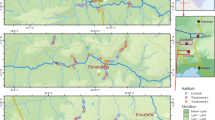
Larval control is a major component in mosquito control programs. This study evaluated the wide-scale application of Bti/Bs biolarvicide (Bacillus thuringiensis var. israelensis [Bti] and Bacillus sphaericus [Bs]) in different aquatic habitats in urban and peri-urban Malindi, Kenya. This study was done from June 2006 to December 2007. The urban and peri-urban area of Malindi town was mapped and categorized in grid cells of 1 km2. A total of 16 1-km2 cells were selected based on presence Community Based Organization dealing with malaria control within the cells. Each of the 16 1-km2 cells was thoroughly searched for the presence of potential larval habitats. All habitats, whether positive or negative for larvae, were treated and rechecked 24 h (1 day), 6 days, and 10 days later for the efficacy of Bti/Bs. Weekly larval sampling was done to determine the mosquito larval dynamics in the aquatic habitats during the study period. Morphological identification of the mosquito larvae showed that Anopheles gambiae s.l. Giles was the most predominant species of the Anopheles and while in the culicines, Cx. quinquefasciatus Say was the predominant species. Anopheles larvae were all eliminated in habitats within a day post-application. For culicine larvae, 38.1% (n = 8) of the habitat types responded within day 1 post-treatment and all the larvae were killed, they turned negative during the days of follow-up. Another 38.1% (n = 8) of the habitat types had culicine larvae but turned negative by day 6, while three habitats (14.3%) had larvae by 6th day but turned negative by 10th day. However during this Bti/Bs application studies, two habitat types, house drainage and cesspits (9.5%), remained positive during the follow-up although the mosquito larvae were significantly reduced. Both early and late instars of Anopheles larvae immediately responded to Bti/Bs application and reached 100% mortality. The early and late instars of culicine responded to the Bti/Bs application but not as fast as the Anopheles larval instars. The early instars Culex, responded with 90.8% mortality at day 1 post-treatment, and the mortality was 99.9% at day 10. Similarly, the late instars Culex followed the same trend and exhibited same mortalities. The weekly sampling in the aquatic habitats showed that there was a 36.3% mosquito larval reduction in the aquatic habitats over the 18-months study period. In conclusion, Bti/Bs biolarvicide are useful in reducing the mosquito larval densities in a wide range of habitats which have a direct impact of adult mosquito populations.


Similar content being viewed by others
References
Barbazan P, Baldet T, Darriet F, Escaffre H, Djoda DH, Hougard JM (1998) Impact of treatments with Bacillus sphaericus on Anopheles populations and the transmission of malaria in Maroua, a large city in a savannah region of Cameroon. J Am Mosq Control Assoc 14:33–39
Becker N (1992) Community participation in the operational use of microbial control agents in mosquito control programmes. Bull Soc Vector Ecol 17:114–118
Charles JF, Nielsen-LeRoux C (2000) Mosquitocidal bacterial toxins: diversity, mode of action and resistance phenomena. Mem Inst Oswaldo Cruz 95:201–206
Charlwood JD, Graves PM (1987) The effect of permethrin impregnated bednets on a population of Anopheles farauti in coastal Papua New Guinea. Med Vet Entomol 1:319–327
Fillinger U, Lindsay S (2006) Suppression of exposure to malaria vectors by an order of magnitude using microbial larvicides in rural Kenya. Trop Med Int Hlth 11:1629–1642
Fillinger U, Knols BGJ, Becker N (2003) Efficacy and efficiency of new bacillus thuringiensis var. israelensis and bacillus sphaericus formulations against Afrotropical anophelines in Western Kenya. Trop Med Int Hlth 8:37–47
Gillies MT, Coetzee M (1987) A supplement to anophelinae of Africa south of Sahara (Afro-tropical region). Publication of the South African Institute of Medical Research 55:1–143
Gunasekaran K, Shriram AN, Elangovan A, Narayanan RJ, Balaraman K (1996) Efficacy of Bacillus sphaericus in different breeding habitats of Culex quinquefasciatus. Southeast Asian J Trop Med Public Health 27:622–627
Hopkins GHE (1952) Mosquitoes of the Ethiopian region, 2nd edn, 1. Larval bionomics of mosquitoes and taxonomy of culicine larvae. British Museum Natural History, London, p 355
Kahindi S, Midega JT, Mwangangi JM, Kibe L, Nzovu J, Luethy P, Githure J, Mbogo C (2008) The efficacy of Vectobac DT and Culinexcombi against mosquito larvae in unused swimming pools in Malindi, Kenya. J Am Mosq Control Assoc 24:538–542
Karch S, Monteny N, Jullien JL, Sinegre G, Coz J (1990) Control of Culex pipiens by Bacillus sphaericus and role of nontarget arthropods in its recycling. J Am Mosq Control Assoc 6:47–54
Karch S, Asidi A, Manzambi M, Salaun JJ (1991) Field trials with Vectolex (Bacillus sphaericus) and Vectolex (Bacillus thuringiensis H-14) against malaria Anopheles gambiae and Culex quinquefasciatus breeding in Zaire. J Am Mosq Control Assoc 7:176–177
Karch S, Asidi A, Manzambi M, Salaun J (1992) Efficacy of Bacillus sphaericus against malaria vector Anopheles gambiae and other mosquitoes in swamps and rice fields in Zaire. J Am Mosq Control Assoc 8:376–380
Keating J, Macintyre K, Mbogo CM, Githure JI, Beier JC (2004) Characterization of potential larval habitats for Anopheles mosquitoes in relation to urban land-use in Malindi, Kenya. Int J Hlth Geog 3:9–15
Kibe LW, Mbogo CM, Keating J, Molyneux S, Githure JI, Beier JC (2006) Community based vector control in Malindi, Kenya. Afr Health Sci 6:240–246
Killeen GF, McKenzie FE, Foy BD, Schieffelin C, Billingsley PF, Beier JC (2000a) The potential impact of integrated malaria transmission control on entomologic inoculation rate in highly endemic areas. Am J Med Hyg 62:545–551
Killeen GF, McKenzie FE, Foy BD, Schieffelin C, Billingsley PF, Beier JC (2000b) A simplified model for predicting malaria Entomologic Inoculation Rates based on Entomologic and Parasitologic parameters relevant to control. Am J Med Hyg 62:535–544
Killeen G, Fillinger U, Kiche I, Gouagna L, Knols B (2002) Eradication of Anopheles gambiae from Brazil: lessons for malaria control in Africa. Lancet Infect Dis 2:618–627
Mireji PO, Keating J, Hassanali A, Mbogo CM, Nyambaka H, Kahindi S, Beier JC (2007) Heavy metals in mosquito larval habitats in urban Kisumu and Malindi, Kenya, and their impact. Ecotoxicol Environ Saf 70:147–153
Mittal PK (2003) Biolarvicides in vector control: challenges and prospects. J Vect Borne Dis 40:20–32
Mulla MS, Darwaseh HA, Zgomba M (1990) Effect of some environmental factors on the efficacy of Bacillus sphaericus 2362 and Bacillus thuringiensis (H-14) against mosquitoes. Bull Soc Vector Ecol 15:166–175
Mulla MS, Su T, Thavara U, Tawatsin A, Kong-ngamsuk W, Phan-Urai P (1999) Efficacy of new formulations of the microbial larvicide Bacillus sphaericus against polluted water mosquitoes in Thailand. J Vector Ecol 24:99–110
Ohaga SO, Ndiege IO, Kubasu SS, Beier JC, Mbogo C (2007) Larvicidal activity of Piper guineense and Spilanthes mauritiana crude-powder against Anopheles gambiae and Culex quinquefasciatus in Kilifi District, Kenya. J Biol Sci 7:1215–1220
Okinyo D (2002) Bio prospecting for phytochemicals for Anopheles gambiae larvae control. Kenyatta University, Msc thesis
Pantuwatana S, Maneeroj R, Upatham ES (1989) Long residual activity of Bacillus sphaericus 1593 against Culex quinquefasciatus larvae in artificial pools. Southeast Asian J Trop Med Public Health 20:421–427
Porter AG, Davidson EW, Liu JW (1993) Mosquitocidal toxins of bacilli and their genetic manipulation for effective biological control of mosquitoes. Microbiol Rev 57:838–861
Robert V, Macintyre K, Keating J, Trape J-F, Duchemin J-B, Warren M, Beier JC (2003) Malaria transmission in urban Africa. Am J Trop Med Hyg 68:169–176
Seyoum A, Kabiru EW, Lwande W, Killeen GF, Hassanali A, Knols BGJ (2002a) Repellency of live potted plants against Anopheles gambiae from human baits in semi-field experimental huts. Am J Trop Med Hyg 67:191–195
Seyoum A, Palsson K, Kung’a S, Kabiru E, Lwande W, Killeen GF, Hassanali A, Knols BG (2002b) Traditional use of mosquito repellent plants in western Kenya and their evaluation in semi-field experimental huts against Anopheles gambaie: ethnobotanical studies and application by thermal expulsion and direct burning. Trans R Soc Trop Med Hyg 96:225–231
Shililu JI, Tewolde GM, Brantly E, Githure JI, Mbogo CM, Beier JC, Fusco R, Novak B (2003) Efficacy of Bacillus thuringiensis israelensis, Bacillus sphaericus and temephos for managing Anopheles larvae in Eritrea. J Am Mosq Control Assoc 19:251–258
Soper FL, Wilson DB (1943) Anopheles gambiae in Brazil. The Rockefeller Foundation, New York
Su T (2008) Evaluation of water-soluble pouches of Bacillus Sphaericus applied as prehatch treatment against Culex Mosquitoes in simulated catch basins. J Am Mosq Control Assoc 24:54–60
Su T, Mulla MS, Zaim M (2003) Laboratory and field evaluations on novaluron, a new IGR, against Culex mosquitoes. J Am Mosq Control Assoc 19:408–418
Sutherland DD, McNelly JJ, Hansen JA (1989) Evaluation of granular Bacillus sphaericus to control Culex in sewage treatments ponds in Cape May. New Jersey Mosquito Control Association 76:84–90
Utzinger J, Tozan Y, Singer BH (2001) Efficacy and cost-effectiveness of environmental management for malaria control. Trop Med Intl Health 6:677–687
WHO (1975) Manual on practical entomology in Malaria. Part II. Methods and techniques. World Health Organization Offset Publication, Geneva, No. 13
WHO (1993) Implementation of the global malaria strategy. WHO Technical Report Series 839, Geneva
WHO (1999) International programme on chemical safety (IPCS): microbial pest control agent Bacillus thuringiensis. Environ Health Criteria I217:1–105
WHO (2004) Global strategic framework for integrated vector management. Document WHO/CDS/CPE/PVC/2004.10. World Health Organization, Geneva
Yohannes M, Haile M, Ghebreyesus TA, Witten KH, Getachew A, Byass P, Lindsay SW (2005) Can source reduction of mosquito larval habitat reduce malaria transmission in Tigray, Ethiopia? Trop Med Int Health 10:1274–1285
Acknowledgements
We are grateful to the scientific and technical teams at the Centre for Geographic Medicine Research Coast, Kilifi for help in design and implementation of this work. We acknowledge the field data collection provided by Benson Jefwa, Hafuzwa Bokia, Daniel Thoya, MwanaAmani Issa, Nyambura Mbugua, Hafswa Hassan, Agnes Kambi, Sarah Konde, Agnes Samuel (Posthumously), Riziki Ramadhani, Kazungu Tuva, Matlida Zawadi, Jackson Kahindi, Wesonga Abdalla, Batuli Ali, Revenge Taura, David Shida, Festus Yaah, and Gabriel Nzai. This work was supported by the Biovision Foundation of Switzerland through International Centre of Insect Physiology and Ecology (ICIPE). This paper has been published with the permission of the Director of the Kenya Medical Research Institute (KEMRI).
Competing interests
The authors declare that they have no competing interests in the choice and use of Culinex® Combi biolarvicide.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mwangangi, J.M., Kahindi, S.C., Kibe, L.W. et al. Wide-scale application of Bti/Bs biolarvicide in different aquatic habitat types in urban and peri-urban Malindi, Kenya. Parasitol Res 108, 1355–1363 (2011). https://doi.org/10.1007/s00436-010-2029-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-010-2029-1