Abstract
Background
Anopheles farauti is one of the major vectors of malaria in the Southwest Pacific region and is responsible for past outbreaks in Australia. With an adaptable biting profile conducive to behavioural resistance to indoor residual spraying (IRS) and insecticide-treated nets (ITNs), its all-night biting behaviour can switch to biting mostly in the early evening. With limited insight into the biting profile of An. farauti populations in areas that have not encountered IRS or ITNs, the aim of this study was to develop insights on the biting behaviour of a malaria control naive population of An. farauti.
Methods
Biting profiles of An. farauti were conducted at Cowley Beach Training Area, in north Queensland, Australia. Initially, encephalitis virus surveillance (EVS) traps were used to document the 24-h biting profile of An. farauti and then human landing collections (HLC) were used to follow the 18.00–06.00 h biting profile. The human landing catches (HLC) were performed at both the end of the wet (April) and dry (October) seasons.
Results
Data exploration using a Random Forest Model shows that time of night is the most important variable for predicting An. farauti biting activity. Temperature was found to be the next important predictor, followed by humidity, trip, collector, and season. The significant effect of time of night and peak in time of night biting, between 19.00 and 20.00 h was also observed in a generalized linear model. The main effect of temperature was significant and non-linear and appears to have a positive effect on biting activity. The effect of humidity is also significant but its relationship with biting activity is more complex. This population’s biting profile is similar to populations found in other parts of its range prior to insecticide intervention. A tight timing for the onset of biting was identified with more variation with the end of biting, which is likely underpinned by an endogenous circadian clock rather than any light intensity.
Conclusion
This study sees the first record of a relationship between biting activity and the decreasing temperature during the night for the malaria vector, Anopheles farauti.
Similar content being viewed by others
Background
Throughout the Southwest Pacific region where malaria occurs, one of the primary vectors is Anopheles farauti [1]. It has a wide distribution, being found from the Moluccas (Indonesia) in the west, throughout Papua New Guinea (PNG), Solomon Islands, Vanuatu and northern Australia. Anopheles farauti is a coastal species rarely found more than 5 km from the coast with brackish swamps being the preferred oviposition site, although it is commonly found in fresh water sites [2].
With regard to malaria transmission, An. farauti has a reputation for being difficult to control in the Solomon Islands using the traditional malaria vector control methods of indoor residual spraying (IRS) [3,4,5] and insecticide-treated nets (ITNs). The feeding behaviour of this species, documented by several studies before the introduction of DDT IRS, shows An. farauti starting to feed at dusk and progressing to a peak around midnight: in the D’Entrecasteaux Islands (PNG) [6], New Britain (PNG) [7] and the Solomon Islands [8, 9]. Following the introduction of DDT IRS into PNG and the Solomon Islands during the 1960s and 1970s, An. farauti shifted its peak biting time forward to between 18.00 and 20.00 h. In New Britain (PNG), after five spray cycles (across two years) with DDT IRS, there was a distinct shift in the peak biting time from the middle part of the night to between 18.00 and 19.00, with 76% of feeding occurring before 21.00 [7]. Similar studies in the Solomon Islands also saw a shift in biting activity moving from midnight to an early night peak followed by a second peak just before sunrise [8, 9].
More recent studies in the Solomon Islands confirm the early night biting peak occurring before 21.00 remains in these populations despite the cessation of DDT spraying in the 1980s [5, 10]. The lack of a reversion to the original phenotype of biting through the night after the IRS selection pressure ended is interesting as there would be populations of An. farauti extant on the islands where malaria control activities did not take place. However, a possible reversion did occur on Buka Island (PNG) where 19 years after the DDT IRS control campaign (1961–1980) ceased, the An. farauti population exhibited a peak biting time of 00.00–01.00 [11, 12].
Molecular identification methods suggest An. farauti in the Solomon Islands to be a single species [4, 13, 14], and so it remains unclear why these populations would not revert back to an original feeding phenotype after the Global Eradication Campaign in the 1970s in the Solomon Islands, but could possibly be attributed the strong selection on small island populations. More recent use of long-lasting insecticidal nets (LLINs) has likely reinforced this early evening biting behaviour [10, 15]. A better understanding of the feeding behaviour of An. farauti in malaria control naive populations may shed some light on this phenomenon.
Despite the use of LLINs in the Southwest Pacific, malaria has been increasing since 2015 with the largest increases seen in the Solomon Islands [16]. Communities in PNG and the Solomon Islands spend much of the early part of the night outdoors [15, 17], and An. farauti, by feeding early, can obtain a bloodmeal without entering houses. Thus, these shifts in peak feeding suggest changes in mosquito biting behaviour to avoid the insecticide, resulting in uncontrolled outdoor malaria transmission. Additionally, day time biting has been recorded with An. farauti [18, 19] using collections from 07.00 to 10.00 [19]; although the numbers collected were small, no specific attempt was made to assess day time biting activity during these early studies.
Unlike PNG and the Solomon Islands, malaria on mainland Australia is not a serious problem [20]. Thus, DDT IRS was never employed for malaria control and anophelines in Australia have not encountered sustained indoor insecticide selection pressures. Anopheles farauti is thought to be the main vector of malaria in Australia [21], with the last outbreak by this mosquito being Plasmodium vivax in 2002 [22]. The host-seeking behaviour and night-biting profile of this species in Australia is not well understood. Additionally, there are other aspects of the host-seeking behaviour of An. farauti that have not been studied in any part of its range, such as the exact time host seeking commences and then ceases. Traditionally, human landing catches (HLC) commence at 18.00 and finish at 06.00, but does host seeking occur before and after these times? What triggers this host-seeking behaviour, does the relationship between light intensity and times of biting in relation to sunset and sunrise have an effect? There are limited studies to date that has explored how light can affect biting behaviour. Also, what effect does temperature and humidity have on host seeking?
The aim of this study is to determine the biting profile of a population of An. farauti that has not encountered indoor malaria-control based insecticide treatments. It was hypothesized that the populations in north Queensland will show host-seeking activities throughout the night with a peak around midnight as has been found in other An. farauti populations prior to indoor insecticide pressure.
Methods
The study site
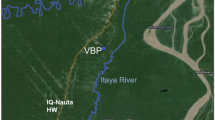
Collections of An. farauti were made at the Cowley Beach Training Area (CBTA), an Australian Defence Force training facility, situated in the wet tropical region in far north Queensland, Australia, approximately 40 km south of Innisfail (Fig. 1). The training area comprises 5081 ha of coastal lowland plains consisting of mixed open forest, melaleuca swamps, estuarine aquatics, rainforest and 8 km of beaches [23]. Tidal flats, potential An. farauti oviposition sites, cover approximately 37% of CBTA and consist mainly of regularly inundated areas with mangroves and tidal creeks [23].
The climate type for the area is monsoonal with a distinct wet (November to April) and dry (May to October) season. There is seasonality in the temperature with May to October being the cooler months and November to April the hotter months with mean minimum and maximum temperatures of 15.8 °C to 28.0 °C and 21.1 °C to 30.7 °C, respectively. Innisfail receives a mean annual rainfall of 3500 mm per year (n = 139 years), making it one of the wettest places in Australia. The mean relative humidity is high (75–80%) and is constant throughout the year [24].
Biting activity
Anopheles farauti biting activity was determined by HLC, a total of 25 nights of collection were conducted at the end of the wet season (April) and 34 nights of collection conducted at the end of the dry season (September, October) from 2014 to 2017. Collections were made at a single site (17° 41′ 14.7″ S 146° 06′ 06.4″ E) 1.4 km inland from the coast and at the boundary of mixed open forest and rainforest 1.5 km south of the primary oviposition site (Fig. 1). During HLC, the collector exposed their lower legs (between ankle and knee) to host-seeking mosquitoes for 50 min each hour from 18.00 to 06.00. Catches were made by two collectors, one collector working from 18.00 to 00.00 and the second collector working from 00.00 to 06.00. The collectors rotated between shifts throughout the collection periods to reduce bias for differences in individual odours and collecting abilities. The collectors monitored for mosquitoes that landed on their legs; using a torch, any anophelines landing were captured using a mouth aspirator. The mosquitoes were then placed into a wax-lined paper cup, covered with mosquito netting labelled for each hour of the collections. Anophelines collected were killed by freezing and the numbers caught for the hour were counted and recorded. To determine if temperature or humidity affected the biting profile, these parameters were recorded on the hour for each hour of collection. To determine the 24-h biting profile, an encephalitis virus surveillance (EVS) trap [25] was set for 24 h at the collection site. The trap was collected each hour and the number of An. farauti collected was counted and recorded. This was replicated over four nights.
Species identification
All Anopheles collected were initially identified by adult morphology to the An. farauti complex [26]. As two other isomorphic species: Anopheles hinesorum and Anopheles torresiensis are known to occur in north Queensland coastal region [27], sub-sets of anophelines from each collection period were preserved in 70–100% ethanol for transport to the laboratory where they were assessed by PCR-based diagnostics to confirm the target study species was An. farauti [28].
Factors associated with biting profiles
To determine the commencement of biting, the time was recorded when the first An. farauti was collected. To determine the cessation of biting, collections were continued after 06.00 and until 20 min had elapsed since the last An. farauti was collected. At each of these time points the temperature, humidity and lumination intensity (lux) (Lutron light meter, Model LX-1108) was recorded.
In order to assess the most important variables recorded that may be associated with the biting activity of An. farauti, data exploration was initially performed to assess overall patterns in the data. As time of night is known to be important in predicting the biting activity of this species, the mean and standard errors of numbers of An. farauti collected in both the wet and dry seasons were plotted. As expected from previous studies, it was shown that there is a non-linear trend in data associated with time of night (Additional file 1: Fig. S1). A Generalized Additive Model (GAM) was fitted to the data to further assess the effects of the other recorded variables, as well as to account for potential effects of collector and temporal factors. This was performed using the gam function in the R package [29] ‘mgcv’ [30]. Also initially fitted was a Random Forest Model using the R package ‘randomForest’ [31], to assess which variables are likely to be the strongest predictors of mosquito biting activity. Based on the results of data exploration, the final GAM that was fit to the data was Number ~ s(Time, k = 12) + s(Temperature) + ti(Time, Temperature, k = 12) + s(Humidity) + s(Date, k = 7, bs = “re”) + Collector.
Results
Species identification
All mosquitoes collected by HLC during the wet and dry seasons (n = 18,006) were determined to be An. farauti s.l. by morphology. A sub-set of the specimens was confirmed as An. farauti by molecular analysis (n = 250), with no other cryptic species present.
Time of night, temperature, humidity and other predictors of An. farauti biting activity
At CBTA, An. farauti commenced feeding early in the night, peaking between 19.00 and 20.00, biting activity then fell off over the remainder of the night (Fig. 2 and Additional file 1: Fig. S1). In the dry season (April), the majority of An. farauti biting activity occurred before midnight (76%) with two biting peaks, the highest at 19.00–20.00 and a lesser peak at 22.00–23.00 (Fig. 2A and Additional file 1: Fig. S1). Similarly, in the wet season (October), 64% of the biting activity occurred before midnight with a biting peak also at 19.00–20.00 (Additional file 1: Fig. S1). A highly fixed timing for the commencement and cessation of biting was found between collection nights (Table 1). Additionally, the light intensity observed for the commencement and cessation of biting was highly consistent in both the wet and dry seasons (Table 1). Overall, light intensity at the cessation of biting in the morning was greater than that at start of biting in the evening. The four outdoor 24-h EVS collections (n = 756) made at CBTA at the collection site suggested An. farauti were not caught between the times of 06.00 and 18.00 (Fig. 3). The biting numbers (total) of An. farauti were higher in the dry (n = 10,821) than the wet (n = 7185) seasons.
Smooths from the Generalized Additive Model. A Surface plot of combined effects of temperature and time of night on number of An. farauti collected. B Partial smoothed effect of time of night on number of An. farauti collected. C Partial smoothed effect of temperature on number of An. farauti collected. D Partial smoothed effect of humidity on number of An. farauti collected. Residuals and standard errors are shown in B-D
Data exploration using a Random Forest Model shows that time of night is clearly the most important variable measured for predicting An. farauti biting activity (Fig. 4). This is followed by the temporal variable, date of which likely reflects fluctuations in the numbers of mosquitoes caught due to other environmental factors not recorded (such as rainfall and wind). Thus, date was included in the GAM as a random effect. Temperature was found to be the next most important predictor, followed by humidity, trip, collector, and season. Since any temporal autocorrelation associated with differences in number of mosquitoes collected should be accounted for by date, trip was not included as a factor in the GAM. Likewise, season was excluded due to both its low predictive power and obvious collinearity with temperature and humidity. The final GAM that was fit to the data had an adjusted R squared of 0.472 and explained 50.9% of the deviance in residuals. All non-random variables fitted were significant and non-linear, with time of night having the most complex relationship with mosquito biting activity (edf = 9.73) as well as being the most significant predictor (P < 2e−16). To visualize these complex non-linear effects, the smooths fitted to the data are presented, as well as the standard errors and means associated with them (Fig. 2). Collector was fit as a parametric (linear effect) allowing the intercept of collector to vary in the model. From this, there were found significant differences in intercept between the two collectors (P = 0.0001, t = − 3.877), with a mean difference of 16.44, after adjusting for other variables. The significant effect of time of night and peak in time of night biting, between 19.00 and 20.00, can be clearly seen in Fig. 2A and B. The main effect of temperature was significant (P = 0.006) and non-linear (edf = 2.36) and appears to have a positive effect on biting activity between 12.4 °C and approximately 22 °C, after adjusting for other variables, above which it has a negative effect when considered as a main effect (Fig. 2C). However, there is also a clear interaction between time of night and temperature, as indicated by the highly significant and non-linear tensor interaction term (P = 6.93e−0.5, edf = 2.46). From Fig. 2A, it can be seen that this interaction predicts that on warmer nights, the biting peak between 19.00 and 20.00 is less pronounced, and the biting profile appears flatter, with mosquitoes being collected more consistently through the evening. The effect of humidity is also significant (P = 0.0002) but its relationship with biting activity is more complex (edf = 7.38) and difficult to interpret (Fig. 2D).
The four coldest and four warmest nights during the study were used to assess the effect of temperature on biting activity. Cold night average temperatures were below 17 °C and warmest nights had average temperatures above 25 °C. The cooler nights occurred during the dry season (spring) when An. farauti densities numbers were higher (n = 2411) and the decreasing temperature throughout the night reduced biting activity. In this, a temperature threshold of 18 °C saw An. farauti biting activity drop rapidly (Additional file 1: Fig. S2). On warmer nights (n = 4) of collection (n = 1957 Anopheles collected), the temperature was consistent throughout the night and An. farauti biting activity peaked between 19.00 and 01.00 (Additional file 1: Fig. S2).
Discussion
Accurate knowledge of the biting profile or the night biting profile of malaria vectors is important as the two main control strategies currently used against malaria vectors remain controlling the host-seeking females with IRS and LLINs. For these measures to be effective, the vector should be seeking a bloodmeal when humans are either indoors and/or under an ITN. Under these conditions the vector should come into contact with the insecticide, either on the walls or the net and die. If the vector feeds before this time, it will likely feed outdoors and avoid contact with insecticide and outdoor transmission will continue despite the intervention measures.
This study of An. farauti at CBTA was carried out over four years in both wet (April) and dry (October) seasons. The night biting profile of this An. farauti population, which has not been subjected to IRS or ITNs appears to show a similar biting profile to other population that were subjected to insecticidal pressure IRS or ITNs [5, 7, 32]., When more extreme ambient temperature is considered, a decreasing temperature below 18 °C through the night reduces biting activity with most activity limited to the warmer early evening window. On warmer nights (above 25 °C), where the temperature is constant, biting appears to occur through the night, similar to other populations in the region that had not been subjected to indoor insecticidal pressure, as in pre-spray PNG [12, 19, 33] and the Solomon Islands [8, 32]. In this, the mean temperature in the equatorial coastal lowlands of PNG and the Solomon Islands is around ~ 25 °C (min 22 °C-max 31 °C) [34] and biting activity does not usually occur at temperatures below 20 °C. The observation in Queensland, a population at a higher latitude and thus encountering a broader temperature fluctuation, sees the first record of a relationship between a reduction in the biting activity of An. farauti and decreasing temperature during the night. Mosquitoes are ectotherms and metabolism is dictated by ambient temperature [35, 36], with tropical mosquitoes sensitive to lower ambient temperatures affecting flight and biting activity. Temperature and biting activity studies on another tropical mosquito also found in Queensland, Australia, Aedes aegypti, suggests biting activity decreases and can even stop at between 15 °C and 18 °C [37] and may help understand the biting profile of An. farauti on these cooler nights.
Anopheles farauti shows a tight coastal distribution with the ability to utilize both brackish and fresh water larval sites [2, 38]. This mosquito can display higher population densities during the dry season as observed in studies in the Solomon Islands [10, 39]. Also seen is a similar seasonal influence in the CBTA study site, where adult productivity is higher during the dry season and this pattern is probably due to larvae being flushed out to the sea by heavy tropical rainfall during the wet season.
Ecological niche modelling of An. farauti in Australia found a strong correlation between parameters of temperature (i.e., minimum, diurnal, seasonal and annual temperature range as well as sea temperature), which, apart from sea temperature, are moderated along the coast by the sea temperature [40]. The observed decrease in female host-seeking activity with cooler ambient temperatures complements a similar finding [40], that these environmental parameters may help define the range for An. farauti and may prevent the species moving inland where larger temperature variation would be encountered, although temperature fluctuations decrease in more equatorial regions.
The tight feeding timing consistency appears more tied to a 24-h clock rather than light intensity and complements the findings of Duffield et al. [41], in that An. farauti daily nocturnal flight activity is underpinned by the circadian clock—a research area in mosquitoes now beginning to unfold with new genomic and transcriptomic tools available [42,43,44,45].
Conclusion
Anopheles farauti is one of the most important malaria vectors in the Southwest Pacific and has shown consistent behavioural resistance to indoor malaria control strategies such as IRS and ITNs, with populations moving to earlier evening biting under this selection pressure.
This population displays biting time plasticity with early biting preference observable when night temperature drops below 18 °C to biting through the night on warmer nights that maintain temperatures above 25 °C. It would be interesting to consider how this feeding time plasticity is controlled and if there is any connection with observed behavioural insecticide resistance in this malaria vector.
Availability of data and materials
The datasets generated and analysed during the current study are available through DRYAD DOI https://doi.org/10.5061/dryad.s1rn8pkc2 and contains a README file and mosquito trapping results in an.xlsx file.
Abbreviations
- IRS:
-
Indoor residual spraying
- ITN:
-
Insecticide treated nets
- EVS:
-
Encephalitis virus surveillance trap
- HLC:
-
Human landing catch
- PNG:
-
Papua New Guinea
- LLIN:
-
Long-lasting insecticidal net
- CBTA:
-
Cowley Beach Training Area
- ADFMIDI:
-
Australian Defence Force Malaria and Infectious Disease Institute
References
Cooper RD, Waterson DGE, Frances SP, Beebe NW, Pluess B, Sweeney AW. Malaria vectors of Papua New Guinea. Int J Parasitol. 2009;39:1495–501.
Cooper RD, Waterson DGE, Frances SP, Beebe NW, Sweeney AW. Speciation and distribution of the members of the Anopheles punctulatus (Diptera: Culicidae) group in Papua New Guinea. J Med Entomol. 2002;39:16–27.
Russell TL, Beebe NW, Cooper RD, Lobo NF, Burkot TR. Successful malaria elimination strategies require interventions that target changing vector behaviours. Malar J. 2013;12:56.
Russell TL, Beebe NW, Bugoro H, Apairamo A, Collins FH, Cooper RD, et al. Anopheles farauti is a homogeneous population that blood feeds early and outdoors in the Solomon Islands. Malar J. 2016;15:7.
Russell TL, Beebe NW, Bugoro H, Apairamo A, Chow WK, Cooper RD, et al. Frequent blood feeding enables insecticide-treated nets to reduce transmission by mosquitoes that bite predominately outdoors. Malar J. 2016;15:9.
Spencer M. Malaria in the D’Entrecasteaux Islands, Papua, with particular reference to Anopheles farauti Laveran. Proc Linnean Soc NSW. 1965;91:115–28.
Sweeney AW. A review of chemical control of malaria vectors in the south west pacific region. In: Laird M, Miles JW, editors. Integrated mosquito control methodologies, vol. 1. London: Academic Press; 1983. p. 143–58.
Slooff R. Assignment report, March 1964 to September 1969. WHO/WPR/47/69. Geneva: World Health Organization; 1969.
Taylor B. Changes in the feeding behaviour of a malaria vector, Anopheles farauti Lav., following the use of DDT as a residual spray in houses in the British Solomon Islands Protectorate. Trans R Entomol Soc Lond. 1975;127:227–92.
Bugoro H, Hii J, Butafa C, Iroofa C, Apairamo A, Cooper R, et al. The bionomics of the malaria vector Anopheles farauti in Northern Guadalcanal, Solomon Islands: issues for successful vector control. Malar J. 2014;13:56.
Spencer M. Pre- and early postspray observations on the anophelines of Buka Island, New Guinea. 1961, Malaria Control Programme, PNG Health Department.
Cooper RD, Frances SP. Malaria vectors on Buka and Bougainville islands, Papua New Guinea. J Am Mosq Control Assoc. 2002;18:100–6.
Ambrose L, Cooper RD, Russell TL, Burkot TR, Lobo NF, Collins FH, et al. Microsatellite and mitochondrial markers reveal strong gene flow barriers for Anopheles farauti in the Solomon Archipelago: implications for malaria vector control. Int J Parasitol. 2014;44:225–33.
Ambrose L, Riginos C, Cooper RD, Leow KS, Ong W, Beebe NW. Population structure, mitochondrial polyphyly and the repeated loss of human biting ability in anopheline mosquitoes from the Southwest Pacific. Mol Ecol. 2012;21:4327–43.
Bugoro H, Cooper RD, Butafa C, Iro’ofa C, Mackenzie DO, Chen CC, et al. Bionomics of the malaria vector Anopheles farauti in Temotu Province, Solomon Islands: issues for malaria elimination. Malar J. 2011;10:133.
WHO. World malaria report 2021. Geneva: World Health Organization; 2021.
Bugoro H, Iro’ofa C, Mackenzie DO, Apairamo A, Hevalao W, Corcoran S, et al. Changes in vector species composition and current vector biology and behaviour will favour malaria elimination in Santa Isabel Province, Solomon Islands. Malar J. 2011;10:287.
Black RH. Observations on the behavior of Anopheles farauti Laveran, an important malaria vector in the territory of Papua New Guinea. Med J Aust. 1955;42:949–55.
Standfast HA. Biting times of nine species of New Guinea Culicidae (Diptera). J Med Entomol. 1967;4:192–6.
Black RH. Malaria in Australia. Canberra: Australian Government Publishing Service; 1972.
Mackerras IM. The Australasian anophelines as vectors of malaria. Med J Aust. 1947;1:1–8.
Hanna JN, Ritchie SA, Eisen DP, Cooper RD, Brookes DL, Montgomery BL. An outbreak of Plasmodium vivax malaria in Far North Queensland, 2002. Med J Aust. 2004;180:24–8.
Buosi P. Environmental Impact Assessment of Defence Training Activities at Cowley Beach Training Area (CBTA). 2004.
Australian Bureau of Meteorology (BOM 2020): http://www.bom.gov.au/climate/averages/tables/cw_032025.shtml.
Rohe D, Fall R. A miniature battery powered CO2 baited light trap for mosquito borne encephalitis surveillance. Bull Soc Vector Ecol. 1979;4:24–7.
Marks EN. An atlas of common Queensland mosquitoes. University of Queensland Bookshop. 1967.
Van Den Hurk AF, Cooper RD, Beebe NW, Williams GM, Bryan JH, Ritchie SA. Seasonal abundance of Anopheles farauti (Diptera. Culicidae) sibling species in far north Queensland, Australia. J Med Entomol. 2000;37:153–61.
Beebe NW, Saul A. Discrimination of all members of the Anopheles punctulatus complex by polymerase chain reaction—restriction fragment length polymorphism analysis. Am J Trop Med Hyg. 1995;53:478–81.
R-Core-Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. 2017.
Wood SN. Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. J R Stat Soc Ser B. 2011;73:3–36.
Liaw A, Wiener M. Classification and regression by randomForest. R News. 2002;2:18–23.
Taylor B. Observations on malaria vectors of the Anopheles punctulatus complex in the British Solomon Islands Protectorate. J Med Entomol. 1975;1:677–87.
Sweeney AW. Variations in density of Anopheles farauti Laveran in the Carteret Islands. P N G Med J. 1968;10:11–8.
McAlpine JR, Keig G, Fall F. Climate of Papua New Guinea. Canberra, 1983.
Heinrich B. Thermoregulation in endothermic insects. Science. 1974;185:747–56.
Suh E, Grossman MK, Waite JL, Dennington NL, Sherrard-Smith E, Churcher TS, et al. The influence of feeding behaviour and temperature on the capacity of mosquitoes to transmit malaria. Nat Ecol Evol. 2020;4:940–51.
Christophers SR. Aedes aegypti (L.), the yellow fever mosquito: its life history, bionomics and structure. London: Cambridge University Press; 1960.
Beebe NW, Cooper RD. Distribution and evolution of the Anopheles punctulatus group (Diptera: Culicidae) in Australia and Papua New Guinea. Int J Parasitol. 2002;32:563–74.
Bugoro H, Hii J, Russell TL, Cooper RD, Chan BK, Iro’ofa C, et al. Influence of environmental factors on the abundance of Anopheles farauti larvae in large brackish water streams in Northern Guadalcanal. Solomon Islands Malar J. 2011;10:262.
Sweeney AW, Beebe NW, Cooper RD, Bauer JT, Peterson AT. Environmental factors associated with distribution and range limits of malaria vector Anopheles farauti in Australia. J Med Entomol. 2006;43:1068–75.
Duffield GE, Acri DJ, George GF, Sheppard AD, Beebe NW, Ritchie SA, Burkot TR. Diel flight activity of wild-caught Anopheles farauti (ss) and An. hinesorum malaria mosquitoes from northern Queensland, Australia. Parasit Vectors. 2019;12:14.
Rund SSC, Hou TY, Ward SM, Collins FH, Duffield GE. Genome-wide profiling of diel and circadian gene expression in the malaria vector Anopheles gambiae. Proc Natl Acad Sci USA. 2011;108:E421–30.
Rund SSC, Gentile JE, Duffield GE. Extensive circadian and light regulation of the transcriptome in the malaria mosquito Anopheles gambiae. BMC Genomics. 2013;14:218.
Baik LS, Nave C, Au DD, Guda T, Chevez JA, Ray A, et al. Circadian regulation of light-evoked attraction and avoidance behaviors in daytime-versus nighttime-biting mosquitoes. Curr Biol. 2020;30:3252-9.e3.
Balmert NJ, Rund SS, Ghazi JP, Zhou P, Duffield GE. Time-of-day specific changes in metabolic detoxification and insecticide resistance in the malaria mosquito Anopheles gambiae. J Insect Physiol. 2014;64:30–9.
Acknowledgements
We thank the administration staff at ADFMIDI for assisting in the logistical preparedness for this study. We are grateful to the range control officers and grounds staff - David Sly at CBTA for your assistance to during this study. We also thank Dr. Simon Hart and Dr. Simone Blomberg for their advice on modelling and data analysis.
Disclaimer
The opinions expressed here are those of the authors and do not necessarily reflect those of the Australian Defence Force and/or extant Defence Force Policy.
Funding
This study was supported by the Australian Defence Force.
Author information
Authors and Affiliations
Contributions
WKC, RDC and NWB conceived the study. WKC and RDC performed the field collections and morphological identifications. WKC performed the molecular experiments. PP contributed to the field collections. LA provided assistance with modelling and data analysis. WKC drafted the first version of the manuscript. RDC, NWB, LA and PP revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
For this research, ethical approval was granted from the Australian Defence Human Research Ethics Committee—Protocol number: 774-14 (10 April, 2015), Australian Defence Force Malaria and Infectious Disease Institute (ADFMIDI) Biosafety committee and ADFMIDI Work Health and Safety committee for conducting human landing catch on this study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Fig. S1.
Anopheles farauti mean (± SE) biting profile from HLC at CBTA for 15 nights each during the wet (Apr) and dry (Oct) seasons between 2014 and 2017. Fig. S2. (A) Anopheles farauti mean biting profile on four of the coldest nights during the study. (B) Anopheles farauti mean biting profile on four of the hottest nights during the study. Mean (± SE) An. farauti collected per person (black) compares with temperature (red).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Chow, W.K., Beebe, N.W., Ambrose, L. et al. Seasonal assessment on the effects of time of night, temperature and humidity on the biting profile of Anopheles farauti in north Queensland, Australia using a population naive to malaria vector control pressures. Malar J 22, 85 (2023). https://doi.org/10.1186/s12936-023-04495-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12936-023-04495-5