Abstract
Background
Malaria burden among under-five children living in endemic areas of Yemen is largely unknown due to the lack of community-based studies. Therefore, this study determined the prevalence and risk factors associated with falciparum malaria among under-five children in rural communities of Al-Mahweet governorate, Yemen.
Methods
This community-based, cross-sectional study recruited 400 under-five children from two rural districts of Al-Mahweet governorate in December 2019. Demographic characteristics (gender, age, education and occupation of the child’s parents, and household size) and risk factors associated with malaria were collected through interviews with children’s caregivers using a structured questionnaire. Finger-prick blood was screened for Plasmodium falciparum and non-falciparum species using rapid diagnostic tests (RDTs), and duplicate Giemsa-stained thick and thin blood films were examined for malaria parasites. The density of asexual P. falciparum stages was also estimated. Data were then analysed, and the agreement between the results of thick-film microscopy and RDTs for diagnosing falciparum malaria was assessed using the kappa index. Statistical significance was set at a P-value of < 0.05.
Results
Plasmodium falciparum was prevalent among 9.8% (95% CI 7.0–13.1) of under-five children in the rural communities of Al-Mahweet, with a median asexual parasite density of 763 ± 2606 parasites/μl of blood (range: 132–4280) and low-to-moderate parasitaemia levels. Approximately one-third of microscopy-confirmed cases were gametocyte carriers. Multivariable logistic regression analysis confirmed that age of three years or older (AOR = 5.6, 95% CI 1.6–19.8; P = 0.007), not sleeping under a mosquito net the previous night of the survey (AOR = 8.0, 95% CI 2.4–27.4; P = 0.001), sleeping outdoors at night (AOR = 4.4, 95% CI 2.0–10.0; P < 0.001), and absence of indoor residual spraying (IRS) during the last year (AOR = 4.2, 95% CI 1.9–9.4; P < 0.001) were the independent predictors of falciparum malaria among under-five children in the rural communities of Al-Mahweet. The observed percentage agreement between thick-film microscopy and RDTs was 98.5%, with a very good agreement (k-index = 0.9) between the two methods for falciparum malaria diagnosis that was statistically significant.
Conclusion
Approximately one in ten under-five children in rural communities of Al-Mahweet is infected with P. falciparum based on microscopy and RDTs. Age of three years or older, not sleeping under mosquito nets, sleeping outdoors at night and absence of IRS can independently predict falciparum malaria among them. The very good agreement between thick-film microscopy and RDTs for diagnosing falciparum malaria in children supports the usefulness of using RDTs in such resource-limited rural communities.
Similar content being viewed by others
Background
Malaria is a major public health problem in the world. According to the latest report by the World Health Organization (WHO), 241 million cases were estimated in 85 malaria-endemic countries, increasing from 227 million in 2019 [1]. In the WHO Eastern Mediterranean Region, 5.7 million cases and 12,330 deaths were estimated in 2020 [1]. Despite the efforts devoted by the National Malaria Control Programme (NMCP) since the early 2000s, the at-risk population was estimated at 64.5% and the reported cases exceeded 164,000 in 2020, with approximately 99% being caused by Plasmodium falciparum [1].
Under-five children are at high risk of contracting malaria and developing severe disease [2]. Apart from symptomatic cases, asymptomatic children can be major reservoirs of malaria, contributing substantially but silently to its transmission in endemic areas [3, 4]. Infection in asymptomatic carriers with microscopic parasitaemia often persists for months and goes undetected and untreated, making them a major gametocyte reservoir for mosquitoes [5, 6]. Children can be a key contributor to the infection of mosquitoes. For instance, a cohort study found that school-aged Ugandan children may be responsible for more than half of all mosquito infections [4]. Moreover, gametocytes from asymptomatic carriers can be more infectious to mosquitoes compared to those from symptomatic patients [7]. Unveiling the burden of asymptomatic malaria among children is instrumental in the success of malaria elimination interventions.
In Yemen, community-based malaria prevalence in under-five children is largely unknown, with few published studies reporting on childhood malaria. In this regard, 18.6% of children in rural districts of Taiz governorate were confirmed to be infected with P. falciparum in the mid-2000s, and falciparum malaria was recently reported among 8% of schoolchildren from Bajil district of Hodeidah governorate [8, 9]. Another study found that suspected severe falciparum malaria may account for up to 40% of paediatric admissions during the peak transmission season in endemic areas, and more than half of confirmed cases can be severe [10]. With the prevalence of malnutrition in endemic areas as a result of the humanitarian crisis imposed by armed conflicts [11], children are at high risk of malaria morbidity and mortality. Therefore, the present study determined the prevalence and risk factors associated with falciparum malaria among under-five children in the rural communities of Al-Mahweet during the peak transmission season. In addition, agreement between rapid diagnostic tests (RDTs) and thick-film microscopy for diagnosing falciparum malaria among rural children was assessed.
Methods
Study design, population and setting
A community-based, cross-sectional study was conducted among under-five children in two rural districts of Al-Mahweet governorate known to be highly endemic for malaria in December 2019. Children of both genders were included in the study if they were residents in the study districts over the year preceding the study and did not receive antimalarial drug(s) one month before the study, and if their parents/guardians gave written informed consent.
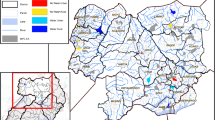
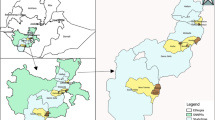
Al-Mahweet is a small mountainous governorate at the coordinates of 15° 28′ N and 43° 32′ E, approximately 111 km to the northwest of Sana'a. It is about 2,382 km2 and is inhabited by approximately 597,000 people [12]. It is endemic for malaria and borders two governorates with the highest burden of malaria in Yemen [13], Hodeidah to the west and Hajjah to the north. The epidemiology of malaria in Al-Mahweet might be influenced by internal displacement from its bordering highest-burden governorates as a result of the armed conflicts. The malaria transmission season in the governorate usually lasts for approximately six months, from November to April [14]. Of its nine districts, Bani Sa’d and Al-Khabt (Fig. 1) are the most afflicted with malaria because of their low altitude (below 1500 m). In 2019, P. falciparum was detected among 15.6% (3235/20764) of febrile patients examined by RDTs and/or microscopy at health facilities in the two districts; of whom, 908/5001 (18.2%) were under-five children according to the data derived from the Electronic Disease Early Warning System (eDEWS) of Yemen (Dr Reema Alyusfi, eDEWS Manager, personal communication). Therefore, these two districts were purposively selected to conduct this study.
Sample size and sampling strategy
A minimum sample size of 348 children was calculated using OpenEpi software, Version 3.01 (www.OpenEpi.com) based on an expected prevalence of 13% [8, 9], a confidence level of 95%, an absolute precision of 5% and a design effect of 2. Based on an expected non-response rate of 15%, the sample was adjusted to 400 children to account for ineligible or missing children due to absenteeism or caregivers’ refusal. A multi-stage cluster sampling strategy was adopted. First, villages of Bani Sa’d and Al-Khabt districts were listed, and 20 villages were randomly selected from the two districts. Second, 20 households with at least one under-five child were randomly selected from each village, followed by random sampling of one child if the household had more than one.
Data and blood collection
Demographic characteristics (gender, age, education and occupation of the child’s parents and household size) and risk factors associated with malaria were collected through face-to-face interviews with children’s caregivers using a structured questionnaire. Axillary temperature was measured using a thermometer. Children with a temperature of ≥ 37.5 °C were considered febrile. Blood drops were collected by finger prick for rapid malaria testing and preparing duplicate thick and thin blood films.
Malaria rapid testing and microscopy
Blood drops were screened for P. falciparum and non-falciparum species using CareStart™ Malaria HRP2/pLDH (Pf/PAN) Combo RDTs (AccessBio, NJ, USA), according to the manufacturer’s instructions. Such RDT kits are currently adopted by the NMCP for malaria diagnosis in the country. Thick and thin blood films were prepared, Giemsa-stained and examined microscopically using standard procedures [15, 16]. Thick films were examined for a minimum of 100 oil-immersion fields before being recorded as negative for malaria parasites. Parasite density per microlitre (µl) of blood was estimated by counting P. falciparum asexual stages against 200 leukocytes on thick blood films, assuming an average leukocyte count of 8000/µl of blood [15, 16]. This procedure was repeated in two other areas of the film, and the average of the three counts was then recorded.
Data analysis
Data were analysed using IBM SPSS Statistics, Version 23.0 (IBM Corp., Armonk, NY, USA). Categorical variables were expressed as frequencies and proportions, while continuous variables were expressed as the median and interquartile range (IQR) for non-normally distributed data. The prevalence of falciparum malaria was reported with its corresponding 95% confidence interval (CI), as determined by microscopy and/or RDTs. Univariate analysis with Pearson's chi-square or Fisher’s exact test was used to test the association of demographic and clinical characteristics as well as risk factors with falciparum malaria among under-five children, reporting the odds ratios (ORs) and corresponding 95% CIs of the associations. Multivariable logistic regression analysis was performed for significant factors in univariate analysis to identify the independent predictors of falciparum malaria among under-five children, together with their adjusted ORs (AORs) and corresponding 95% CIs. Agreement between the results of thick-film microscopy and RDTs for diagnosing falciparum malaria was assessed using the kappa (k) index, where the agreement was interpreted as poor (k < 0.20), fair (k = 0.20–0.40), moderate (k > 0.40–0.60), good (k > 0.60–0.80) or very good (k > 0.80) [17]. Statistical significance was set at a P-value of < 0.05.
Results
Characteristics of the study population
The majority of children included in this study were males (54.2%), aged three years or older (62.5%), with a median (IQR) age of 3 (2) years, and living in households of six members or more (63.5%). Most children’s fathers had primary education (37.0%) or were uneducated (37.0%) and were labourers (68.8%), while the majority of children’s mothers were uneducated (83.2%) and unemployed (99.2%) (Table 1).
Prevalence of falciparum malaria among under-five children
Falciparum malaria was prevalent among 9.8% (95% CI 7.0–13.1) of under-five children in rural communities of Al-Mahweet, where microscopy confirmed infection among 9.3% (95% CI 6.6–12.5) and RDTs were positive among 8.8% (95% CI 6.2–12.0) of children (Table 2).
Parasite density and gametocyte carriage
The median asexual parasite density among children with microscopy-confirmed falciparum malaria was 763 ± 2606 parasites/μl of blood (range: 132–4280), with low and moderate parasitaemia levels being prevalent among 51.4% and 48.6% of infected children, respectively. Of children with falciparum malaria, 32.2% were gametocyte carriers (Table 3).
Association of children’s demographics and clinical characteristics with falciparum malaria
The prevalence of falciparum malaria was significantly higher among children aged three years or older (OR = 8.2, 95% CI 2.5–27.3; P < 0.001) than in younger children (14.4% vs. 2.0%, respectively). In contrast, no significant association was observed between falciparum malaria and gender, household size, parental educational status, or parental employment status. Likewise, no significant association was observed between falciparum malaria and fever, sweating, chills, vomiting, or jaundice (Table 4).
Risk factors associated with falciparum malaria among under-five children
Not sleeping under a mosquito net the previous night of the survey (OR = 11.8, 95% CI 3.6–39.0; P < 0.001), sleeping outdoors at night (OR = 4.5, 95% CI 2.2–9.2; P < 0.001), absence of indoor residual spraying (IRS) during the last year (OR = 3.4, 95% CI 1.7–6.8; P < 0.001) were significantly associated with falciparum malaria among under-five children in Al-Mahweet governorate (Table 5).
Independent predictors of falciparum malaria among under-five children
Multivariable logistic regression analysis confirmed that age of three years or older (AOR = 5.6, 95% CI 1.6–19.8; P = 0.007), not sleeping under a mosquito net the previous night of the survey (AOR = 8.0, 95% CI 2.4–27.4; P = 0.001), sleeping outdoors at night (AOR = 4.4, 95% CI 2.0–10.0; P < 0.001), and absence of IRS during the last year (AOR = 4.2, 95% CI 1.9–9.4; P < 0.001) were the independent predictors of falciparum malaria among under-five children in the rural communities of Al-Mahweet (Table 6).
Agreement between thick-film microscopy and RDTs for malaria diagnosis
The results of thick-film microscopy and RDTs were concordant in all cases, except for two positive cases using RDTs and four positive cases by thick-film microscopy. The observed percentage agreement between the two methods was 98.5%, and there was very good agreement (k = 0.9) between thick-film microscopy and RDTs for falciparum malaria diagnosis that was statistically significant (Table 7).
Discussion
The vision of the NMCP’s strategic plan was to make Yemen malaria-free by the end of 2018 [18], but the disease remains a major public health problem in the country. Since 2015, the efforts to control and eliminate malaria have been hampered by the armed conflict and its repercussions, including the deteriorated health system, humanitarian crisis and internal displacement. This is the first study to report on the prevalence and risk factors associated with falciparum malaria in under-five children at the community level in Al-Mahweet governorate, Yemen. The prevalence of P. falciparum among 9.8% of under-five children in the rural communities of Al-Mahweet governorate is quite alarming, particularly with the asymptomatic nature of infection in the majority of surveyed children. Because this study has been conducted before the coronavirus disease 2019 (COVID-19) pandemic, the situation might be even worse as a result of the negative impact of the pandemic on the prevention and control of infectious diseases, including malaria. The prevalence of falciparum malaria among children in the present study is slightly higher than that (8%) recently reported among schoolchildren in Bajil district of Hodeidah [9], but lower than the microscopy-confirmed prevalence of 18.6% reported for P. falciparum among children in two rural districts of Taiz in the mid-2000s [8]. The absence of infection with P. vivax among under-five children in the present study is consistent with the very low prevalence of less than 0.25% recently reported among schoolchildren in Bajil [9].
The prevalence of falciparum malaria among under-five children in the present study is comparable to the microscopy-based prevalence of 11.7% reported among under-five children in southern highland Rwanda in community- and facility-based surveys [19]. Nonetheless, the polymerase chain reaction (PCR)-based prevalence among Rwandan children increased to 16.7% [19], highlighting the need for molecular surveillance of P. falciparum infection among children in the rural communities of Al-Mahweet for the true burden of infection to be uncovered. Compared to the present study, a higher prevalence was reported among under-five children in Tanzania (15.9% by RDTs) [20], Uganda (19.7% by microscopy) [21], sub-Saharan African countries (18.8–24.2% by microscopy and RDTs) [22], Nigeria (27% by microscopy) [23] and Malawi (> 37% by RDTs) [24]. However, a lower prevalence was reported among under-five children in Kenya (4.8% by microscopy) and Madagascar (7.8% by RDTs) [25, 26].
Gametocyte carriage by approximately one-third of under-five children with microscopically confirmed P. falciparum in the rural communities of Al-Mahweet poses a major challenge to malaria control and elimination efforts. This gametocyte reservoir may considerably contribute to the transmission of infection in the community [5, 6, 27], considering that under-five children have been estimated to comprise 14% of the Yemeni population in 2020 [28]. Because gametocytes were detected microscopically in the present study, the reservoir of sub-microscopic gametocyte carriage can even be larger and is yet to be assessed using molecular surveys. It is important to identify and treat sub-microscopic gametocyte carriers with transmission-blocking drugs to eliminate the disease. Yemen’s NMCP has updated the national antimalarial treatment policy through the integration of low-dose primaquine (PQ) with first- and second-line treatments of uncomplicated falciparum malaria [29]. However, gametocytes among asymptomatic carriers and sub-microscopic gametocyte carriage could compromise this transmission-blocking strategy [30, 31].
The independent prediction of falciparum malaria in children aged three years or older in the present study is consistent with those recently reported for under-five children in Rwanda, Uganda and Malawi [19, 21, 24] but inconsistent with that reported for Nigerian children [23]. In contrast to the present finding, age was not a predictor of falciparum malaria among Yemeni schoolchildren in Bajil [9]. The lower risk of infection with P. falciparum in younger children could be partly explained by the greater care they are given, reducing their exposure to mosquito bites. Furthermore, age-related differences in infection of children with P. falciparum could be attributed, among other factors, to variations in acquired immunity, transmission intensity and seasonality [32, 33]. The absence of association between the gender of under-five children and falciparum malaria in the present study is consistent with that reported for under-five children in Uganda [21]. Likewise, gender did not predict falciparum malaria among schoolchildren in Bajil [9]. Gender indifferences regarding infection with P. falciparum among under-five children in the present study could be explained by similar exposure patterns to mosquito bites among male and female children in rural communities. On the other hand, the non-significant association between the educational and employment status of fathers and mothers with falciparum malaria among under-five children is inconsistent with those reported for children infected with malaria in African countries [26, 34, 35]. However, the lack of association could be attributed to the very large proportion of parents with the same low levels of education and employment type.
The absence of a significant association between fever or other clinical features and falciparum malaria among under-five children in the present study is inconsistent with the finding among schoolchildren in Hajr valley in Hadhramout [36], where fever was significantly associated with malaria. However, the finding of the present study is consistent with the observations among children with malaria in Rwanda, Uganda and Papua New Guinea [19, 37, 38]. The large proportion of asymptomatic cases among under-five children in the present study (28 out of 39 microscopic cases) poses a big challenge to malaria elimination, hindering the strategy of using a single dose of PQ for interrupting transmission.
Vector control through mass distribution of long-lasting insecticidal nets (LLINs) is one of the most important interventions to protect local communities at risk of malaria. In Yemen, LLINs were first introduced and distributed to pregnant women and under-five children in 2006 [14], and since then, this strategy has been increasingly escalated in endemic areas by the NMCP. However, more than half of under-five children in the present study were reported to not have slept under mosquito nets during the night preceding the survey. The low utilization of mosquito nets by targeted communities undermines the effectiveness of LLINs as a prevention and control intervention. In this regard, 42.5% of under-five children with access to LLINs were found to have slept under them in Hodeidah governorate [39]. The reasons for not sleeping under mosquito nets despite having access need to be investigated and addressed to foster the desired impact of LLINs on malaria control and elimination. In accord with the low utilization of mosquito nets, falciparum malaria among children in the present study was found to be independently predicted by not sleeping under mosquito nets. Children who did not sleep under mosquito nets were eightfold more likely to have been infected with P. falciparum compared to those who did. Sleeping under mosquito nets was found to be significantly associated with reduced malaria prevalence among under-five children in African countries [19, 23, 24]. In contrast, sleeping under mosquito nets was not significantly associated with a reduction in the prevalence of falciparum malaria among Yemeni schoolchildren in Bajil [9] and under-five children in Uganda [21].
In Yemen, IRS was scaled up as a vector control intervention in 2007 to reinforce efforts to control and eliminate malaria in endemic areas of the country [14]. In the present study, children living in households not sprayed with residual insecticides within the year preceding the survey were fourfold more likely to have been infected with P. falciparum compared to those living in sprayed households. The lack of IRS during the year preceding the survey independently predicted falciparum malaria among under-five children in Al-Mahweet rural communities, highlighting the need to assess the household coverage with IRS in targeted areas and take action accordingly. In agreement with the present study, lacking IRS was found to be significantly associated with a higher risk of malaria among Ugandan under-five children [21]. Combining IRS and insecticide-treated nets could be more effective for controlling malaria than either intervention alone [40], and such a combination is recommended in areas of low-to-moderate transmission to reduce seasonal malaria transmission and support elimination efforts [41]. Understanding vector bionomics and transmission dynamics is vital to tailor effective interventions for malaria prevention and control in the rural areas of Al-Mahweet. In another context, an earlier study in Taiz revealed the predominance of endophilic vectors of malaria and recommended the reinforcement of vector control measures that target indoor biting vectors [42].
Sleeping outdoors at night was an independent predictor of infection with P. falciparum among under-five children in the present study, with children sleeping outdoors being 4.4-fold more likely to have been infected compared to those sleeping indoors. Given that sleeping outside houses is a common practice in rural communities of malaria-endemic areas of the country because of the hot climate, understanding the exophilic/exophagic behaviours of mosquitoes would help prevent outdoor malaria transmission. Sleeping patterns in the community can affect vector-human contact and could significantly increase malaria transmission. Therefore, parents should be educated not to leave their children to sleep outdoors without using LLINs, particularly with the finding that none of the under-five children in the present study was reported to be protected with mosquito repellents. The absence of a significant association between residence close to water or garbage collections and infection of under-five children with P. falciparum in the present study could be attributed to the almost similar geographical and ecological aspects of rural areas. In contrast, residence near water collections was an independent predictor of falciparum malaria among schoolchildren in Bajil [9]. In another context, residents near water collections in Hadhramout governorate, east of Yemen, were at a significantly higher risk of malaria [43].
Given that the strongest risk factors of malaria in under-five children were related to malaria control measures rather than natural topographical or other reasons, there is a need to assess the factors negatively influencing the performance of the NMCP in implementing such interventions. With the great global emphasis on expanding the use of current malaria control interventions together with international funding for them, the strong association between malaria prevalence and poor malaria control measures should raise concerns about the status of malaria control in such rural districts. Accordingly, gaps in coverage, access and utilization of LLINs besides household coverage with IRS should be identified and addressed, considering the potential impact of armed conflicts and the humanitarian crisis on malaria control efforts.
RDTs have become essential tools for malaria control and elimination in rural areas and primary healthcare settings, where microscopy is unavailable or impractical, by enabling immediate diagnosis and prompt treatment. The significant and very good agreement between thick-film microscopy and RDTs for diagnosing falciparum malaria among under-five children in rural communities of Al-Mahweet supports using RDTs in remote and rural areas of the governorate where electricity is unavailable. However, the difference between the results of microscopy and RDTs in six cases justifies the need to assess the sensitivity and specificity of RDTs, preferably compared to PCR. Apart from being time-consuming, the sensitivity of microscopy may overlook infections with low parasite densities and its specificity is affected by the individual capacities of microscopists [44, 45]. Accordingly, it may underestimate the burden of malaria, particularly in asymptomatic low-parasitaemia or sub-microscopic infections. RDTs are useful in augmenting malaria diagnosis by addressing the disadvantages of microscopy, particularly in remote malaria-endemic areas. In the context of the escalating humanitarian crisis and complex emergency, integrated community case management (iCCM) was launched to train healthcare workers in treating under-five children for pneumonia, diarrhoea and uncomplicated malaria in rural and hard-to-reach communities of Yemen in 2017 [46]. However, the success of iCCM as a strategy to control malaria relies on the availability of high-quality RDTs to diagnose malaria in febrile children [47].
The present study is limited by adopting microscopy and RDTs for malaria diagnosis. As a result, overlooking sub-microscopic infections and false positivity of RDTs could not be ruled out. However, it provides preliminary data on the burden of malaria among under-five children in rural communities of Al-Mahweet. Another limitation could be introduced by the possible variations in cluster sizes because of having no information about the population size per cluster to adopt the probability proportional to size sampling approach. As a conservative approach, we used a sample design effect of 2.0 when calculating the sample size to account for the uneven distribution of the outcome in clusters. The findings of the present study can guide public health authorities in tailoring interventions to reduce malaria burden. Malaria prevalence among under-five children in the present study underscores the need for molecular surveillance of malaria among such an at-risk population to uncover the burden of sub-microscopic infections and develop appropriate elimination strategies accordingly.
Conclusions
Approximately one in ten under-five children in rural communities of Al-Mahweet is infected with P. falciparum based on microscopy and RDTs. Age of three years or older, not sleeping under mosquito nets, sleeping outdoors at night and absence of IRS are the independent predictors of falciparum malaria among under-five children. The very good agreement between thick-film microscopy and RDTs for diagnosing falciparum malaria among children supports the usefulness of using RDTs in such resource-limited rural communities.
Availability of data and materials
Data are available within the manuscript and can be provided by the corresponding author.
Abbreviations
- AOR:
-
Adjusted odds ratio
- CI:
-
Confidence interval
- COVID-19:
-
Coronavirus diseases 2019
- eDEWS:
-
Electronic Disease early warning system
- HRP2:
-
Histidine-rich protein 2
- iCCM:
-
Integrated community case management
- IQR:
-
Interquartile range
- IRS:
-
Indoor residual spraying
- LLIN:
-
Long-lasting insecticidal net
- NMCP:
-
National Malaria control programme
- OR:
-
Odds ratio
- PCR:
-
Polymerase chain reaction
- pLDH:
-
Parasite lactate dehydrogenase
- PQ:
-
Primaquine
- RDT:
-
Rapid diagnostic test
- SPSS:
-
Statistical package for the social sciences
- WHO:
-
World Health Organization
References
WHO. World Malaria Report 2021. Geneva: World Health Organization 2021.
WHO. WHO guidelines for malaria. Geneva: World Health Organization. WHO/UCN/GMP/2021.01 2021.
Lindblade KA, Steinhardt L, Samuels A, Kachur SP, Slutsker L. The silent threat: asymptomatic parasitemia and malaria transmission. Expert Rev Anti Infect Ther. 2013;11:623–39.
Andolina C, Rek JC, Briggs J, Okoth J, Musiime A, Ramjith J, et al. Sources of persistent malaria transmission in a setting with effective malaria control in eastern Uganda: a longitudinal, observational cohort study. Lancet Infect Dis. 2021;21:1568–78.
Alves FP, Gil LH, Marrelli MT, Ribolla PE, Camargo EP, Da Silva LH. Asymptomatic carriers of Plasmodium spp. as infection source for malaria vector mosquitoes in the Brazilian Amazon. J Med Entomol. 2005;42:777–9.
Lin JT, Saunders DL, Meshnick SR. The role of submicroscopic parasitemia in malaria transmission: what is the evidence? Trends Parasitol. 2014;30:183–90.
Gouagna LC, Ferguson HM, Okech BA, Killeen GF, Kabiru EW, Beier JC, et al. Plasmodium falciparum malaria disease manifestations in humans and transmission to Anopheles gambiae: a field study in Western Kenya. Parasitology. 2004;128(Pt 3):235–43.
Alkadi HO, Al-Maktari MT, Nooman MA. Chloroquine-resistant Plasmodium falciparum local strain in Taiz governorate. Republic Yemen Chemotherapy. 2006;52:166–70.
Alwajeeh TS, Abdul-Ghani R, Allam AF, Farag HF, Khalil SSM, Shehab AY, et al. Uncomplicated falciparum malaria among schoolchildren in Bajil district of Hodeidah governorate, west of Yemen: association with anaemia and underweight. Malar J. 2020;19:358.
Al-Taiar A, Jaffar S, Assabri A, Al-Habori M, Azazy A, Al-Mahdi N, et al. Severe malaria in children in Yemen: two site observational study. BMJ. 2006;333:827.
Dureab F, Al-Falahi E, Ismail O, Al-Marhali L, Al Jawaldeh A, Nuri NN, et al. An overview on acute malnutrition and food insecurity among children during the conflict in Yemen. Children. 2019;6:77.
CSO. Statistical Year Book for 2017, 2017. https://www.cso-yemen.com/content.php?lng=english&cid=131 Accessed 4 April 2022.
IOM. Malaria in Yemen: needs assessment. Amman: International Organization for Migration; 2017.
NMCP/MoPHP. Yemen Malaria Control Programme Review 2013. Sana'a: National Malaria Control Programme/Ministry of Public Health and Population; 2014.
Cheesbrough M. District laboratory practice in tropical Countries: Part 1. London: Cambridge University Press; 2010.
WHO. Basic malaria microscopy. Part 1. Learner's guide, 2nd edn. Geneva: World Health Organization; 2010.
Altman GA. Practical Statistics for Medical Research. London: Chapman and Hall; 1992.
NMCP/MoPHP. The National Strategy for Malaria Control and Elimination 2014–2018. Sana’a: National Malaria Control Programme/Ministry of Public Health and Population; 2014.
Gahutu JB, Steininger C, Shyirambere C, Zeile I, Cwinya-Ay N, Danquah I, et al. Prevalence and risk factors of malaria among children in southern highland Rwanda. Malar J. 2011;10:134.
Mwaiswelo RO, Mmbando BP, Chacky F, Molteni F, Mohamed A, Lazaro S, et al. Malaria infection and anemia status in under-five children from Southern Tanzania where seasonal malaria chemoprevention is being implemented. PLoS ONE. 2021;16: e0260785.
Roberts D, Matthews G. Risk factors of malaria in children under the age of five years old in Uganda. Malar J. 2016;15:246.
Yang D, He Y, Wu B, Deng Y, Li M, Yang Q, et al. Drinking water and sanitation conditions are associated with the risk of malaria among children under five years old in sub-Saharan Africa: A logistic regression model analysis of national survey data. J Adv Res. 2020;21:1–13.
Oguoma VM, Anyasodor AE, Adeleye AO, Eneanya OA, Mbanefo EC. Multilevel modelling of the risk of malaria among children aged under five years in Nigeria. Trans R Soc Trop Med Hyg. 2020;115:482–94.
Gaston RT, Ramroop S. Prevalence of and factors associated with malaria in children under five years of age in Malawi, using malaria indicator survey data. Heliyon. 2020;6: e03946.
Clouston SA, Yukich J, Anglewicz P. Social inequalities in malaria knowledge, prevention and prevalence among children under 5 years old and women aged 15–49 in Madagascar. Malar J. 2015;14:499.
Sultana M, Sheikh N, Mahumud RA, Jahir T, Islam Z, Sarker AR. Prevalence and associated determinants of malaria parasites among Kenyan children. Trop Med Health. 2017;45:25.
Koepfli C, Yan G. Plasmodium gametocytes in field studies: Do we measure commitment to transmission or detectability? Trends Parasitol. 2018;34:378–87.
United Nations Department of Economic and Social Affairs. World population prospects 2019.https://population.un.org/wpp/Download/Files/1_Indicators%20(Standard)/EXCEL_FILES/1_Population/WPP2019_POP_F07_1_POPULATION_BY_AGE_BOTH_SEXES.xlsx. Accessed 6 July 2022.
NMCP/MoPHP. National Malaria Strategic Plan 2020–2024. Sana’a: National Malaria Control Programme/Ministry of Public Health and Population; 2020.
Birkholtz LM, Coetzer TL, Mancama D, Leroy D, Alano P. Discovering new transmission-blocking antimalarial compounds: challenges and opportunities. Trends Parasitol. 2016;32:669–81.
Birkholtz LM, Alano P, Leroy D. Transmission-blocking drugs for malaria elimination. Trends Parasitol. 2022;38:390–403.
Doolan DL, Dobaño C, Baird JK. Acquired immunity to malaria. Clin Microbiol Rev. 2009;22:13–36.
Carneiro I, Roca-Feltrer A, Griffin JT, Smith L, Tanner M, Schellenberg JA, et al. Age-patterns of malaria vary with severity, transmission intensity and seasonality in sub-Saharan Africa: a systematic review and pooled analysis. PLoS ONE. 2010;5: e8988.
Snyman K, Mwangwa F, Bigira V, Kapisi J, Clark TD, Osterbauer B, et al. Poor housing construction associated with increased malaria incidence in a cohort of young Ugandan children. Am J Trop Med Hyg. 2015;92:1207–13.
Zgambo M, Mbakaya BC, Kalembo FW. Prevalence and factors associated with malaria parasitaemia in children under the age of five years in Malawi: a comparison study of the 2012 and 2014 Malaria Indicator Surveys (MISs). PLoS ONE. 2017;12: e0175537.
Bin Mohanna MA, Bin Ghouth AS, Rajaa YA. Malaria signs and infection rate among asymptomatic schoolchildren in Hajr Valley. Yemen East Mediterr Health J. 2007;13:35–40.
Hetzel MW, Morris H, Tarongka N, Barnadas C, Pulford J, Makita L, et al. Prevalence of malaria across Papua New Guinea after initial roll-out of insecticide-treated mosquito nets. Trop Med Int Health. 2015;20:1745–55.
Yeka A, Nankabirwa J, Mpimbaza A, Kigozi R, Arinaitwe E, Drakeley C, et al. Factors associated with malaria parasitemia, anemia and serological responses in a spectrum of epidemiological settings in Uganda. PLoS ONE. 2015;10(3): e0118901.
Al-Eryani SMA, Mahdy MAK, Al-Mekhlafi AM, Abdul-Ghani R. Access to and use of long-lasting insecticidal nets and factors associated with non-use among communities in malaria-endemic areas of Al Hudaydah governorate in the Tihama region, west of Yemen. Malar J. 2017;16:244.
Kleinschmidt I, Schwabe C, Shiva M, Segura JL, Sima V, Mabunda SJA, et al. Combining indoor residual spraying and insecticide-treated net interventions. Am J Trop Med Hyg. 2009;81(3):519–24.
WHO. Indoor residual spraying: an operational manual for indoor residual spraying (IRS) for malaria transmission control and elimination, 2nd edn. Geneva: World Health Organization; 2015.
Al-Eryani SM, Kelly-Hope L, Harbach RE, Briscoe AG, Barnish G, Azazy A, et al. Entomological aspects and the role of human behaviour in malaria transmission in a highland region of the Republic of Yemen. Malar J. 2016;15:130.
Bamaga OA, Mahdy MA, Mahmud R, Lim YA. Malaria in Hadhramout, a southeast province of Yemen: prevalence, risk factors, knowledge, attitude and practices (KAPs). Parasit Vectors. 2014;7:351.
Snounou G, Viriyakosol S, Jarra W, Thaithong S, Brown KN. Identification of the four human malaria parasite species in field samples by the polymerase chain reaction and detection of a high prevalence of mixed infections. Mol Biochem Parasitol. 1993;58(2):283–92.
McManus DP, Bowles J. Molecular genetic approaches to parasite identification: their value in diagnostic parasitology and systematics. Int J Parasitol. 1996;26:687–704.
Miller NP, Zunong N, Al-Sorouri TAA, Alqadasi YM, Ashraf S, Siameja C. Implementing integrated community case management during conflict in Yemen. J Glob Health. 2020;10: 020601.
WHO/UNICEF. Joint Statement: Integrated Community Case Management (iCCM). An equity-focused strategy to improve access to essential treatment services for children. New York: UNICEF 2012.
Acknowledgements
We thank the parents/guardians for kindly consenting to include their children in the study. We also thank the NMCP for donating RDTs.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
MAAA, RA and MAKM designed the study. MAAA conducted the fieldwork. MAAA, RA and MAKM analysed the data. MAAA, RA and MAKM interpreted the results. RA drafted the manuscript. MAAA, RA, MAKM and MAA edited and revised the manuscript. All authors read and approved the final submission of the manuscript.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
This study was approved by the Research Ethics Committee of the Faculty of Medicine and Health Sciences, Sana’a University, Sana’a, Yemen. Written informed consent was obtained from all children’s parents/ guardians who participated voluntarily after explaining the objectives of the study. Confidentiality of the participants was assured. Feedback about the results of the study was given to the parents/ guardians, and those found positive were referred to the Case Management Unit of the NMCP in the governorate to be treated according to the national antimalarial treatment policy.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Al-Quhaiti, M.A.A., Abdul-Ghani, R., Mahdy, M.A.K. et al. Malaria among under-five children in rural communities of Al-Mahweet governorate, Yemen. Malar J 21, 344 (2022). https://doi.org/10.1186/s12936-022-04371-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12936-022-04371-8