Abstract
Background
A study was conducted prior to implementing a cluster-randomized controlled trial (CRT) of a lethal house lure strategy in central Côte d’Ivoire to provide baseline information on malaria indicators in 40 villages across five health districts.
Methods
Human landing catches (HLC) were performed between November and December 2016, capturing mosquitoes indoors and outdoors between 18.00 and 08.00 h. Mosquitoes were processed for entomological indicators of malaria transmission (human biting, parity, sporozoite, and entomological inoculation rates (EIR)). Species composition and allelic frequencies of kdr-w and ace-1R mutations were also investigated within the Anopheles gambiae complex.
Results
Overall, 15,632 mosquitoes were captured. Anopheles gambiae sensu lato (s.l.) and Anopheles funestus were the two malaria vectors found during the survey period, with predominance for An. gambiae (66.2%) compared to An. funestus (10.3%). The mean biting rate for An. gambiae was almost five times higher than that for An. funestus (19.8 bites per person per night for An. gambiae vs 4.3 bites per person per night for An. funestus) and this was evident indoors and outdoors. Anopheles funestus was more competent to transmit malaria parasites in the study area, despite relatively lower number tested for sporozoite index (4.14% (63/1521) for An. gambiae vs 8.01% (59/736) for An. funestus; χ2 = 12.216; P < 0.0001). There were no significant differences between the proportions infected outdoors and indoors for An. gambiae (4.03 vs 4.13%; χ2 = 0.011; P = 0.9197) and for An. funestus (7.89 vs 8.16%; χ2 = 2.58e−29; P = 1). The majority of both infected vectors with malaria parasites harboured Plasmodium falciparum (93.65% for An. gambiae and 98. 31% for An. funestus). Overall, the EIR range for both species in the different districts appeared to be high (0.35–2.20 infected bites per human per night) with the highest value observed in the district of North-Eastern-Bouaké. There were no significant differences between transmission occurring outdoor and indoor for both species. Of the An. gambiae s.l. analysed, only An. gambiae sensu stricto (14.1%) and Anopheles coluzzii (85.9%) were found. The allelic frequencies of kdr and ace-1R were higher in An. gambiae (0.97 for kdr and 0.19 for ace-1R) than in An. coluzzii (0.86 for kdr and 0.10 for ace-1R) (P < 0.001).
Conclusion
Despite universal coverage with long-lasting insecticidal nets (LLINs) in the area, there was an abundance of the malaria vectors (An. gambiae and An. funestus) in the study area in central Côte d’Ivoire. Consistent with high insecticide resistance intensity previously detected in these districts, the current study detected high kdr frequency (> 85%), coupled with high malaria transmission pattern, which could guide the use of Eave tubes in the study areas.
Similar content being viewed by others
Background
Malaria is caused by protozoan parasites belonging to the Plasmodium genus, which are transmitted by the female Anopheles mosquito during blood feeding. Over the last 10 years, considerable efforts have been made to control malaria in many parts of the world, especially in sub-Saharan Africa. This has led to the decline in malaria transmission in many parts of Africa [1, 2]. According to the last World Malaria Report [3], the significant progress in malaria control can be attributed to a scale-up of vector control interventions, as well as improved diagnostic testing, rapid and efficient treatment of malaria patients. However, despite these considerable efforts to reduce transmission, malaria remains one of the major causes of morbidity and mortality in sub-Saharan Africa [1, 4]. Vector control relies on a handful of insecticides used for indoor residual spraying (IRS) and treatment of long-lasting insecticidal nets (LLINs) and insecticide resistance has been widely detected in malaria vectors across the continent [5,6,7,8]. The situation is particularly worrying with an increase in intensity and mechanisms of insecticide resistance detected over time [8, 9]. There is a pressing need for effective, sustainable tools or strategies for malaria control.
The observation that host-seeking African malaria vectors predominantly enter human dwellings through open eaves motivated the development of the EaveTubes technology [10]. EaveTubes are an innovative delivery system where insecticide-treated inserts are placed in tubes installed in the eaves of houses. These inserts enable the transfer of a high dose of insecticide capable of killing even strongly insecticide-resistant Anopheles mosquitoes [11]. EaveTubes, in combination with screening of windows and doors, were found to reduce malaria transmission in a cluster-randomized controlled trial (CRT) conducted in central Côte d’Ivoire between 2016 and 2019 [12]. EaveTubes present a mechanism to expose the mosquito population to alternative classes of insecticide presenting a delivery method that could be utilized for insecticide resistance management [10, 11].
Collecting baseline data on entomological parameters, including vector densities, malaria sporozoite rates and insecticide resistance phenotypes, would be valuable data that will justify the choice for EaveTubes as appropriate intervention in the area. The current study was conducted prior to the start of the CRT across all study villages in central Côte d’Ivoire.
Methods
Study site
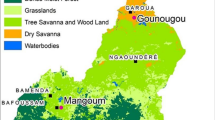
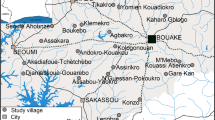
The study was conducted in 40 villages across five health districts (Béoumi, Southern-Bouaké, North-Eastern-Bouaké, North-Western-Bouaké, Sakassou). All districts were covered with a high rate (> 80%) of standard pyrethroids-based LLINs (Permanet 2.0 and OlysetNet). Malaria transmission in these areas occurs year-round with a peak during the wet season (April-November). The main malaria vector, Anopheles gambiae sensu lato (s.l.) was highly resistant to almost all public health classes of insecticides [13], with 125.8 bites per human per night and entomological inoculation rates (EIR), reaching 459.9 infected bites per human per night in some rural places of the districts [14]. Anopheles funestus s.l. and Anopheles nili s.l. were also present, but less abundant.
For the CRT, 40 village (clusters) were identified within a 60 km radius around the city of Bouaké. The villages were selected to have 100–600 houses, of which at least 80% had corrugated iron roof and brick-made walls, suitable for installation of EaveTubes. Villages were at least 2 km apart from each other.
Mosquito collection
To assess malaria transmission indicators, a cross-sectional survey was conducted between November and December 2016 (the beginning of the dry season), to collect adult mosquitoes within homes by human landing catches (HLC). Volunteers were recruited within the study villages. They sat with their legs uncovered attracting mosquitoes around and collecting those landing on their legs using glass haemolysis tubes plugged with cotton. Captures were done in each village over two consecutive nights by two mosquito collectors (one indoors and one outdoors) in five randomly selected households. For each capture point, one volunteer collected mosquitoes from 18:00 to 00:00 h and a second volunteer took over from 00:00 to 08:00. Volunteers rotated from a capture point to another to account for any possible differences in individual attractiveness to mosquitoes. The mosquitoes collected were kept in cool boxes and transported to the laboratory for processing the next morning.
Identification and processing of mosquitoes
Mosquitoes were first identified using morphological identification key [15]. Only known malaria vector species in Côte d’Ivoire (An. gambiae and An. funestus) [14] were analysed, although other rare Anopheles with potential for malaria transmission were collected. Due to the large numbers of An. gambiae and An. funestus captured during the HLC, only a sub-set of samples was analysed.
For this sub-set, two to four females of An. gambiae and An. funestus were randomly selected per sampling hour and per site and their ovaries were dissected to determine parity status [16]. Of the parous female mosquitoes, up to 60 per village, when available, were randomly selected to be processed for sporozoite infection by quantitative polymerase chain reaction (qPCR) assay [17]. The same sub-sample was also tested for molecular identification of species [18] and to detect the Knockdown resistance gene L1014F (kdr-w) [19] and the acetylcholinesterase gene G119S (ace-1R) mutations [20].
Data analysis
Indoor and outdoor human biting rates (HBR) measured were the mean number of vector bites received per person per night of collection (b/p/n). The result was obtained by the number of anophelines captured at each sampling point divided by the total number of sampling nights and the average number of collectors. Parity rate (PR) was the proportion of parous mosquitoes over the total dissected. The Plasmodium sporozoite rate (SR) in each vector species population was the number of mosquitoes infected with sporozoites in the head-thorax, divided by the total number of mosquitoes tested. The nightly EIR was the number of infectious bites per person per night and defined as the product of HBR and SR. It is conventionally the product of the daily HBR and the SR from the caught mosquitoes. For this study, nightly EIR was calculated using the following formula:
In (2), the first (x) term is \(HBR\) and the second (y) is \(SR\). This approach was used because the SR was estimated assuming that all non-parous mosquitoes were sporozoite negative.
Data were analysed in R (version 4.0.3). The Wilcoxon (W) test was used to compare the differences in vector species for HBR and EIR between sampling locations in households and among health districts. The Pearson’s Chi-square (χ2) test was used to compare parity and sporozoite rates. For all statistics, a p value below 0.05 was considered as statistically significant.
The allelic frequencies of the two resistance genes (kdr L1014F and ace-1R G119S) in An. gambiae sibling species were tested to Hardy–Weinberg equilibrium (HWE) conformity using the exact HW test and also compared.
Ethics clearance
This study followed the ethics principles recommended by the Côte d’Ivoire Ministry of Health ethics committee (ref: 039/MSLS/CNER-dkn), the Pennsylvania State University’s Human Research Protection Program under the Office for Research Protections (ref.: STUDY00003899 and STUDY00004815), and the London School of Hygiene and Tropical Medicine ethical review board (No. 11223).
Verbal and written informed consent from all participants were obtained in the local language prior to their enrolment in the study. Volunteer mosquito collectors were well trained on how to collect mosquitoes without being bitten. They received vaccination against yellow fever and the project offered treatment of confirmed malaria cases free of charge, according to the national malaria control programme policy.
Results
Mosquito species composition, density and human biting pattern
A total of 15,632 female mosquitoes were captured using HLC, of which 66.2% (10,350) were An. gambiae and 1,615 (10.3%) were An. funestus (Table 1 and Fig. 1A). There was a relatively equal preference towards biting both indoors and outdoors for both vectors and began biting from the early evening (from 19.00 onwards) to reach a peak around 02.00 (An. gambiae) or 03:00 (An. funestus) (Fig. 2). Biting then decreased steadily, and by dawn (06:00) it fell below 0.2 b/p/n. Overall, the mean biting rate for An. gambiae (22.13 b/p/n) was significantly higher (six-fold) than that for An. funestus (3.51 b/p/n) (P < 0.01) and this was evident both indoors and outdoors, except in North-eastern-Bouaké (8.4 vs 6.34 b/p/n; W = 2,236.5, P = 0.368) (Table 2). Overall, the biting patterns indoors and outdoors were similar for An. gambiae and An. funestus (P > 0.05) (Table 2).
Parity rate
Parity rates were high for both species caught indoors and outdoors; it averaged 89–91% for An. gambiae and 97–98% for An. funestus, with overall a significant difference (P > 0.05) between the two species. There were no significant differences in the parity rates indoors and outdoors across health districts (P > 0.05) (Table 3).
Plasmodium sporozoite rate
Overall, infection rate for An. funestus (8.01%) was significantly higher (two-fold) than for An. gambiae (4.14%) (χ2 = 12.216; P < 0.0001). There was no significant difference between the proportion infected outdoors and indoors for An. gambiae (4.03 vs 4.13%; χ2 = 0.011; P = 0.9197), and for An. funestus (7.89 vs 8.16%; χ2 = 2.58e−29; P = 1) (Table 4).
The majority of An. gambiae infected with malaria parasites harboured Plasmodium falciparum (93.65%), and a few had Plasmodium malariae (6.35%) (Table 5). There was no Plasmodium ovale detected in any of the samples tested for An. gambiae. Almost all An. funestus analysed were infected with P. falciparum (98.31%) and only one individual had P. ovale (1.69%), with no An. funestus testing positive for P. malariae (Table 5). Within the An. gambiae complex, the proportions of sporozoite rate in parous individuals for An. gambiae sensu stricto (s.s.) were similar to Anopheles coluzzii (P > 0.05) (Fig. 3).
Entomological inoculation rate
The EIRs ranged 0.21–2.20 for An. gambiae and 0.02–1.11 for An. funestus across health districts. The overall transmission for An. gambiae (0.77 ib/h/n) was two-fold higher than for An. funestus (0.38 ib/p/n) (W = 1,263; P = 3.92.10–06), without differences indoors and outdoors with either species (P > 0.05) (Table 6).
Frequencies of the kdr 1014F and ace-1.R 119S alleles in Anopheles gambiae species complex
Out of 1,374 An. gambiae s.l. mosquitoes analysed by PCR, 1,350 were successfully identified to species (< 2% failure rate). Both An. gambiae s.s. (n = 190; 14.1%) and An. coluzzii (n = 1,160; 85.9%) were found within the An. gambiae complex analysed. For both kdr and ace-1R genes, the allelic frequencies were higher in parous individuals of An. gambiae s.s. than in An. coluzzii (P < 0.001) (Table 7).
Discussion
Here we have provided a descriptive analysis of the entomological indicators relevant to malaria transmission in central Côte d’Ivoire, prior to the start of a CRT evaluating a new malaria vector control intervention.
The human malaria vector species that were found in the study area at the time of sampling (November–December 2016) were An. gambiae and An. funestus, with An. gambiae being more abundant. The predominance of An. gambiae could be explained by the presence of breeding sites favourable to An. gambiae (e.g., rice paddy fields, vegetable plots, marshes) throughout the study area [21,22,23].This aligns with previous studies conducted in the same area, and elsewhere in Côte d’Ivoire, which reported the predominance of An. gambiae among local malaria vectors [24, 25]. With An. funestus, swampy marshes along rivers were the main breeding sites as also observed in previous study in the areas [26].
Anopheles gambiae s.s. and An. coluzzii were the only members of An. gambiae complex identified in the study area. Anopheles coluzzii found in high proportion (85.90%) was consistent with previous findings in the area of Bouaké [13, 23, 27] but contrasts with other studies in the northern savannah of the country, where An. gambiae was more prevalent [24, 28]. The difference observed is likely due to variations in mosquito larval habitats; An. coluzzii tends to exploit more permanent breeding sites, including those created by the type of irrigation for rice cultivation found in Bouaké and the surrounding area. Permanent availability of breeding sites, due to intensive and perennial agricultural practices could have led to the presence of An. coluzzii [29].
The increase in biting activity for both species coinciding with the time when many people would be going to bed was found with a peak in biting around 02:00 for An. gambiae and 03:00 for An. funestus. This is similar to previous entomological studies conducted in same area around Bouaké [22] as well as the northern part of Cote d’Ivoire [24, 30] and elsewhere in Africa [31]–33]. These biting profiles highlight the utility of LLINs as a personal protective measure against host-seeking malaria vectors. However, the fact that outdoor biting An. gambiae mosquitoes were found in similar proportion to indoor biting mosquitoes is a sign that people are at risk of malaria transmission when they are outside in the evenings. It further highlights the need for novel strategies or tools to target outdoor malaria transmission [34, 35].
Mean parity rates and sporozoite rates were high in both species, especially in An. funestus, indicating a high prevalence of older female mosquitoes, which had already gone through several cycles of blood feeding. Despite lower numbers, the overall sporozoite index rate for An. funestus was higher than An. gambiae, indicating that it is still an important malaria vector in the area. These results are consistent with findings from previous studies in northern Côte d’Ivoire [24, 30], and show a need to better characterize the biology and ecology of An. funestus in this area [26], as well as careful monitoring of the epidemiological significance of An. funestus in malaria transmission.
The mean nightly EIR for both species in this study was 1.20 infected bites per person per night between November and December 2016. By extrapolation, this global nightly estimated infected bites could correspond to 438 infected bites per person per year. Meta-analysis from a pool of studies conducted in various epidemiological settings across Africa reported EIRs ranging 1 to 1,000 infected bites per person per year and that an annual EIR high than 200 per person per year was consistently associated with malaria prevalence averaging > 80% [31]. Similarity, in a baseline epidemiological study conducted at a similar time, in the same area, prevalence was reported to be 73.9% [12]. The area around Bouaké can therefore be considered as highly endemic for malaria. Moreover, EIR in the study area was equally high indoors and outdoors and varied across health districts in both vector species, possibly linked to the high vector abundance in the area [14]. The similarity between indoor and outdoor transmission of malaria is inconsistent with LLIN use in the area [9].
Consistent with recent studies carried out in the area of Bouaké [7, 9, 13, 36], there was a high frequency of both kdr and ace1R genes in An. gambiae and An. coluzzii, with a higher frequency for An. gambiae; probably due to selection pressure through the use of insecticide. The lower frequency the resistance alleles in An. coluzzii was associated with higher proportion of heterozygous, implying that An. gambiae is better adapted to insecticide pressure as evidenced elsewhere in Côte d’Ivoire [8, 37] and other parts of sub-Saharan Africa [24, 38, 39].
Resolving the problem posed by outdoor transmission of malaria has become critical [34, 40] LLINs and IRS are effective strategies controlling malaria but unfortunately they can only operate indoors [41, 42]. Once again the high outdoor transmission of malaria in this study triggers the urgent search for innovative tools or strategies to overcome outdoor transmission of malaria.
Conclusion
Densities of An. gambiae and An. funestus were high in central Côte d’Ivoire prior to the start of a CRT evaluating a new method of malaria vector control. The density of An. gambiae was higher than for An. funestus, although An. funestus had overall higher rate of infection with Plasmodium parasites (sporozoite index). However, malaria transmission indicator based on the number of infected bite per person per night (EIR) for An. gambiae was consistently higher than for An. funestus, without differences indoors and outdoors with either species, despite universal coverage of LLINs in the area. Owing to its resistance breaking potential, the claim is to evaluate EaveTubes in areas of high insecticide resistance and where the force of malaria transmission is intense.
Consistent with high insecticide resistance intensity previously detected in these districts, the current study detected high Kdr frequency (> 85%), coupled with high malaria transmission pattern, which could guide the use of EaveTubes in the study areas.
Availability of data and materials
The datasets supporting the conclusions of this manuscript are included within the manuscript and its additional files, and are available from the corresponding author on reasonable request.
Abbreviations
- Ace-1 R :
-
Acetylcholinesterase-1 resistance
- ace-1 R G119S:
-
G119S mutation in ace-1R
- CRT:
-
Cluster-Randomized controlled Trial
- EIR:
-
Entomological Inoculation Rate
- HBR:
-
Human Biting Rates
- HLC:
-
Human Landing Catches
- HWE:
-
Hardy–Weinberg Equilibrium
- IPR:
-
Institut Pierre Richet
- IRS:
-
Indoor Residual Spraying. L1014F kdr: West knockdown resistance
- LLINs:
-
Long Lasting Insecticidal Nets
- OR:
-
Odds ratio
- PR:
-
Parity Rate
- qPCR:
-
Quantitative Polymerase Chain Reaction
- R:
-
Resistant
- S:
-
Susceptible
- SR:
-
Sporozoite Rate
- VCPEC:
-
Vector Control Evaluation Centre
- WHO:
-
World Health Organization
References
Bhatt S, Weiss DJ, Mappin B, Dalrymple U, Cameron E, Bisanzio D, et al. Coverage and system efficiencies of insecticide-treated nets in Africa from 2000 to 2017. Elife. 2015;4:e09672.
Ranson H, N’Guessan R, Lines J, Moiroux N, Nkuni Z, Corbel V. Pyrethroid resistance in African anopheline mosquitoes: what are the implications for malaria control? Trends Parasitol. 2011;27:91–8.
WHO. World malaria report 2021. Geneva: World Health Organization; 2021.
WHO. World malaria report 2020: 20 years of global progress and challenges. Geneva: World Health Organization; 2020. p. 299.
Riveron JM, Irving H, Ndula M, Barnes KG, Ibrahim SS, Paine MJI, et al. Directionally selected cytochrome P450 alleles are driving the spread of pyrethroid resistance in the major malaria vector Anopheles funestus. Proc Natl Acad Sci USA. 2013;110:252–7.
Mulamba C, Riveron JM, Ibrahim SS, Irving H, Barnes KG, Mukwaya LG, et al. Widespread pyrethroid and DDT resistance in the major malaria vector Anopheles funestus in East Africa Is driven by metabolic resistance mechanisms. PLoS ONE. 2014;9: e110058.
Koffi AA, Ahoua Alou LP, Adja MA, Chandre F, Pennetier C. Insecticide resistance status of Anopheles gambiae s.s population from M’Bé: a WHOPES-labelled experimental hut station, 10 years after the political crisis in Côte d’Ivoire. Malar J. 2013;12:151.
Kouassi BL, Edi C, Tia E, Konan LY, Akré MA, Koffi AA, et al. Susceptibility of Anopheles gambiae from Côte d’Ivoire to insecticides used on insecticide-treated nets: evaluating the additional entomological impact of piperonyl butoxide and chlorfenapyr. Malar J. 2020;19:454.
Oumbouke WA, Pignatelli P, Barreaux AMG, Tia IZ, Koffi AA, Ahoua Alou LP, et al. Fine scale spatial investigation of multiple insecticide resistance and underlying target-site and metabolic mechanisms in Anopheles gambiae in central Côte d’Ivoire. Sci Rep. 2020;10:15066.
Knols BGJ, Farenhorst M, Andriessen R, Snetselaar J, Suer RA, Osinga AJ, et al. Eave tubes for malaria control in Africa: an introduction. Malar J. 2016;15:404.
Andriessen R, Snetselaar J, Suer RA, Osinga AJ, Deschietere J, Lyimo IN, et al. Electrostatic coating enhances bioavailability of insecticides and breaks pyrethroid resistance in mosquitoes. Proc Natl Acad Sci USA. 2015;112:12081–6.
Sternberg ED, Cook J, Alou LPA, Assi SB, Koffi AA, Doudou DT, et al. Impact and cost-effectiveness of a lethal house lure against malaria transmission in central Côte d’Ivoire: a two-arm, cluster-randomised controlled trial. Lancet. 2021;397:805–15.
Camara S, Koffi AA, Ahoua Alou LP, Koffi K, Kabran J-PK, Koné A, et al. Mapping insecticide resistance in Anopheles gambiae (s.l.) from Côte d’Ivoire. Parasit Vectors. 2018;11:19.
Diakité NR, Guindo-Coulibaly N, Adja AM, Ouattara M, Coulibaly JT, Utzinger J, et al. Spatial and temporal variation of malaria entomological parameters at the onset of a hydro-agricultural development in central Côte d’Ivoire. Malar J. 2015;14:340.
Gillies MT, Coetzee M. The Anophelinae of Africa south of the Sahara (Afrotropical region). Johannesburg S Afr Inst Med Res. 1987;55:143.
Detinova TS. Age-grouping methods in diptera of medical importance with special reference to some vectors of malaria. Monogr Ser World Health Organ. 1962;47:13–191.
Mangold KA, Manson RU, Koay ESC, Stephens L, Regner M, Thomson RB, et al. Real-time PCR for detection and identification of Plasmodium spp. J Clin Microbiol. 2005;43:2435–40.
Favia G, Lanfrancotti A, Spanos L, Sidén-Kiamos I, Louis C. Molecular characterization of ribosomal DNA polymorphisms discriminating among chromosomal forms of Anopheles gambiae s.s.: An gambiae s.s. rDNA polymorphisms. Insect Mol Biol. 2001;10:19–23.
Bass C, Nikou D, Donnelly MJ, Williamson MS, Ranson H, Ball A, et al. Detection of knockdown resistance (kdr) mutations in Anopheles gambiae: a comparison of two new high-throughput assays with existing methods. Malar J. 2007;6:111.
Bass C, Nikou D, Vontas J, Williamson MS, Field LM. Development of high-throughput real-time PCR assays for the identification of insensitive acetylcholinesterase (ace-1R) in Anopheles gambiae. Pestic Biochem Physio. 2010;96:80–5.
Dossou-yovo J, Doannio J, Rivière F, Duval J. Rice cultivation and malaria transmission in Bouaké city (Côte d’Ivoire). Acta Trop. 1994;57:91–4.
Dossou-Yovo J, Doannio JMC, Diarrassouba S, Chauvancy G. [The impact of rice fields on the transmission of malaria in Bouake, Cote d’Ivoire](in French). Bull Soc Path Exot. 1998;91:327–33.
Zoh DD, Yapi A, Adja MA, Guindo-Coulibaly N, Kpan DMS, Sagna AB, et al. Role of Anopheles gambiae s.s. and Anopheles coluzzii (Diptera: Culicidae) in human malaria transmission in rural areas of Bouaké, in Côte d’Ivoire. J Med Entomol. 2020;57:1254–61.
Zogo B, Soma DD, Tchiekoi BN, Somé A, Ahoua Alou LP, Koffi AA, et al. Anopheles bionomics, insecticide resistance mechanisms, and malaria transmission in the Korhogo area, northern Côte d’Ivoire: a pre-intervention study. Parasite. 2019;26:40.
Yokoly FN, Zahouli JBZ, Small G, Ouattara AF, Opoku M, de Souza DK, et al. Assessing Anopheles vector species diversity and transmission of malaria in four health districts along the borders of Côte d’Ivoire. Malar J. 2021;20:409.
Byrne I, Chan K, Manrique E, Lines J, Wolie RZ, Trujillano F, et al. Technical workflow development for integrating drone surveys and entomological sampling to characterise aquatic larval habitats of Anopheles funestus in agricultural landscapes in Côte d’Ivoire. J Environ Public Health. 2021;2021:3220244.
Koffi AA, Ahoua Alou LP, Djenontin A, Kabran JPK, Dosso Y, Kone A, et al. Efficacy of Olyset® Duo, a permethrin and pyriproxyfen mixture net against wild pyrethroid-resistant Anopheles gambiae s.s. from Côte d’Ivoire: an experimental hut trial. Parasite. 2015;22:28.
Zogo B, Koffi AA, Alou LPA, Fournet F, Dahounto A, Dabiré RK, et al. Identification and characterization of Anopheles spp. breeding habitats in the Korhogo area in northern Côte d’Ivoire: a study prior to a Bti-based larviciding intervention. Parasit Vectors. 2019;12:146.
Gimonneau G, Pombi M, Choisy M, Morand S, Dabiré RK, Simard F. Larval habitat segregation between the molecular forms of the mosquito Anopheles gambiae in a rice field area of Burkina Faso. West Africa Med Vet Entomol. 2012;26:9–17.
Adja AM, N’Goran KE, Kengne P, Koudou GB, Toure M, Koffi AA, et al. [Vectorial transmission of malaria in shrubby Savannah area at Ganse, Ivory Coast](in French). Med Trop (Mars). 2006;66:449–55.
Killeen GF, Githure JI, Beier JC. Short report: entomologic inoculation rates and Plasmodium falciparum malaria prevalence in Africa. Am J Trop Med Hyg. 1999;61:109–13.
Kilama M, Smith DL, Hutchinson R, Kigozi R, Yeka A, Lavoy G, et al. Estimating the annual entomological inoculation rate for Plasmodium falciparum transmitted by Anopheles gambiae s.l. using three sampling methods in three sites in Uganda. Malar J. 2014;13:111.
Conn JE, Norris DE, Donnelly MJ, Beebe NW, Burkot TR, Coulibaly MB, et al. Entomological monitoring and evaluation: diverse transmission settings of ICEMR Projects will require local and regional malaria elimination strategies. Am J Trop Med Hyg. 2015;93:28–41.
Russell TL, Govella NJ, Azizi S, Drakeley CJ, Kachur SP, Killeen GF. Increased proportions of outdoor feeding among residual malaria vector populations following increased use of insecticide-treated nets in rural Tanzania. Malar J. 2011;10:80.
Musiime AK, Smith DL, Kilama M, Rek J, Arinaitwe E, Nankabirwa JI, et al. Impact of vector control interventions on malaria transmission intensity, outdoor vector biting rates and Anopheles mosquito species composition in Tororo. Uganda Malar J. 2019;18:445.
Wolie RZ, Koffi AA, Ahoua Alou LP, Sternberg ED, N’Nan-Alla O, Dahounto A, et al. Evaluation of the interaction between insecticide resistance-associated genes and malaria transmission in Anopheles gambiae sensu lato in central Côte d’Ivoire. Parasit Vectors. 2021;14:581.
Alou LPA, Koffi AA, Adja MA, Assi SB, Kouassi PK, N’Guessan R. Status of pyrethroid resistance in Anopheles gambiae ss M form prior to the scaling up of long lasting insecticidal nets (LLINs) in Adzope. Eastern Cote d’Ivoire Parasit Vectors. 2012;5:289.
Koukpo CZ, Fassinou AJYH, Ossè RA, Agossa FR, Sovi A, Sewadé WT, et al. The current distribution and characterization of the L1014F resistance allele of the kdr gene in three malaria vectors (Anopheles gambiae, Anopheles coluzzii, Anopheles arabiensis) in Benin (West Africa). Malar J. 2019;18:175.
Soma DD, Zogo BM, Somé A, Tchiekoi BN, Hien DFS, Pooda HS, et al. Anopheles bionomics, insecticide resistance and malaria transmission in southwest Burkina Faso: a pre-intervention study. PLoS ONE. 2020;15:e0236920.
Moshi IR, Manderson L, Ngowo HS, Mlacha YP, Okumu FO, Mnyone LL. Outdoor malaria transmission risks and social life: a qualitative study in South-Eastern Tanzania. Malar J. 2018;17:397.
Dossou-Yovo J, Guillet P, Rogier C, Chandre F, Carnevale P, Assi S-B, et al. Protective efficacy of lambda-cyhalothrin treated nets in Anopheles gambiae pyrethroid resistance areas of Côte d’Ivoire. Am J Trop Med Hyg. 2005;73:859–64.
Bhatt S, Weiss DJ, Cameron E, Bisanzio D, Mappin B, Dalrymple U, et al. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature. 2015;526:207–11.
Acknowledgements
We would like to thank the technical staff at the Institut Pierre Richet, Bouaké, Côte d’Ivoire for their valued support during mosquito collection surveys and laboratory analysis. The authors are very grateful to colleagues from various disciplines from Côte d’Ivoire, especially from the Unité de Recherche et de Pédagogie de Génétique, UFR Biosciences, Université Félix Houphouët-Boigny, Abidjan, for their useful contribution. We also thank the volunteer mosquito collectors in villages for their participation towards the study.
Funding
This study is supported by a grant to the Pennsylvania State University from the Bill & Melinda Gates Foundation (OPP1131603), for evaluating the impact of an intervention composed of household screening plus a novel insecticide delivery system called In2Care EaveTubes.
Author information
Authors and Affiliations
Contributions
RZW, AAK and RN designed the study. RZW, LAT, YN, IZT, WAO and AAPL conducted the field and laboratory. RZW and AD analysed the data. RZW wrote the manuscript. AAK, AAPL, ONA, EDS, JC, SPAN, TBM and RN supervised the study and revised the manuscript. All authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethical clearance and consent information are included within the manuscript.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wolie, R.Z., Koffi, A.A., Ayuk-Taylor, L. et al. Entomological indicators of malaria transmission prior to a cluster-randomized controlled trial of a ‘lethal house lure’ intervention in central Côte d’Ivoire. Malar J 21, 188 (2022). https://doi.org/10.1186/s12936-022-04196-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12936-022-04196-5