Abstract
Background
A better understanding of vector distribution and malaria transmission dynamics at a local scale is essential for implementing and evaluating effectiveness of vector control strategies. Through the data gathered in the framework of a cluster randomized controlled trial (CRT) evaluating the In2Care (Wageningen, Netherlands) Eave Tubes strategy, the distribution of the Anopheles vector, their biting behaviour and malaria transmission dynamics were investigated in Gbêkê region, central Côte d’Ivoire.
Methods
From May 2017 to April 2019, adult mosquitoes were collected monthly using human landing catches (HLC) in twenty villages in Gbêkê region. Mosquito species wereidentified morphologically. Monthly entomological inoculation rates (EIR) were estimated by combining the HLC data with mosquito sporozoite infection rates measured in a subset of Anopheles vectors using PCR. Finally, biting rate and EIR fluctuations were fit to local rainfall data to investigate the seasonal determinants of mosquito abundance and malaria transmission in this region.
Results
Overall, Anopheles gambiae, Anopheles funestus, and Anopheles nili were the three vector complexes found infected in the Gbêkê region, but there was a variation in Anopheles vector composition between villages. Anopheles gambiae was the predominant malaria vector responsible for 84.8% of Plasmodium parasite transmission in the area. An unprotected individual living in Gbêkê region received an average of 260 [222–298], 43.5 [35.8–51.29] and 3.02 [1.96–4] infected bites per year from An. gambiae, An. funestus and An. nili, respectively. Vector abundance and malaria transmission dynamics varied significantly between seasons and the highest biting rate and EIRs occurred in the months of heavy rainfall. However, mosquitoes infected with malaria parasites remained present in the dry season, despite the low density of mosquito populations.
Conclusion
These results demonstrate that the intensity of malaria transmission is extremely high in Gbêkê region, especially during the rainy season. The study highlights the risk factors of transmission that could negatively impact current interventions that target indoor control, as well as the urgent need for additional vector control tools to target the population of malaria vectors in Gbêkê region and reduce the burden of the disease.
Similar content being viewed by others
Background
Malaria is still a major public health problem in sub-Saharan Africa despite improvements in the diagnosisof the pathogens and large-scale deployment of vector control tools, such as long-lasting insecticidal nets (LLINs) and indoor residual spraying (IRS). According to the World Malaria Report 2021, a slight upward trend in malaria incidence was observed in 2020, after stagnation, between 2015 and 2019 [1]. A number of factors may contribute to this, including the growing problem of insecticide-resistant mosquitoes [2, 3], outdoor malaria transmission [4, 5], residual transmission [6, 7], gaps in control management [8] and disruption to services during the COVID-19 pandemic [1].
Today, the greatest burden of malaria occurs across in the World Health Organization (WHO) African region, with an estimated 228 million malaria cases and 602,000 malaria deaths [1]. Malaria in sub-Saharan Africa is transmitted by a range of Anopheles mosquitoes [9, 10] and transmission dynamics can be highly heterogenous [11, 12]. Although the whole sub-Saharan region is exposed to malaria transmission, the risk of infection and disease varies greatly across the continent and even within small geographical areas [11, 13]. This high heterogeneity, influenced by ecological factors, such as climate, physical geography, land use, human behaviour and other social factors [14,15,16], needs to be considered when planning and implementing vector control strategies.
In Côte d’Ivoire, malaria transmission is perennial, albeit with a sharp increase during the wet season [17, 18]. The Plasmodium species responsible for human malaria are mainly transmitted by the primary vectors Anopheles gambiae sensu stricto and Anopheles coluzzii [19]. Anopheles funestus sensu lato (s.l.) and Anopheles nili s.l. are secondary vectors [17, 20]. In some localities of western Côte d’Ivoire, these secondary vectors have played a significant role in malaria transmission largely due to their predominantly anthropophilic and endophilic tendencies [20]. Malaria incidence was estimated at more than 287 cases per thousand and 15,913 deaths in 2020 [1]. Recently in Gbêkê region, central Côte d’Ivoire the incidence of malaria infection has been estimated at 2.29 per child-year [21].
Vector control by the national malaria control programme (NMCP) is based on sustaining high LLIN access and use, via universal coverage campaigns supplemented with continuous distribution from antenatal care campaigns and the expanded programme for immunization; targeted IRS in high transmission areas since 2020 and treatment. Since 2010, the NMCP, with the support of the Global Fund to Fight AIDS, Tuberculosis and Malaria (Global Fund), started scaling up mass distribution of LLINs to achieved universal coverage. Unfortunately, the large scale use of pyrethroid insecticides in public health as well as in agriculture has resulted in mosquitoes building up high resistance to the insecticide [22,23,24], making the pyrethroid-treated nets less effective [25,26,27]. In the face of increasing pyrethroid resistance, many African countries NMCPs are challenged with finding new ways to prevent malaria transmission. Urgent action is required to slow or prevent the development and further spread of insecticide resistance, including the use of two or more compounds of different insecticide classes to make a single product or development and evaluation of new interventions strategies that aim to maintain effective vector control [28].
In the Gbêkê region, central Côte d’Ivoire the In2Care (Wageningen, Netherlands) Eave Tubes, a new tool for the targeted delivery of insecticides against mosquitoes, attempting to enter houses through the eaves have been evaluated in a large-scale cluster randomized trial (CRT) between May 2017 and April 2019 [21]. The project, which was conducted in 40 villages, was designed to test whether modification of houses through the addition of window screening and eave tubes, provides additional protection against malaria in areas with intense pyrethroid-resistance above and beyond universal coverage of pyrethroid-only LLINs [29]. The epidemiological results of the study published recently, showed an impressive drop of 38% in malaria case incidence in children living in clusters with intervention [21]. Through the data gathered within this trial in the 20 control villages, vector distribution, their behaviour and malaria transmission dynamic were updated in Gbêkê region, under natural conditions with universal coverage of pyrethroid-only bed nets.
Methods
Study area and trial design
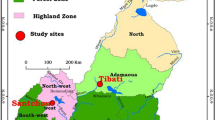
The study was carried out in the Gbêkê region in central Côte d’Ivoire. This region is characterized by wet savannah with a single annual rainy season (April to November) followed by a long dry season (December to March). There was an average annual rainfall of 1223 mm and an average temperature of 26.3 C during the study period. It is highly malaria endemic area with year-round transmission, and malaria cases are almost entirely attributable to Plasmodium falciparum [30, 31]. Members of the An. gambiae complex (An. coluzzii and An. gambiae s.s.) are the main vectors [30]. Members of the An. funestus were also present as secondary vectors [21]. The local malaria vector populations are highly resistant to almost all classes of insecticides used for vector control [22, 23, 32].
The eave tube study design was a two-armed, cluster-randomized controlled trial with 20 villages (clusters) per arm. Villages in the control arm received universal coverage of LLINs, while the villages in the intervention arm received universal coverage of LLINs plus the screening plus eave tube (SET) intervention free of charge [29]. In the framework of this study, only the data from control villages were analysed to show the natural transmission dynamics.
Mosquito sampling
Each month between May 2017 and April 2019, mosquitoes were sampled using human landing catches (HLC) both indoors and outdoors for one night at four randomly selected houses in the 20 study villages (Fig. 1) to estimate the variation in their abundance over time. Collections began at 06:00 pm with one person sitting inside of the house in the living room area and one sitting outside of the house. Every hour, each team were supervised by research technicians to ensure that they were awake and working according to protocol. Every two hours, the two capturers were rotated between indoor and outdoor collection sites to minimize the bias due to their attractiveness. At 01:00 am, a second capturer team took over and continued the capture until 08:00 am. Mosquitoes collected were brought back to the laboratory, and were identified using a species key based on morphological traits[33]. Mosquitoes were stored individually in tubes with silica gel and kept at − 20 °C pending further laboratory processing. Non-Anopheles species were discarded after recording numbers caught.
Monthly rainfall and temperature data during the study period was obtained from the National Weather Service of Bouake airport. The data consist of monthly mean of the daily rainfall and temperature in the region.
Determining Plasmodium spp infection in female mosquitoes
A random subset of 59,901 captured An. gambiae s.l. and 10,059 An. funestus and 1,991 An. nili females were dissected to determine parity. Mosquito DNA was extracted from the head and thorax of each specimen in a random sample of up to 60 parous females per village per monthly survey using cetyl trimethyl ammonium bromide (CTAB) 2% method [34]. Quantitative Polymerase Chain Reaction (qPCR) was used to assess sporozoite prevalence as described by Mangold et al. [35].
Data analysis
Human landing catch captures were done monthly and then the data were pooled every two months for analysis. The human biting rate (HBR, the number of Anopheles vectors collected per person per night), the sporozoite rate (SR, the number of vectors positive for sporozoites over the number of vectors tested) and the entomological inoculation rate (EIR, is the number of infective bites per person per night). EIR was calculated by multiplying the HBR by the SR as described by Sternberg et al. All non-parous mosquitoes were considered negative for the calculation of sporozoite rates and EIRs.
A separate rate was determinedfor HBR, SR and EIR for each species.
Statistical analysis for the comparison of HBR, SRs and EIR between species, seasons (rainy/dry), year and collection positions (indoor/outdoor) were performed using R software version 4.1.2, and figures with GraphPad Prism 7 software.
To assess the difference in HBR and EIR, a generalized linear mixed model (GLMM) fitting a negative binomial distribution was applied using the lme4 package. SRs were compared using a binomial mixed effect model (function “glmer” from the package lme4) [36]. The fixed variables were the Anopheles species, collection position (indoor/outdoor), season (rainy/dry) and year. The villages and month of collection were considered as a random intercept to adjust for sampling variations across villages and years.
Results
Species composition and vector distribution
A total of 157,645 mosquitoes belonging to four genera were collected over 4,880 sampling person-nights using HLC methods (Table 1). Of these, 71,207 (45.2%) were collected indoors and 86,438 (54.8%) outdoors. Mosquitoes collected included Anopheles, Aedes, Culex and Mansonia species (Table 1). Of the Anophelines collected, 94.5% (128,632/136,049) were malaria vectors comprised of An. gambiae, An. funestus and An. nili.
Overall, An. gambiae s.l. was the most common malaria vector, and accounted for more than 84.5% (108,761/128,632) of all malaria vectors. The other vectors were An. funestus (12.0% (15,417/128,632)) and An. nili 3.5% (4,454/128,632) (Table 2).
The relative abundance and species composition of the malaria vectors varied from one village to another (Table 3; Fig. 2). In most of the study villages An. gambiae predominated throughout the year with more than 70% of the catch, followed by An. funestus and An. nili. Exceptions were seen in 3 villages, An. funestus and An. nili were main malaria vectors found in Kouassi Atienkro with 55.5% and 24.0% of the catch, respectively. In M’Mebo An. gambiae (48.6% of catch) and An. funestus (47.9% of the catch) were equally present. In Gare Kan village, An. gambiae was the most abundantly represented with 62.7% of malaria vector collected, but An. funestus (20.2%) and An. nili (17.2%) were also found at a comparable rate (Table 3; Fig. 2).
Dynamics of malaria transmission
Seasonal abundance and biting patterns of Anopheles mosquitoes
The monthly abundance of human-biting Anopheles species during the study period are shown in Fig. 3. The mean Anopheles caught per human, per night were: An. gambiae = 18.0 [95% CI 11.0–26.8], An. funestus = 2.5 [95% CI 0.8–4.1] and An. nili = 0.5 [95% CI − 0.1–1.5] (Table 2). Significantly greater numbers of An. gambiae were collected across the study area compared to the An. funestus group (RR [95%] = 12.6 [12.0–13.1] p < 0.001) and the An. nili group (RR [95%] = 56.4 [53.0–60.1], p < 0.001) (Table 2). Overall, An. gambiae and An. nili biting rates were significantly higher outdoors compared to indoors (OR [95% CI] = 1.2 [1.16–1.23], p < 0.001) suggesting an exophilic tendency for these species in this study. In contrast, the highest biting rates in An. funestus group were recorded indoors compared to outdoors (OR [95% CI] = 0.9 [0.86–0.98], p = 0.014) confirming that An. funestus tends to be endophagic (Table 2).
During the sampling period, more vectors (of the An. gambiae, An. funestus and An. nili group) were recorded during the rainy season (April to November) than in the dry season (December to March) (RR [95%] = 4.0 [2.2–7.3], p < 0.001), but vectors biting rates peaked in August and September corresponding to rainiest months (Fig. 3).
Mean density of An. gambiae per human per night was 7.0 [5.7–8.3] during the dry season, but increased three-fold (24.9 [22.5–27.3]; p = 0.010) in the rainy season. Overall, densities of An. funestus and An. nili were very low and were closely correlated with monthly rainfall patterns (Fig. 3). Both vectors were almost undetectable during the dry seasons (December to March) (Fig. 3).
Abundance of malaria vectors varied from one year to the other (Table 4). Overall the mean density of An. gambiae per person per night decreased significantly over the two years of data collection, from 22.1 [18.3–25.9] in year 1 period to 15.7 [13.0–18.4] in the year 2 of the trial period (RR [95%] = 0.58 [0.55–0.60], p < 0.001). There was no difference in An. funestus biting rates between years (p = 0.051; Table 4). For An. nili, the highest densities were observed during the first year of data collection (RR [95%] = 16.3 [13.3–20]. Anopheles gambiae, An. funestus and An. nili were recorded as biting all night long. However, An. gambiae peak of biting time was recorded between 02:00 am and 03:00 am indoors and outdoors, while that of An. funestus was recorded one hour later (between 04:00 am and 05:00 am) (Fig. 4). Anopheles funestus was also recorded biting predominantly indoors during the night. An. nili showed earlier biting activity (beginning at 11:00 pm) than An. gambiae and An. funestus, with biting densities increased between 00:00 am and 01:00 am, and then decreased during the second part of the night (Fig. 4).
Parity and sporozoite infection rate
We dissected 71,951 Anopheles for determination of parous rate. Overall, Anopheles parous rate was 84.9%. Parous rate was 83.3%, 88.9% and 97.2% for An. gambiae, An. funestus and An. nili, respectively (Table 2).
A total of 14,490 Anopheles mosquitoes (An. gambiae, An funestus and An nili) were analysed to assess for the presence of Plasmodium spp., with 703 found infected, giving an overall sporozoite rate of 4.8% [95% CI 4.5–5.2]. Infective Anopheles mosquitoes were found in all twenty study villages with infection rates ranging from 2.5% to 10.4% (Table 3). Most infections were with Plasmodium falciparum (94.6%), and the remaining (5.4%) were infections with Plasmodium ovale and Plasmodium malariae. The sporozoite rates did not differ significantly between malaria vectors collected indoors 5.1% [4.2–6.0] or outdoors 4.4% [3.7–5.2], (OR [95% CI] = 1.1 [1.0–1.3], p = 0.06). Overall sporozoite rate varied significantly among Anopheles spp. (p < 0.01) and fluctuated across the seasons with the highest rates observed in the rainy season (OR [95% CI] = 1.8 [1.4–2.4], p < 0.001, Table 2). The sporozoite rate recorded for An. gambiae during rainy season (5.1% [95% CI 4.6–5.6]) were significantly higher compared to dry season (2.4% [95% CI 1.7–3.1]); (OR [95% CI] = 0.4 [0.3–0.6], p < 0.0001). However, the sporozoite rate recorded for An. funestus in rainy season 5.8% [95% CI 5.0–6.5] and dry season 5.9% [95% CI 0.3–11.5] did not indicate seasonal variation (OR [95% CI] = 1.7 [1.0–3.2], p = 0.10). The An. nili appeared to contribute to transmission mainly in the rainy season (Table 2).
When considering the collection years, the sporozoite rate of An. gambiae, recorded in year 1 (3.7% [95% CI 3.2–4.2]) was significantly lower than that of the year 2 (5.5% [95% CI 5.0–6.2]) (OR [95% CI] = 0.7 [0.5–0.8], p < 0.0001). For An. funestus and in An. nili no significant difference of sporozoite rate wasfound between the two years (p > 0.05; Table 4).
Entomological inoculation rate (EIR)
In Gbêkê region, malaria transmission occurred all year long (Fig. 5), with variation in transmission intensities across the seasons (Table 2). From May 2017 to April 2019, the average annual EIR was estimated at 260.0 infective bite/per person/per year for An. gambiae; 43.5 ib/p/yr for An. funestus and 3.0 ib/p/yr for the An. nili, respectively. Monthly EIR was higher in the rainy season compared to the dry season, for both An. gambiae (30.6 [95% CI 27.3–33.9] vs. 3.9 [95% CI 2.4–5.4]) and An. funestus (5.4 [95% CI 4.7–6.1] vs. 0.1 [95% CI 0.0–0.2]; Table 2). Transmission intensity reached its peak in August–September, with an average of 46.0 ib/p/m for An. gambiae, 9.4 ib/p/m for An. funestus and 1.1 ib/p/m for An. nili (Fig. 5). Overall, An. gambiae was the major malaria vector responsible for 84.8% of total transmission, followed by An. funestus: 14.9% of transmission (Table 2). These vectors were responsible for malaria transmission in both the rainy season and the dry season. Anopheles nili (0.98%) also played an active role in the transmission of malaria parasites in rainy season, although its importance is far less than that of An. gambiae and An. funestus.
Discussion
This study was conducted to characterize Anopheles vector distribution and malaria transmission dynamics, and vector biting behaviour in Gbêkê region, central Côte d’Ivoire. High species diversity grouped into six genera of mosquitoes was recorded in the study area. The diversity and abundance of mosquito fauna observed in this study might result from favorable environmental conditions for the developement of mosquito in the study area.[37].
The study of malaria transmission revealed that three common African Anopheles vector, An. gambiae, An. funestus and An. nili, are involved and sustain parasite transmission to local communities. Anopheles gambiae is the primary vector in the area, accounting for 84.8% infective bites. These data indicate high Plasmodium infection rates in An. funestus, affirming its role as the important vector in the location particulary in the village of Kouassi Atienkro, M’Mebo and Gare Kan. Anopheles nili were also found infected with malaria parasites, but it was present at a very low density. Infection levels recorded with An gambiae during this study were close to those previously recorded in the same region of Côte d’Ivoire [31, 38]. However, the involvement of An. funestus and An. nili in malaria transmission alongside An. gambiae in the area contrasted with the recent findings, where transmission was mainly sustained by An. gambiae [30, 31]. Indeed, these species responsible for all the Plasmodium ssp transmission recorded in this study have previously been incriminated in malaria transmission in Côte d’Ivoire [17, 19, 20, 39, 40].
This study demonstrated that the malaria vector species and abundance and malaria transmission intensity in the Gbêkê region varied significantly according to the season. Anopheles gambiae was present all year long; however, it was found at very low density during the dry season but became very abundant in the rainy season. Indeed, the larval habitats of this species are known to increase in number and productivity in rainy season but appear to diminish significantly during the dry season [41]. Anopheles funestus densities decreased also during the dry season. This is expected for An. gambiae, but is surprising for An. funestus. It has been described that An. funestus reaches its peak of abundance during the dry season in Savannah areas [42]. The larvae of this species is commonly found in large, permanent or semi-permanent bodies of fresh water such as swamps, large ponds and lake edge, preferentially with emergent vegetation on its margins [43]. In the study area, overall, the natural swamps and marshes are the most important potential breeding sites, and the extent of these habitats depends predominantly on the rains, explaining the low density of An. funestus during the dry season. Nevertheless, the high sporozoite rate recorded for An funestus in the dry season despite its low density suggested that this species strongly contributes to maintain malaria transmission during this season. Anopheles nili is also present in the area during the rainy season, although at a very low density. It was collected more particularly in certain villages (Kouassi Atienkro, M’Mebo and Gare Kan) close to several rivers and the water level of these rivers is kept high in rainy season. This reflects the presence of larval habitats favourable to the development of this species [20, 44]. But An. nili has greatly diminished in the year 2 of the collection of mosquitoes even though there was abundant rain. This is because excessive rainfall could also flush out breeding sites thus reduces the mosquito population [45, 46].
High risk of malaria transmission was recorded in Gbêkê region probably due to the presence of several vectors harbouring the Plasmodium parasite. These results estimated that unprotected individual living in Gbêkê region could receive an average of more than 321 infective bites per year from three major vector species (An. gambiae, An. funestus and An. nili) despite high coverage of LLINs. This high EIRs are consistent with previous work [31, 38], have also been reported from other regions of Côte d'Ivoire [17, 19]. Such levels of transmission recorded in the country, are relatively high when put in African context [47, 48]. The risk of being bitten by malaria vector mosquitoes was found to be up to ninefold higher during the rainy season compared to dry season. The increase in EIR in the rainy season could be explained by the increase in vectors densities and sporozoite rate during this season. Similar observations were reported elsewhere [42]. It could also be that environmental temperature plays a role as temperatures during the dry season are potentially above the optimum for malaria transmission [49, 50], contributing further to the observed seasonality.
Hourly mosquito captures showed that malaria vector populations began host searching at around 06:00 pm –07:00 pm, peaked at 00:00 am–03:00 am and then declined to negligible levels by 06:00 am–07:00 am. The biting time does not indicate a shift in host seeking towards dusk or dawn when people are unprotected by their bed nets. However, it was observed that An. gambiae seems more likely to feed outdoors than indoors that is in accordance with others results recorded in northern Côte d’Ivoire [17]. Endophagy is usually the expected dominant behaviour in An. gambiae [51,52,53]. It would appear that insecticide pressure from IRS and ITNs is selecting for mosquito vector populations which are increasingly outdoor feeding [54,55,56]. Some studies have shown that social patterns and human behavior (in terms of sleeping hours, outdoor activities and ITN use) may determine exposure to Anopheles mosquitoes and have an effect on transmission [57, 58]. Previous findings in the study area revealed that peoples spend a substantialamount of time outdoors [58] so there are potentially many opportunities for exposure when householders are not necessarily indoors and protected by LLINs. Indoor vector control measures alone (such as LLINs and IRS) could target a significant part of the vector population but are unable to stop transmission [59]. Hence, malaria control in high endemic areas needs to be strengthened with complementary tools to alleviate the burden of the disease. One limitation of the study was the use of qPCR which has been shown to overestimate the sporozoite rate [60].
This study has allowed a better understanding of malaria transmission dynamics and vector biting behaviour in Gbêkê region following the universal coverage of LLINs. The study highlights the risk factors of transmission that could negatively impact current interventions that target indoor control. Considering an aim of malaria elimination in Côte d’Ivoire and particulary in the Gbêkê region, it is increasingly urgent to research and develop novel vector control tools or complementary strategies particularly designed to suppress its very large malaria vector populations and the behaviour of vector populations.
Conclusions
Malaria transmission in the Gbêkê area was mainly due to An. gambiae, while An. funestus group and An. nili complex played minor roles. This is the first report on the contribution of the An. nili as a secondary vector of malaria transmission in the area. The entomological indicators of malaria transmission were high despite the presence of standard LLINs. Additional vector control tools are urgently needed to complement current malaria control interventions.
Availability of data and materials
The datasets used and/or analysed during the current study are available At Intitut Pierre Richet/Institut national de santé Publique and will be made available on reasonable request.
Abbreviations
- CRT:
-
Cluster randomized trial
- HLC:
-
Human landing catches
- EIR:
-
Entomological inoculation rate
- SR:
-
Sporozoite infection rate
- LLINs:
-
Long-lasting insecticide nets
- IRS:
-
Indoor residual spraying
- NMCP:
-
National malaria control programme
- SET:
-
Sreening plus eave tube
- PCR:
-
Polymerase chain reaction
References
WHO. World malaria report 2021. Geneva: World Health Organization; 2021.
Ranson H, N’Guessan R, Lines J, Moiroux N, Nkuni Z, Corbel V. Pyrethroid resistance in African anopheline mosquitoes: what are the implications for malaria control? Trends Parasitol. 2011;27:91–8.
Corbel V, N’Guessan R, Brengues C, Chandre F, Djogbenou L, Martin T, et al. Multiple insecticide resistance mechanisms in Anopheles gambiae and Culex quinquefasciatus from Benin. West Africa Acta Trop. 2007;101:207–16.
Keïta M, Doumbia S, Sissoko I, Touré M, Diawara SI, Konaté D, et al. Indoor and outdoor malaria transmission in two ecological settings in rural Mali: implications for vector control. Malar J. 2021;20:127.
Denz A, Njoroge MM, Tambwe MM, Champagne C, Okumu F, van Loon JJA, et al. Predicting the impact of outdoor vector control interventions on malaria transmission intensity from semi-field studies. Parasit Vectors. 2021;14:64.
Killeen GF. Characterizing, controlling and eliminating residual malaria transmission. Malar J. 2014;13:330.
Carnevale P, Manguin S. Review of Issues on residual malaria transmission. J Infect Dis. 2021;223:S61-80.
Kokwaro G. Ongoing challenges in the management of malaria. Malar J. 2009;8:S2.
Sinka ME, Pironon S, Massey NC, Longbottom J, Hemingway J, Moyes CL, et al. A new malaria vector in Africa: predicting the expansion range of Anopheles stephensi and identifying the urban populations at risk. Proc Natl Acad Sci USA. 2020;117(24900–8):10.
Afrane YA, Bonizzoni M, Yan G. Secondary malaria vectors of sub-Saharan Africa: threat to malaria elimination on the continent ? In: Rodriguez-Morales AJ, editor. Current topics in malaria. London: IntechOpen; 2016.
Duchemin J-B, Macintyre K, Warren M, Keating J, Robert V, Beier JC, et al. Malaria transmission in urban sub-Saharan Africa. Am J Trop Med Hyg. 2003;68:169–76.
Noor AM, Kinyoki DK, Mundia CW, Kabaria CW, Mutua JW, Alegana VA, et al. The changing risk of Plasmodium falciparum malaria infection in Africa: 2000–10: a spatial and temporal analysis of transmission intensity. Lancet. 2014;383:1739–47.
Selvaraj P, Wenger EA, Gerardin J. Seasonality and heterogeneity of malaria transmission determine success of interventions in high-endemic settings: a modeling study. BMC Infect Dis. 2018;18:413.
Castro MC. Malaria transmission and prospects for malaria eradication: the role of the environment. Cold Spring Harb Perspect Med. 2017;7:a025601.
Stryker JJ, Bomblies A. The impacts of land use change on malaria vector abundance in a water-limited, highland region of Ethiopia. EcoHealth. 2012;9:455–70.
Chan K, Tusting LS, Bottomley C, Saito K, Djouaka R, Lines J. Malaria transmission and prevalence in rice-growing versus non-rice-growing villages in Africa: a systematic review and meta-analysis. Lancet Planet Health. 2022;6:e257–69.
Zogo B, Soma DD, Tchiekoi BN, Somé A, Alou LPA, Koffi AA, et al. Anopheles bionomics, insecticide resistance mechanisms, and malaria transmission in the Korhogo area, northern Côte d’Ivoire: a pre-intervention study. Parasite. 2019;26:40.
Koudou BG, Doumbia M, Janmohamed N, Tschannen AB, Tanner M, Hemingway J, et al. Effects of seasonality and irrigation on malaria transmission in two villages in Côte d’Ivoire. Ann Trop Med Parasitol. 2010;104:109–21.
Assouho KF, Adja AM, Guindo-Coulibaly N, Tia E, Kouadio AMN, Zoh DD, et al. Vectorial transmission of malaria in major districts of Côte d’Ivoire. J Med Entomol. 2020;57:908–14.
Adja AM, N’goran EK, Koudou BG, Dia I, Kengne P, Fontenille D, et al. Contribution of Anopheles funestus, An. gambiae and An. nili (Diptera: Culicidae) to the perennial malaria transmission in the southern and western forest areas of Côte d’Ivoire. Ann Trop Med Parasitol. 2011;105:13–24.
Sternberg ED, Cook J, Alou LPA, Assi SB, Koffi AA, Doudou DT, et al. Impact and cost-effectiveness of a lethal house lure against malaria transmission in central Côte d’Ivoire: a two-arm, cluster-randomised controlled trial. Lancet. 2021;397:805–15.
Camara S, Koffi AA, Ahoua Alou LP, Koffi K, Kabran J-PK, Koné A, et al. Mapping insecticide resistance in Anopheles gambiae (s.l.) from Côte d’Ivoire. Parasit Vectors. 2018;11:19.
Zoh DD, Ahoua Alou LP, Toure M, Pennetier C, Camara S, Traore DF, et al. The current insecticide resistance status of Anopheles gambiae (s.l.) (Culicidae) in rural and urban areas of Bouaké Côte d’Ivoire. ParasitVectors. 2018;11:118.
Koffi AA, Alou LPA, Kabran J-PK, N’Guessan R, Pennetier C. Re-visiting insecticide resistance status in Anopheles gambiae from Côte d’Ivoire: a nation-wide informative survey. PLoS ONE. 2013;8:e82387.
N’Guessan R, Corbel V, Akogbéto M, Rowland M. Reduced efficacy of insecticide-treated nets and indoor residual spraying for malaria control in pyrethroid resistance area. Benin Emerg Infect Dis. 2007;13:199–206.
Strode C, Donegan S, Garner P, Enayati AA, Hemingway J. The impact of pyrethroid resistance on the efficacy of insecticide-treated bed nets against African Anopheline mosquitoes: systematic review and meta-analysis. PLoS Med. 2014;11:e1001619.
Churcher TS, Lissenden N, Griffin JT, Worrall E, Ranson H. The impact of pyrethroid resistance on the efficacy and effectiveness of bednets for malaria control in Africa. eLife. 2016;5:e16090.
WHO. Global malaria programme. Global plan for insecticide resistance management in malaria vectors. Geneva: World Health Organization; 2012.
Sternberg ED, Cook J, Ahoua Alou LP, Aoura CJ, Assi SB, Doudou DT, et al. Evaluating the impact of screening plus eave tubes on malaria transmission compared to current best practice in central Côte d’Ivoire: a two armed cluster randomized controlled trial. BMC Public Health. 2018;18:894.
Adja AM, Zoh DD, Sagna AB, Kpan DMS, Guindo-Coulibaly N, Yapi A, et al. Diversity of Anopheles gambiae sl, Giles (Diptera: Culicidae) larval habitats in urban areas and malaria transmission in Bouaké Côte d’Ivoire. Vector-Borne Zoonotic Dis. 2021;21:593–601.
Zoh DD, Yapi A, Adja MA, Guindo-Coulibaly N, Kpan DMS, Sagna AB, et al. Role of Anopheles gambiae s.s. and Anopheles coluzzii (Diptera: Culicidae) in human malaria transmission in rural areas of Bouaké, in Côte d’Ivoire. J Med Entomol. 2020;57:1254–61.
Koffi AA, Ahoua Alou LP, Adja MA, Chandre F, Pennetier C. Insecticide resistance status of Anopheles gambiae ss population from M’Bé: a WHOPES-labelled experimental hut station, 10 years after the political crisis in Côte d’Ivoire. Malar J. 2013;12:151.
Gillies MT, Coetzee M. A supplement to the Anophelinae of Africa south of the Sahara (Afrotropical Region). S Afr Inst Med Res. 1987;55:1–143.
Yahouédo GA, Cornelie S, Djègbè I, Ahlonsou J, Aboubakar S, Soares C, et al. Dynamics of pyrethroid resistance in malaria vectors in southern Benin following a large scale implementation of vector control interventions. Parasit Vectors. 2016;9:385.
Mangold KA, Manson RU, Koay ESC, Stephens L, Regner M, Thomson RB, et al. Real-time PCR for detection and identification of Plasmodium spp. J Clin Microbiol. 2005;43:2435–40.
Brooks ME, Kristensen K, van Benthem KJ, Magnusson A, Berg CW, Nielsen A, et al. glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R J. 2017;9:378–400.
Ndenga BA, Simbauni JA, Mbugi JP, Githeko AK, Fillinger U. Productivity of malaria vectors from different habitat types in the Western Kenya Highlands. PLoS ONE. 2011;6:e19473.
Dossou-yovo J, Doannio JM, Rivière F, Chauvancy G. Malaria in Côte d’Ivoire wet savannah region: the entomological input. Trop Med Parasitol. 1995;46:263–9.
Koudou BG, Tano Y, Doumbia M, Nsanzabana C, Cissé G, Girardin O, et al. Malaria transmission dynamics in central Côte d’Ivoire: the influence of changing patterns of irrigated rice agriculture. Med Vet Entomol. 2005;19:27–37.
Yokoly FN, Zahouli JB, Small G, Ouattara AF, Opoku M, de Siuza DK, et al. Assessing Anopheles vector species diversity and transmission of malaria in four health districts along the borders of Côte d’Ivoire. Malar J. 2021;20:409.
Mourou J-R, Coffinet T, Jarjaval F, Cotteaux C, Pradines E, Godefroy L, et al. Malaria transmission in Libreville: results of a one year survey. Malar J. 2012;11:40.
Soma DD, Zogo BM, Somé A, Tchiekoi BN, de Hien DF, S, Pooda HS, et al. Anopheles bionomics, insecticide resistance and malaria transmission in southwest Burkina Faso: a pre-intervention study. PLoS ONE. 2020;15:e0236920.
Debrah I, Afrane YA, Amoah LE, Ochwedo KO, Mukabana WR, Zhong D, et al. Larval ecology and bionomics of Anopheles funestus in highland and lowland sites in western Kenya. PLoS ONE. 2021;16:e0255321.
Ossè RA, Tokponnon F, Padonou GG, Sidick A, Aïkpon R, Fassinou A, et al. Involvement of Anopheles nili in Plasmodium falciparum transmission in North Benin. Malar J. 2019;18:152.
Yiga V, Nampala H, Tumwiine J. Analysis of the model on the effect of seasonal factors on malaria transmission dynamics. J Appl Math. 2020;2020:e8885558.
Tompkins AM, Ermert V. A regional-scale, high resolution dynamical malaria model that accounts for population density, climate and surface hydrology. Malar J. 2013;12:65.
Gething PW, Patil AP, Smith DL, Guerra CA, Elyazar IRF, Johnston GL, et al. A new world malaria map: Plasmodium falciparum endemicity in 2010. Malar J. 2011;10:378.
Beier JC, Killeen GF, Githure JI. Entomologic inoculation rates and Plasmodium falciparum malaria prevalence in Africa. Am J Trop Med Hyg. 1999;61:109–13.
Beck-Johnson LM, Nelson WA, Paaijmans KP, Read AF, Thomas MB, Bjørnstad ON. The importance of temperature fluctuations in understanding mosquito population dynamics and malaria risk. R Soc Open Science. 2017;4:160969.
Stresman GH. Beyond temperature and precipitation: ecological risk factors that modify malaria transmission. Acta Trop. 2010;116:167–72.
Akogbéto MC, Salako AS, Dagnon F, Aïkpon R, Kouletio M, Sovi A, et al. Blood feeding behaviour comparison and contribution of Anopheles coluzzii and Anopheles gambiae, two sibling species living in sympatry, to malaria transmission in Alibori and Donga region, northern Benin. West Africa Malar J. 2018;17:307.
Tuno N, Kjaerandsen J, Badu K, Kruppa T. Blood-feeding behavior of Anopheles gambiae and Anopheles melas in Ghana, western Africa. J Med Entomol. 2010;47:28–31.
Sinka ME, Bangs MJ, Manguin S, Coetzee M, Mbogo CM, Hemingway J, et al. The dominant Anopheles vectors of human malaria in Africa, Europe and the Middle East: occurrence data, distribution maps and bionomic précis. Parasit Vectors. 2010;3:117.
Russell TL, Govella NJ, Azizi S, Drakeley CJ, Kachur SP, Killeen GF. Increased proportions of outdoor feeding among residual malaria vector populations following increased use of insecticide-treated nets in rural Tanzania. Malar J. 2011;10:80.
Carrasco D, Lefèvre T, Moiroux N, Pennetier C, Chandre F, Cohuet A. Behavioural adaptations of mosquito vectors to insecticide control. Curr Opin Insect Sci. 2019;34:48–54.
Durnez L, Coosemans M. Residual transmission of malaria: an old issue for new approaches. In: Manguin S, editor. Anopheles mosquitoes—new insights into malaria vectors. London: IntechOpen; 2013.
Rodríguez-Rodríguez D, Katusele M, Auwun A, Marem M, Robinson LJ, Laman M, et al. Human behavior, livelihood, and malaria transmission in two sites of Papua New Guinea. J Infect Dis. 2021;223:S171–86.
Barreaux AMG, Oumbouke WA, Brou N, Tia IZ, Ahoua Alou LP, Doudou DT, et al. The role of human and mosquito behaviour in the efficacy of a house-based intervention. Philos Trans R Soc Lond B Biol Sci. 2021;376:20190815.
Griffin JT, Hollingsworth TD, Okell LC, Churcher TS, White M, Hinsley W, et al. Reducing Plasmodium falciparum malaria transmission in Africa: a model-based evaluation of intervention strategies. PLoS Med. 2010;7:e1000324.
Hendershot AL, Esayas E, Sutcliffe AC, Irish SR, Gadisa E, Tadesse FG, et al. A comparison of PCR and ELISA methods to detect different stages of Plasmodium vivax in Anopheles arabiensis. Parasit Vectors. 2021;14:473.
Acknowledgements
We thank all participants of the study, especially technicians at the VCPEC-IPR for their technical assistance. We are also, grateful to the villagers of all sites for their kind collaboration.
Funding
This study was made possible by the support of the Pennsylvania State University from the Bill & Melinda Gates Foundation (OPP1131603).
Author information
Authors and Affiliations
Contributions
RN, AAK, EDS, MBT, JC and LPAA conceived and designed the study. LPAA, RZW, EDS, IZT, WAO, FHAY and SC participated in the data collection, laboratory and data management work. RN, AAK, EDS and LPAA supervised the study. SC and LPAA analysed the data. SC and AAK wrote the manuscript. All authors revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The trial was reviewed and approved by the Côte d’Ivoire Ministry of Health ethics committee (039/MSLS/CNER-dkn). We also obtained written and verbal informed consent from all trial participants. Mosquito collectors were immunized against yellow fever and medical supervision was provided during the trial. Confirmed malaria cases were treated free of charge for illness according to national policies.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Koffi, A.A., Camara, S., Ahoua Alou, L.P. et al. Anopheles vector distribution and malaria transmission dynamics in Gbêkê region, central Côte d’Ivoire. Malar J 22, 192 (2023). https://doi.org/10.1186/s12936-023-04623-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12936-023-04623-1