Abstract
Background
In rural Burkina Faso, the primary malaria vector Anopheles gambiae sensu lato (s.l.) primarily feeds indoors at night. Identification of factors which influence mosquito house entry could lead to development of novel malaria vector control interventions. A study was therefore carried out to identify risk factors associated with house entry of An. gambiae s.l. in south-west Burkina Faso, an area of high insecticide resistance.
Methods
Mosquitoes were sampled monthly during the malaria transmission season using CDC light traps in 252 houses from 10 villages, each house sleeping at least one child aged five to 15 years old. Potential risk factors for house entry of An. gambiae s.l. were measured, including socio-economic status, caregiver’s education and occupation, number of people sleeping in the same part of the house as the child, use of anti-mosquito measures, house construction and fittings, proximity of anopheline aquatic habitats and presence of animals near the house. Mosquito counts were compared using a generalized linear mixed-effect model with negative binomial and log link function, adjusting for repeated collections.
Results
20,929 mosquitoes were caught, of which 16,270 (77.7%) were An. gambiae s.l. Of the 6691 An. gambiae s.l. identified to species, 4101 (61.3%) were An. gambiae sensu stricto and 2590 (38.7%) Anopheles coluzzii. Having a metal-roof on the child’s sleeping space (IRR = 0.55, 95% CI 0.32–0.95, p = 0.03) was associated with fewer malaria vectors inside the home.
Conclusion
This study demonstrated that the rate of An. gambiae s.l. was 45% lower in sleeping spaces with a metal roof, compared to those with thatch roofs. Improvements in house construction, including installation of metal roofs, should be considered in endemic areas of Africa to reduce the burden of malaria.
Similar content being viewed by others
Background
Despite large reductions in the malaria burden across sub-Saharan Africa from 2000 to 2015 [1], some countries continue to experience extremely high malaria transmission [2]. In Africa, malaria transmission is highly efficient because of the wide distribution of Anopheles gambiae sensu lato (s.l.), an effective malaria vector that readily feeds on people indoors at night, where about 79% of malaria transmission typically occurs [3]. The indoor density of malaria mosquitoes is dependent on numerous environmental and household factors, including the abundance and proximity of aquatic habitats of malaria mosquitoes [4, 5], presence of large domesticated animals who may serve as alternative blood sources [6], typology of houses [7, 8], use of anti-mosquito measures in the house [5], number of residents [9] and variability in the attractiveness of individual people [10] (Fig. 1).
Environmental and household factors affecting the abundance of malaria vectors indoors. Indoor malaria vector abundance is affected by environmental risk factors such as weather conditions, proximity and productivity of natural and human-made larval habitats, presence of livestock and animals that may divert or attract malaria vectors, outdoor activities such as cooking, sleeping or playing which may increase biting (especially where outdoor early evening biting is a problem). Indoor malaria vector density can be reduced by features of the house construction (e.g. screening, closed eaves) and by use of personal protective measures such as ITNs and household insecticides. Increased human density indoors increases the odour plume of carbon dioxide and other attractants which can attract malaria vectors towards an inhabited house
Burkina Faso is an area of intense seasonal malaria transmission, and cases are increasing [11,12,13] despite high coverage of vector control tools, including three national insecticide-treated net (ITN) mass distribution campaigns in 2010, 2013 and 2016 [14]. Resistance to pyrethroids, the main insecticide class used for treating ITNs, is high in An. gambiae s.l., and research conducted in the study area suggests that exposure to ITNs may have no impact on the lifelong survival of malaria vectors [15]. New tools are urgently needed to reduce the burden of malaria in Burkina Faso and other countries in sub-Saharan Africa.
Several studies have demonstrated that malaria mosquito house entry can be reduced through simple changes to house design, such as closing eaves and screening windows and doors [16]. The use of personal protective measures such as ITNs and spatial repellents may also reduce transmission [17, 18]. There is a lack of evidence, of whether such methods will reduce house entry of malaria vectors in settings of high insecticide resistance, such as the study site in south-west Burkina Faso. A risk factor survey was conducted to identify variables associated with indoor density of An. gambiae s.l. during the malaria transmission season in an area of intense malaria transmission in south-west Burkina Faso. Findings from this study might identify potential opportunities for improving malaria control in Burkina Faso and other countries in sub-Saharan Africa experiencing persistently high malaria transmission.
Methods
Study site
The study was conducted in Banfora Health District, in the Cascades Region, south-west Burkina Faso (Fig. 2). This is an area of Sudanian savannah covering 6295 km2 with an estimated population of 407,073 inhabitants [13]. Malaria transmission is intense and seasonal, occurring mainly during the rainy season, from May to November [19]. Plasmodium falciparum accounts for 90% of cases [19]. The main malaria vectors are An. gambiae sensu stricto (s.s.) and Anopheles coluzzii [20]. In 2016, approximately 1 year before this study took place, a universal coverage campaign distributed ITNs with permethrin or deltamethrin (Sumitomo Chemical, Vestergaard and BASF) at a rate of one net for every two people at risk. No additional ITNs were distributed by the study. No indoor residual spraying was conducted. Families typically live alongside their extended family in compounds, each led by a compound head.
Compounds typically consist of multiple single room buildings arranged in a circular or semi-circular fashion around a shared open space, with sleeping areas, kitchens and toilets existing as separate structures [21]. Polygyny is common, with multiple wives and their children often living in the same compound. Children typically sleep with their mother but once they are old enough (~ 10 years) boys and girls are separated and move into another single room house in the compound.
Study design
The study was nested in a cohort study of risk factors for P. falciparum infection in children aged five to 15 years [22]. This study reports on the household and environmental risk factors associated with the density of An. gambiae s.l. in the children’s sleeping space during the peak malaria transmission period from 24 July to 28 December 2017.
Recruitment of study cohort
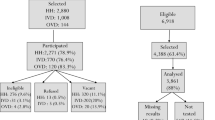
Sampling and recruitment of the study cohort is described elsewhere [23]. In brief, a random sample of 10 villages were selected from a list of villages in the study area using a two-stage process. Firstly, five health centres in the study area were selected, each with a catchment radius of 10 km. Secondly, two villages, at least 3 km apart, were selected from each catchment area. An enumerated list of children in the study villages was obtained from the Banfora Demographic and Health Surveillance System. From each village, a random sample of 30 children aged 5 to 15 years were chosen. Each child was selected from a separate house, and, where possible, a separate compound. Children were included in the study if they were of the appropriate age, were likely to remain resident in the village over the duration of the transmission season and the caregiver provided written informed consent to participate in the study. Children received a curative dose of artemisinin-based combination therapy and 252 children who were successfully cleared of P. falciparum infection (confirmed by polymerase chain reaction, PCR) were included in the cohort study and this current study reports on the entomological surveillance from the children’s sleeping space.
Entomological surveillance
CDC light traps (John Hock, Gainsville, USA) were used to estimate indoor mosquito densities in the study child’s sleeping space. These traps were placed with the bulb 1.5 m above the floor, approximately 0.5 m from the foot end of a bed with an ITN occupied by the study child. Houses were sampled from 19.00 h to 06.00 h every 4 weeks. Two villages (Nofesso and Ouangolodougou) were inaccessible for two weeks at the start of the study period due to flooding. Mosquitoes were taken to the laboratory in cool boxes and killed by freezing. Mosquitoes were identified morphologically using established keys [23]. The presence of circumsporozoites protein (CSP) in An. gambiae s.l. were identified using an enzyme-linked immunosorbent assay [24] and An. gambiae s.l. females were typed to species by PCR [25, 26]. If less than 100 An. gambiae s.l. were caught per house then all were typed to species by PCR, but if the number was greater than 100, a third of the mosquitoes were randomly sampled for PCR analysis.
Risk factor assessment
In June, a questionnaire was administered to the caregiver of the study child to collect information on ethnicity, education level and occupation of caregivers, ITN use during the previous night, use of other protective measures (e.g. insecticide knockdown spray, mosquito coils, traditional spatial repellent), number of people sleeping in the same part of the house as the study child, roof, wall and floor construction of the child’s sleeping space, whether the eaves (the gap between the top of the wall and the roof) were open or closed and presence of mosquito screening. Information was also collected from the head of the child’s household (typically the child’s father) on asset ownership and household characteristics, following standard procedures used in the Burkina Faso Demographic and Health Survey (DHS questionnaire, Additional File 1) [27]. The DHS questionnaire specifically referred to the construction of the head of household’s house, which may or may not have reflected the construction of the study child’s sleeping space due to the social structure in the study area. The number and type of large domestic animals (cattle, goats, sheep, pig, dog, donkeys or horses) tethered within 5 m of the sleeping space was recorded. The sleeping space was geo-located using a handheld global positioning system (GARMIN eTrex 20). Larval surveys were carried out in each village in September, during the peak of the transmission season. All water bodies within 1 km of the sleeping space were mapped, including irrigated fields, streams and ponds, puddles, and foot or hoof prints. The presence of anopheline larvae was recorded with a dipper.
Data management and statistical analysis
Data were collected on Android personal digital assistants programmed using the KoboCollect system and included drop down boxes and consistency checks to reduce data entry errors. Following cleaning, the dataset was locked and saved in Microsoft Access. The primary outcome was the number of female An. gambiae s.l. collected in each child’s sleeping space per night. QGIS Geographic Information System (QGIS Development Team (2019), Open Source Geospatial Foundation Project) was used to determine distances between the child’s sleeping space and aquatic habitats. Principal component analysis (PCA) was used to calculate the socio-economic status (SES) factor score of the head of the child’s household. SES factor scores were ranked, and households divided into five equal wealth quintiles, from 1, the poorest, to 5, the least poor. The entomological inoculation rate (EIR) or estimated number of infectious bites per study child during the transmission season was calculated using the formula EIR = MaSd where Ma is the human biting rate, estimated from the arithmetic mean number of female An. gambiae s.l. caught per light trap night across the transmission season, where S is the proportion of female An. gambiae s.l. found to be CSP positive by village and d is the number of days in the transmission season. Mean values were compared using a t-test and proportions compared using chi-squared tests. A generalized linear mixed-effect model with a negative binomial distribution, to account for overdispersion, and log link function was used to identify risk factors associated with the mean number of An. gambiae s.l. per catch night per sleeping space each month. Risk factors were selected a priori based on importance for malaria vector house entry. These were SES quintile of the household head, ITN use, use of other protective measures, number of people sleeping in the same part of the house as the child, roof, floor and wall material in the sleeping space, eaves (open or closed), presence of large domesticated animals within 5 m of the sleeping space and presence of habitats positive for anopheline larvae within 300 m of the child’s sleeping space. A random effect for study child ID number was used to account for repeated measures on the same sleeping space and village was included as a fixed effect. Univariate analysis was conducted followed by construction of a simple multivariate model in which every risk factor was included, irrespective of whether the variable was significant in the univariate model. Interactions were tested between a subset of variables that were thought to be biologically relevant to explore. Means and 95% confidence intervals were calculated. Statistical analysis was carried out in Stata 15 (Statacorp, Texas, USA). The study is reported following STROBE guidelines [28].
Results
As reported elsewhere [22], a total of 20,929 mosquitoes were caught from 1151 trap collections in 252 children’s sleeping spaces, with 16,270 of these being An. gambiae s.l. (77.7%). Of the 6691 An. gambiae s.l. identified to species (excluding 924 lost and non-identified samples), 4101 were An. gambiae s.s. (61.3%) and 2590 An. coluzzii (38.7%). Malaria vector abundance rose in July after the start of the rains in May, reaching a peak in August, before declining to low levels in November and December. 3.3% of An. gambiae s.l. were CSP positive and the overall EIR in the study area was 80.4 infective bites/child over the six-month transmission season. The village-level EIR ranged from 40.8 in Timperba to 191.9 in Tondoura.
The ethnic composition of the study population was Gouin (38.9%, 98/252), Karaboro (21.8%, 55/252), Mossi (11.5%, 29/252), Turka (9.1%, 23/252), Fulani (6.3%, 16/252), Senoufo (4.4%, 11/252) and other ethnic groups (7.9%, 20/252; Table 1). Caregivers were predominantly illiterate (79.0%, 199/252) and farmers (95.2%, 240/252). 80.6% (203/252) of caregivers reported that their child slept under an ITN the previous night, while 15.9% (40/252) reported using mosquito coils and 6.4% (16/252) insecticide knockdown spray. Children’s sleeping spaces were constructed with predominantly brick walls (57.9%, 146/252), cement or tiled floors (70.6%, 178/252), metal roofs (75.8%, 191/252) and open eaves (54.8%, 138/252). Window screening was rare (0.4%, 1/252). 67.1% (169/252) of households had large domestic animals (cattle, goats, sheep, dogs, pig, donkeys or horses) within 5 m of the house. 50.4% (127/252) of child’s sleeping spaces were located within 300 m of an aquatic habitat containing anopheline larvae.
Sleeping spaces with metal roofs were more likely to have walls and floors made of finished materials and open eaves than thatch roof sleeping spaces. 81.7% (156/191) of sleeping spaces with a metal roof had a cement or tiled floor compared to 42.3% (22/52) of those with a thatch roof (p < 0.001). Metal roof sleeping spaces were also more likely to have brick or cement walls (78.0%, 149/191) compared to thatch roof sleeping spaces (55.8%, 29/52, p < 0.001). Sleeping spaces with a metal roof were also more likely to have open eaves (66.0%, 126/191) than sleeping spaces with a thatch roof (23.1%, 12/52 and 28.8%, 15/52 respectively, p < 0.001 and p = 0.003). Children living in sleeping spaces with a thatch roof were more likely to share the same part of the house with greater than 12 people (38.5%, 20/52), than children living in sleeping spaces with a metal roof (26.2%, 50/191; p < 0.001). There was no association between metal roof sleeping spaces and distance from the nearest anopheline larvae positive habitat (99/191, 51.8% of those in metal roof houses lived within 300 m of a positive anopheline habitat, versus 23/52, 44.2% of those in thatch roof houses, p = 0.38).
There did not appear to be any strong trend between SES quintile of the household head and roof material, floor material or wall material of the child’s sleeping space (Table 2). Children living in poorer households were, however, more likely to have open eaves in their sleeping space (78.3% of quintile 1) than richer households (26.7% of quintile 5; p < 0.001).
In the final multivariate model, having a metal roof (IRR = 0.55, 95% CI 0.32–0.95, p = 0.03) was associated with fewer malaria vectors indoors, after adjusting for the other risk factors including SES of the household head (Table 3). There was no association between malaria vector density and SES of the household head, use of ITNs or other personal protection measures, the number of people living in the same part of the house as the study child, floor or wall material, eave status, presence of domestic animals within 5 m or presence of anopheline positive larval habitats within 300 m of the child’s sleeping space.
Discussion
The study findings demonstrate highly intense transmission of malaria in Banfora Health District with a person sleeping without an ITN experiencing a seasonal EIR varying from 40.8 infectious bites per person in Timperba village to 191.9 in Toundoura village [27]. Reported ITN use was high with 80.6% of caregivers reporting that the study child slept under an ITN the previous night. The incidence rate of An. gambiae s.l. in metal roof sleeping spaces was almost half that in thatch roof houses (IRR = 0.55, 95% CI 0.32–0.95, p = 0.03) and no other significant risk factors were identified.
Finding fewer malaria vectors indoors in metal roof houses compared to thatch roof houses may be a result of the indoor climate of the different typologies of houses. Metal roof houses tend to be hotter and less humid than thatch roof houses which can reduce the survivorship of malaria vectors resting indoors [29]. Alternatively, metal-roofs may simply be a marker for a better-quality home that is less porous to mosquitoes since metal roof houses are often better built, with fewer mosquito entry points, than thatched-roofed houses. This study found metal-roof houses were more likely to have floors and walls made of finished materials, than thatch-roof houses, although the proportion of metal roof houses with open eaves was higher. This was an unusual finding since metal roofs and closing of the eaves are often implemented together.
Reduced malaria vector density in metal-roof houses compared to thatch-roof houses has been reported in several studies, including a Tanzanian study where metal-roof houses had 33% less Anopheles arabiensis than thatch-roof houses [30], and a Ugandan study where there were 38–43% fewer An. gambiae s.l. in metal-roof houses [31]. Results are, however, contradictory in other studies. In The Gambia, metal-roof houses were not associated with fewer mosquitoes [6], and in an experimental study, metal roof houses with closed eaves and mud walls had similar numbers of mosquitoes as thatch-roofed houses with open eaves and mud walls [7]. It may be that there is a trade off between the killing effect of metal roofs due to the hostile indoor climate, and a heating effect of the roof, which can increase carbon dioxide production from humans and therefore attract more malaria vectors [7, 32].
Ultimately, whether a metal-roof house has more or less mosquitoes than a thatch-roof house will depend on how porous the house is to mosquitoes and the extent of ventilation [16]. Further research is needed to understand the importance of different house construction features on indoor climate and vector entry.
The study has several limitations. Firstly, ITN use the previous night was assessed by asking the caregiver, which may be prone to social desirability bias [33]. The use of an ITN will usually vary over the transmission season, but we only measured use during the baseline survey. This may have impacted on our ability to identify an association between ITN usage and indoor density of malaria vectors. Secondly, the study did not collect information on all possible risk factors for malaria vector house entry. For example, whether the doors were kept open until late in the evening was not included in the risk factors assessed. The analysis did not adjust for unmeasured risk factors, which may have confounded the associations observed.
The cohort study in which this entomological study was nested did not identify strong risk factors for P. falciparum infection, with only overnight travel and higher SES factor score being associated with higher rates of P. falciparum infection [22]. It is difficult to reconcile the entomological and epidemiological findings and further studies are needed. It is perhaps unsurprising that the risk factors for malaria vector density and P. falciparum infection in children differed, since higher indoor vector density does not automatically imply higher infection risk. The indoor density of malaria vectors may be less important in this study area due to the observation of increasing outdoor biting with some studies suggesting ~ 54% of An. gambiae s.l. host seeking outdoors [34] or more simply, it does not accurately reflect the transmission intensity experienced by a child sleeping under a net. Research also suggests that the study communities spend more time outside in the peri-domestic environment during peak biting times than previously thought [35].
What are the implications of the study findings for vector control and future research? The study highlights the potential of improved housing to reduce malaria transmission and supports the results of systematic reviews and multi-country research studies on this topic [36, 37]. Housing improvements tend to be implemented as a package and, in line with this, our study found that metal-roof sleeping spaces were more likely to have floors and walls made of finished materials than thatch-roof sleeping spaces. Improving house construction should be a focus for malaria reduction, with increasing evidence in support of screened, self-closing doors, closed eaves, raising buildings off the ground, screened windows on either side of building for ventilation and solid roofs [16, 38, 39]. As well as contributing to the development agenda, there is also evidence that improved housing can reduce risk of other major causes of death in children including diarrhoea, growth failure and anaemia [40]. While other vector control tools such as dual-active ingredient ITNs are now being deployed in the study area, the study results highlight the importance of non-insecticidal interventions such as house improvement to increase long-term resilience against malaria and for insecticide resistance management.
Conclusion
This study in south-west Burkina Faso demonstrates a 45% reduction in indoor density of malaria vectors in sleeping spaces with a metal roof compared to those sleeping spaces with thatch roofs. The study adds to the growing evidence base supporting the use of housing improvement against malaria.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CI:
-
Confidence interval
- CSP:
-
Circumsporozoite protein
- DHS:
-
Demographic and Health Survey
- ITN:
-
Insecticide treated-net
- IRR:
-
Incidence rate ratio
- PCR:
-
Polymerase chain reaction
- SES:
-
Socio-economic status
References
Bhatt S, Weiss DJ, Cameron E, Bisanzio D, Mappin B, Dalrymple U, et al. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature. 2015;526:207–11.
WHO. World Malaria Report 2019. Geneva: World Health Organization; 2019.
Sherrard-Smith E, Skarp J, Beale A, Fornadel C, Norris L, Moore SJ, et al. Mosquito feeding behaviour and how it influences residual malaria transmission across Africa. Proc Natl Acad Sci USA. 2019;116:15086–95.
Hast MA, Stevenson JC, Muleba M, Chaponda M, Kabuya J-B, Mulenga M, et al. Risk factors for household vector abundance using indoor CDC light traps in a high malaria transmission area of northern Zambia. Am J Trop Med Hyg. 2019;101:126–36.
McCann RS, Messina JP, MacFarlane DW, Bayoh MN, Gimnig JE, Giorgi E, et al. Explaining variation in adult Anopheles indoor resting abundance: the relative effects of larval habitat proximity and insecticide-treated bed net use. Malar J. 2017;16:288.
Kirby MJ, Green C, Milligan PM, Sismanidis C, Jasseh M, Conway DJ, et al. Risk factors for house-entry by malaria vectors in a rural town and satellite villages in the Gambia. Malar J. 2008;7:2.
Jatta E, Jawara M, Bradley J, Jeffries D, Kandeh B, Knudsen JB, et al. How house design affects malaria mosquito density, temperature, and relative humidity: an experimental study in rural Gambia. Lancet Planet Health. 2018;2:e498–508.
Mburu MM, Juurlink M, Spitzen J, Moraga P, Hiscox A, Mzilahowa T, et al. Impact of partially and fully closed eaves on house entry rates by mosquitoes. Parasit Vectors. 2018;11:383.
Kaindoa EW, Mkandawile G, Ligamba G, Kelly-Hope LA, Okumu FO. Correlations between household occupancy and malaria vector biting risk in rural Tanzanian villages: implications for high-resolution spatial targeting of control interventions. Malar J. 2016;15:199.
Lindsay SW, Adiamah JH, Miller JE, Pleass RJ, Armstrong JRM. Variation in attractiveness of human subjects to malaria mosquitoes (Diptera: Culicidae) in the Gambia. J Med Entomol. 1993;30:368–73.
Ministère de la Santé Burkina Faso. Annuaire statistique 2016. Ouagadougou: Ministère de la Santé Burkina Faso; 2017.
Ministère de la Santé Burkina Faso. Annuaire statistique 2017. Ouagadougou: Ministère de la Santé Burkina Faso; 2018.
Ministère de la Santé Burkina Faso. Annuaire statistique 2018. Ouagadougou: Ministère de la Santé Burkina Faso; 2019.
Louis VR, Schoeps A, Tiendrebéogo J, Beiersmann C, Yé M, Damiba MR, et al. An insecticide-treated bed-net campaign and childhood malaria in Burkina Faso. Bull World Health Organ. 2015;93:750–8.
Hughes A, Lissenden N, Viana M, Toé KH, Ranson H. Anopheles gambiae populations from Burkina Faso show minimal delayed mortality after exposure to insecticide-treated nets. Parasit Vectors. 2020;13:17.
Lindsay SW, Davies M, Alabaster G, Altamirano H, Jatta E, Jawara M, et al. Recommendations for building out mosquito-transmitted diseases in sub-Saharan Africa: the DELIVER mnemonic. Phil Trans R Soc Lond B Biol Sci. 2020;376:20190814.
Maia MF, Kliner M, Richardson M, Lengeler C, Moore SJ. Mosquito repellents for malaria prevention. Cochrane Database Syst Rev. 2018;2:CD011595.
Pryce J, Richardson M, Lengeler C. Insecticide-treated nets for preventing malaria. Cochrane Database Syst Rev. 2018;11:Cd00063.
Tiono AB, Kangoye DT, Rehman AM, Kargougou DG, Kabore Y, Diarra A, et al. Malaria incidence in children in South-West Burkina Faso: comparison of active and passive case detection methods. PLoS ONE. 2014;9:e86936.
Tiono AB, Guelbeogo MW, Sagnon NF, Nebie I, Sirima SB, Mukhopadhyay A, et al. Dynamics of malaria transmission and susceptibility to clinical malaria episodes following treatment of Plasmodium falciparum asymptomatic carriers: results of a cluster-randomized study of community-wide screening and treatment, and a parallel entomology study. BMC Infect Dis. 2013;13:535.
Guglielmo F, Ranson H, Sagnon NF, Jones C. The issue is not ‘compliance’ exploring exposure to malaria vector bites through social dynamics in Burkina Faso. Anthropol Med. 2021. https://doi.org/10.1080/13648470.2021.1884185.
Yaro JB, Ouedraogo A, Ouedraogo ZA, Diarra A, Lankouande M, Agboraw E, et al. A cohort study to identify risk factors for Plasmodium falciparum infection in Burkinabe children: implications for other high burden high impact countries. Malar J. 2020;19:371.
Gillies MT, Coetzee M. A supplement to the Anophelinae of Africa south of the Sahara. Johannessburg, South Africa. S Afr Inst Med Res. 1987;55:1–143.
Wirtz RA, Duncan JF, Njelesani EK, Schneider I, Brown AE, Oster CN, et al. ELISA method for detecting Plasmodium falciparum circumsporozoite antibody. Bull World Health Organ. 1989;67:535–42.
Fanello C, Santolamazza F, Della Torre A. Simultaneous identification of species and molecular forms of the Anopheles gambiae complex by PCR-RFLP. Med Vet Entomol. 2002;16:461–4.
Scott JABW, Collins FH. Identification of single specimens of the Anopheles gambiae complex by the polymerase chain reaction. Am J Trop Med Hyg. 1993;4:520–9.
Institut National de la Statistique et de la Démographie, Programme National de Lutte contre le Paludisme, ICF International. Enquête sur les Indicateurs du Paludisme (EIPBF) 2014. Burkina Faso, 2015.
von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453–7.
Lindsay SW, Jawara M, Mwesigwa J, Achan J, Bayoh N, Bradley J, et al. Reduced mosquito survival in metal-roof houses may contribute to a decline in malaria transmission in sub-Saharan Africa. Sci Rep. 2019;9:7770.
Kaindoa EW, Finda M, Kiplagat J, Mkandawile G, Nyoni A, Coetzee M, et al. Housing gaps, mosquitoes and public viewpoints: a mixed methods assessment of relationships between house characteristics, malaria vector biting risk and community perspectives in rural Tanzania. Malar J. 2018;17:298.
Rek JC, Alegana V, Arinaitwe E, Cameron E, Kamya MR, Katureebe A, et al. Rapid improvements to rural Ugandan housing and their association with malaria from intense to reduced transmission: a cohort study. Lancet Planet Health. 2018;2:e83–94.
Knudsen JB, Pinder M, Jatta E, Jawara M, Yousuf MA, Søndergaard AT, et al. Measuring ventilation in different typologies of rural Gambian houses: a pilot experimental study. Malar J. 2020;19:273.
Krezanoski PJ, Bangsberg DR, Tsai AC. Quantifying bias in measuring insecticide-treated bednet use: meta-analysis of self-reported vs objectively measured adherence. J Glob Health. 2018;8:010411.
Sanou A. The ecology and behaviour of insecticide resistant malaria vectors and implications for control in Burkina Faso. Thesis, University of Glasgow, 2020.
Guglielmo F, Sanou A, Churcher T, Ferguson HM, Ranson H, Sherrard-Smith E. Quantifying individual variability in exposure risk to mosquito bites in the Cascades region, Burkina Faso. Malar J. 2021;20:44.
Tusting LS, Bottomley C, Gibson H, Kleinschmidt I, Tatem AJ, Lindsay SW, et al. Housing improvements and malaria risk in sub-Saharan Africa: a multi-country analysis of survey data. PLoS Med. 2017;14:e1002234.
Tusting LS, Ippolito MM, Willey BA, Kleinschmidt I, Dorsey G, Gosling RD, et al. The evidence for improving housing to reduce malaria: a systematic review and meta-analysis. Malar J. 2015;14:e209.
Sternberg ED, Cook J, Alou LPA, Assi SB, Koffi AA, Doudou DT, et al. Impact and cost-effectiveness of a lethal house lure against malaria transmission in central Cote d’Ivoire: a two-arm, cluster-randomised controlled trial. Lancet. 2021;397:805–15.
Carrasco-Tenezaca M, Jawara M, Abdi MY, Bradley J, Brittain OS, Ceesay S, et al. The relationship between house height and mosquito house entry: an experimental study in rural Gambia. J R Soc Interface. 2021;18:20210256.
Tusting LS, Gething PW, Gibson HS, Greenwood B, Knudsen J, Lindsay SW, et al. Housing and child health in sub-Saharan Africa: a cross-sectional analysis. PLoS Med. 2020;17:e1003055.
Acknowledgements
The authors wish to thank the CNRFP staff, community members, opinion leaders, the community health workers, research assistants, field supervisors and workers whose cooperation and help made this study possible. Thanks also to Federica Guglielmo for helpful conversations on community structures in the study site.
Funding
This project was supported by the Wellcome Trust (Wellcome Trust Collaborative Award “Improving the efficacy of malaria prevention in an insecticide resistant Africa (MiRA)” to the Liverpool School of Tropical Medicine grant agreement number 200222/Z/15/Z). SWL and ALW are supported by the Global Challenges Research Fund and Biotechnology and Biological Sciences Research Council (BB/R00532X/1) award to the BOVA Network (Building Out Vector borne diseases in sub-Saharan Africa). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. JB received support from the UK MRC and the UK DFID (#MR/R010161/1) under the MRC/DFID Concordat agreement and as part of the EDCTP2 programme supported by the European Union.
Author information
Authors and Affiliations
Contributions
Conceived and designed the study: SWL, ALW, ABT, NFS, EW, HR. Conducted field and laboratory work: JBY, ABT, KHT, AS, WMG. Conducted data analysis: JBY, ALW, SWL, ABT, AO, EA, JB. Contributed to and approved the final manuscript: JBY, ALW, SWL, ABT, AO NFS, HR, KHT, AS, WMG, EW, EA. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Permission to enter the communities was sought from village leaders. The caregivers of study participants provided informed consent (or assent of child if aged 12–15 years) to participate in the cohort study and for collection of mosquitoes from the child’s sleeping space. Study documents were approved by the Burkina Faso Ministry of Health Research Ethics Committee (Deliberation No 2016-12-137), CNRFP Institutional Bioethics Committee (No2016/000007/MS/SG/CNRFP/CIB), Durham University Department of Biosciences Ethics Committee (SBBS/EC/MIRA) and Liverpool School of Tropical Medicine Ethical Committee (Protocol number: 16/047). The study was conducted in compliance with principles set out by the International Conference on Harmonization Good Clinical Practice, the Declaration of Helsinki and the regulatory requirements of Burkina Faso.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests. All authors declare that they had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Questionnaire administered to head of household on asset ownership and household characeristics.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yaro, J.B., Tiono, A.B., Sanou, A. et al. Risk factors associated with house entry of malaria vectors in an area of Burkina Faso with high, persistent malaria transmission and high insecticide resistance. Malar J 20, 397 (2021). https://doi.org/10.1186/s12936-021-03926-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12936-021-03926-5