Abstract
Background
In spite of the global effort to eliminate malaria, it remains the most significant vector-borne disease of humans. Plasmodium falciparum is the dominant malaria parasite in sub-Saharan Africa. However, Plasmodium vivax is becoming widely spread throughout Africa. The overuse of vector control methods has resulted in a remarkable change in the behaviour of mosquito that feeds on human as well as on vector composition. The aim of this study was to identify Anopheles mosquito species in vivax malaria endemic regions and to investigate their role in P. vivax circumsporozoite protein (Pvcsp) allele diversity.
Methods
Mosquito samples were collected from Central Sudan (Rural Khartoum and Sennar) and Eastern Sudan (New Halfa, Kassala state) using pyrethrum spray catch (PSC) and CDC light traps. Mosquitoes were identified using appropriate morphological identification keys and Anopheles gambiae complex were confirmed to species level using molecular analysis. A subset of blood-fed anopheline mosquitoes were dissected to determine the presence of natural infection of malaria parasites. In addition, the rest of the samples were investigated for the presence of Pvcsp gene using nested-PCR.
Results
A total of 1037 adult anopheline mosquitoes were collected from New Halfa (N = 467), Rural Khartoum (N = 132), and Sennar (N = 438). Morphological and molecular identification of the collected mosquitoes revealed the presence of Anopheles arabiensis (94.2%), Anopheles funestus (0.5%), and Anopheles pharoensis (5.4%). None of the dissected mosquitoes (N = 108) showed to be infected with malaria parasite. Overall P. vivax infectivity rate was 6.1% (63/1037) by Pvcsp nested PCR. Co-dominance of An. arabiensis and An. pharoensis is reported in Sennar state both being infected with P. vivax.
Conclusion
This study reported P. vivax infection among wild-caught anopheline mosquitoes in Central and Eastern Sudan. While An. arabiensis is the most abundant vector observed in all study areas, An. funestus was recorded for the first time in New Halfa, Eastern Sudan. The documented Anopheles species are implicated in Pvcsp allele diversity. Large-scale surveys are needed to identify the incriminated vectors of P. vivax malaria and determine their contribution in disease transmission dynamics.
Similar content being viewed by others
Background
The estimated malaria cases in 2019 was 229 million cases occurred worldwide resulting in 409,000 malaria related death, owing the deadliest parasite (Plasmodium falciparum), predominantly in sub-Saharan Africa [1]. According to the World malaria report in 2020, there were 2015 million cases in 2019, mostly (94% of total cases) in African continent [1]. Plasmodium falciparum is considered to be the most important malaria species responsible for more than 99.7% of malaria cases [2] followed by P. vivax [3], a generally considered less pathogenic parasite causing a benign type of malaria. However, the “benign tertian malaria” description of vivax malaria has been challenged by recent reports and documentation of severe P. vivax infections and even deaths [4, 5]. Plasmodium vivax stands for about half infections outside Africa [6,7,8], representing 75% of malaria cases in the WHO Region of the Americas, 53% in the WHO Region of South-East Asia [1], and 40% in the Eastern Mediterranean Region [7]. However, its presence in Africa has not well documented and reported because of the very high endemicity of P. falciparum and for the accepted paradigm that Africans are protected from P. vivax infection by genetic factors [9, 10].
Plasmodium vivax parasite exploits the human Duffy antigen/chemokine receptor (DARC) to invade the red blood cell [11]. Duffy antigen is rarely expressed in the African populations [12], so infection prevalence was thought to be less in Africa due to negativity of Duffy binding protein among its population [8, 13]. However, several studies revealed that infection may persist in individuals lacking this receptor [14, 15].
In Sudan, P. falciparum is responsible for 91.2% of malaria cases while P. vivax makes 8.8% of the cases [16, 17]. However, during the recent years, the number P. vivax infections is increased throughout the country with an overall prevalence of 26.6% [18], and prevalence of 40%, 38% in White Nile and Gezira states, respectively [15, 19]. The role of Anopheles mosquitoes in transmitting malaria parasites depends on several factors including their preference to feed on humans [20] and their innate susceptibility to the Plasmodium [21, 22]. The main malaria vectors in Africa belong to three major groups of vectors, the Anopheles gambiae complex, the Anopheles funestus group, and the Anopheles nili complex [23, 24]. Methods of mosquito control still rely heavily on the use of long-lasting insecticidal nets (LLINs) and indoor residual spraying (IRS) [25, 26], that target indoor resting vectors. An updated study of anopheline mosquitoes and their behaviour is much needed to guide the vector control operations [27, 28].
The vast majority of studies stated that Anopheles arabiensis is the main if not the only malaria vector in Sudan [29,30,31,32]. However, few studies encountered the presence of other Anopheles species at malaria foci in Sudan, such as An. funestus sensu stricto (s.s.) [33], Anopheles pharoensis [34] and An. nili sensu lato (s.l.) [35].
Plasmodium circumsporozoite protein (CSP) is an abundant surface protein expressed on the surface of sporozoite and oocyst [36] its expression starting on day seven onwards post mosquito infection [37]. This protein is implicated in salivary gland invasion in mosquitoes, sporozoite maturation, and hepatocyte invasion in humans [38]. Plasmodium vivax circumsporozoite (Pvcsp) gene has three distinctive variants (VK 210, VK 247, and P. vivax-like) [39,40,41].
The present study was conducted to identify the anopheline mosquito fauna in regions where P. vivax malaria is endemic and to investigate their possible role in P. vivax circumsporozoite protein (Pvcsp) gene allele diversity.
Methods
Study areas and mosquito collection
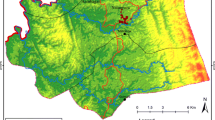
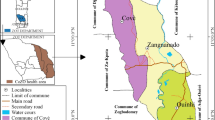
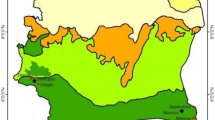
The study was conducted in two regions, Central Sudan (Gezura Slanj, Rural Khartoum state 15.7938° N, 32.8987° E, Sennar state 13.0317° N, 33.9750°) and Eastern Sudan (New Halfa, Kassala state 15.3288° N, 35.5986° E) (Fig. 1). The collection of the adult anopheline mosquito samples was carried out between September 2015 to February of 2016 using standard Pyrethrum Spray Catch (PSC), and CDC light traps. In each study area, two endemic malaria sites were chosen randomly for mosquito adult collection. The collection period was three consecutive nights per month per site.
Samples processing and morphological identification
The collected adult mosquitoes were sorted out according to genus level. All female anopheline mosquitoes were divided into blood-fed, unfed, and gravid. A subset of blood-fed samples, collected by CDC light traps, was kept alive and dissected immediately using dissecting stereomicroscope and sterilized dissecting needles to detect the natural infection of Plasmodium parasites according to [42], while the rest of the samples, in addition to carcasses of freshly dissected samples, were preserved as dry specimens in labelled eppendorf tubes containing silica gel and stored at room temperature until morphological identification and molecular analysis. Samples were identified to species level using standard entomological keys [23, 43].
DNA extraction and Pvcsp nested-PCR
Genomic DNA was extracted from whole individual mosquito using the Livak method [44]. Samples of An. gambiae complex were subjected to molecular identification following the protocol of Scott et al. [45], with slight modification on the cycling number to be set as 36 cycles.
Nested-PCR was performed to detect Pvcsp gene following [46, 47] with minor modifications. Cycling condition for the outer PCR was as follows: 95 ºC initial denaturation for 3 min, 37 cycles of: denaturation at 94 ºC for 30 s, annealing at 58 ºC for 1 min, 72 ºC elongation for 1 min, and final elongation was performed at 72 ºC for 10 min. The nested PCR was set as follows: 95 ºC initial denaturation for 3 min, 15 cycles of: 94 ºC for 30 s, 63.8 ºC for 1 min, 72 ºC for 2 min, cycles of: 94 ºC for 30 s, 64 ºC for 1 min, 72 ºC for 2 min and final elongation at 72 ºC for 10 min.
PCR products were separated in 1% agarose gel stained with ethidium bromide and observed under UV using BioDocAnalyze gel image documentation system (Biometra Analytika Jena Company, Germany).
Results
A total of 1037 adult female anopheline mosquitoes were collected from three study sites; New Halfa (N = 467), Rural Khartoum (N = 132), and Sennar (N = 438). Out of the total, 644 mosquitoes were blood-fed, 393 were unfed, while 117 were gravid (Table 1). The combined morphological and molecular identification revealed that the species composition of the collected mosquito was predominately An. arabiensis 94.2% (976/1037), An. pharoensis 5.4% (56/1037), and An. funestus s.s. 0.5% (5/1037) (Table 1).
A subset (N = 108) of blood-fed mosquitoes were dissected to detect natural infection with malaria parasite (P. falciparum and P. vivax). None was found infected with sporozoites. CSP gene analysis showed that sixty-three samples showed presence of Pvcsp gene, with overall infectivity rate (6.1%). Details of numbers of infected mosquito species among study sites are presented in Table 1.
Six Pvcsp allelic variants were found in two study areas. In New Halfa, five allelic variants (700 bp, 300 bp, 250 bp, 200 bp and 100 bp) were detected (Fig. 2), while in Sennar state, two allelic variants (400 bp and 300 bp) were detected in mosquito samples (Fig. 2).
Discussion
The present study was conducted to identify the species of Anopheles in regions endemic for P. vivax malaria and to investigate their role in P. vivax circumsporozoite protein (Pvcsp) gene allele diversity.
Updating the existed knowledge about the vector composition and relative density in malaria endemic areas are essential entomological and epidemiological indicators for the disease burden, transmission season, and monitoring the vector control methods [48,49,50].
Previous studies in Sudan [5, 31, 51] reported that P. falciparum is responsible for more than 95% of clinical malaria cases while revealing 3% were due to P. vivax. However, recently there an increasing numbers P. vivax cases reported in many parts of the country [18].
Results of this study demonstrated that An. arabiensis was the most abundant Anopheles species, followed by An. pharoensis, and the least was An. funestus, supporting that An. arabiensis is the principal malaria vector in Sudan. For the first time, An. pharoensis was found positive for P. vivax. In addition to Lewis in 1956 [35], no further published data from Sudan had suggested a role for An. pharoensis, which is considered to be a secondary vector. Other studies in Sennar [31], and Gezira state [30] reported An. arabiensis to harbouring malaria parasites. In contrast to Himeidan et al. and Lewis [52, 53], who had recorded the presence of An. pharoensis and Anopheles multicolour in New Halfa, this study showed the presence of An. funestus in the same area but in a small number.
In this study, sporozoites were not observed in the dissected blood fed mosquitoes. This result is similar to previous studies conducted in Sennar and Khartoum [31, 54]. One explanation for this finding could be due to the fact that fresh blood fed mosquitoes may have been freshly infected by early stages of Plasmodium parasites during their sexual life cycle such as mature gametes, zygotes, ookinetes, oocysts. Appearance of sporozoites usually requires approximately two weeks from the time of ingesting infected blood meal with malaria parasites by mosquito vectors [37].
In this study, P. vivax was not detected in any mosquito collected in Rural Khartoum in accordance with a previous study conducted in Khartoum state [55]. In the present study, six distinct variants of P. vivax were identified (five alleles and two alleles in New Halfa Sennar, repectively) and a marked difference in infectivity rates of the identified mosquitoes (An. arabiensis versus An. pharoensis) was demonstrated in the two study sites. This variation in Pvcsp allelic distribution perhaps is due to adaptation of parasites to local mosquito species thus transmit distinct P. vivax variants/haplotypes with different efficiency [56, 57]. A better understanding of co-evolutionary dynamics between co-dominant mosquitoes and parasites will facilitate the identification of molecular mechanisms related to disease transmission and provide important data to guide malaria control. Plasmodium vivax is becoming a serious health problem exhibiting a wide range of hosts children adults and even pregnant women [58]. It has also been detected in asymptomatic individuals [59, 60]. The underestimation of P. vivax malaria infections could be attributed to misdiagnosis of the infection using rapid diagnostic tests (RDTs) [61], or the presence of hypnozoites which cannot be detected using RDTs [62].
Assessment of the impact of vector control interventions on malaria transmission requires more data about entomological indicators including the identification of the vector composition, distribution, and density [63].
Conclusion
The findings of this study are very alarming mainly because it showed the expansion of the efficient malaria vector distribution, An. funestus, into Eastern Sudan. The study also confirms the role of Anopheles species in Pvcsp allele diversity in Sudan. These findings suggest changes in malaria epidemiology in Sudan that requires further entomological, parasitological, and epidemiological studies to accurately determine the distribution and density of malaria vectors countrywide, and to investigate their role in the malaria transmission. Additionally, the potential association between vector species and different Plasmodium species they transmit, need to be investigated thoroughly. Furthermore, the susceptibility of the malaria vectors in Sudan to the currently applied vector control tools must be urgently investigated.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Abbreviations
- PSC:
-
Pyrethrum Spray Catch
- s. s.:
-
Sensu stricto
- s. l.:
-
Sensu lato
- CSP:
-
Circumsporozoite protein
- Pvcsp:
-
Plasmodium vivax Circumsporozoite protein
- WHO:
-
World Health Organization
- DARC:
-
Duffy antigen/chemokine receptor
- LLINs:
-
Long-lasting insecticidal nets
- IRS:
-
Indoor residual spraying
- SNP:
-
Single nucleotide polymorphism
- IGS:
-
Intergenic spacer region
- PCR:
-
Polymerase chain reaction
- UV:
-
Ultra violet
- RDTs:
-
Rapid diagnostic tests
- VK:
-
P. vivax Csp gene variant
References
WHO. World Malaria Report 2020. Geneva, World Health Organization, 2020. https:// https://www.who.int/publications/i/item/9789240015791.
WHO. World Malaria Report 2018. Geneva, World Health Organization, 2018. https://www.who.int/malaria/publications/world-malaria-report-2018/en/.
Guerra C, Howes R, Patil A, Gething P, Van Boeckel T, Temperley W, et al. The international limits and population at risk of Plasmodium vivax transmission in 2009. PLoS Negl Trop Dis. 2010;4:e774.
Tjitra E, Anstey N, Sugiarto P, Warikar N, Kenangalem E, Karyana M, et al. Multidrug-resistant Plasmodium vivax associated with severe and fatal malaria: a prospective study in Papua. Indonesia PLoS Med. 2008;5:e128.
Mahgoub H, Gasim G, Musa I, Adam I. Severe Plasmodium vivax malaria among Sudanese children at New Halfa Hospital. Eastern Sudan Parasit Vectors. 2012;5:154.
WHO. World Malaria Report 2016. Geneva, World Health Organization, 2016. https://www.who.int/malaria/publications/world-malaria-report-2016/report/en/.
WHO. World Malaria Report 2017. Geneva, World Health Organization, 2017. https://www.who.int/malaria/publications/world-malaria-report-2017/en/.
Howes R, Reiner R Jr, Battle K, Longbottom J, Mappin B, Ordanovich D, et al. Plasmodium vivax transmission in Africa. PLoS Negl Trop Dis. 2015;9:e0004222.
Miller L, Mason S, Clyde D, McGinniss M. The resistance factor to Plasmodium vivax in blacks - The Duffy-blood-group genotype. FyFy N Engl J Med. 1976;295:302–4.
Zimmerman P. Plasmodium vivax infection in Duffy-negative people in Africa. Am J Trop Med Hyg. 2017;97:636–8.
Mendis K, Sina B, Marchesini P, Carter R. The neglected burden of Plasmodium vivax malaria. Am J Trop Med Hyg. 2001;64(1–2 Suppl):97–106.
Howes R, Patil A, Piel F, Nyangiri O, Kabaria C, Gething P, et al. The global distribution of the Duffy blood group. Nat Commun. 2011;2:266.
Twohig K, Pfeffer D, Baird J, Price R, Zimmerman P, Hay S, et al. Growing evidence of Plasmodium vivax across malaria-endemic Africa. PLoS Negl Trop Dis. 2019;13:e0007140.
Ryan J, Stoute J, Amon J, Dunton R, Mtalib R, Koros J, et al. Evidence for transmission of Plasmodium vivax among a duffy antigen negative population in Western Kenya. Am J Trop Med Hyg. 2006;75:575–81.
Abdelraheem M, Albsheer M, Mohamed H, Amin M, Mahdi Abdel Hamid M. Transmission of Plasmodium vivax in Duffy-negative individuals in central Sudan. Trans R Soc Trop Med Hyg. 2016;110:258–60.
FMoH. Sudan malaria diagnosis and treatment protocol 2017. Khartoum, 2017. https://reliefweb.int/sites/reliefweb.int/files/resources/sudan_malaria_treatment_protocol_final.21_nov_docx.pdf
Hussien M, Abdel Hamid M, Elamin E, Hassan A, Elaagip A, Salama A, et al. Antimalarial drug resistance molecular makers of Plasmodium falciparum isolates from Sudan during 2015–2017. PLoS ONE. 2020;15:e0235401.
Elgoraish A, Elzaki S, Ahmed R, Ahmed A, Fadlalmula H, Abdalgader Mohamed S, et al. Epidemiology and distribution of Plasmodium vivax malaria in Sudan. Trans R Soc Trop Med Hyg. 2019;trz044.
Suliman M, Hamad B, Albasheer M, Elhadi M, Amin Mustafa M, Elobied M, et al. Molecular evidence of high proportion of Plasmodium vivax malaria infection in White Nile area in Sudan. J Parasitol Res. 2016;2016:2892371.
WHO. Global Strategic Framework for Integrated Vector Management. Geneva, World Health Organization, 2004.
Blandin S, Levashina E. Mosquito immune responses against malaria parasites. Curr Opin Immunol. 2004;16:16–20.
Yassine H, Osta M. Anopheles gambiae innate immunity. Cell Microbiol. 2010;12:1–9.
Gillies M, Coetzee M. A supplement to the Anophelinae of Africa south of the Sahara (Afrotropical Region). Publ S Afr Inst Med Res. 1987;55:1–143.
Sinka M, Bangs M, Manguin S, Rubio-Palis Y, Chareonviriyaphap T, Coetzee M, et al. A global map of dominant malaria vectors. Parasit Vectors. 2012;5:69.
Noor A, Mutheu J, Tatem A, Hay S, Snow R. Insecticide-treated net coverage in Africa: mapping progress in 2000–07. Lancet. 2009;373:58–67.
WHO. Global technical strategy for malaria 2016–2030. Geneva, World Health Organization, 2015. https://www.who.int/malaria/areas/global_technical_strategy/en/.
Mabaso M, Sharp B, Lengeler C. Historical review of malarial control in southern African with emphasis on the use of indoor residual house-spraying. Trop Med Int Health. 2004;9:846–56.
Bayoh M, Mathias D, Odiere M, Mutuku F, Kamau L, Gimnig J, et al. Anopheles gambiae: historical population decline associated with regional distribution of insecticide-treated bed nets in western Nyanza Province. Kenya Malar J. 2010;9:62.
Haridi A. Partial exophily of Anopheles gambiae species B in the Khashm Elgirba area in eastern Sudan. Bull World Health Organ. 1972;46:39–46.
Ahmed, I. Malaria Transmission in Abu Ushar Village, Gezira Irrigated Area, Central Sudan. University of Khartoum. 2003; MSc. thesis. http://khartoumspace.uofk.edu/handle/123456789/11390.
Elmahdi Z, Nugud A, Elhassan I. Estimation of malaria transmission intensity in Sennar state, central Sudan. East Mediterr Health J. 2012;18:951–6.
Ageep T, Damiens D, Alsharif B, Ahmed A, Salih E, Ahmed F, et al. Participation of irradiated Anopheles arabiensis males in swarms following field release in Sudan. Malar J. 2014;13:484.
Makhawi A, Aboud M, El Raba’a F, Osman O, Elnaiem D. Identification of Anopheles species of the Funestus group and their role in malaria transmission in Sudan. J Appl Industry Sci. 2015;3:58–62.
el Gaddal A, Haridi A, Hassan F, Hussein H. Malaria control in the Gezira-Managil Irrigated Scheme of the Sudan. J Trop Med Hyg. 1985;88:153–9.
Lewis D. The anopheline mosquitoes of the Sudan. Bull Entomol Res. 1956;47:475–94.
Coppi A, Natarajan R, Pradel G, Bennett B, James E, Roggero M, et al. The malaria circumsporozoite protein has two functional domains, each with distinct roles as sporozoites journey from mosquito to mammalian host. J Exp Med. 2011;208:341–56.
Kojin B, Adelman Z. The Sporozoite’s Journey Through the mosquito: A critical examination of host and parasite factors required for salivary gland invasion. Front Ecol Evol. 2019;7:284.
Arnot D, Barnwell J, Tam J, Nussenzweig V, Nussenzweig R, Enea V. Circumsporozoite protein of Plasmodium vivax: gene cloning and characterization of the immunodominant epitope. Science. 1985;230:815–8.
Cassiano G, StortiMelo L, Póvoa M, Galardo A, Rossit A, Machado R. Development of PCR-RFLP assay for the discrimination of Plasmodium species and variants of P.vivax (VK210, VK247 and P. vivax-like) in Anopheles mosquitoes. Acta Trop. 2011;118:118–22.
de Almeida SE, Sucupira I, de Oliveira MB, Catete C, de Souza R, Dos Santos A, et al. VK210 and VK247 genotypes of Plasmodium vivax in anopheline mosquitoes from Brazilian Amazon. Sci Rep. 2019;9:9391.
Gonzalez J, Hurtado S, Arevalo-Herrera M, Herrera S. Variants of the Plasmodium vivax circumsporozoite protein (VK210 and VK247) in Colombian isolates. Mem Instit Oswaldo Cruz. 2001;96:709–12.
WHO. Manual on practical entomology in malaria. Geneva, World Health Organization, 1975. https://apps.who.int/iris/handle/10665/42481.
Gillies M, De Meillon B. The Anophelinae of Africa south of the Sahara (Ethiopian Zoogeographical Region). Publ S Afr Inst Med Res. 1968;54:1–343.
Livak K. Organization and mapping of a sequence on the Drosophila melanogaster X and Y chromosomes that is transcribed during spermatogenesis. Genetics. 1984;107:611–34.
Scott J, Brogdon W, Collins F. Identification of single specimens of the Anopheles gambiae complex by the polymerase chain reaction. Am J Trop Med Hyg. 1993;49:520–9.
Imwong M, Pukrittayakamee S, Grüner A, Rénia L, Letourneur F, Looareesuwan S, et al. Practical PCR genotyping protocols for Plasmodium vivax using Pvcs and Pvmsp1. Malar J. 2005;4:20.
Lopez A, Ortiz A, Coello J, Sosa-Ochoa W, Torres R, Banegas E, et al. Genetic diversity of Plasmodium vivax and Plasmodium falciparum in Honduras. Malar J. 2012;11:391.
Collins F, Besansky N. Vector biology and the control of malaria in Africa. Science. 1994;264:1874–5.
Coetzee M, Craig M, le Sueur D. Distribution of African malaria mosquitoes belonging to the Anopheles gambiae complex. Parasitol Today. 2000;16:74–7.
Drake J, Beier J. Ecological niche and potential distribution of Anopheles arabiensis in Africa in 2050. Malar J. 2014;13:213.
Abdalla S, Malik E, Ali K. The burden of malaria in Sudan: incidence, mortality and disability–adjusted life–years. Malar J. 2007;6:97.
Himeidan Y, Dukeen M, El-Rayah A. Adam I Anopheles arabiensis: abundance and insecticide resistance in an irrigated area of eastern Sudan. East Mediterr Health J. 2004;10:167–74.
Lewis D. Observations on Anopheles gambiae and other mosquitoes at Wadi Halfa. Trans R Soc Trop Med Hyg. 1944;38:215–29.
El Sayed B, Arnot D, Mukhtar M, Baraka O, Dafalla A, Elnaiem D, et al. A study of the urban malaria transmission problem in Khartoum. Acta Trop. 2000;75:163–71.
Ali T. Occurrence and distribution of phenotypic and genotypic resistant strain of Anopheles arabiensis to insecticides and Plasmodium sporozoites infection in Khartoum State, Sudan. PhD Thesis, Sudan University of Science & Technology. 2015. http://repository.sustech.edu/handle/123456789/12954.
Joy D, Gonzalez-Ceron L, Carlton J, Gueye A, Fay M, McCutchan T, et al. Local adaptation and vector-mediated population structure in Plasmodium vivax malaria. Mol Biol Evol. 2008;25:1245–52.
González-Cerón L, Rodríguez M, Nettel-Cruz J, Hernández-Ávila J, Malo-García I, Santillán-Valenzuela F, et al. Plasmodium vivax CSP-Pvs25 variants from southern Mexico produce distinct patterns of infectivity for Anopheles albimanus versus An. pseudopunctipennis, in each case independent of geographical origin. Parasit Vectors. 2019;12:86.
Rayis D, Ahmed M, Omer E, Adam I. Plasmodium vivax malaria among pregnant women in Eastern Sudan. Asian Pacific J Trop Dis. 2016;6:2021–3.
Motshoge T, Ababio G, Aleksenko L, Read J, Peloewetse E, Loeto M, et al. Molecular evidence of high rates of asymptomatic P. vivax infection and very low P. falciparum malaria in Botswana. BMC Infect Dis. 2016;16:520.
Björkman A. Asymptomatic low-density malaria infections: a parasite survival strategy? Lancet Infect Dis. 2018;18:485–6.
El-Amin E, Elbashir M, Elamin O, Mukhtar Y, Abdo H, Abdul-Rahman I, et al. The underlying aetiologies of coma in febrile Sudanese children. Trans R Soc Trop Med Hyg. 2013;107:307–12.
Lin J, Saunders D, Meshnick S. The role of submicroscopic parasitemia in malaria transmission: what is the evidence? Trends Parasitol. 2014;30:183–90.
Gnanguenon V, Govoetchan R, Agossa F, Ossè R, Oke-Agbo F, Azondekon R, et al. Transmission patterns of Plasmodium falciparum by Anopheles gambiae in Benin. Malar J. 2014;13:444.
Acknowledgements
The authors would like to thank all health workers in respective study areas who actively participated in the field work and facilitated accessing the endemic sites.
Funding
This research received partial funding from Third World Academy of Sciences (TWAS), Italy (Research Grant Agreement No. 19–225 RG/BIO/AF/AC_G-FR3240310127).
Author information
Authors and Affiliations
Contributions
MMAH and MMA: conception and design of project, OFA, AA and MMA: Sample collection and preservation. OFA: conducted the lab work. OFA, AE, GMP and MMAH: analysis and interpretation of data. OFA, AE, GMP, AA and MMAH: drafted and corrected the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Abdelwhab, O.F., Elaagip, A., Albsheer, M.M. et al. Molecular and morphological identification of suspected Plasmodium vivax vectors in Central and Eastern Sudan. Malar J 20, 132 (2021). https://doi.org/10.1186/s12936-021-03671-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12936-021-03671-9