Abstract
Resveratrol (3,5,4′-trihydroxy-trans-stilbene) is a natural phenol that is present in the skin of the grape, blueberry, raspberry, mulberry, and peanut. This substance is synthesized in these plants following injury or exposure to pathogens. Resveratrol is used as a dietary supplement for a long time and its effects have been assessed in animal models of human disorders. It has potential beneficial effects in diverse pathological conditions such as diabetes mellitus, obesity, hypertension, neoplastic conditions, Alzheimer's disease, and cardiovascular disorders. Notably, resveratrol has been found to affect the expression of several genes including cytokine coding genes, caspases, matrix metalloproteinases, adhesion molecules, and growth factors. Moreover, it can modulate the activity of several signaling pathways such as PI3K/AKT, Wnt, NF-κB, and Notch pathways. In the current review, we summarize the results of studies that reported modulatory effects of resveratrol on the expression of genes and the activity of signaling pathways. We explain these results in two distinct sections of non-neoplastic and neoplastic conditions.
Similar content being viewed by others
Introduction
Resveratrol (3,5,4′-trihydroxy-trans-stilbene) is a natural phenol that is synthesized by numerous plants following injury or exposure to pathogens [1]. The skin of the grape, blueberry, raspberry, mulberry, and peanut is regarded as a source of resveratrol [2]. Resveratrol is used as a dietary supplement and its effects have been assessed in animal models of human disorders (Fig. 1). Resveratrol is a pan-assay interference agent that makes positive impacts in various laboratory tests [3]. These effects are mediated through its interactions with biomolecules on cell membranes [4]. In plants, resveratrol is synthesized by the enzyme resveratrol synthase [5].
In humans, resveratrol can be administered through buccal delivery being absolved via the saliva. Yet, buccal delivery is not an efficient route since it has low aqueous solubility [6]. Moreover, high amounts of hepatic glucuronidation and sulfonation further limit the bioavailability of resveratrol [7]. Resveratrol is glucuronidated and sulfonated in the intestinal and hepatic tissues. Its sulfonation in the intestine is induced by microbial activity [8]. While the half-life of resveratrol is about 8–14 min, sulphate and glucuronide resveratrol metabolites have half-lives of more than 9 h [9].
This agent has been found to alter the expression of several genes in different pathological conditions. In the current review, we summarize the results of studies that reported modulatory effects of resveratrol on the expression of genes and the activity of signaling pathways. We explain these results in two distinct sections of non-neoplastic and neoplastic conditions. The main focus of this manuscript is on studies that reported modulatory effects of resveratrol on PI3K/AKT signaling pathway.
Effects of resveratrol on gene expression in non-neoplastic conditions
Cardiac diseases
In order to assess the protective effects of resveratrol against cardiac hypertrophy, Guan et al. have exposed male rats to Male rats were exposed to chronic intermittent hypoxia (CIH). CIH has resulted in the elevation of heart weight/body weight and left ventricle weight/body weight ratios as well as left ventricular remodeling. Moreover, authors have reported elevation of the apoptosis index, up-regulation of oxidative biomarkers, increase in autophagy marker Beclin-1, and down-regulation of p62 in the CIH group. Intragastric administration of resveratrol has enhanced cardiac function, amended cardiac hypertrophy, and reversed CIH-induced changes in oxidative stress and apoptosis. Mechanistically, PI3K/AKT-associated suppression of the mTOR pathway has been identified as the mediator of effects of resveratrol autophagy activation following CIH stimulation [14]. In an experiment in aged rats, Lin et al. have shown swimming exercise training, resveratrol treatment, or a combination of both can improve heart function. Authors have also reported a slight increase in the activity of the PI3K/AKT pathway in rats subjected to exercise training and resveratrol treatment. Yet, the activity of SIRT1 in the aged rat hearts has been only with resveratrol treatment. Besides, rats exposed to both interventions exhibited activation of both SIRT1 and PI3K/AKT pathways and inhibition of FOXO3 accumulation [15]. Table 1 describes the impact of resveratrol on the expression of genes in the context of cardiovascular disorders.
Based on the anti-thrombotic and anti-inflammatory effects of resveratrol, this agent is also suggested to decreases COVID-19-associated mortality, which is due to activation of thrombotic and inflammatory cascades [18].
Central nervous system (CNS) disorders
Resveratrol has been found to have neuroprotective effects against early brain injury (EBI) following subarachnoid hemorrhage (SAH). Experiments in rat models have shown that intraperitoneal administration of this agent decreases mortality and brain edema following SAH. Moreover, resveratrol has enhanced neurological scores in these animals. Histological studies have shown the effect of resveratrol in the reduction of neuronal pyknosis and swelling. Moreover, resveratrol has enhanced expressions of beclin-1, LC3-II, LC3-II/LC3-I, and Bcl-2, while decreasing p-AKT, p-mTOR, p62, cleaved caspase-3, caspase-9, and BAX levels. Further studies have verified the effects of resveratrol in the induction of autophagy. Therefore, the neuroprotective effect of resveratrol is exerted through the regulation of autophagy and apoptosis via modulating the AKT/mTOR pathway [19].
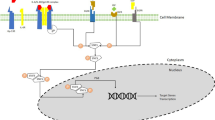
Neuroprotective effects of resveratrol have also been investigated in a rat model of middle cerebral artery occlusion. Resveratrol has remarkably enhanced neurological function, decreased cerebral infarct size, reduced neuron injury, and diminished neuron apoptosis. Mechanistically, resveratrol up-regulates p-JAK2, p-STAT3, p-AKT, p-mTOR, and BCL-2 levels, while down-regulating cleaved caspase-3 and BAX levels. Taken together, resveratrol protects against cerebral ischemia/reperfusion injury through induction of the activities of JAK2/STAT3 and PI3K/AKT/mTOR pathways [20]. Another experiment has shown that resveratrol reduces neurological deficit scores and MPO activity and suppresses induction of IL-1β, TNFα, and COX2 inflammatory markers. In addition, resveratrol attenuates ischemic brain injury following cerebral artery occlusion via modulation of PI3K/AKT signaling pathway [21] (Fig. 2). Through upregulating heme oxygenase-1 (HO-1) via the PI3K/AKT/Nrf2 axis, resveratrol can attenuate the cytotoxic effects of amyloid-β1–42 in PC12 cells [22]. Moreover, through activating PP2A and PI3K/AKT induced-inhibition of GSK-3β, resveratrol can inhibit Tau phosphorylation in the rat brain [23]. Thus, resveratrol may be considered as an anti-Alzheimer's disease substance. Table 2 describes the impact of resveratrol on the expression of genes in the context of CNS disorders.
Resveratrol could activate the PI3K/AKT pathway [25]. On the other hand, this mentioned pathway could increase the Nrf2 translocation, finally induce transcription of anti-oxidative enzymes involved in inhibiting apoptosis. Moreover, GSK-3β could inhibit the Nrf2-ARE, then the transcription of antioxidant enzymes is induced. Interestingly, resveratrol by inactivating JAK-STAT or the NF-kB pathways could decrease ROS production and cell death [34, 35]
A clinical trial in patients with Alzheimer's disease has shown measurable levels of resveratrol and its major metabolites in plasma and cerebrospinal fluid of patients following treatment with this substance. However, brain volume loss has been promoted by treatment with resveratrol [33].
Diabetic complications
The beneficial effects of resveratrol on cardiac function have been assessed in an animal model of diabetic cardiomyopathy. Resveratrol has suppressed high glucose-associated apoptosis of ventricular myocytes in neonatal rats. Moreover, resveratrol has reversed the effects of high glucose in reduction of cell viability, inhibition of AKT and FoxO3a phosphorylation, and suppression of cytoplasmic transfer of FoxO3a. The protective effects of resveratrol have been abolished by a PI3K inhibitor, indicating that the therapeutic effect of this agent is mediated through inhibition of apoptosis via the PI3K/AKT/FoxO3a cascade [36]. Another study has shown that resveratrol through up-regulating mmu-miR-363-3p via the PI3K/AKT pathway can reverse high-fat diet-induced insulin resistance [37]. Resveratrol has also shown protective effects against high glucose-associated apoptosis and senescence of nucleus pulposus cells. Functionally, resveratrol inhibits the production of reactive oxygen species (ROS) and activates PI3K/AKT pathway under the high glucose condition [38]. The protective effects of resveratrol against diabetic nephropathy are exerted through modulation of PI3K/AKT/FoxO3a pathway, attenuation of the high glucose-induced oxidative stress, and reduction of apoptosis [39]. Resveratrol-induced suppression of PKC expression has also been shown to counteract NOX-associated endothelial to mesenchymal transition in endothelial cells of retina following exposure to high glucose [40]. Table 3 describes the impact of resveratrol on the expression of genes in the context of diabetic complications.
Gastrointestinal disorders
Resveratrol has been shown to exert protective effects against radiation-induced intestinal damage. This agent has amended the intestinal oxidative stress markers, malondialdehyde and glutathione levels, and enzymatic activity of catalase. Additionally, resveratrol has decreased the production of proinflammatory molecules TNF-α, NF-κB, and IL-1β in the intestine. These effects have been accompanied by down-regulation of PI3K, AKT, and mTOR in the intestinal tissue of irradiated animals. Therefore, resveratrol can be used as a potential adjuvant in radiotherapeutic regimens [43]. Moreover, resveratrol via the PI3K/AKT-mediated Nrf2 pathway could protect intestinal cells against oxidative stress [44]. The protective effects of resveratrol against liver fibrosis have been verified in different studies. Resveratrol can regulate the activity of hepatic stellate cells via modulating NF-κB and PI3K/AKT pathways [45]. Moreover, resveratrol via the miR-20a-mediated activation of the PTEN/PI3K/AKT pathway can inhibit LF [46]. Table 4 describes the impact of resveratrol on the expression of genes in the context of gastrointestinal disorders.
Other disorders
Resveratrol has also been shown to inhibit ox-LDL-stimulated expression of TLR4 in activated platelets. This effect has been similarly seen in LPS-activated and puromycin-pretreated platelets. Mechanistically, resveratrol attenuates ox-LDL-stimulated phosphorylation of NF-κB and STAT3. Moreover, the suppressive impact of resveratrol on TLR4 expression has been correlated with the inhibition of phosphorylation of AKT. Combined administration of resveratrol and a PI3K inhibitor synergistically inhibits AKT phosphorylation and TLR4 expression. Besides, resveratrol has increased the expression of sirtuin 1 and phosphorylation of AMPK, which was decreased by ox-LDL. Besides, resveratrol has been shown to reduce platelet aggregation and adhesion and CD40L expression in ox-LDL-exposed platelets. Therefore, resveratrol can inhibit the TLR4-associated inflammatory responses in ox-LDL-induced platelets and might be used as an option for the treatment of thrombosis and atherosclerotic conditions [48]. In addition, a certain formulation of resveratrol-loaded nanoparticles has been shown to inhibit LPS-induced accumulation of leukocytes in the bronchoalveolar fluid. This effect has been accompanied by improvement of respiratory function, prevention of accumulation of leukocytes and neutrophils, and reduction of IL-6, KC, MIP-1α, MIP-2, MCP-1, and RANTES levels in lung tissues. Additionally, the mentioned formulation could inhibit MDA levels and SOD activity and block ERK and PI3K/AKT pathways after LPS stimulation [49]. In addition, resveratrol through suppression of PI3K/Nrf2/HO-1 pathway could inhibit oxidative stress, inflammation, and cell apoptosis and alleviate acute lung injury in septic rats [50]. The protective effect of resveratrol against sepsis-induced changes in the myocardium has been shown to be exerted through suppression of NF-kB and induction of the PI3K/AKT/mTOR pathway [51]. Table 5 describes the impact of resveratrol on the expression of genes in the context of other disorders.
Effects of resveratrol on gene expression in neoplastic conditions
Hematological malignancies
Resveratrol can combat multidrug resistance (MDR) in leukemia. This substance has been shown to enhance the anti-proliferative effect of bestatin in the K562/ADR leukemia cell line. Concurrent treatment of leukemic cells with bestatin and resveratrol has decreased IC50 values of bestatin and increased activity of caspase-3 and caspase-8, indicating the potential effect of resveratrol in the enhancement of bestatin-induced apoptosis. Resveratrol has enhanced intracellular levels of bestatin via suppressing P-gp function and decreasing the expression level of P-gp, therefore increasing the anti-proliferative effect of bestatin in K562/ADR cells. Mechanistically, resveratrol has been shown to decrease AKT and mTOR phosphorylation without affecting the phosphorylation of JNK or ERK1/2 [59]. Moreover, resveratrol can regulate apoptosis and proliferation of leukemia cells through modulation of PTEN/PI3K/AKT [60]. Table 6 describes the impact of resveratrol on the expression of genes in the context of hematological malignancies.
Gastrointestinal cancers
Resveratrol has protective effects against bile acid-induced gastric intestinal metaplasia. Resveratrol has been shown to decrease the expression of CDX2 and enhance the activity of FoxO4 in gastric cell lines. Based on the bioinformatics and chromatin-immunoprecipitation analyses, FoxO4 has been shown to bind with the promoter region of CDX2. These effects are mediated through the enhancement of nuclear translocation phospho-FoxO4. In addition, resveratrol enhances FoxO4 phosphorylation via modulation of the PI3K/AKT pathway. Taken together, resveratrol can decrease bile acid-induced gastric intestinal metaplasia via the PI3K/AKT/p-FoxO4 cascade. Thus, it has a protective effect against bile acid-induced gastric intestinal metaplasia particularly those associated with bile acid reflux [63]. In addition, through regulating the PTEN/ PI3K/AKT pathway, resveratrol could induce cell cycle arrest in human gastric cancer cells [64]. Besides, via MARCH-1-induced regulation of the PTEN/AKT pathway, resveratrol can inhibit the malignant progression of hepatocellular carcinoma [65]. Resveratrol can also up-regulate connexin43 and inhibit the AKT pathway, therefore sensitizing colorectal cancer cells to cetuximab [66]. Table 7 describes the impact of resveratrol on the expression of genes in the context of gastrointestinal cancers.
Reproductive system cancers
Resveratrol has been shown to decrease expression levels of MTA1, a constituent of the nucleosome remodeling and deacetylating (NuRD) complex which is up-regulated in numerous malignancies [75]. Moreover, resveratrol can enhance acetylation and reactivation of PTEN through suppression of the MTA1/HDAC complex, leading to blockage of the AKT pathway. Further experiments in the orthotopic model of prostate cancer have verified the effects of resveratrol in the enhancement of PTEN expression, reduction of p-AKT levels, in suppression of proliferation. Therefore, resveratrol can decrease the activity of survival pathways of prostate cancer via modulating the MTA1/HDAC axis [76]. In ovarian cancer cells, resveratrol can induce apoptosis and impair glucose uptake via AKT/GLUT1 axis [77]. Moreover, resveratrol has been shown to induce cell death via ROS‑dependent inactivation of Notch1/PTEN/AKT cascade [78]. Table 8 describes the impact of resveratrol on the expression of genes in the context of reproductive system cancers.
A phase I clinical study in the prostate cancer pathogenesis has demonstrated potential use of resveratrol could for delaying cancer recurrence. Pulverized muscadine grape skin which comprises resveratrol could delay recurrence of prostate cancer through increasing the PSA doubling time. Yet, the obtained results have not been statistically significant [81].
Lung cancer
Resveratrol has been shown to inhibit the expression of XRCC1 and increase the etoposide-associated apoptosis in non-small cell lung cancer (NSCLC) cells. Thus, the inhibitory role of resveratrol on the expression of XRCC1 improves the sensitivity of these cells to etoposide [82]. Moreover, through suppressing the PI3K/AKT-HK2 pathway, resveratrol can play a role in the clinical prevention and treatment of NSCLC [47]. Resveratrol also activates SIRT1 and stimulates protective autophagy in NSCLC cells through suppression of AKT/mTOR and induction of p38-MAPK [83]. Finally, resveratrol can sensitize lung cancer cells to TRAIL via suppressing the AKT/NF-κB pathway [84]. Table 9 describes the impact of resveratrol on the expression of genes in the context of lung cancer.
Other cancers
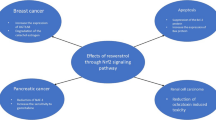
Resveratrol has been shown to suppress the proliferation of both parental and vemurafenib-resistant melanoma cell lines. Moreover, it can reduce AKT phosphorylation in these cells. Therefore, it can reverse vemurafenib resistance in patients receiving BRAF inhibitors [86]. Moreover, by inhibiting the PI3K/AKT/mTOR pathway, it could promote autophagy and suppress the growth of melanoma cells [87]. Resveratrol has also been shown to sensitize breast cancer cells to docetaxel-induced cytotoxicity via inhibiting docetaxel-mediated activation of the HER-2/AKT axis [88]. In addition, resveratrol can promote the anti-tumor effects of rapamycin in papillary thyroid cancer via modulation of the PI3K/AKT/mTOR pathway [89]. Table 10 describes the impact of resveratrol on the expression of genes in the context of cancers (Fig. 3).
Treatment with resveratrol could decrease expression of miR-21 and finally decrease cancer cell survival; these events have been occurred after enhancing PTEN expression and blocking PI3K/AKT and mTOR pathways [94]. Also, resveratrol could decrease cancer cell survival and proliferation via inhibiting the ERK1/2 pathway [96, 100]
A clinical study in women with high risk of breast cancer development has shown that serum levels of total trans-resveratrol and glucuronide metabolite are enhanced following consumption of both 5 and 50 mg trans-resveratrol twice daily for 12 weeks. Moreover, this treatment has led to reduction of RASSF-1α methylation parallel with increasing concentrations of serum trans-resveratrol [99].
Discussion
Several clinical trials have assessed the efficacy, safety, and pharmacokinetics of resveratrol [101]. It has potential beneficial effects in diverse pathological conditions such as diabetes mellitus, obesity, hypertension, neoplastic conditions, Alzheimer's disease, and cardiovascular disorders [101]. However, the therapeutic efficacy of resveratrol seems to be dependent on several factors [102]. For instance, the efficacy of resveratrol has been higher in certain types of cancer compared with others. Moreover, additional clinical trials should be conducted to assess the effects of resveratrol in the treatment of Alzheimer's disease and stroke. Studies in the context of cardiovascular disorders have shown beneficial effects of resveratrol. However, these effects depend on demographics features, since it has not been effective in extremely overweight persons, even has been harmful in schizophrenic patients [103].
Another important note is that the optimal dosage of resveratrol which can induce the maximum beneficial effects without raising toxic effects remains to be identified. A number of studies have reported toxic and adverse effects after consumption of resveratrol [104]. Thus, widespread investigations on the long-term effects of resveratrol in human subjects are needed. Moreover, the interactions between resveratrol and other therapeutic agents should be assessed [104]. A possible adverse effect of resveratrol might be mediated by down-regulation of Akt which induces ROS generation and endothelial cell injury in a dose-dependent manner [105]. Moreover, resveratrol has been shown to alter redox state of human endothelial cells and cause cellular death through a mitochondrial-dependent route [106].
Notably, resveratrol has been found to affect the expression of several genes including cytokine coding genes, caspases, matrix metalloproteinases, adhesion molecules, and growth factors [101]. In addition to the mentioned protein coding genes, evidence from in vitro and in vivo assays has shown the direct effects of resveratrol on several non-coding genes and possible implication of these transcripts in the therapeutic effects of resveratrol [107]. Moreover, it can modulate the activity of several signaling pathways such as PI3K/AKT, Wnt, NF-κB, and Notch pathways [101]. Among the mentioned pathways, the regulatory effects of resveratrol on the activity of the PI3K/AKT pathway have been better appraised in different contexts. In the context of neoplastic conditions, resveratrol not only inhibits malignant behavior of cells and epithelial-mesenchymal transition but also sensitizes neoplastic cells to anti-cancer drugs such as rapamycin [89], doxorubicin [67], vemurafenib [86], cetuximab [66], etoposide [82] and docetaxel [88]. Therefore, it can be used as an adjuvant to enhance the efficacy of several types of anti-cancer modalities ranging from conventional chemotherapeutic agents to targeted therapies. The effects of resveratrol in the suppression of growth of cancer stem cells have been validated in some types of cancers particularly glioblastoma [91]. This property of resveratrol should be appraised in other cancers to find whether it can be used as a drug to combat tumor metastasis and recurrence.
An important issue in the clinical application of resveratrol is the identification of the best route and formulations of this agent. A certain nanoformulation of resveratrol has been proved to be an effective approach for improving the protective effects of resveratrol against lung injury, proposing that the modified-release preparation of this substance can be effective in this situation [49]. Further studies are needed to appraise the efficacy of this formulation in other conditions.
Conclusion
Taken together, resveratrol has several therapeutic effects including modulation of immune responses and ROS formation, suppression of malignant behavior of cancer cells, and sensitization of these cells to anti-cancer drugs. Increasing the bioavailability of this agent and identification of the most appropriate route of administration of this agent are important changes that should be addressed before the extensive application of resveratrol in clinical settings.
Data availability
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
References
Frémont L. Biological effects of resveratrol. Life Sci. 2000;66(8):663–73 (Epub 2000/02/19. eng).
Shrikanta A, Kumar A, Govindaswamy V. Resveratrol content and antioxidant properties of underutilized fruits. J Food Sci Technol. 2015;52(1):383–90 (Epub 05/04. eng).
Baell J, Walters MA. Chemistry: chemical con artists foil drug discovery. Nature. 2014;513(7519):481–3 (Epub 2014/09/26. eng).
Ingólfsson HI, Thakur P, Herold KF, Hobart EA, Ramsey NB, Periole X, et al. Phytochemicals perturb membranes and promiscuously alter protein function. ACS Chemical Biol. 2014;9(8):1788–98 (Epub 2014/06/06. eng).
Valletta A, Iozia LM, Leonelli F. Impact of environmental factors on stilbene biosynthesis. Plants. 2021;10(1):90.
Madhav NV, Shakya AK, Shakya P, Singh K. Orotransmucosal drug delivery systems: a review. J Control Release. 2009;140(1):2–11 (Epub 2009/08/12. eng).
Walle T, Hsieh F, DeLegge MH, Oatis JE Jr, Walle UK. High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab Dispos. 2004;32(12):1377–82 (Epub 2004/08/31. eng).
Luca SV, Macovei I, Bujor A, Miron A, Skalicka-Woźniak K, Aprotosoaie AC, et al. Bioactivity of dietary polyphenols: the role of metabolites. Crit Rev Food Sci Nutr. 2020;60(4):626–59 (Epub 2019/01/08. eng).
Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006;5(6):493–506 (Epub 2006/05/30. eng).
Poulsen MM, Fjeldborg K, Ornstrup MJ, Kjær TN, Nøhr MK, Pedersen SB. Resveratrol and inflammation: challenges in translating pre-clinical findings to improved patient outcomes. Biochim Biophy Acta. 2015;1852(6):1124–36.
Aluyen JK, Ton QN, Tran T, Yang AE, Gottlieb HB, Bellanger RA. Resveratrol: potential as anticancer agent. J Diet Suppl. 2012;9(1):45–56.
Bastianetto S, Ménard C, Quirion R. Neuroprotective action of resveratrol. Biochim Biophy Acta. 2015;1852(6):1195–201.
Khan MA, Chen H-C, Wan X-X, Tania M, Xu A-H, Chen F-Z, et al. Regulatory effects of resveratrol on antioxidant enzymes: a mechanism of growth inhibition and apoptosis induction in cancer cells. Mol Cells. 2013;35(3):219–25.
Guan P, Sun Z-M, Wang N, Zhou J, Luo L-F, Zhao Y-S, et al. Resveratrol prevents chronic intermittent hypoxia-induced cardiac hypertrophy by targeting the PI3K/AKT/mTOR pathway. Life Sci. 2019;233: 116748.
Lin C-H, Lin C-C, Ting W-J, Pai P-Y, Kuo C-H, Ho T-J, et al. Resveratrol enhanced FOXO3 phosphorylation via synergetic activation of SIRT1 and PI3K/Akt signaling to improve the effects of exercise in elderly rat hearts. Age. 2014;36(5):1–10.
Chong E, Chang S-L, Hsiao Y-W, Singhal R, Liu S-H, Leha T, et al. Resveratrol, a red wine antioxidant, reduces atrial fibrillation susceptibility in the failing heart by PI3K/AKT/eNOS signaling pathway activation. Heart Rhythm. 2015;12(5):1046–56.
Zhang X, Huang L, Hua L, Feng H, Shen B. Resveratrol protects myocardial apoptosis induced by ischemia-reperfusion in rats with acute myocardial infarction via blocking P13K/Akt/e-NOS pathway. Eur Rev Med Pharmacol Sci. 2019;23(4):1789–96.
Giordo R, Zinellu A, Eid AH, Pintus G. Therapeutic potential of resveratrol in COVID-19-associated hemostatic disorders. Molecules. 2021. https://doi.org/10.3390/molecules26040856.
Guo D, Xie J, Zhao J, Huang T, Guo X, Song J. Resveratrol protects early brain injury after subarachnoid hemorrhage by activating autophagy and inhibiting apoptosis mediated by the Akt/mTOR pathway. NeuroReport. 2018;29(5):368.
Hou Y, Wang K, Wan W, Cheng Y, Pu X, Ye X. Resveratrol provides neuroprotection by regulating the JAK2/STAT3/PI3K/AKT/mTOR pathway after stroke in rats. Genes Diseases. 2018;5(3):245–55.
Lei J, Chen Q. Resveratrol attenuates brain damage in permanent focal cerebral ischemia via activation of PI3K/Akt signaling pathway in rats. Neurol Res. 2018;40(12):1014–20.
Hui Y, Chengyong T, Cheng L, Haixia H, Yuanda Z, Weihua Y. Resveratrol attenuates the cytotoxicity induced by amyloid-β 1–42 in PC12 cells by upregulating heme oxygenase-1 via the PI3K/Akt/Nrf2 pathway. Neurochem Res. 2018;43(2):297–305.
Shati AA, Alfaifi MY. Trans-resveratrol inhibits tau phosphorylation in the brains of control and cadmium chloride-treated rats by activating PP2A and PI3K/Akt induced-inhibition of GSK3β. Neurochem Res. 2019;44(2):357–73.
Park D-J, Kang J-B, Shah F-A, Koh P-O. Resveratrol modulates the Akt/GSK-3β signaling pathway in a middle cerebral artery occlusion animal model. Lab Anim Res. 2019;35(1):1–8.
Abdel-Aleem GA, Khaleel EF, Mostafa DG, Elberier LK. Neuroprotective effect of resveratrol against brain ischemia reperfusion injury in rats entails reduction of DJ-1 protein expression and activation of PI3K/Akt/GSK3b survival pathway. Arch Physiol Biochem. 2016;122(4):200–13.
Wang N, He J, Pan C, Wang J, Ma M, Shi X, et al. Resveratrol activates autophagy via the AKT/mTOR signaling pathway to improve cognitive dysfunction in rats with chronic cerebral hypoperfusion. Front Neurosci. 2019;13:859.
Huang N, Zhang Y, Chen M, Jin H, Nie J, Luo Y, et al. Resveratrol delays 6-hydroxydopamine-induced apoptosis by activating the PI3K/Akt signaling pathway. Exp Gerontol. 2019;124: 110653.
Fan Y, Li Y, Huang S, Xu H, Li H, Liu B. Resveratrol-primed exosomes strongly promote the recovery of motor function in SCI rats by activating autophagy and inhibiting apoptosis via the PI3K signaling pathway. Neurosci Lett. 2020;736: 135262.
Bai X, Guo X, Zhang F, Zheng L, Ding W, Yang S. Resveratrol combined with 17 β-estradiol prevents IL-1 β induced apoptosis in human nucleus pulposus via the PI3K/AKT/Mtor and PI3K/AKT/GSK-3 β pathway. J Invest Surg. 2020. https://doi.org/10.1080/08941939.2019.1705941.
Yang S-D, Ma L, Yang D-L, Ding W-Y. Combined effect of 17β-estradiol and resveratrol against apoptosis induced by interleukin-1β in rat nucleus pulposus cells via PI3K/Akt/caspase-3 pathway. PeerJ. 2016;4: e1640.
Han X, Leng X, Zhao M, Wu M, Chen A, Hong G, et al. Resveratrol increases nucleus pulposus matrix synthesis through activating the PI3K/Akt signaling pathway under mechanical compression in a disc organ culture. 2017. Biosci Rep. https://doi.org/10.1042/BSR20171319.
Gao J, Zhang Q, Song L. Resveratrol enhances matrix biosynthesis of nucleus pulposus cells through activating autophagy via the PI3K/Akt pathway under oxidative damage. 2018. Biosci Rep. https://doi.org/10.1042/BSR20180544.
Turner RS, Thomas RG, Craft S, van Dyck CH, Mintzer J, Reynolds BA, et al. A randomized, double-blind, placebo-controlled trial of resveratrol for Alzheimer disease. Neurology. 2015;85(16):1383–91.
Ma C, Wang Y, Dong L, Li M, Cai W. Anti-inflammatory effect of resveratrol through the suppression of NF-κB and JAK/STAT signaling pathways. Acta Biochim Biophys Sin. 2015;47(3):207–13.
Xu F, Wang Y, Cui W, Yuan H, Sun J, Wu M, et al. Resveratrol prevention of diabetic nephropathy is associated with the suppression of renal inflammation and mesangial cell proliferation: possible roles of Akt/NF-B pathway. Int J Endocrinol. 2014. https://doi.org/10.1155/2014/289327.
Wu Z, Huang A, Yan J, Liu B, Liu Q, Zhang J, et al. Resveratrol ameliorates cardiac dysfunction by inhibiting apoptosis via the PI3K/Akt/FoxO3a pathway in a rat model of diabetic cardiomyopathy. J Cardiovasc Pharmacol. 2017;70(3):184–93.
Shu L, Zhao H, Huang W, Hou G, Song G, Ma H. Resveratrol Upregulates mmu-miR-363-3p via the PI3K-Akt pathway to improve insulin resistance induced by a high-fat diet in mice. Diabetes Metab Syndr Obes Targets Ther. 2020;13:391.
Wang W, Li P, Xu J, Wu X, Guo Z, Fan L, et al. Resveratrol attenuates high glucose-induced nucleus pulposus cell apoptosis and senescence through activating the ROS-mediated PI3K/Akt pathway. 2018. Biosci Rep. https://doi.org/10.1042/BSR20171454.
Liu M-H, Yuan C, He J, Tan T-P, Wu S-J, Fu H-Y, et al. Resveratrol protects PC12 cells from high glucose-induced neurotoxicity via PI3K/Akt/FoxO3a pathway. Cell Mol Neurobiol. 2015;35(4):513–22.
Giordo R, Nasrallah GK, Posadino AM, Galimi F, Capobianco G, Eid AH, et al. Resveratrol-elicited pkc inhibition counteracts nox-mediated endothelial to mesenchymal transition in human retinal endothelial cells exposed to high glucose. Antioxidants. 2021;10(2):224.
Zhao Y, Song W, Wang Z, Wang Z, Jin X, Xu J, et al. Resveratrol attenuates testicular apoptosis in type 1 diabetic mice: Role of Akt-mediated Nrf2 activation and p62-dependent Keap1 degradation. Redox Biol. 2018;14:609–17.
Li X, Yang S, Wang L, Liu P, Zhao S, Li H, et al. Resveratrol inhibits paclitaxel-induced neuropathic pain by the activation of PI3K/Akt and SIRT1/PGC1α pathway. J Pain Res. 2019;12:879.
Radwan RR, Karam HM. Resveratrol attenuates intestinal injury in irradiated rats via PI3K/Akt/mTOR signaling pathway. Environ Toxicol. 2020;35(2):223–30.
Zhuang Y, Wu H, Wang X, He J, He S, Yin Y. Resveratrol attenuates oxidative stress-induced intestinal barrier injury through PI3K/Akt-mediated Nrf2 signaling pathway. Oxid Med Cell Longev. 2019. https://doi.org/10.1155/2019/7591840.
Zhang DQ, Sun P, Jin Q, Li X, Zhang Y, Zhang YJ, et al. Resveratrol regulates activated hepatic stellate cells by modulating NF-κB and the PI3K/Akt signaling pathway. J Food Sci. 2016;81(1):H240–5.
Zhu L, Mou Q, Wang Y, Zhu Z, Cheng M. Resveratrol contributes to the inhibition of liver fibrosis by inducing autophagy via the microRNA-20a-mediated activation of the PTEN/PI3K/AKT signaling pathway. Int J Mol Med. 2020;46(6):2035–46.
Zhang H, Sun Q, Xu T, Hong L, Fu R, Wu J, et al. Resveratrol attenuates the progress of liver fibrosis via the Akt/nuclear factor-κB pathways. Mol Med Rep. 2016;13(1):224–30.
Sun J, Zhang M, Chen K, Chen B, Zhao Y, Gong H, et al. Suppression of TLR4 activation by resveratrol is associated with STAT3 and Akt inhibition in oxidized low-density lipoprotein-activated platelets. Eur J Pharmacol. 2018;836:1–10.
de Oliveira MTP, de Sá CD, de Souza ÉT, Guterres SS, Pohlmann AR, Silva PMR, et al. Orally delivered resveratrol-loaded lipid-core nanocapsules ameliorate LPS-induced acute lung injury via the ERK and PI3K/Akt pathways. Int J Nanomed. 2019;14:5215.
Wang Y, Wang X, Zhang L, Zhang R. Alleviation of acute lung injury in rats with sepsis by resveratrol via the phosphatidylinositol 3-kinase/nuclear factor-erythroid 2 related factor 2/heme oxygenase-1 (PI3K/Nrf2/HO-1) pathway. Med Sci Monit. 2018;24:3604.
Shang X, Lin K, Yu R, Zhu P, Zhang Y, Wang L, et al. Resveratrol protects the myocardium in sepsis by activating the phosphatidylinositol 3-kinases (PI3K)/AKT/mammalian target of rapamycin (mTOR) pathway and inhibiting the nuclear factor-κB (NF-κB) signaling pathway. Med Sci Monit. 2019;25:9290.
Nakajima S, Ishimaru K, Kobayashi A, Yu G, Nakamura Y, Oh-Oka K, et al. Resveratrol inhibits IL-33–mediated mast cell activation by targeting the MK2/3–PI3K/Akt axis. Sci Rep. 2019;9(1):1–11.
Xu X, Liu X, Yang Y, He J, Gu H, Jiang M, et al. Resveratrol inhibits the development of obesity-related osteoarthritis via the TLR4 and PI3K/Akt signaling pathways. Connect Tissue Res. 2019;60(6):571–82.
Shen J, Qu C, Xu L, Sun H, Zhang J. Resveratrol exerts a protective effect in chronic unpredictable mild stress-induced depressive-like behavior: involvement of the AKT/GSK3β signaling pathway in hippocampus. Psychopharmacology. 2019;236(2):591–602.
Yang H, Chen Q, Sun F, Zhao N, Wen L, Li L, et al. Down-regulation of the klf5-c-Myc interaction due to klf5 phosphorylation mediates resveratrol repressing the caveolin-1 transcription through the PI3K/PKD1/Akt pathway. PLoS ONE. 2017;12(12): e0189156.
Chen B, Xue J, Meng X, Slutzky JL, Calvert AE, Chicoine LG. Resveratrol prevents hypoxia-induced arginase II expression and proliferation of human pulmonary artery smooth muscle cells via Akt-dependent signaling. Am J Physiol Lung Cell Mol Physiol. 2014;307(4):L317–25.
Liu M, Cheng L, Li X, Ding H, Wang H, Wang M, et al. Resveratrol reverses myogenic induction supression caused by high glucose through activating SIRT1/AKT/FOXO1 pathway. 2020.
Eo SH, Cho HS, Kim SJ. Resveratrol regulates type II collagen and COX-2 expression via the ERK, p38 and Akt signaling pathways in rabbit articular chondrocytes. Exp Ther Med. 2014;7(3):640–8.
Wang L, Wang C, Jia Y, Liu Z, Shu X, Liu K. Resveratrol increases anti-proliferative activity of bestatin through downregulating P-glycoprotein expression via inhibiting PI3K/Akt/mTOR pathway in K562/ADR cells. J Cell Biochem. 2016;117(5):1233–9.
Meng J, Liu G, Song J, Chen L, Wang A, Gao X, et al. Preliminary results indicate resveratrol affects proliferation and apoptosis of leukemia cells by regulating PTEN/PI3K/AKT pathway. Eur Rev Med Pharmacol Sci. 2019;23(10):4285–92.
Guan H, You Z, Wang C, Fang F, Peng R, Mao L, et al. MicroRNA-200a suppresses prostate cancer progression through BRD4/AR signaling pathway. Cancer Med. 2019;8(4):1474–85.
Sui T, Ma L, Bai X, Li Q, Xu X. Resveratrol inhibits the phosphatidylinositide 3-kinase/protein kinase B/mammalian target of rapamycin signaling pathway in the human chronic myeloid leukemia K562 cell line. Oncol Lett. 2014;7(6):2093–8.
Lu W, Ni Z, Jiang S, Tong M, Zhang J, Zhao J, et al. Resveratrol inhibits bile acid-induced gastric intestinal metaplasia via the PI3K/AKT/p-FoxO4 signalling pathway. Phytother Res. 2020. https://doi.org/10.1002/ptr.6915.
Jing X, Cheng W, Wang S, Li P, He L. Resveratrol induces cell cycle arrest in human gastric cancer MGC803 cells via the PTEN-regulated PI3K/Akt signaling pathway. Oncol Rep. 2016;35(1):472–8.
Dai H, Li M, Yang W, Sun X, Wang P, Wang X, et al. Resveratrol inhibits the malignant progression of hepatocellular carcinoma via MARCH1-induced regulation of PTEN/AKT signaling. Aging. 2020;12(12):11717.
Wang Y, Wang W, Wu X, Li C, Huang Y, Zhou H, et al. Resveratrol sensitizes colorectal cancer cells to cetuximab by connexin 43 upregulation-induced Akt inhibition. Front Oncol. 2020;10:383.
Xu J, Liu D, Niu H, Zhu G, Xu Y, Ye D, et al. Resveratrol reverses Doxorubicin resistance by inhibiting epithelial-mesenchymal transition (EMT) through modulating PTEN/Akt signaling pathway in gastric cancer. J Exp Clin Cancer Res. 2017;36(1):1–14.
Liu Y-Z, Wu K, Huang J, Liu Y, Wang X, Meng Z-J, et al. The PTEN/PI3K/Akt and Wnt/β-catenin signaling pathways are involved in the inhibitory effect of resveratrol on human colon cancer cell proliferation. Int J Oncol. 2014;45(1):104–12.
Yuan L, Zhou M, Huang D, Wasan HS, Zhang K, Sun L, et al. Resveratrol inhibits the invasion and metastasis of colon cancer through reversal of epithelial-mesenchymal transition via the AKT/GSK-3β/Snail signaling pathway. Mol Med Rep. 2019;20(3):2783–95.
Liu MH, Lin XL, Li J, He J, Tan TP, Wu SJ, et al. Resveratrol induces apoptosis through modulation of the Akt/FoxO3a/Bim pathway in HepG2 cells. Mol Med Rep. 2016;13(2):1689–94.
Chai R, Fu H, Zheng Z, Liu T, Ji S, Li G. Resveratrol inhibits proliferation and migration through SIRT1 mediated post-translational modification of PI3K/AKT signaling in hepatocellular carcinoma cells. Mol Med Rep. 2017;16(6):8037–44.
Li D, Wang G, Jin G, Yao K, Zhao Z, Bie L, et al. Resveratrol suppresses colon cancer growth by targeting the AKT/STAT3 signaling pathway. Int J Mol Med. 2019;43(1):630–40.
Zeng Y-H, Zhou L-Y, Chen Q-Z, Li Y, Shao Y, Ren W-Y, et al. Resveratrol inactivates PI3K/Akt signaling through upregulating BMP7 in human colon cancer cells. Oncol Rep. 2017;38(1):456–64.
Patel KR, Brown VA, Jones DJ, Britton RG, Hemingway D, Miller AS, et al. Clinical pharmacology of resveratrol and its metabolites in colorectal cancer patients. Cancer Res. 2010;70(19):7392–9 (Epub 2010/09/16. eng).
Kai L, Samuel SK, Levenson AS. Resveratrol enhances p53 acetylation and apoptosis in prostate cancer by inhibiting MTA1/NuRD complex. Int J Cancer. 2010;126(7):1538–48.
Dhar S, Kumar A, Li K, Tzivion G, Levenson AS. Resveratrol regulates PTEN/Akt pathway through inhibition of MTA1/HDAC unit of the NuRD complex in prostate cancer. Biochim Biophys Acta. 2015;1853(2):265–75.
Gwak H, Haegeman G, Tsang BK, Song YS. Cancer-specific interruption of glucose metabolism by resveratrol is mediated through inhibition of Akt/GLUT1 axis in ovarian cancer cells. Mol Carcinog. 2015;54(12):1529–40.
Kim TH, Park JH, Woo JS. Resveratrol induces cell death through ROS-dependent downregulation of Notch1/PTEN/Akt signaling in ovarian cancer cells. Mol Med Rep. 2019;19(4):3353–60.
Ye M, Tian H, Lin S, Mo J, Li Z, Chen X, et al. Resveratrol inhibits proliferation and promotes apoptosis via the androgen receptor splicing variant 7 and PI3K/AKT signaling pathway in LNCaP prostate cancer cells. Oncol Lett. 2020;20(5):1.
Wang Z, Wu L, Tong S, Hu X, Zu X, Li Y, et al. Resveratrol suppresses the epithelial-to-mesenchymal transition in PC-3 cells by down-regulating the PI3K/AKT signaling pathway. Anim Cells Syst. 2016;20(2):77–85.
Paller CJ, Rudek MA, Zhou XC, Wagner WD, Hudson TS, Anders N, et al. A phase I study of muscadine grape skin extract in men with biochemically recurrent prostate cancer: safety, tolerability, and dose determination. Prostate. 2015;75(14):1518–25.
Ko JC, Syu JJ, Chen JC, Wang TJ, Chang PY, Chen CY, et al. Resveratrol enhances etoposide-induced cytotoxicity through down-regulating ERK 1/2 and AKT-mediated X-ray repair cross-complement group 1 (XRCC 1) protein expression in human non-small-cell lung cancer cells. Basic Clin Pharmacol Toxicol. 2015;117(6):383–91.
Wang J, Li J, Cao N, Li Z, Han J, Li L. Resveratrol, an activator of SIRT1, induces protective autophagy in non-small-cell lung cancer via inhibiting Akt/mTOR and activating p38-MAPK. Onco Targets Ther. 2018;11:7777.
Rasheduzzaman M, Jeong J-K, Park S-Y. Resveratrol sensitizes lung cancer cell to TRAIL by p53 independent and suppression of Akt/NF-κB signaling. Life Sci. 2018;208:208–20.
Clinton SK, Giovannucci EL, Hursting SD. The World Cancer Research Fund/American Institute for Cancer Research Third Expert Report on diet, nutrition, physical activity, and cancer: impact and future directions. J Nutr. 2020;150(4):663–71.
Luo H, Umebayashi M, Doi K, Morisaki T, Shirasawa S, Tsunoda T. Resveratrol overcomes cellular resistance to vemurafenib through dephosphorylation of akt in BRAF-mutated melanoma cells. Anticancer Res. 2016;36(7):3585–9.
Wang M, Yu T, Zhu C, Sun H, Qiu Y, Zhu X, et al. Resveratrol triggers protective autophagy through the ceramide/Akt/mTOR pathway in melanoma B16 cells. Nutr Cancer. 2014;66(3):435–40.
Vinod B, Nair H, Vijayakurup V, Shabna A, Shah S, Krishna A, et al. Resveratrol chemosensitizes HER-2-overexpressing breast cancer cells to docetaxel chemoresistance by inhibiting docetaxel-mediated activation of HER-2–Akt axis. Cell Death Discov. 2015;1(1):1–9.
Bian P, Hu W, Liu C, Li L. Resveratrol potentiates the anti-tumor effects of rapamycin in papillary thyroid cancer: PI3K/AKT/mTOR pathway involved. Arch Biochem Biophys. 2020;689: 108461.
Chen JM, Bai JY, Yang KX. Effect of resveratrol on doxorubicin resistance in breast neoplasm cells by modulating PI3K/Akt signaling pathway. IUBMB Life. 2018;70(6):491–500.
Jiao Y, Li H, Liu Y, Guo A, Xu X, Qu X, et al. Resveratrol inhibits the invasion of glioblastoma-initiating cells via down-regulation of the PI3K/Akt/NF-κB signaling pathway. Nutrients. 2015;7(6):4383–402.
Gong C, Xia H. Resveratrol suppresses melanoma growth by promoting autophagy through inhibiting the PI3K/AKT/mTOR signaling pathway. Exp Ther Med. 2020;19(3):1878–86.
Hu W, Yang E, Ye J, Han W, Du ZL. Resveratrol protects neuronal cells from isoflurane-induced inflammation and oxidative stress-associated death by attenuating apoptosis via Akt/p38 MAPK signaling. Exp Ther Med. 2018;15(2):1568–73.
Zhou C, Ding J, Wu Y. Resveratrol induces apoptosis of bladder cancer cells via miR-21 regulation of the Akt/Bcl-2 signaling pathway. Mol Med Rep. 2014;9(4):1467–73.
Dai Z, Lei P, Xie J, Hu Y. Antitumor effect of resveratrol on chondrosarcoma cells via phosphoinositide 3-kinase/AKT and p38 mitogen-activated protein kinase pathways. Mol Med Rep. 2015;12(2):3151–5.
Zhao Y, Tang H, Zeng X, Ye D, Liu J. Resveratrol inhibits proliferation, migration and invasion via Akt and ERK1/2 signaling pathways in renal cell carcinoma cells. Biomed Pharmacother. 2018;98:36–44.
Chang C-H, Lee C-Y, Lu C-C, Tsai F-J, Hsu Y-M, Tsao J-W, et al. Resveratrol-induced autophagy and apoptosis in cisplatin-resistant human oral cancer CAR cells: a key role of AMPK and Akt/mTOR signaling. Int J Oncol. 2017;50(3):873–82.
Graham RM, Hernandez F, Puerta N, De Angulo G, Webster KA, Vanni S. Resveratrol augments ER stress and the cytotoxic effects of glycolytic inhibition in neuroblastoma by downregulating Akt in a mechanism independent of SIRT1. Exp Mol Med. 2016;48(2):e210.
Zhu W, Qin W, Zhang K, Rottinghaus GE, Chen YC, Kliethermes B, et al. Trans-resveratrol alters mammary promoter hypermethylation in women at increased risk for breast cancer. Nutr Cancer. 2012;64(3):393–400 (Epub 2012/02/16. eng).
Chen X, Hu X, Li Y, Zhu C, Dong X, Zhang R, et al. Resveratrol inhibits Erk1/2-mediated adhesion of cancer cells via activating PP2A–PTEN signaling network. J Cell Physiol. 2019;234(3):2822–36.
Singh AP, Singh R, Verma SS, Rai V, Kaschula CH, Maiti P, et al. Health benefits of resveratrol: evidence from clinical studies. Med Res Rev. 2019;39(5):1851–91 (Epub 2019/02/12. eng).
Berman AY, Motechin RA, Wiesenfeld MY, Holz MK. The therapeutic potential of resveratrol: a review of clinical trials. NPJ Precis Oncol. 2017. https://doi.org/10.1038/s41698-017-0038-6 (Epub 2017/10/11. eng).
Zortea K, Franco VC, Francesconi LP, Cereser KM, Lobato MIR, Belmonte-de-Abreu PS. Resveratrol supplementation in schizophrenia patients: a randomized clinical trial evaluating serum glucose and cardiovascular risk factors. Nutrients. 2016;8(2):73.
Shaito A, Posadino AM, Younes N, Hasan H, Halabi S, Alhababi D, et al. Potential adverse effects of resveratrol: a literature review. Int J Mol Sci. 2020. https://doi.org/10.3390/ijms21062084 (Epub 2020/03/22. eng).
Pasciu V, Posadino AM, Cossu A, Sanna B, Tadolini B, Gaspa L, et al. Akt downregulation by flavin oxidase-induced ROS generation mediates dose-dependent endothelial cell damage elicited by natural antioxidants. Toxicol Sci. 2010;114(1):101–12 (Epub 2009/12/18. eng).
Posadino AM, Cossu A, Giordo R, Zinellu A, Sotgia S, Vardeu A, et al. Resveratrol alters human endothelial cells redox state and causes mitochondrial-dependent cell death. Food Chem Toxicol. 2015;78:10–6 (Epub 2015/02/07. eng).
Giordo R, Wehbe Z, Posadino AM, Erre GL, Eid AH, Mangoni AA, et al. Disease-associated regulation of non-coding RNAs by resveratrol: molecular insights and therapeutic applications. Front Cell Dev Biol. 2022;10:894305.
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
SGF wrote the manuscript and revised it. MT, SAA designed and supervised the study. HS, ZB, BMS, SFT, SGB and BMH collected the data and designed the tables and figures. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participant
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ghafouri-Fard, S., Bahroudi, Z., Shoorei, H. et al. Disease-associated regulation of gene expression by resveratrol: Special focus on the PI3K/AKT signaling pathway. Cancer Cell Int 22, 298 (2022). https://doi.org/10.1186/s12935-022-02719-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12935-022-02719-3