Abstract
Background
Delayed cancer diagnosis and inefficient cancer prognosis determination are problems faced in cancer diagnosis and treatment. MicroRNAs (miRs), especially miR-212, have shown a promise in cancer diagnosis and prognosis. Herein, we performed a systematic review and meta-analysis to assess the prognostic and diagnostic value of miR-212 level in cancer and evaluated its association with patient characteristics.
Methods
A fully electronic literature search using related keywords was performed in PubMed, Scopus, Web of Science, Embase, and ScienceDirect databases by June 6, 2021, with no time or language restriction. Meta-analysis was performed to pool survival prognosis data using hazard ratio (HR), association using odds ratio (OR), and diagnostic data using sensitivity, specificity, and diagnostic odds ratio (DOR). Sub-group analysis and meta-regression were performed as appropriate.
Results
Results of 28 studies on 1880 patients showed a poor cancer prognosis with high levels of miR-212 in pancreatic ductal adenocarcinoma (PDAC, HR = 2.451 [1.447–4.149]), and a poor cancer prognosis with low levels of miR-212 in other cancers (HR = 2.514 [2.162–2.923]). Higher alpha-fetoprotein (AFP) level and Edmondson-Steiner grade were factors associated with miR-212 low level incidence. Diagnostic odds ratio 10.688 (3.644–31.348) and SROC AUC of 0.84 confirmed high diagnostic performance of miR-212.
Conclusion
Our systematic review and meta-analysis results confirm miR-212 high value in cancer prognosis and diagnosis. High level of miR-212 showed poor prognosis in PDAC and low level of miR-212 showed poor prognosis in other cancers. in conclusion, miR-212 could be a novel potential biomarker in cancer diagnosis and prognosis.
Similar content being viewed by others
Introduction
Cancer is a leading cause of death by causing about 10 million deaths in 2020 worldwide [1]. One potential justification for this high mortality rate is the delayed diagnosis; the highest mortality rates belong to lung, colorectal, hepatic, stomach, and breast cancers, all of which usually present with vague symptoms, especially in the early stages [1, 2]. In addition to delayed diagnosis, ineffective methods to determine the cancer prognosis could cause management flaws leading to under- or over-treatment of patients, both of which impose an economic burden on the patients and health system.
MicroRNAs (miRs) are a main member of small non-coding mRNAs; they act as modulatory tools that work post-transcriptionally to regulate mRNA translation by attaching to the 3'-untranslated region [3, 4]. Genetic codes of miR-212 are located on chromosome 17p13.3. MiR-212 can regulate the cell cycle, proliferation, differentiation, and apoptosis; thus, it can have an oncogenic function or act as a tumor suppressor gene [4]. miRs dysregulation is shown in several cancers, affects patient prognosis and treatment outcomes [2]. miR-212 is one of them which is shown to be altered in hepatocellular carcinoma (HCC), gastric carcinoma, pancreatic ductal adenocarcinoma (PDAC), colorectal carcinoma (CRC), breast cancer, prostate cancer, renal cell carcinoma (RCC), non-small cell lung cancer (NSCLC), etc. Some of these studies assessed prognostic value by finding correlations between miR-212 levels with patient survival, disease-free survival, and recurrence-free survival. Some studies proposed diagnostic power for miR-212 by calculating sensitivity, specificity, and other related factors diagnostic measures. Herein, we systematically reviewed the literature to provide a better view of its prognostic and diagnostic roles, and we powered our findings by multiple meta-analyses on data extracted from these studies.
Methods
A systematic review and meta-analysis were performed and reported according to The Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) statement [5].
Search strategy
Without any time or language restriction, we searched Pubmed, Scopus, Web of Science, Embase, and ScienceDirect on June 6, 2021, using Medical Subject Headings (MeSH), entry terms, and related keywords to miR-212 and cancer. The search was ameliorated by hand search in google scholar and references of included studies. The detailed search strategy is provided in Additional file 1.
Inclusion criteria
Original human research papers were included in this study. For prognostic evaluation papers providing hazard ratio (HR) or enough data to estimate HR of cancer death comparing patients with low and high levels of miR-212 were included, for patient characteristics association with miR-212 level evaluation papers providing odds ratio (OR) or enough data to calculate OR of patient characteristics comparing patients with low and high level of miR-212 were included, and for diagnostic evaluation papers providing numbers of true and false positive and negative samples using miR-212 level as determinant or enough data to estimate these were included in this study.
Data extraction
First author name, publication year, country of study, type of assessed miR-212, cancer type, tumor stage, Specimen, and miR-212 assay method were extracted from all studies. For articles included in the prognostic evaluation, crude and adjusted HRs were extracted, and in case of no reported HR, it was extracted from the Kaplan–Meier curve using the Guyot method [6]. For association evaluation, the number of cancer patients in each cell of 2 × 2 table dividing patients in two categories of each characteristic in low and high miR-212 level was extracted from studies. Finally, for diagnostic evaluation, numbers of true positive, false positive, false negative, and true negative were extracted from the articles in three types: reported by the study, obtained from the receiver operating characteristic (ROC) curve using Youden index method [7] and obtained from the ROC curve using Index of Union method [8].
Quality assessment
Quality assessment was performed using the Newcastle–Ottawa scale (NOS) for the assessment of the quality of nonrandomized studies in meta-analyses [9, 10] for studies included for prognostic and association evaluation and QUADAS-2 tool for the quality assessment of diagnostic accuracy studies [11] for studies included for diagnostic evaluation. For NOS, a score < 4 was considered low quality, a score 4–6 was deemed to be medium quality, and a score of > 6 was regarded as high quality. Search (S. R. and M. S.), screening and full-text assessment (S. R. and M. S.), data extraction (S. R. and M. S.), and Quality assessment (S. R. and M. M.) were all performed by two investigators separately. Disagreements were resolved through discussion or consultation from the third reviewer (A. S.).
Statistical analysis
Cochran’s Q test and Higgins’s I2 and p-value [12, 13] were used to evaluate heterogenicity. In case of low heterogenicity defined by I2 < 50 and p-value > 0.05 fixed-effects model was used to pool data; in other conditions, random-effects model was applied. In order to solve observed inconsistency in the included studies email was sent to the corresponding author.
For prognostic evaluation, Comprehensive Meta Analysis software (Version 3.3.070, November 20, 2014) was used to calculate pooled HR. For studies not reporting HR, we digitized the Kaplan–Meier curve using Web Plot Digitizer [14] and estimated HR using coxph function of survival package (Version 2.42-3) using R (Version 4.1.1) in RStudio (Version 1.4.1717) on estimated individual patient data provided by R code in R studio based on the algorithm published by Guyot et al. [6]. For overall survival sub-group analysis and meta-regression were used to find the source of heterogenicity. Sensitivity analysis was performed through one-study-removed analysis, which calculates the pooled effect size of studies after omitting one study each time. Publication bias was assessed using Begg’s funnel plot [15] and Begg’s [15] and Eager’s [16] tests. Nonsignificant p-value of Begg’s and Eager’s tests shows no publication bias. Also, due to the observed different effect of pancreatic cancer on miR-212, publication bias assessment was also performed on studies after removing studies on pancreatic cancer.
For association evaluation of patient characteristics and low miR-212 level incidence, OR calculation and pooling from the number of patients based on characteristics in low miR-212 and high miR-212 was performed using Comprehensive Meta-Analysis software.
For diagnostic evaluation, the numbers of true positive, false positive, false negative and true negative were extracted from articles or calculated using article-provided sensitivity and specificity. To lower the heterogenicity and threshold effect induced by various optimal cut-off determination methods used in different studies, sensitivity and specificity and number of true and false positive and negative were also extracted based on the numbers obtained by Web Plot Digitizer from ROC curves utilizing two methods: higher Youden Index [7], which is the most commonly used method to determine optimal cut-off leading to the point that has the highest summation of sensitivity and specificity, and lower Index of Union [8] which selects the point where sensitivity and specificity are both most near to area under the curve (AUC) and thus to each other [8]. Meta-analysis of Diagnostic and Screening Tests (Meta-DiSc®) software (Version 1.4, Madrid, Spain) was used to pool the studies [17]. Pooled sensitivity, specificity, positive likelihood ratio (PLR), negative likelihood ratio (NLR), and diagnostic odds ratio (DOR) and 95% confidence intervals were calculated using the DerSimonian-Laird method. ROC plane and summary ROC (SROC) curve were drawn. The Moses-Shapiro-Littenberg model was used to investigate the constancy of DOR. Not significant p-value of the Moses-Shapiro-Littenberg model shows a constant DOR; thus, symmetrical curve in SROC curve is the preferred curve; otherwise, asymmetrical curve is preferred. SROC AUC curve was drawn applying the exponential of the constant of the Moses-Shapiro-Littenberg model. To investigate the threshold effect, which mainly happens on account of applying different cut-offs in different studies, “Spearman correlation coefficient between the logit of sensitivity and logit of 1-specificity” was calculated [17]. No significant and considerable positive Spearman correlation shows no threshold effect. For further assessment of miR-212 diagnostic value in cancer, Fagan’s nomogram was applied using function nomogram in UncertainInterval package (Version 0.7.0), considering pretest probability of 0.2. Results of Fagan’s nomogram show probability of disease for an individual with a positive test in a population with a prevalence of 20% and probability of disease for an individual with negative test in the same population. To assess publication bias among studies included in the diagnostic evaluation, Deek's funnel plot was drawn and assessed using metabin, metabias, and funnel functions of meta package (Version 4.19-1) in RStudio.
Results
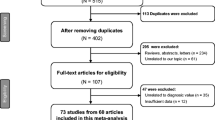
Performed search provided 874 records, including 173 articles from Pubmed, 295 from Scopus, 187 from Web of Science, 192 from Embase, and 27 from Science Direct. After the removal of duplicate records, 521 articles were left. Title and abstract of articles were screened, resulting in the exclusion of 470 studies. The full textes of 51 remaining articles were assessed resulting in exclusion of 21 articles (12 articles due to not reporting OR and HR or clinical signifance of miR-212, 8 articles investigated a set of microRNAs but not miR-212 solely, and one study investigated diagnosis of the presence of metastasis instead of cancer). Ultimately 28 studies were included in the systematic review and meta-analysis.
Totally 1880 patients from 28 studies were included in this systematic review and meta-analysis. Pooled analysis of prognostic dimension of miR-212 on cancer survival was performed on 1479 patients from 18 studies, pooled analysis of the association between low miR-212 level incidence and patient characteristics were performed on 1438 patients from 19 studies, and pooled analysis of the diagnostic value of miR-212 in cancer diagnosis was performed on 416 patients from 8 studies.
Studies included in the prognostic evaluation Fig. 1 were performed in China between 2013 and 2020. Five studies were performed on HCC, three on CRC, two on PDAC, two on gastric cancer, and six on other cancers. Studies included in the diagnostic evaluation were performed between 2013 and 2020. Three studies were performed in China, two in Iran, two in the USA, and one in the UK. Each study was performed on a particular organ tumor, and the biofluid samples of plasma, serum, tissue, urine, and bile were used to examine miR-212 levels.
PRISMA flow diagram of the selection process [59]
The prognostic dimension of miR-212 in cancer patients was investigated using HR, showing higher mortality of low miR-212 patients compared to high miR-212 patients (Table 2A). HR for overall survival (OS) was 2.081 (1.593, 2.717) based on the data of 1479 patients from 18 studies showing higher mortality in low miR-212 patients (Fig. 2A). The source of heterogenicity observed by I2 = 68.884 was investigated through sub-group analysis (Table 2A and Additional file 2: Figures S5–S13). In the analysis sub-grouped by cancer type, I2 was 0 for HCC, CRC, and PDAC. In all studies, lower miR-212 showed poorer prognosis, but in two studies on PDAC; so, another sub-group analysis was performed dividing studies in PDAC and other cancers where I2 was 0 for both sub-groups and pooled HR was 0.408 (0.241, 0.691) for PDAC and 2.514 (2.162, 2.923) for other cancers (Fig. 2B). In meta-regression analysis, significant regression was observed when studies were tagged by cancer type and sample size. Larger sample size was associated with higher mortality. Also, pooled HR for adjusted overall survival, disease-free survival (DFS), adjusted disease-free survival, and recurrence-free survival (RFS) were all higher than 1.8 and significant (Table 2A and Additional file 2: Figures S14–S17). Sensitivity analysis did not show a considerable difference in pooled effect size after removing any study; however, omitting studies on PDAC resulted in a slight increase in HR (Fig. 2C). NOS results showed high quality of all included studies in prognostic and association evaluation (Table 1). To investigate publication bias, Begg's funnel plot (Fig. 2D) was drawn, and Begg’s and Eager’s tests were performed. Publication bias was rejected by no significant p-value at Begg and Mazumdar rank correlation (p-value = 0.850) and Eager’s regression intercept (p-value = 0.261). To have a broader investigation of publication bias Begg’s and Eager’s tests were performed on studies after removing studies on PDAC, resulting in p-values of 0.242 and 0.909 for Begg’s and Eager’s tests respectively (Additional file 2: Figure S4). Also, Duval and Tweedie’s trim and fill was performed on studies after removing studies on PDAC, confirming study results in cancers other than PDAC by an effect size of 2.514 (2.162–2.923) (Additional file 2: Figure S4).
Prognostic evaluation of cancer patients in low miR-212 level patients compared with high miR-212 patients: forrest plot of overall survival (OS) (A), forrest plot of OS sub-grouped by cancer type in two groups of Pancreatic ductal adenocarcinoma (PDAC) and other cancer types (B), sensitivity analysis of overall survival using one-study-removed method (C), and Begg’s funnel plot of publication bias (D)
Pooled association between low miR-212 level incidence and patient characteristics is shown in Table 2B and Additional file 2: Figures S18–S37. Higher tumor (T), nodes (N), and metastases (M) (TNM) stage was associated with the incidence of low miR-212 level; however, larger tumor size and distant metastasis did not show any association with miR-212 level. Higher serum alpha-fetoprotein (AFP) level showed a significant association with lower miR-212 level. The association was significant in pooling all studies or pooling studies considered 400 mg serum AFP level as cut-off. While pooling studies considering 200 as serum AFP level cut-off did not show a significant association. Presence of venous infiltration and higher Edmondson-Steiner grade were other factors being associated with low miR-212 level incidence.
Evaluation of diagnostic value based on the numbers obtained preferably using Youden Index showed pooled values of sensitivity 0.716 (0.669–0.759), specificity 0.790 (0.748–0.827), positive likelihood ratio 3.249 (1.849–5.709), negative likelihood ratio 0.378 (0.226–0.631), and diagnostic odds ratio 10.688 (3.644–31.348) and SROC AUC of 0.84 confirming high diagnostic performance of miR-212 (Table 2C and Fig. 3). Numbers preferably obtained using the Index of Union confirmed the findings (Table 2C and Additional file 2: Figures S39). Results of subgroup analysis and meta-regression are provided in Table 2C. Meta-regression did not show significant regression in any considered factor. Indicated by Spearman correlation coefficient, no positive correlation between the logit of sensitivity and logit of 1-specificity concluded no threshold effect in analysis using numbers preferably obtained by Youden Index (− 0.048, p-value = 0.911) and Index of Union (− 0.762 p-value = 0.028). Results of QUADAS-2 are demonstrated in Additional file 2: Figure S40 and S41. Fagan’s nomogram based on pooled sensitivity and specificity acquired by numbers preferably obtained by Youden Index showed a positive predictive value of 46.2%, meaning 0.46 probability of having cancer in an individual from a tested population with 20% cancer prevalence with a positive miR-212 cancer test (Additional file 2: Figure S42 and S43). Also, the negative post-test result was found to be 8.25% showing an individual from the same population with a negative test result has a probability of 0.08 to be affected by cancer. Deek’s funnel plot did not show any publication bias using numbers obtained preferably by Youden Index (p-value = 0.798, Fig. 3F) and Index of Union (p-value = 0.652, Additional file 2: Figure S39F).
Diagnostic evaluation of cancer using miR-212 level as determinant based on numbers preferably obtained by Youden index: forrest plot of sensitivity (A), specificity (B), positive likelihood ratio (LR) (C), negative LR (D), and diagnostic odds ratio (OR) (E), Deek's funnel plot (F), receiver operating characteristic (ROC) plane (G) and Summary ROC (SROC) curve (H)
Discussion
Timely cancer detection and accurate estimation of prognosis are crucial for appropriate patient management. The prognostic and diagnostic value of miRs is trending as they could be our potential solution. miR-212 is one of many miRs with aberrant expression in different cancer patients; Herein, we analyzed its diagnostic accuracy and prognostic value as the existing studies reported valuable but inconsistent results. Given the unique pathophysiology of each cancer and even different subtypes of particular cancer, it would be more helpful to assess miR-212 based on cancer type and subtype; however, due to relatively scarce standard studies on every single cancer, we aimed to evaluate its importance in a metanalytic overview.
Our prognostic assessment showed that the HR of low levels of miR-212 to its high levels was 2.081 (1.593, 2.717, I2 = 68.884) in predicting OS. To eliminate heterogeneity, we used subgroup analysis which detected PDAC studies as the heterogeneity source. Subgroup analysis illustrated a poorer prognosis of low miR-212 levels in cancers other than PDAC (HR = 2.514); however, PDAC was an exception (HR = 0.408). But why a specific miR is increased in one cancer and decreased in another? It should be noted that one miR potentially can interact with 200 genes [4]. For instance, the Retinoblastoma (Rb)-1 tumor suppressor gene is the target of miR-212 in PDAC (increased miR-212 suppresses Rb1 that promotes cell growth), but Rb binding protein-2 (RBP2) is the target in gastric carcinoma and HCC (decreased miR-212 upregulates RBP2 which promotes cell growth) [4]. When miR-212 can target tumor suppressor genes or their products, it is theoretically plausible for them to be a cancer biomarker with diagnostic and prognostic value. About PDAC, different studies showed consistent results; Yue et al. observed increased levels of both miR-212 and hypoxia-induced factor-1α (HIF-1α) in PDAC patients [18]. They powered their findings via in vitro analysis of the miR-212 promoter region, which possesses five hypoxia response elements, potentially able to bind HIF-1α. Schultz et al. and Wu et al. also found elevated miR-212 in PDAC patients [4, 19]. Several studies indicated that lower miR-212 is tied to worse outcomes in HCC patients [20,21,22,23]. Dou et al. found that HCC-related OS and DFS are predictable via miR-212 levels combined with Forkhead box protein-A1 (FOXA1), which was more reliable than each alone [21]. FOXA1 promotes cell proliferation and apoptosis with an established role in HCC development and post-transcriptionally down-regulated by miR-212-3p [21, 24]. The miR-212 also suppresses the connective tissue growth factor (CTGF) (which promotes tumoral angiogenesis) and histone-H3 lysine-4 demethylase of RBP2 (which is upregulated in HCC) [20, 22, 23]. Similarly, some of the mentioned pathways and some other pathways are declared to be involved in breast cancer, renal cell carcinoma, prostate cancer, nasopharyngeal carcinoma, etc. The relevant targeted genes and their relative function are shown in Additional file 2: Table S1.
Significant pooled association of low miR-212 level incidence with Higher serum AFP level and higher Edmondson-Steiner grade confirm miR-212 value in HCC.
MiRs express abnormally in several cancers; they possess less complex transcriptional and translational modifications than proteins and miRs [2]. They are stable in a wide range of pH and resist degradation with several freezes and thaw procedures [3, 25]. These features make them suitable candidates for cancer diagnosis and treatment. Our diagnostic outcomes resulted from PDAC, HCC, breast cancer, gastric cancer, bladder cancer, prostate cancer, and non-small cell lung cancer studies. In diagnostic evaluation, while using the Youden index, the pooled sensitivity and specificity were 0.716 (0.669–0.759) and 0.790 (0.748–0.827), respectively. DOR is a single number that indicates the diagnostic accuracy of the intended test by dividing PLR to NLR, thus combining both sensitivity and specificity into a single number. Pooled DOR using the Youden index was 10.69 (3.64–31.35), showing that miR-212 could be a potential biomarker in cancer diagnosis. Notably, using multiple miRs as a diagnostic panel could increase diagnostic accuracy to the point that it is applicable in clinical settings, as seen in several studies [26, 27]. Of note, Bagheri et al. reported no additional benefits of using a panel of miRs (miR-223, miR-212, and SNORD37) to detect non-small cell lung cancer, emphasizing the usefulness of miRs as a compound diagnostic tool can be changed based on the type of cancer [3]. This study also encourages future experiments to assess several miRs to find an optimal panel of miRs for each cancer.
Additionally, using miRs based on clinical data (family history, social history, medication history, etc.) and assessing them in specific biofluids (sputum, saliva, bile, urine, etc.) can remarkably increase clinical diagnostic accuracy [25, 27]. Cote et al. showed that miR-212 levels in bile had higher sensitivity, specificity, and DOR than plasma levels for PDAC [27]. Another solution to clinically optimize the diagnostic accuracy of miR-212 is to use it in a high-risk population for each specific cancer [27]. Recently, the diagnostic value of other miRs like miR-375, miR-21, miR-34a, and miR-155 have been studied with relatively similar diagnostic values [28,29,30]. Future diagnosis of cancer can be revolutionized by proper use of miRs, which accordingly could guide us toward more efficient treatment.
The present review has some limitations: all prognostic articles were from China; thus, more studies are needed in other races to generalize the reported findings. Also, miR-212 diagnostic accuracy and prognostic value differ by type of cancer and even cancer subtypes.
In conclusion, miR-212 can help us diagnose cancers with a prolonged latency period and determine cancer prognosis.
Availability of data and material
Not applicable.
References
WHO. Cancer Fact-sheet Geneva [updated 21 Sep 2021]. https://www.who.int/news-room/fact-sheets/detail/cancer. Accessed 17 Oct 2021.
Wang Y, Gao X, Wei F, Zhang X, Yu J, Zhao H, et al. Diagnostic and prognostic value of circulating miR-21 for cancer: a systematic review and meta-analysis. Gene. 2014;533(1):389–97.
Bagheri A, Khorshid HRK, Mowla SJ, Mohebbi HA, Mohammadian A, Yaseri M, et al. Altered miR-223 expression in sputum for diagnosis of non-small cell lung cancer. Avicenna J Med Biotechnol. 2017;9(4):189.
Wu Z, Zhou L, Ding G, Cao L. Overexpressions of miR-212 are associated with poor prognosis of patients with pancreatic ductal adenocarcinoma. Cancer Biomark. 2017;18(1):35–9.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ (Clin Res Ed). 2021;372:n71.
Guyot P, Ades AE, Ouwens MJ, Welton NJ. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan-Meier survival curves. BMC Med Res Methodol. 2012;12:9.
Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3(1):32–5.
Unal I. Defining an optimal cut-point value in ROC analysis: an alternative approach. Comput Math Methods Med. 2017;2017:3762651.
Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–5.
Modesti PA, Reboldi G, Cappuccio FP, Agyemang C, Remuzzi G, Rapi S, et al. Panethnic differences in blood pressure in Europe: a systematic review and meta-analysis. PloS ONE. 2016;11(1):7601.
Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155(8):529–36.
Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–58.
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ (Clin Res Ed). 2003;327(7414):557–60.
Rohatgi A. Webplotdigitizer: version 4.4. 2020. https://www.automerisio/WebPlotDigitizer
Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–101.
Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ (Clin Res Ed). 1997;315(7109):629–34.
Zamora J, Abraira V, Muriel A, Khan K, Coomarasamy A. Meta-DiSc: a software for meta-analysis of test accuracy data. BMC Med Res Methodol. 2006;6:31.
Yue H, Liu L, Song Z. miR-212 regulated by HIF-1α promotes the progression of pancreatic cancer. Exp Ther Med. 2019;17(3):2359–65.
Schultz NA, Andersen KK, Roslind A, Willenbrock H, Wøjdemann M, Johansen JS. Prognostic microRNAs in cancer tissue from patients operated for pancreatic cancer—five microRNAs in a prognostic index. World J Surg. 2012;36(11):2699–707.
Chen J-q, Ou Y-l, Huang Z-p, Hong Y-g, Tao Y-p, Wang Z-g, et al. MicroRNA-212-3p inhibits the proliferation and invasion of human hepatocellular carcinoma cells by suppressing CTGF expression. Sci Rep. 2019;9(1):1–10.
Dou C, Wang Y, Li C, Liu Z, Jia Y, Li Q, et al. MicroRNA-212 suppresses tumor growth of human hepatocellular carcinoma by targeting FOXA1. Oncotarget. 2015;6(15):13216.
Tu H, Wei G, Cai Q, Chen X, Sun Z, Cheng C, et al. MicroRNA-212 inhibits hepatocellular carcinoma cell proliferation and induces apoptosis by targeting FOXA1. Onco Targets Ther. 2015;8:2227.
Wang F, Wang J, Ju L, Chen L, Cai W, Yang J. Diagnostic and prognostic potential of serum miR-132/212 cluster in patients with hepatocellular carcinoma. Ann Clin Biochem. 2018;55(5):576–82.
Yuan Z, Ye M, Qie J, Ye T. FOXA1 promotes cell proliferation and suppresses apoptosis in hcc by directly regulating miR-212-3p/FOXA1/AGR2 signaling pathway. Onco Targets Ther. 2020;13:5231.
Duell EJ, Lujan-Barroso L, Sala N, Deitz McElyea S, Overvad K, Tjonneland A, et al. Plasma microRNAs as biomarkers of pancreatic cancer risk in a prospective cohort study. Int J Cancer. 2017;141(5):905–15.
Pu X, Ding G, Wu M, Zhou S, Jia S, Cao L. Elevated expression of exosomal microRNA-21 as a potential biomarker for the early diagnosis of pancreatic cancer using a tethered cationic lipoplex nanoparticle biochip. Oncol Lett. 2020;19(3):2062–70.
Cote GA, Gore AJ, McElyea SD, Heathers LE, Xu H, Sherman S, et al. A pilot study to develop a diagnostic test for pancreatic ductal adenocarcinoma based on differential expression of select miRNA in plasma and bile. Am J Gastroenterol. 2014;109(12):1942.
Hou Y, Wang J, Wang X, Shi S, Wang W, Chen Z. Appraising microRNA-155 as a noninvasive diagnostic biomarker for cancer detection: a meta-analysis. Medicine. 2016;95(2):e2450.
Imani S, Zhang X, Hosseinifard H, Fu S, Fu J. The diagnostic role of microRNA-34a in breast cancer: a systematic review and meta-analysis. Oncotarget. 2017;8(14):23177.
Yan J, She Q, Shen X, Zhang Y, Liu B, Zhang G. Potential role of MicroRNA-375 as biomarker in human cancers detection: a meta-analysis. BioMed Res Int. 2017. https://doi.org/10.1155/2017/1875843.
Meng X, Wu J, Pan C, Wang H, Ying X, Zhou Y, et al. Genetic and epigenetic down-regulation of microRNA-212 promotes colorectal tumor metastasis via dysregulation of MnSOD. Gastroenterology. 2013;145(2):426-36 e1-6.
Qi B, Liu SG, Qin XG, Yao WJ, Lu JG, Guo L, et al. Overregulation of microRNA-212 in the poor prognosis of esophageal cancer patients. Genet Mol Res. 2014;13(3):7800–7.
Dou C, Wang Y, Li C, Liu Z, Jia Y, Li Q, et al. MicroRNA-212 suppresses tumor growth of human hepatocellular carcinoma by targeting FOXA1. Oncotarget. 2015;6(15):13216–28.
Li D, Li Z, Xiong J, Gong B, Zhang G, Cao C, et al. MicroRNA-212 functions as an epigenetic-silenced tumor suppressor involving in tumor metastasis and invasion of gastric cancer through down-regulating PXN expression. Am J Cancer Res. 2015;5(10):2980–97.
Tu H, Wei G, Cai Q, Chen X, Sun Z, Cheng C, et al. MicroRNA-212 inhibits hepatocellular carcinoma cell proliferation and induces apoptosis by targeting FOXA1. Onco Targets Ther. 2015;8:2227–35.
Gu C, Wang Z, Jin Z, Li G, Kou Y, Jia Z, et al. MicroRNA-212 inhibits the proliferation, migration and invasion of renal cell carcinoma by targeting X-linked inhibitor of apoptosis protein (XIAP). Oncotarget. 2017;8(54):92119–33.
Jiang C, Wang H, Zhou L, Jiang T, Xu Y, Xia L. MicroRNA-212 inhibits the metastasis of nasopharyngeal carcinoma by targeting SOX4. Oncol Rep. 2017;38(1):82–8.
Lv ZD, Yang DX, Liu XP, Jin LY, Wang XG, Yang ZC, et al. MiR-212-5p suppresses the epithelial-mesenchymal transition in triple-negative breast cancer by targeting Prrx2. Cell Physiol Biochem. 2017;44(5):1785–95.
Tang TT, Huan LT, Zhang SJ, Zhou H, Gu L, Chen XH, et al. MicroRNA-212 functions as a tumor-suppressor in human non-small cell lung cancer by targeting SOX4. Oncol Rep. 2017;38(4):2243–50.
Wu ZR, Zhou LJ, Ding GP, Cao LP. Overexpressions of miR-212 are associated with poor prognosis of patients with pancreatic ductal adenocarcinoma. Cancer Biomark. 2017;18(1):35–9.
Zhou Y, Ji Z, Yan W, Zhou Z, Li H. The biological functions and mechanism of miR-212 in prostate cancer proliferation, migration and invasion via targeting Engrailed-2. Oncol Rep. 2017;38(3):1411–9.
Qu HW, Jin Y, Cui ZL, Jin XB. MicroRNA-212 participates in the development of prostate cancer by upregulating BMI1 via NF-κB pathway. Eur Rev Med Pharmacol Sci. 2018;22(11):3348–56.
Tong Z, Meng X, Wang J, Wang L. MicroRNA-212 inhibits the proliferation and invasion of human renal cell carcinoma by targeting FOXA1. Mol Med Rep. 2018;17(1):1361–7.
Wang F, Wang J, Ju LL, Chen L, Cai WH, Yang JL. Diagnostic and prognostic potential of serum miR-132/212 cluster in patients with hepatocellular carcinoma. Ann Clin Biochem. 2018;55(5):576–82.
Chen JQ, Ou YL, Huang ZP, Hong YG, Tao YP, Wang ZG, et al. MicroRNA-212-3p inhibits the proliferation and invasion of human hepatocellular carcinoma cells by suppressing CTGF expression. Sci Rep. 2019;9(1):9820.
Mou TY, Zhang RR, Wang YN. MiRNA-212 acts as a tumor-suppressor in colorectal carcinoma through targeting SOX4. Eur Rev Med Pharmacol Sci. 2019;23(24):10751–60.
Yue H, Liu L, Song ZG. miR-212 regulated by HIF-1 promotes the progression of pancreatic cancer. Exp Ther Med. 2019;17(3):2359–65.
Azar M, Shanehbandi D, Mansouri M, Sarand SP, Asadi M, Akbari M, et al. Altered expression levels of miR-212, miR-133b and miR-27a in tongue squamous cell carcinoma (TSCC) with clinicopathological considerations. Gene Rep. 2020;19:6.
Kang Y, Yanfei CUI, Ming TAN. MicroRNA-212 suppresses cell proliferation in nasopharyngeal carcinoma by targeting ELF3. Oncol Lett. 2020;19(4):2902–8.
Shao JP, Su F, Zhang SP, Chen HK, Li ZJ, Xing GQ, et al. miR-212 as potential biomarker suppresses the proliferation of gastric cancer via targeting SOX4. J Clin Lab Anal. 2020;34(12):3511.
Yuan Z, Ye M, Qie J, Ye T. FOXA1 promotes cell proliferation and suppresses apoptosis in HCC by directly regulating miR-212-3p/FOXA1/AGR2 signaling pathway. Onco Targets Ther. 2020;13:5231–40.
Zhang L, Zhang Y, Wang SS, Tao L, Pang LJ, Fu RT, et al. MiR-212-3p suppresses high-grade serous ovarian cancer progression by directly targeting MAP3K3. Am J Transl Res. 2020;12(3):875–88.
Miah S, Dudziec E, Drayton RM, Zlotta AR, Morgan SL, Rosario DJ, et al. An evaluation of urinary microRNA reveals a high sensitivity for bladder cancer. Br J Cancer. 2012;107(1):123–8.
Cote GA, Gore AJ, McElyea SD, Heathers LE, Xu H, Sherman S, et al. A pilot study to develop a diagnostic test for pancreatic ductal adenocarcinoma based on differential expression of select miRNA in plasma and bile. Am J Gastroenterol. 2014;109(12):1942–52.
Ramalinga M, Roy A, Srivastava A, Bhattarai A, Harish V, Suy S, et al. MicroRNA-212 negatively regulates starvation induced autophagy in prostate cancer cells by inhibiting SIRT1 and is a modulator of angiogenesis and cellular senescence. Oncotarget. 2015;6(33):34446–57.
Bagheri A, Khorram Khorshid HR, Mowla SJ, Mohebbi HA, Mohammadian A, Yaseri M, et al. Altered miR-223 expression in sputum for diagnosis of non-small cell lung cancer. Avicenna J Med Biotechnol. 2017;9(4):189–95.
Damavandi Z, Torkashvand S, Vasei M, Soltani BM, Tavallaei M, Mowla SJ. Aberrant expression of breast development-related MicroRNAs, miR-22, miR-132, and miR-212, in breast tumor tissues. J Breast Cancer. 2016;19(2):148–55.
Pu X, Ding G, Wu M, Zhou S, Jia S, Cao L. Elevated expression of exosomal microRNA–21 as a potential biomarker for the early diagnosis of pancreatic cancer using a tethered cationic lipoplex nanoparticle biochip. Oncol Lett. 2020;19(3):2062–70.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):097.
Acknowledgements
Not applicable.
Funding
No financial support was received for this study.
Author information
Authors and Affiliations
Contributions
Conceptualization: SR, MS, AS; literature search: SR, MS, MM. Analysis: SR, MS; writing-original draft: SR, MS, MM; writing-review and editing: AS. Approval: all authors. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
Authors claim no competing interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Detailed search strategy.
Additional file 2:
Table S1, S2 and Figure S1–S43.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Raji, S., Sahranavard, M., Mottaghi, M. et al. MiR-212 value in prognosis and diagnosis of cancer and its association with patient characteristics: a systematic review and meta-analysis. Cancer Cell Int 22, 163 (2022). https://doi.org/10.1186/s12935-022-02584-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12935-022-02584-0