Abstract
Mitochondrial pyruvate carrier 1 (MPC1) is a key metabolic protein that regulates the transport of pyruvate into the mitochondrial inner membrane. MPC1 deficiency may cause metabolic reprogramming. However, whether and how MPC1 controls mitochondrial oxidative capacity in cancer are still relatively unknown. MPC1 deficiency was recently found to be strongly associated with various diseases and cancer hallmarks. We utilized online databases and uncovered that MPC1 expression is lower in many cancer tissues than in adjacent normal tissues. In addition, MPC1 expression was found to be substantially altered in five cancer types: breast-invasive carcinoma (BRCA), kidney renal clear cell carcinoma (KIRC), lung adenocarcinoma (LUAD), pancreatic adenocarcinoma (PAAD), and prostate adenocarcinoma (PRAD). However, in KIRC, LUAD, PAAD, and PRAD, high MPC1 expression is closely associated with favourable prognosis. Low MPC1 expression in BRCA is significantly associated with shorter overall survival time. MPC1 expression shows strong positive and negative correlations with immune cell infiltration in thymoma (THYM) and thyroid carcinoma (THCA). Furthermore, we have comprehensively summarized the current literature regarding the metabolic reprogramming effects of MPC1 in various cancers. As shown in the literature, MPC1 expression is significantly decreased in cancer tissue and associated with poor prognosis. We discuss the potential metabolism-altering effects of MPC1 in cancer, including decreased pyruvate transport ability; impaired pyruvate-driven oxidative phosphorylation (OXPHOS); and increased lactate production, glucose consumption, and glycolytic capacity, and the underlying mechanisms. These activities facilitate tumour progression, migration, and invasion. MPC1 is a novel cancer biomarker and potentially powerful therapeutic target for cancer diagnosis and treatment. Further studies aimed at slowing cancer progression are in progress.
Similar content being viewed by others
Background
Mitochondria are involved in bioenergetic, biosynthetic, and signalling organelle functions [1]. Mitochondria function as energy factories for cells and are essential for the activity, function, and viability of eukaryotic cells [2,3,4]. They are ubiquitous intracellular semiautonomous organelles responsible for bioenergetic metabolism, ageing, and apoptosis. Due to the high energy demand, mitochondria are especially important for oxidative phosphorylation (OXPHOS) [5, 6]. Metabolic reprogramming is a crucial hallmark of many diseases, including cancer. Metabolic adaptations are involved in tumour initiation and proliferation [7,8,9]. Mitochondrial dysfunction plays a crucial role in cancer metabolism, proliferation, and progression [10].
Metabolic alterations result in oncogenic metabolites in some malignancies [2, 11]. Metabolic reprogramming helps provide ATP and essential macromolecules for protein and nucleotide biosynthesis in cancer cells [12,13,14,15]. Cancer cells undergo metabolic reprogramming that enhances metabolic plasticity, enables the tumour to survive in a nutrient-scarce environment [16], and facilitates survival, proliferation, and metastasis [7, 17, 18]. Tumour cells typically display several, but not necessarily all, hallmarks of cancer, such as decreased glucose uptake, opportunistic nutrient acquisition, glycolysis, increased nitrogen assimilation, aberrant regulation of metabolically driven genes, and increased lactate production [7, 19, 20]. Aerobic glycolysis, also known as the Warburg effect [13], is one of the earliest altered metabolism phenotypes seen in tumour cells [21, 22]. Most cancers exhibit the Warburg effect while retaining mitochondrial respiration [23]. Aerobic glycolysis end products such as lactate may contribute to microenvironment alterations and facilitate tumorigenesis and cancer progression [22, 24, 25].
Alterations in mitochondria and mitochondrial DNA have been reported in many cancers [26,27,28]. Therefore, mitochondria may be an attractive therapeutic target for these cancers. Mitochondrial pyruvate carrier (MPC) is a conserved protein complex located in the mitochondrial membrane [29, 30]. The complex contains MPC1 and MPC2 proteins and plays a crucial function in transporting pyruvate, the end product of glycolysis, from the cytosol into mitochondria [31, 32]. Downregulation of MPC1 is associated with poor cancer prognosis, and MPC1 is particularly interesting as a potential therapeutic target. The MPC1 HUGO Gene Nomenclature Committee (HGNC) ID is 21606, and its genomic location is shown in Fig. 1a. MPC1 was distributed in mitochondria, the cytosol, the extracellular space, and the nucleus (Fig. 1b). The genomic information for MPC1 is presented in the form of a gene card (https://www.genecards.org/). Together, MPC1 and MPC2 function as crucial mitochondrial pyruvate transporters. MPC1 and MPC2 form a protein complex in the inner mitochondrial membrane [33]. Importantly, the MPC1 pyruvate transporter acts as a bridge between glycolysis and the tricarboxylic acid (TCA) cycle. The loss of MPC1 in humans may result in impaired mitochondrial pyruvate uptake and pyruvate oxidation [33]. Pathway enrichment analyses have indicated that MPC1 interacts with many proteins. The top 13 proteins that interact with MPC1 are shown in Fig. 1c. Some biological processes regulated by MPC1 are glucose energy metabolism, pyruvate metabolism and the TCA cycle, respiratory electron transport, ATP synthesis via chemiosmotic coupling, heat production via uncoupling proteins, and glucose metabolism.
Overexpression of MPC1 promotes metabolic flux through the mitochondrial membrane and inhibits the Warburg effect without compromising glucose consumption or maximum cell concentration [34]. In contrast, MPC1 deficiency, glutamine breakdown, increased urea, and increased pyruvate-alanine cycle activity all lead to better regulation of gluconeogenesis and maintenance of euglycaemia [35]. A high-fat diet in mice may increase hepatic MPC1 expression and activity [35], which contribute to gluconeogenesis and hyperglycaemia [36]. MPC1 may function in fatty acid oxidation to meet biological energy demands [35]. The overexpression of MPC1 significantly reduces lactate production [34].
Decreased MPC1 expression may function in tumour progression
MPC1 dysregulation is associated with cancer. Notably, Bensard et al. [9] demonstrated that MPC1 has a close relationship with the glycolytic metabolic phenotype and stem cell markers in the cancer initiation process. MPC1 is downregulated in various cancers. Aberrant expression of MPC1 is also involved in cancer-associated metabolic dysregulation. For example, MPC1 regulates mitochondrial respiratory capacity in renal cell carcinoma (RCC) [37], prostate cancer [38], hepatocellular carcinoma (HCC) [39], and cholangiocarcinoma [40].
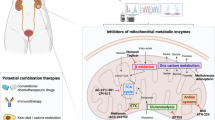
MPC1 is downregulated in many tumour cells, which affects tumour mitochondrial respiratory capacity. In human renal cell carcinoma, the upstream regulator peroxisome proliferator-activated receptor-gamma coactivator 1 alpha (PGC-1α) targets and recruits oestrogen-related receptor-alpha (ERR-α). The accumulation of ERR-α activates the proximal MPC1 promoter, thus causing MPC1 overexpression. In addition, PGC-1α deficiency inhibits MPC1 expression, therefore decreasing pyruvate transport and impairing pyruvate-driven OXPHOS in RCC [41] (Fig. 2). PGC-1α is downregulated in HCC, leading to decreased MPC1 expression. In the liver, MPC1 binds nuclear respiratory factor 1 (NRF1) and promotes mitochondrial biogenesis. In HCC, a decrease in proliferator-activated receptor gamma coactivator-1 alpha(PGC1α)attenuates NRF1 and MPC1 expression, thus promoting HCC progression [39]. In prostate cancer, MPC1 deficiency significantly increases lactate production, glucose consumption, and glycolytic capacity [38]. In cholangiocarcinoma, PGC1α reverses the Warburg effect by switching Warburg effect-related aerobic glycolysis to OXPHOS and upregulating MPC1 and pyruvate dehydrogenase E1α subunit expression [40], which facilitate tumour migration and invasion (Figs. 2, 3).
MPC1 functions as a clinical indicator of many cancers
Correlations of MPC1 expression with infiltrating immune cells across cancers
To explore the mRNA expression level in tumours and adjacent normal tissues, we utilized the Oncomine database (https://www.oncomine.org/). The results implied that MPC1 is differentially expressed in various cancers; for example, MPC1 is expressed at lower levels in bladder, colorectal, oesophageal, gastric, kidney, and liver cancers than in adjacent normal tissues (Fig. 4a). To investigate the MPC1 expression level in The Cancer Genome Atlas (TCGA) database, TMIER 2.0 online analysis was employed in this study. The investigation demonstrated that MPC1 was lower in bladder urothelial carcinoma (BLCA), breast invasive carcinoma (BRCA), cervical squamous cell carcinoma and endocervical adenocarcinoma (CESC), cholangiocarcinoma (CHOL), colon adenocarcinoma (COAD), oesophageal carcinoma (ESCA), head and neck squamous cell carcinoma (HNSC), kidney chromophobe (KICH), kidney renal clear cell carcinoma (KIRC), kidney renal papillary cell carcinoma (KIRP), liver hepatocellular carcinoma (LIHC), lung adenocarcinoma (LUAD), lung squamous cell carcinoma (LUSC), prostate adenocarcinoma (PRAD), rectum adenocarcinoma (READ), stomach adenocarcinoma (STAD), thyroid carcinoma (THCA), and uterine corpus endometrial carcinoma (UCEC) than in adjacent normal tissues (Fig. 4b). Furthermore, we explored the correlation between MPC1 expression and patient prognosis. The results indicated that MPC1 was significantly differentially expressed in many cancers, such as BRCA, KIRC, LUAD, pancreatic adenocarcinoma (PAAD), and PRAD. However, in KIRC (P = 2.4e-05, Fig. 5a), LUAD (P = 0.0085, Fig. 5b), PAAD (P = 0.028, Fig. 5c), and PRAD (P = 0.044, Fig. 5d), high expression of MPC1 was closely associated with favourable prognosis. Low expression of MPC1 in BRCA was significantly associated with shorter overall survival time (P = 0.046, Fig. 5e). In addition, MPC1 expression was deeply affected in five cancer types: BRCA, KIRC, LUAD, PAAD, and PRAD. However, in KIRC, LUAD, PAAD, and PRAD, high expression of MPC1 was closely associated with favourable prognosis. Because our aim was to determine the expression patterns and prognostic value of MPC1 across cancers, we analysed the expression status of MPC1 at the mRNA level and then explored its relationship with prognosis across cancers using the TCGA database. MPC1 showed significantly different expression levels across cancers. Differential expression of MPC1 indicated different prognoses in various cancers. Based on an analysis of public data, the MPC1 mRNA expression level is a potential prognostic indicator of cancer. However, because of tumour heterogeneity, patient prognoses may be different. These studies imply that aberrant MPC1 expression has the potential to become a novel detection biomarker and prognostic indicator in various cancers.
MPC1 functions in T cell homeostasis
To estimate tumour-associated immune cell infiltration and MPC1 expression across cancers, we performed TIMER2.0 online analysis (http://timer.comp-genomics.org/), and the results demonstrated that MPC1 expression showed a significant relationship with immune purity and immune cell infiltration in 26 cancers, as illustrated in Fig. 6a. Specifically, B cell, CD8+ T cell, dendritic cell (DC), macrophage, and neutrophil infiltration showed a significant association with MPC1 expression across cancers. In addition, in HNSC, LUSC, ovarian cancer (OV), thymoma (THYM), THCA, and tenosynovial giant cell tumour (TGCT), there were strong correlations between the immune cell infiltration level and MPC1 expression. In addition, we further investigated the association of MPC1 expression with immune cell infiltration levels in the cancer types with the strongest positive and negative correlations and determined that MPC1 expression showed strong positive correlations with the levels of infiltrating B cells (r = 0.647, P = 7.57e-15), CD8+ T cells (r = 0.54, P = 5.59e-10), CD4+ T cells (r = 0.569, P = 7.57e-11), macrophages (r = 0.529, P = 1.50e-09), neutrophils (r = −0.067, P = 4.78e-01) and DCs (r = 0.661, P = 1.16e-15) in THYM (Fig. 6b). Similarly, there were negative correlations with immune purity (r = −0.149, P = 8.31e-03) and the levels of infiltrating B cells (r = −0.209, P = 3.71e-06), CD8+ T cells (r = −0.076, P = 9.50e-02), CD4+ T cells (r = −0.271, P = 1.17 e-09), macrophages (r = −0.103, P = 2.23e-02), neutrophils (r = −0.318, P = 6.07e-13), and DCs (r = −0.35, P = 1.92e-15) in THCA (Fig. 6c). These results indicate that MPC1 plays an important role in immune infiltration in various cancers.
The association of MPC1 expression with immune infiltration. a MPC1 expression and the immune infiltration landscape in 26 cancers. b MPC1 expression is positively associated with tumour purity and immune cell infiltration in THYM. c MPC1 expression is negatively associated with tumour purity and immune cell infiltration in THCA
MPC1 function as a key indicator of many cancers in the clinic
Metabolic reprogramming is considered a hallmark of cancer and leads to tumour development [42]. Major metabolic aberration is associated with the metabolic environment of cancer [43]. Alternations in cancer metabolism further interact with cellular signalling and epigenetics to promote carcinogenesis and tumour development [44, 45]. MPC1 also functions as a clinical indicator of cancers, including lung cancer [46], gastric cancer [47], colorectal carcinoma (CRC) [9], intrahepatic cholangiocarcinoma (ICC) [48], RCC [49], and glioblastoma (GBM) [41]. In lung cancer, MPC1 expression is lower in LUAD tissue than in non-tumour tissue and is remarkably associated with favourable prognosis [46]. In prostate cancer, MPC1 expression is significantly decreased; this decrease is closely associated with unfavourable prognosis [38]. Similarly, Zhou et al. [47] found that MPC1 expression is decreased in gastric cancer tumour tissues compared with non-tumour tissues and that lower MPC1 expression predicts poor prognosis. Lower MPC1 expression is associated with advanced tumour stage, greater invasion depth, and lymph node metastasis [47]. Bensard et al. [9] found that MPC1 and MPC2 are decreased in human COAD, a result that is recapitulated in early-stage adenomas. Schell et al. [50] demonstrated that MPC1 has low expression in various cancers; they also verified that low expression of MPC1 is closely associated with cancer onset and poor prognosis in colon cancer. They used xenografts to validate that reintroduction of appropriate MPC1 expression reduces tumour growth. Similarly, Tian et al. [51] explored the TCGA and Gene Expression Omnibus databases and confirmed that MPC1 is downregulated in CRC compared with adjacent normal tissues. In addition, they also discovered that MPC1 expression gradually decreased from normal tissues to primary CRC tissues to CRC metastasis tissues [51]. Sandoval et al. [52] utilized TCGA data and found that MPC1 has low expression in COAD tumours with adenomatous polyposis coli (APC) deletions. APC is a tumour suppressor and dictates intestinal cell differentiation fate by regulating the pyruvate metabolism process. In ICC tissues, the expression of MPC1 as measured by immunohistochemistry is lower than that in non-tumour tissues, a finding that is closely associated with poor prognosis [48]. In RCC, Tang et al. [49] measured MPC1 expression in 10 pairs of RCC and corresponding adjacent non-cancerous tissues by qPCR and western blotting. The results indicated that MPC1 expression was significantly decreased in tumour tissues compared to that in non-cancerous tissues. Chai et al. [41] demonstrated that MPC1 is significantly downregulated in GBM tumour tissues, which was associated with poor prognosis, including poor response to temozolomide, based on TCGA database analysis.
Decreased MPC1 expression promotes tumour progression
Cellular metabolism provides a permissive environment that generates metabolic intermediates regulating cellular growth [53,54,55] and proliferation [9]. Cancer cell invasion of adjacent tissues and metastasis to distant sites are complex [56], multiscale processes. Invasion and metastasis contribute to high recurrence risk, poor prognosis, and low survival [57, 58]. Cancer metabolism contributes to cancer cell viability and growth [59]. MPC1 deletion promotes tumour initiation as well as a proliferative and protumorigenic phenotype in genetic tumour models [9]. Loss of MPC1 blocks mitochondrial pyruvate oxidation, which facilitates aerobic glycolysis in colon cancer cells [9]. Ohashi et al. [48] found that MPC1 induces epithelial-to-mesenchymal transition in human ICC cell lines. In addition, low MPC1 expression is related to the invasion and metastasis of ICC cell lines.
MPC1 promotes metastatic capacity through metabolic reprogramming. MPC1 is significantly depressed in CRC [51], both in human tumour tissues and in mouse models. The MPC1 expression level in metastatic CRC is lower than that in primary CRC [51]. Decreased MPC1 expression promotes β-catenin nuclear transcription, enhances the Wnt/β-catenin pathway, and facilitates the expression of the cancer metastasis-related proteins MMP7, E-cadherin, Snail, and MYC, thus promoting CRC liver metastasis. Decreased MPC1 expression enhances lung cancer cell invasion and migration abilities via matrix metalloproteinase (MMP) pathways, and MMP2, MMP3, and MMP7 are significantly overexpressed in an MPC1 knockdown mouse model [46]. Invadopodia formation in MPC1-deficient groups is remarkably increased [46]. In lung cancer, MPC1 works with mitochondrial signal transducer and activator of transcription 3 (mito-STAT3) to reduce cytoplasmic STAT3. Inhibiting STAT3 phosphorylation attenuates malignant progression in lung cancer cells [46]. In mouse xenograft tumours, the overexpression of MPC1 reduces the tumour growth rate and tumour size [46]. MPC1 expression is significantly decreased in prostate cancer specimens [38]. Overexpression of MPC1 significantly inhibits the invasion ability of prostate tumour cells. Chicken ovalbumin upstream promoter transcription factor II (COUP-TFII), an MPC1 upstream regulator, represses MPC1 expression and facilitates growth and metastasis of prostate cancer cells [38]. MPC1 is also decreased in glioblastoma cell lines [60]. In an in vitro RCC experiment, MPC1 deficiency markedly increased the malignancy of RCC. MPC1 functions as an upstream regulator of hypoxia-inducible factor 1-alpha (HIF1α) and plays a key role in the hypoxia/HIF1α axis in oxygen-deficiency conditions. Decreased MPC1 expression facilitates the mobility and invasion of RCC cells through upregulation of MMPs [49]. Consistent with previous studies, the average weight and volume of RCC xenograft tumours in the MPC1-deficient groups were significantly greater than those in the MPC1 overexpression groups [49] (Fig. 3).
The post-translational regulation of MPC1 in cancer
Metabolic and epigenomic alterations facilitate cancer development and progression [61, 62]. Metabolic remodelling and changes to the epigenome (including acetylation) have an important bidirectional regulatory mechanism in cancer [21, 63, 64]. Upon pyruvate transportation, sirtuin 3 (SIRT3) binds and stabilizes MPC1 through deacetylation, which enhances mitochondrial inner member transport of pyruvate [65] (Fig. 2). MPC1 is acetylated at the K45 and K46 acetylation sites. Under high glucose conditions, increased SIRT3-MPC1 binding and MPC1 deacetylation inhibit colon cancer cell growth [65]. MPC1 also plays an important role in enhancing cholangiocarcinoma cell migration and invasion capabilities [47]. In gastric cancer cell lines, the overexpression of MPC1 may attenuate proliferation, migration, and invasion. Taken together, these findings indicate that MPC1 is involved in various tumour progression processes and might be a promising target for cancer treatment.
MPC1 and the stemness phenotype
The overexpression of MPC1 in CRC significantly inhibits the stemness and proliferation abilities of tumour suppressor-deficient intestinal stem cells [9]. LUAD cell lines with lentiviral vector-mediated MPC1 overexpression exhibited smaller volumes and numbers of tumour spheres. Decreasing the expression of MPC1, like MPC1 knockdown and MPC1 silencing, resulted in significantly increased tumour size and volume [46]. In addition, MPC1 deficiency significantly increased cancer stem cell markers such as Nanog homeobox (NANOG), octamer-binding transcription factor 4 (OCT4), and SRY-box transcription factor 2 (SOX2) in LUAD cells in vivo [46]. In gastric cancer, the overexpression of MPC1 decreases the stem cell-like properties and sphere formation capability of tumour cells, and stemness markers such as NANOG, OCT4, SOX2, and β-actin are significantly decreased [47]. In colon cancer cells, MPC1 deficiency promotes stem cell-like gene expression, whereas MPC1 overexpression inhibits stemness ability [9]. Schell et al. [6, 50] found that rescuing the expression of MPC enhances mitochondrial pyruvate oxidation. Specifically, increased MPC1 significantly impedes colony formation in soft agar, spheroid formation, and xenograft growth.
MPC1 mediates metabolic processes
Metabolic substrates are essential for multiple pathological and physiological regulatory processes [31, 66, 67]. The targeting of a large number of molecules and regulatory pathways to slow disease progression, especially in cancers and Parkinson's disease, is under investigation. Studies have suggested MPC as a novel therapeutic target in Parkinson's disease progression [31]. A mechanism involving the MPC complex was found to be involved in enhancing autophagy via mammalian target of rapamycin (mTOR) activation and inhibiting neuroinflammation [31]. Aberrant expression of the MPC complex contributes to pyruvate transport abnormalities and is significantly correlated with cancer cell energy production, also called the Warburg effect [13, 68]. In the early stages of CRC, the glycolytic metabolic phenotype can be detected, featuring low expression of MPC1 [9]. In human renal cell carcinoma, decreased MPC1 expression might lead to impaired mitochondrial respiratory capacity in renal cell carcinoma cells through the upstream gene regulation of PGC1α [37]. These studies suggest that MPC1 is essential for chronic disease and cancer-associated phenotypes and functions as a master regulator of disease progression.
MPC1 is involved in thermoregulation
MPC1 is involved in brown adipose tissue (BAT)-mediated energy metabolism. BAT transforms chemical energy into heat, which helps maintain a warm body temperature and regulates energy metabolism. MPC1 deficiency in BAT increases ketogenesis, causing an increase in 3-hydroxybutyrate in the blood under cold conditions. A significant decrease in MPC1 and MCP2 expression occurs in the BAT of male high-fat-diet mice [69].
MPC1 functions in T cell homeostasis
In T cell homeostasis, MPC1 deficiency affects multiple pathways in early thymocyte T cell β-selection and positive selection [70]. MPC1 deficiency results in a significant decrease in specific αβ T cell numbers, and both reduces and activates peripheral T cell populations [70]. The pyruvate oxidation of T cell precursors is a crucial energy metabolic process for optimal αβ T cell maturation in thymic development [70].
MPC1 is associated with drug resistance
Cancers with strong resistance to current treatments have a poor prognosis. Chai et al. [41] demonstrated the involvement of MPC1 in temozolomide drug resistance, which is closely related with poor prognosis. Oka et al. [71] showed that the relapse of patients with multiple myeloma treated with bortezomib is strongly associated with decreased MPC-1 expression. In addition, Kuroda et al.[72] classified myeloma cells according to phenotype and found that intermediate myeloma cells (MPC1+CD49e− CD45−) were remarkably suppressed. They also detected a large number of residual immature myeloma cells (MPC1− CD49e− CD45−/+) after 3 or 4 cycles of vincristine, doxorubicin, and dexamethasone chemotherapy [72]. However, the need for clinical MPC1-targeting therapies remains urgent.
Conclusions
MPC1, located in the mitochondrial inner membrane, functions in transporting pyruvate from the cytoplasm into mitochondria [29]. To sustain tumour growth, cancer cells need to adapt to the acidic tumour microenvironment [42, 73, 74]. Decreased MPC1 expression facilitates a reduction in the conversion of pyruvate into circulating lactate and plays an important role in regulating cancer-associated metabolism. Specifically, the inhibition of MPC1 may attenuate pyruvate transport into the mitochondrial inner membrane. OXPHOS activity is thus significantly inhibited, and lactate production, glucose consumption, and glycolytic capacity are strongly enhanced, supporting the tumour microenvironment [75,76,77,78]. Moreover, MPC1 deficiency may contribute to the growth, invasion, and metastasis of cancer cells.
Targeting MPC1 may provide novel insight into the design and assessment of drugs for treating cancers, which may be a promising cancer treatment strategy for patients.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author upon reasonable request.
Abbreviations
- MPC1:
-
Mitochondrial pyruvate carrier 1
- OXPHOS:
-
Oxidative phosphorylation
- MPC:
-
Mitochondrial pyruvate carrier
- TCA:
-
Tricarboxylic acid
- HCC:
-
Hepatocellular carcinoma
- PGC-1α:
-
Peroxisome proliferator-activated receptor-gamma coactivator 1 alpha
- ERR-α:
-
Oestrogen-related receptor-alpha
- NRF1:
-
Nuclear respiratory factor 1
- BLCA:
-
Bladder urothelial carcinoma
- BRCA:
-
Breast-invasive carcinoma
- CESC:
-
Cervical squamous cell carcinoma and endocervical adenocarcinoma
- CHOL:
-
Cholangiocarcinoma
- COAD:
-
Colon adenocarcinoma
- ESCA:
-
Oesophageal carcinoma
- HNSC:
-
Head and neck squamous cell carcinoma
- KICH:
-
Kidney chromophobe
- KIRC:
-
Kidney renal clear cell carcinoma
- KIRP:
-
Kidney renal papillary cell carcinoma
- LIHC:
-
Liver hepatocellular carcinoma
- LUAD:
-
Lung adenocarcinoma
- LUSC:
-
Lung squamous cell carcinoma
- PRAD:
-
Prostate adenocarcinoma
- READ:
-
Rectum adenocarcinoma
- STAD:
-
Stomach adenocarcinoma
- THCA:
-
Thyroid carcinoma
- UCEC:
-
Uterine corpus endometrial carcinoma
- OV:
-
Ovarian cancer
- THYM:
-
Thymoma
- CRC:
-
Colorectal carcinoma
- ICC:
-
Intrahepatic cholangiocarcinoma
- RCC:
-
Renal cell carcinoma
- GBM:
-
Glioblastoma
- TCGA:
-
The Cancer Genome Atlas
- APC:
-
Adenomatous polyposis coli
- MMP:
-
Matrix metalloproteinase
- mito-STAT3:
-
Mitochondrial signal transducer and activator of transcription 3
- COUP-TFII:
-
Chicken ovalbumin upstream promoter transcription factor II
- HIF1α:
-
Hypoxia-inducible factor 1-alpha
- SIRT3:
-
Sirtuin 3
- BAT:
-
Brown adipose tissue
- NANOG:
-
NANOG homeobox
- SOX2:
-
SRY-box transcription factor 2
- OCT4:
-
Octamer-binding transcription factor 4
- mTOR:
-
Mammalian target of rapamycin
References
Vyas S, Zaganjor E, Haigis MC. Mitochondria and cancer. Cell. 2016;166:555–66.
Zong WX, Rabinowitz JD, White E. Mitochondria and cancer. Mol Cell. 2016;61:667–76.
Bock FJ, Tait SWG. Mitochondria as multifaceted regulators of cell death. Nat Rev Mol Cell Biol. 2020;21:85–100.
Sung AY, Floyd BJ, Pagliarini DJ. Systems biochemistry approaches to defining mitochondrial protein function. Cell Metab. 2020;31:669–78.
Zielonka J, Joseph J, Sikora A, Hardy M, Ouari O, Vasquez-Vivar J, et al. Mitochondria-targeted triphenylphosphonium-based compounds: syntheses, mechanisms of action, and therapeutic and diagnostic applications. Chem Rev. 2017;117:10043–120.
Schell JC, Wisidagama DR, Bensard C, Zhao H, Wei P, Tanner J, et al. Control of intestinal stem cell function and proliferation by mitochondrial pyruvate metabolism. Nat Cell Biol. 2017;19:1027–36.
Pavlova NN, Thompson CB. The emerging hallmarks of cancer metabolism. Cell Metab. 2016;23:27–47.
DeBerardinis RJ, Chandel NS. Fundamentals of cancer metabolism. Sci Adv. 2016;2:e1600200.
Bensard CL, Wisidagama DR, Olson KA, Berg JA, Krah NM, Schell JC, et al. Regulation of tumor initiation by the mitochondrial pyruvate carrier. Cell Metab. 2020;31:284-300.e7.
Feng LR, Wolff BS, Liwang J, Regan JM, Alshawi S, Raheem S, et al. Cancer-related fatigue during combined treatment of androgen deprivation therapy and radiotherapy is associated with mitochondrial dysfunction. Int J Mol Med. 2020;45:485–96.
Porporato PE, Filigheddu N, Pedro JMB, Kroemer G, Galluzzi L. Mitochondrial metabolism and cancer. Cell Res. 2018;28:265–80.
Martinez-Outschoorn UE, Peiris-Pagés M, Pestell RG, Sotgia F, Lisanti MP. Cancer metabolism: a therapeutic perspective. Nat Rev Clin Oncol. 2017;14:11–31.
Liberti MV, Locasale JW. The warburg effect: how does it benefit cancer cells? Trends Biochem Sci. 2016;41:211–8.
Zhang X, Zhao H, Li Y, Xia D, Yang L, Ma Y, et al. The role of YAP/TAZ activity in cancer metabolic reprogramming. Mol Cancer. 2018;17:134.
Campbell SL, Wellen KE. Metabolic signaling to the nucleus in cancer. Mol Cell. 2018;71:398–408.
Chen H, Chan DC. Mitochondrial dynamics in regulating the unique phenotypes of cancer and stem cells. Cell Metab. 2017;26:39–48.
Kim J, DeBerardinis RJ. Mechanisms and implications of metabolic heterogeneity in cancer. Cell Metab. 2019;30:434–46.
Cao Y. Adipocyte and lipid metabolism in cancer drug resistance. J Clin Invest. 2019;129:3006–17.
Schwartz L, Seyfried T, Alfarouk KO, Da Veiga Moreira J, Fais S. Out of warburg effect: an effective cancer treatment targeting the tumor specific metabolism and dysregulated ph. Semin Cancer Biol. 2017;43:134–8.
Zhu J, Thompson CB. Metabolic regulation of cell growth and proliferation. Nat Rev Mol Cell Biol. 2019;20:436–50.
Danhier P, Bański P, Payen VL, Grasso D, Ippolito L, Sonveaux P, et al. Cancer metabolism in space and time: beyond the warburg effect. Biochim Biophys Acta Bioenerg. 2017;1858:556–72.
Ganapathy-Kanniappan S. Molecular intricacies of aerobic glycolysis in cancer: current insights into the classic metabolic phenotype. Crit Rev Biochem Mol Biol. 2018;53:667–82.
Koppenol WH, Bounds PL, Dang CV. Otto warburg’s contributions to current concepts of cancer metabolism. Nat Rev Cancer. 2011;11:325–37.
Lu J, Tan M, Cai Q. The warburg effect in tumor progression: mitochondrial oxidative metabolism as an anti-metastasis mechanism. Cancer Lett. 2015;356:156–64.
Orang AV, Petersen J, McKinnon RA, Michael MZ. Micromanaging aerobic respiration and glycolysis in cancer cells. Mol Metab. 2019;23:98–126.
DeBalsi KL, Hoff KE, Copeland WC. Role of the mitochondrial DNA replication machinery in mitochondrial DNA mutagenesis, aging and age-related diseases. Ageing Res Rev. 2017;33:89–104.
Hsu CC, Tseng LM, Lee HC. Role of mitochondrial dysfunction in cancer progression. Exp Biol Med. 2016;241:1281–95.
Srinivasan S, Guha M, Kashina A, Avadhani NG. Mitochondrial dysfunction and mitochondrial dynamics-the cancer connection. Biochim Biophys Acta Bioenerg. 2017;1858:602–14.
Taylor EB. Functional properties of the mitochondrial carrier system. Trends Cell Biol. 2017;27:633–44.
Rauckhorst AJ, Taylor EB. Mitochondrial pyruvate carrier function and cancer metabolism. Curr Opin Genet Dev. 2016;38:102–9.
Quansah E, Peelaerts W, Langston JW, Simon DK, Colca J, Brundin P. Targeting energy metabolism via the mitochondrial pyruvate carrier as a novel approach to attenuate neurodegeneration. Mol Neurodegener. 2018;13:28.
Herzig S, Raemy E, Montessuit S, Veuthey JL, Zamboni N, Westermann B, et al. Identification and functional expression of the mitochondrial pyruvate carrier. Science. 2012;337:93–6.
Bricker DK, Taylor EB, Schell JC, Orsak T, Boutron A, Chen YC, et al. A mitochondrial pyruvate carrier required for pyruvate uptake in yeast, Drosophila, and humans. Science. 2012;337:96–100.
Bulté DB, Palomares LA, Parra CG, Martínez JA, Contreras MA, Noriega LG, et al. Overexpression of the mitochondrial pyruvate carrier reduces lactate production and increases recombinant protein productivity in CHO cells. Biotechnol Bioeng. 2020;117:2633–47.
Gray LR, Sultana MR, Rauckhorst AJ, Oonthonpan L, Tompkins SC, Sharma A, et al. Hepatic mitochondrial pyruvate carrier 1 is required for efficient regulation of gluconeogenesis and whole-body glucose homeostasis. Cell Metab. 2015;22:669–81.
Zou S, Lang T, Zhang B, Huang K, Gong L, Luo H, et al. Fatty acid oxidation alleviates the energy deficiency caused by the loss of MPC1 in MPC1(+/−) mice. Biochem Biophys Res Commun. 2018;495:1008–13.
Koh E, Kim YK, Shin D, Kim KS. MPC1 is essential for PGC-1α-induced mitochondrial respiration and biogenesis. Biochem J. 2018;475:1687–99.
Wang L, Xu M, Qin J, Lin SC, Lee HJ, Tsai SY, et al. MPC1, a key gene in cancer metabolism, is regulated by COUPTFII in human prostate cancer. Oncotarget. 2016;7:14673–83.
Wang C, Dong L, Li X, Li Y, Zhang B, Wu H, et al. The PGC1α/NRF1-MPC1 axis suppresses tumor progression and enhances the sensitivity to sorafenib/doxorubicin treatment in hepatocellular carcinoma. Free Radic Biol Med. 2020;163:141–52.
Dan L, Wang C, Ma P, Yu Q, Gu M, Dong L, et al. PGC1α promotes cholangiocarcinoma metastasis by upregulating PDHA1 and MPC1 expression to reverse the warburg effect. Cell Death Dis. 2018;9:466.
Chai Y, Wang C, Liu W, Fan Y, Zhang Y. MPC1 deletion is associated with poor prognosis and temozolomide resistance in glioblastoma. J Neurooncol. 2019;144:293–301.
Zhao H, Yang L, Baddour J, Achreja A, Bernard V, Moss T, et al. Tumor microenvironment derived exosomes pleiotropically modulate cancer cell metabolism. Elife. 2016;5:e10250.
Anderson NM, Mucka P, Kern JG, Feng H. The emerging role and targetability of the TCA cycle in cancer metabolism. Protein Cell. 2018;9:216–37.
Frezza C. Metabolism and cancer: the future is now. Br J Cancer. 2020;122:133–5.
Park JH, Pyun WY, Park HW. Cancer metabolism: phenotype, signaling and therapeutic targets. Cells. 2020;9:2308.
Zou H, Chen Q, Zhang A, Wang S, Wu H, Yuan Y, et al. MPC1 deficiency accelerates lung adenocarcinoma progression through the STAT3 pathway. Cell Death Dis. 2019;10:148.
Zhou X, Xiong ZJ, Xiao SM, Zhou J, Ding Z, Tang LC, et al. Overexpression of MPC1 inhibits the proliferation, migration, invasion, and stem cell-like properties of gastric cancer cells. Onco Targets Ther. 2017;10:5151–63.
Ohashi T, Eguchi H, Kawamoto K, Konno M, Asai A, Colvin H, et al. Mitochondrial pyruvate carrier modulates the epithelial–mesenchymal transition in cholangiocarcinoma. Oncol Rep. 2018;39:1276–82.
Tang XP, Chen Q, Li Y, Wang Y, Zou HB, Fu WJ, et al. Mitochondrial pyruvate carrier 1 functions as a tumor suppressor and predicts the prognosis of human renal cell carcinoma. Lab Invest. 2019;99:191–9.
Schell JC, Olson KA, Jiang L, Hawkins AJ, Van Vranken JG, Xie J, et al. A role for the mitochondrial pyruvate carrier as a repressor of the warburg effect and colon cancer cell growth. Mol Cell. 2014;56:400–13.
Tian GA, Xu CJ, Zhou KX, Zhang ZG, Gu JR, Zhang XL, et al. MPC1 deficiency promotes CRC liver metastasis via facilitating nuclear translocation of β-catenin. J Immunol Res. 2020;2020:8340329.
Sandoval IT, Delacruz RG, Miller BN, Hill S, Olson KA, Gabriel AE, et al. A metabolic switch controls intestinal differentiation downstream of Adenomatous polyposis coli (APC). Elife. 2017;6:e22706.
Vander Heiden MG, DeBerardinis RJ. Understanding the intersections between metabolism and cancer biology. Cell. 2017;168:657–69.
Vazquez A, Kamphorst JJ, Markert EK, Schug ZT, Tardito S, Gottlieb E. Cancer metabolism at a glance. J Cell Sci. 2016;129:3367–73.
Sancho P, Barneda D, Heeschen C. Hallmarks of cancer stem cell metabolism. Br J Cancer. 2016;114:1305–12.
Winkler J, Abisoye-Ogunniyan A, Metcalf KJ, Werb Z. Concepts of extracellular matrix remodelling in tumour progression and metastasis. Nat Commun. 2020;11:5120.
Klein CA. Cancer progression and the invisible phase of metastatic colonization. Nat Rev Cancer. 2020;20:681–94.
Sun W, Ren Y, Lu Z, Zhao X. The potential roles of exosomes in pancreatic cancer initiation and metastasis. Mol Cancer. 2020;19:135.
Lieu EL, Nguyen T, Rhyne S, Kim J. Amino acids in cancer. Exp Mol Med. 2020;52:15–30.
Xiao B, Fan Y, Ye M, Lv S, Xu B, Chai Y, et al. Downregulation of COUP-TFII inhibits glioblastoma growth via targeting MPC1. Oncol Lett. 2018;15:9697–702.
Filippakopoulos P, Knapp S. Targeting bromodomains: epigenetic readers of lysine acetylation. Nat Rev Drug Discov. 2014;13:337–56.
Menzies KJ, Zhang H, Katsyuba E, Auwerx J. Protein acetylation in metabolism—metabolites and cofactors. Nat Rev Endocrinol. 2016;12:43–60.
Reid MA, Dai Z, Locasale JW. The impact of cellular metabolism on chromatin dynamics and epigenetics. Nat Cell Biol. 2017;19:1298–306.
Kimmelman AC, White E. Autophagy and tumor metabolism. Cell Metab. 2017;25:1037–43.
Liang L, Li Q, Huang L, Li D, Li X. Sirt3 binds to and deacetylates mitochondrial pyruvate carrier 1 to enhance its activity. Biochem Biophys Res Commun. 2015;468:807–12.
Rauckhorst AJ, Gray LR, Sheldon RD, Fu X, Pewa AD, Feddersen CR, et al. The mitochondrial pyruvate carrier mediates high fat diet-induced increases in hepatic TCA cycle capacity. Mol Metab. 2017;6:1468–79.
Shen JL, Li CL, Wang M, He LL, Lin MY, Chen DH, et al. Mitochondrial pyruvate carrier 1 mediates abscisic acid-regulated stomatal closure and the drought response by affecting cellular pyruvate content in Arabidopsis thaliana. BMC Plant Biol. 2017;17:217.
Compan V, Pierredon S, Vanderperre B, Krznar P, Marchiq I, Zamboni N, et al. Monitoring mitochondrial pyruvate carrier activity in real time using a BRET-based biosensor: investigation of the warburg effect. Mol Cell. 2015;59:491–501.
Burrell JA, Richard AJ, King WT, Stephens JM. Mitochondrial pyruvate carriers are not required for adipogenesis but are regulated by high-fat feeding in brown adipose tissue. Obesity. 2020;28:293–302.
Ramstead AG, Wallace JA, Lee SH, Bauer KM, Tang WW, Ekiz HA, et al. Mitochondrial pyruvate carrier 1 promotes peripheral T cell homeostasis through metabolic regulation of thymic development. Cell Rep. 2020;30:2889-99.e6.
Oka S, Ono K, Nohgawa M. Clinical effects of CD33 and MPC-1 on the prognosis of multiple myeloma treated with bortezomib. Leuk Lymphoma. 2019;60:2152–7.
Kuroda Y, Sakai A, Okikawa Y, Munemasa S, Katayama Y, Hyodo H, et al. The maturation of myeloma cells correlates with sensitivity to chemotherapeutic agents. Int J Hematol. 2005;81:335–41.
Rinaldi G, Rossi M, Fendt SM. Metabolic interactions in cancer: cellular metabolism at the interface between the microenvironment, the cancer cell phenotype and the epigenetic landscape. Wiley Interdiscip Rev Syst Biol Med. 2018;10:e1397.
Corbet C, Feron O. Cancer cell metabolism and mitochondria: nutrient plasticity for TCA cycle fueling. Biochim Biophys Acta Rev Cancer. 2017;1868:7–15.
Ashton TM, McKenna WG, Kunz-Schughart LA, Higgins GS. Oxidative phosphorylation as an emerging target in cancer therapy. Clin Cancer Res. 2018;24:2482–90.
Joseph M, Timothy Y, Chi VD, Vander H, Michael W, Krystle N, et al. Drugging OXPHOS dependency in cancer. Cancer Discov. 2019;9:OF10.
Deng P, Haynes CM. Mitochondrial dysfunction in cancer: potential roles of ATF5 and the mitochondrial UPR. Semin Cancer Biol. 2017;47:43–9.
Bajzikova M, Kovarova J, Coelho AR, Boukalova S, Oh S, Rohlenova K, et al. Reactivation of dihydroorotate dehydrogenase-driven pyrimidine biosynthesis restores tumor growth of respiration-deficient cancer cells. Cell Metab. 2019;29:399-416.e10.
Acknowledgements
Not applicable.
Funding
This research was funded by the National Natural Science Foundation of China (81790631) and a Zhejiang University Academic Award for Outstanding Doctoral Candidates (2020055).
Author information
Authors and Affiliations
Contributions
C Xue and GL Li participated in original draft preparation; ZY Bao and ZY Zhou collected and analysed the data; and LJ Li reviewed and edited the manuscript. All authors have read and agreed to the published version of the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Xue, C., Li, G., Bao, Z. et al. Mitochondrial pyruvate carrier 1: a novel prognostic biomarker that predicts favourable patient survival in cancer. Cancer Cell Int 21, 288 (2021). https://doi.org/10.1186/s12935-021-01996-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12935-021-01996-8