Abstract
During decades, the research field of cancer metabolism was based on the Warburg effect, described almost one century ago. Lately, the key role of mitochondria in cancer development has been demonstrated. Many mitochondrial pathways including oxidative phosphorylation, fatty acid, glutamine, and one carbon metabolism are altered in tumors, due to mutations in oncogenes and tumor suppressor genes, as well as in metabolic enzymes. This results in metabolic reprogramming that sustains rapid cell proliferation and can lead to an increase in reactive oxygen species used by cancer cells to maintain pro-tumorigenic signaling pathways while avoiding cellular death. The knowledge acquired on the importance of mitochondrial cancer metabolism is now being translated into clinical practice. Detailed genomic, transcriptomic, and metabolomic analysis of tumors are necessary to develop more precise treatments. The successful use of drugs targeting metabolic mitochondrial enzymes has highlighted the potential for their use in precision medicine and many therapeutic candidates are in clinical trials. However, development of efficient personalized drugs has proved challenging and the combination with other strategies such as chemocytotoxic drugs, immunotherapy, and ketogenic or calorie restriction diets is likely necessary to boost their potential. In this review, we summarize the main mitochondrial features, metabolic pathways, and their alterations in different cancer types. We also present an overview of current inhibitors, highlight enzymes that are attractive targets, and discuss challenges with translation of these approaches into clinical practice. The role of mitochondria in cancer is indisputable and presents several attractive targets for both tailored and personalized cancer therapy.

Similar content being viewed by others
Facts
-
Mitochondrial metabolism is reprogrammed in cancer, providing attractive targets for therapy.
-
Although targeting mitochondrial metabolism proves challenging, several inhibitors of key enzymes are currently in clinical trials.
-
Combination strategies and novel drugs against metabolic pathways may provide a potential advantage for precision medicine in cancer.
Open questions
-
Why is it challenging to develop efficient inhibitors for mitochondrial metabolic enzymes?
-
Which are the mitochondrial enzymes that need more specific and less toxic inhibitors?
-
Will combination therapies, including immunotherapy/conventional chemotherapeutics/ketogenic or calorie restriction diets together with inhibition of mitochondrial metabolism improve the survival of cancer patients?
Introduction: Tumor metabolism
Cancer cells are characterized by the ability to proliferate uncontrollably in contrast to normal cells, which are tightly regulated. To maintain rapid cell proliferation, tumor cells activate and/or modify metabolic pathways to obtain more energy, known as metabolic reprogramming. This research field has gained special interest during the latest decade due to new insights into its fundamental importance and the development of novel biochemical and molecular tools. In 2011, Douglas Hanahan and Robert A. Weinberg included metabolic reprogramming as one of the capabilities acquired during malignant transformation, i.e., one of the hallmarks of cancer [1].
In the 1920s, Otto Warburg described that tumor cells consume high amounts of glucose to produce lactate via “aerobic glycolysis” regardless of the presence of oxygen, generating only two molecules of ATP per molecule of glucose, versus the 36 ATP molecules produced during oxidative phosphorylation (OXPHOS) in normal cells [2, 3]. To overcome the 18-fold difference in efficiency, glycolysis is activated by upregulation of glucose transporters (including GLUT1) to increase glucose uptake and by overexpression of the rate-limiting enzyme in glycolysis, hexokinase-2, and lactate dehydrogenase (LDHA). The increase in glycolytic flux results in accumulation of glycolytic intermediates that supply different biosynthetic pathways to fulfill the demands of proliferation, e.g. the pentose phosphate pathway (PPP) for production of ribose and cytosolic nicotinamide adenine dinucleotide phosphate (NADPH) used for nucleotide synthesis and antioxidants; as well as one carbon metabolism necessary for mitochondrial NADPH production, methylation, and nucleotide synthesis [4].
The metabolic alterations in tumor cells are mainly driven by aberrant activation of the phosphatidylinositol-3 kinase-protein kinase B-mammalian target of rapamycin (PI3K-AKT-mTOR) pathway and activation of oncogenes such as MYC, RAS, and hypoxia-inducible factor 1 (HIF-1), as well as mutations or deactivation of tumor suppressor genes including p53 and PTEN (phosphatase and tensin homolog) (Supplementary Box 1).
The Warburg effect and the relevance of OXPHOS
Warburg hypothesized in 1956 that tumor cells rely more on aerobic glycolysis due to an injury in respiration, which led to the misconception that cancer cells have a defective oxidative metabolism. However, Warburg’s experiments indicated that tumors consume oxygen at a lower rate compared to their increased glucose consumption [3]. Several studies have demonstrated that many cancer cells have the capacity to oxidize glucose via OXPHOS in their fully functional mitochondria (Supplementary Table 1). Moreover, inhibition of glycolysis does not prevent tumorigenesis. For instance, inhibition of the pyruvate kinase M2 isoform, responsible for the last step of glycolysis, still results in tumor formation in a breast cancer model [5]. Furthermore, inhibiting LDHA increases mitochondrial respiration in mammary tumor cells, proving that oxidative metabolism still is functional [6]. Therefore, cancer cells may depend equally or predominantly on OXPHOS for ATP supply, with the exemption of tumors with mutations in the tricarboxylic acid (TCA) cycle enzyme genes SDH, IDH, and FH, important during mitochondrial respiration. However, tumors carrying such mutations are still dependent on mitochondrial activity, and are remodeling their metabolism to optimize production of reactive oxygen species (ROS) and produce TCA cycle intermediates necessary for cell proliferation [7, 8].
Mitochondria
Mtochondria are the organelles responsible for energy production in cells. The discovery of the mitochondrial genome (mtDNA) and phylogenetic gene analysis showed that mitochondria are descendants of an endosymbiotic α-proteobacteria (Supplementary Box 2). They consist of an outer (OMM) and an inner membrane (IMM), limiting the intermembrane mitochondrial space, and an electron-dense matrix. The IMM folds in the matrix forming the cristae, which contain the mitochondria respiratory machinery and whose density varies depending on the energy demand. The OMM is permeable, allowing diffusion of molecules up to 5 kDalton via the porin voltage dependent anion channel, receiving signals that will be transmitted into mitochondria [9]. It also serves as a contact site for interaction with other organelles including the endoplasmic reticulum, lysosomes, peroxisomes, endosomes, the plasma membrane, and lipid droplets [10].
Mitochondria are highly dynamic and stay in constant turnover through mitogenesis, mitophagy, and fusion–fission processes (Supplementary Box 3 and Supplementary Fig. 1). They control a vast number of cellular processes related to metabolism, apoptosis, redox homeostasis, calcium signaling, and iron metabolism, thus their integrity is fundamental for the cell (Supplementary Box 4).
Mitochondrial metabolism
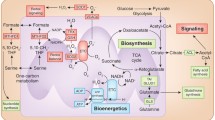
Mitochondria are crucial bioenergetic hubs. Virtually all metabolic fuels can be entirely oxidized to CO2, water, and ATP through the convergence into acetyl coenzyme A (acetyl-CoA), and its funneling into catabolic processes. The major mitochondrial metabolic pathways are the TCA cycle, fatty acid oxidation (FAO), the electron transport chain (ETC), and OXPHOS, all involved in catabolism of biomolecules and energy production. In addition, mitochondria provide precursors for many biomolecules and adapt to different metabolic conditions by modifying nuclear transcription [11]. All these processes have potential roles in development and maintenance of malignant phenotypes and understanding how they function in different cancer types may provide insights for new therapeutic approaches.
Inhibitors of mitochondrial metabolic enzymes in precision medicine of cancer
Cancer represents the second leading cause of deaths worldwide. The main therapeutic approaches include surgery, chemotherapy, and radiation. However, the lack of specifity of chemotherapeutic agents translates into systemic toxicity and, with time, into relapse. Moreover, the heterogeneity of cancer is also associated with chemotherapy resistance, challenging effective treatment.
Immunotherapy has emerged as a promising approach to target cancer, including checkpoint blockage, adoptive T cell transfer, and cancer vaccines. The work of James P. Allison and Tasuku Honjo on cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) and programmed cell death protein 1 (PD-1) for cancer therapy was awarded with the Nobel Prize in Physiology or Medicine in 2018 (www.nobelprize.org). Although immunotherapy is proven to be more successful than the most effective chemotherapeutic drugs, it can also cause severe side effects.
The increased knowledge on the deregulation of cancer metabolism has set focus on precision medicine for targeting of each individual tumor type. However, there are several reasons why metabolism-targeted therapy is challenging. First, it is difficult to develop specific inhibitors as many metabolic enzymes have several isoforms with high structural similarity, e.g., glutaminase 1 and 2 [12] as well as pyruvate kinase 1 and 2 [13]. Second, many of these enzymes have hydrophobic pockets in their active sites, which are difficult to target [14]. Third, cancer cells can reprogram their metabolism, and thus, blocking one pathway by targeting a key enzyme could result in activation of another metabolic hub [15]. Fourth, inhibition of metabolism may cause adverse effects in normal cells.
Even though there are difficulties to identify specific compounds targeting metabolic enzymes, several pro-drugs are currently in clinical trials for treatment of both solid and hemaetological tumors. In the following sections we discuss the importance of mitochondrial metabolism in cancer and available inhibitors for each pathway, highlighting the need for more specific compounds for translation to clinical use.
Mitochondrial metabolic pathways in cancer development
The tricarboxylic acid (TCA) cycle
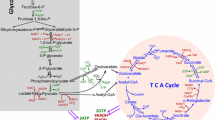
The tricarboxylic acid (TCA) cycle, also named Krebs cycle or citric acid cycle, is a central hub for generation of energy and macromolecules for biosynthetic purposes, as well as for redox balance. The TCA cycle reactions occur in the mitochondrial matrix, allowing oxidation of different fuels (Fig. 1). Carbons derived from glucose, fatty acids, or glutamine, enter the TCA cycle and produce NADH and flavine adenine dinucleotide (FADH2), which in turn transfer electrons to the ETC in the IMM, generating CO2 and ATP. The multiple substrates that supply the TCA cycle together with metabolic reprogramming allow cancer cells to survive in different situations of energy demand. This is dictated by a combination of mutations in oncogenes, tumor suppressor genes, as well as in the tumor microenvironment (TME) [1]. In hypoxia, most cancer cells convert pyruvate into lactate, fueling the TCA cycle with glutamine [16].
Carbons derived from glucose, fatty acids, and glutamine enter the TCA cycle, which produces NADH and FADH2, necessary for the formation of ATP through the electron transport chain (ETC). Glucose is processed into pyruvate via glycolysis, resulting in lactate by lactate dehydrogenase (LDH) or enters the mitochondria through the mitochondrial pyruvate carrier (MPC). Once in the mitochondria, pyruvate will be converted into acetyl-CoA by pyruvate dehydrogenase (PDH). Fatty acids are transferred to the mitochondria by carnitine palmitoyltransferases (CPT1/2) and provide acetyl-CoA to the TCA cycle through β-oxidation. Glutamine enters mitochondria through the solute carrier family 1 member 5 (SCL1A5) transporter and is converted into glutamate by glutaminases (GLS) and into α-ketoglutarate (α-KG) by glutamate dehydrogenase (GDH).
Furthermore, the TCA cycle provides intermediates for the production of lipids, proteins, and nucleotides. These need to be replaced to keep the TCA cycle functioning, a process called anaplerosis. Two anaplerotic pathways are glutaminolysis [17], producing α-ketoglutarate from glutamine, and pyruvate carboxylation, generating oxalacetate from glucose derived pyruvate [18]. Both energy production and biosynthetic pathways need to converge to maintain the pool of metabolic intermediates during low or high energy consumption, i.e., amid fasting or exercise, respectively.
Mutations in TCA cycle enzymes in cancer
Mutations in genes encoding enzymes of the TCA cycle have been associated with cancer progression, especially succinate dehydrogenase (SDH), fumarate hydratase (FH), and isocitrate dehydrogenase (IDH) (Supplementary Table 2). Moreover, these mutations cause abnormal accumulation of different metabolites, so called oncometabolites, resulting in deregulation of signaling promoting cancer progression.
Succinate dehydrogenase (SDH) is a hetero-tetrameric protein, formed by the catalytic subunits SDHA, SDHB, and the ubiquinone-binding and membrane-anchorage subunits SDHC and SDHD, which catalyze oxidation from succinate to fumarate. In addition, it participates as part of Complex II of the ETC, reducing ubiquinone to ubiquinol. Mutations in SDH subunits and in the SDH assembly factor 2 have been correlated with hereditary paraganglioma, pheochromocytoma [19, 20], gastrointestinal stromal tumors, renal cell carcinoma (RCC), thyroid tumors, testicular seminoma, and neuroblastoma [21,22,23,24,25].
Fumarate hydratase (FH) is responsible for the hydration of fumarate to malate. Patients harboring FH mutations have predisposition to develop multiple cutaneous and uterine leiomyomas, hereditary leiomyomatosis as well as renal cell cancer [26], and they may also be associated with ovarian and leydig cell tumors [27]. Mutations in SDH and FH result in the accumulation of succinate and fumarate, respectively. Both SDH and FH are considered tumor suppressor genes [28].
Isocitrate dehydrogenase (IDH), which comes in three isoforms, IDH1, IDH2, and IDH3, catalyzes the oxidative carboxylation of isocitrate to produce α-ketoglutarate (α-KG). Different cancers have mutations in IDH1 and IDH2, including a fraction of acute myeloid leukemia, low-grade glioma, secondary glioblastoma, chondrosarcoma, and cholangiocarcinoma [29,30,31,32]. Contrary to FH and SDH, IDH mutations result in gain of function, converting α-KG to the oncometabolite 2-hydroxyglutarate (2-HG). Additional information regarding mutations in other TCA cycle enzymes as well as oncometabolites is presented in Supplementary Boxes 5 and 6.
Inhibitors of TCA cycle enzymes
Several potential therapeutic strategies to target the TCA cycle for cancer treatment are summarized in Fig. 2. Although loss of function mutations of the FH or SDH enzymes are challenging to target, several small compounds have shown to be successful inhibitors for enzymes with gain of function, while others need improvement. Inhibition of mutant IDH by AGI-5198 reduces 2-HG formation and induces differentiation of glioma cells [33]. Notably, AG-221 [34] and AG-881 [35] are in clinical trials for treatment of acute myelogenous leukemia carrying IDH2 or IDH1/2 mutations, respectively. Similarly CPI-613, targeting both the α-KG dehydrogenase complex as well as pyruvate dehydrogenase, is in phase I/II clinical trials for leukemias, lymphomas, and small cell lung cancer (SCLC) [36, 37].
Glucose-derived pyruvate enters mitochondria via MPC1/2 transporters and is converted to acetyl-CoA via PDH. Inhibition of mitochondria pyruvate transporter using UK5099 attenuates OXPHOS. Alterations in enzymes of the TCA cycle results in production of oncometabolites. These include 2-HG, fumarate, and succinate (dark red). Inhibitors of these enzymes include AGI-5198, AG-221, and AG-881 for IDH, and CPI-613 for KGDHC. Both NADH and FADH2 produced in the TCA cycle transfer electrons to Complex I and II. Electrons then pass through a series of redox reactions producing energy for transportation of protons to the IMS, generating enough energy to produce ATP. Abbreviations: TCA tricarboxylic acid, ETC electron transport chain, MPC1/2 mitochondrial pyruvate carrier 1/2, PDH pyruvate dehydrogenase, CS citrate synthase, ACO2 aconitase 2, IDH isocitrate dehydrogenase, KGDHC α-ketoglutarate dehydrogenase complex, SCS succinyl-CoA synthetase, SDH succinate dehydrogenase, FH fumarate hydratase, MDH malate dehydrogenase, 2-HG 2-hydroxyglutarate, IMS intermembrane mitochondrial space, IMM inner mitochondrial membrane, Mt matrix mitochondrial matrix, FADH2 flavin adenine dinucleotide.
The electron transport chain (ETC) and oxidative phosphorylation (OXPHOS)
The ETC, also known as the respiratory chain, consists of four complexes (CI-IV) bound to the IMM and two electron transfer carriers (ubiquinone and cytochrome c) [38, 39]. The redox reactions result in an electrochemical gradient that releases enough energy for ATP formation via OXPHOS. Both NADH and FADH2 are electron donors, while oxygen is the final electron acceptor. Protons are pumped from the mitochondrial matrix into the intermembrane space by complexes I, III, and IV. Next, Complex V (the ATP synthase) uses the proton motive force generated to deliver protons to the mitochondrial matrix as well as catalyzes the conversion of ADP + Pi to ATP [40] (Fig. 2 and Supplementary Box 7).
Mutations in the ETC
Mitochondrial DNA encodes thirteen subunits of complexes I, III, IV, and V. Several tumor types including colon and rectum adenocarcinoma, ovarian cancer, acute myeloid leukemia, and glioblastoma show somatic mtDNA mutations that affect Complex I (NADH dehydrogenase), III (cytochrome b), or IV (cytochrome c oxidase) [41]. Mitochondrial mutations have also been attributed to tumorigenesis in prostate cancer and hepatocellular carcinoma [42, 43]. Mutations in SDH (Complex II) result in ROS production and accumulation of succinate, which in turn inhibits HIF prolyl hydroxylase, thus activating the HIF pathway [28].
Inhibitors of the ETC
The ETC has long been exploited for therapy, with development of inhibitors for each complex, some of them approved as drugs for certain diseases (Fig. 2). Complex I can be inhibited by tamoxifen, increasing hydrogen peroxide production. This compound is used for treating pre-menopausal hormone-positive breast cancer [44]. Metformin also inhibiting Complex I, is approved for type-2 diabetes and is the most prescribed drug worldwide. Several clinical trials are conducted for its use in colorectal, breast, and prostate cancer [45, 46]. IACS-010759, a new promising inhibitor of Complex I, is in clinical trials for acute myeloid leukemia and certain solid tumors [47]. Rotenone and methyl-4-phenylpyridinium also inhibit Complex I, but are neurotoxic. However, deguelin, a rotenone analog, has potential as a chemotherapeutic drug [48]. There are several experimental inhibitors of Complex II, such as malonate, nitropropionic acid, thenoyltrifluoro-acetona, troglitazone, 3-bromopyruvate, and α-tocopheryl succinate, a vitamine E derivative [49]. To inhibit Complex III, antimycin A is used in experimental research while resveratrol has enrolled clinical trials for different types of cancer [50]. Atovaquone, approved for treatment of pneumocystis pneumonia and malaria, inhibits Complex III both in parasites and human cells and is in clinical trials in combination with chemotherapeutic drugs in non small cell lung cancer (NSCLC) [51]. Complex IV can be inhibited by doxorubicin, a DNA-intercalating chemotherapeutic drug, and the porphyrin photosensitizer photofrin, which is approved for esophageal cancer and NSCLC [52]. N-(4-Hydroxyphenyl) retinamide (fenretinid) is in clinical trials for different tumor types, including ovarian cancer, B-cell non-Hodgkin lymphoma, and breast cancer [53]. No promising inhibitors have been reported to date for Complex V, except for oligomycin, only suitable for experimental use [49]. Employing mitochondrial uncouplers is an alternative approach to impair ETC function. Compounds including niclosamide, nitazoxanide, oxyclozanide, FCCP/CCCP, BAM15, or SR4 short-circuit ATP synthesis by transporting protons across the IMM. Niclosamide is in phase I/II clinical trials for prostate and colon cancer, while nitazoxanide is in phase II for different forms of advanced cancers [54].
One carbon metabolism
One carbon metabolism is a group of connected cytosolic and mitochondrial reactions that comprise the methionine and the folate cycles (Fig. 3). These reactions provide one carbon units in the form of methyl groups to several metabolic pathways and are responsible for the synthesis of thymidine, methionine, serine/glycine, and purine [55]. While the methionine cycle occurs in the cytosol, folate-dependent one carbon metabolism synthesizes serine/consumes glycine in the cytosol and catabolizes serine/produces glycine in mitochondria [56].
Cytosolic and mitochondrial one carbon metabolism pathway indicating the available inhibitors for different enzymes. Folate is converted to DHF and further to THF via DHFR, a process that consumes NADPH. Serine is converted by SHMT1 (cytosolic) or SHMT2 (mitochondrial) to glycine. The one carbon unit resulting from the reaction is transferred to THF forming 5,10-methylene-THF, which is oxidized by cytosolic MTHFD1 or mitochondrial MTHFD2/MTHFD2L to 10-formyl-THF via the intermediate 5,10-methenyl-THF, generating NAD(P)H. The one carbon unit in 10-formyl-THF can be converted either to formate, generating ATP from ADP by MTHFD1 or MTHFD1L, or metabolized to THF and released as a CO2 via ALDH1L2. Formate is present in mitochondria and is used as substrate for the bidirectional enzyme MTHFD1 to form 10-formyl-THF, for de novo purine synthesis, and 5,10-methylene-THF for thymidylate synthesis as well as for the methionine cycle. Several inhibitors have been identified that target one carbon metabolism, including methotrexate and aminopterin for DHFR, SHIN1/2 for SHMT1/2, and LY345899 and DS18561882 for MTHFD1/2. Abbreviations: DHF dihydrofolate, THF tetrahydrofolate, DHFR dihydrofolate reductase, SHMT1/2 serine hydroxymethyltransferase 1/2, THF tetrahydrofolate, MTHFD2 methylenetetrahydrofolate dehydrogenase 2, MTHFD1/2L methylenetetrahydrofolate dehydrogenase 1/2 like, ALDH1L2 aldehyde dehydrogenase 1 family member L2 also known as 10-formyl-THF dehydrogenase, TYMS thymidylate synthase.
Aminopterin, a folate analog, was one of the first chemotherapeutic agents inducing leukemia remission [57]. It is a precursor of methotrexate, which is still widely used today for treatment of cancer as well as other diseases. Methotrexate is an inhibitor of dihydrofolate reductase, synthesizing tetrahydrofolate, the active form of folic acid. The products derived from methotrexate intracellular metabolism, polyglutamates, also inhibit two of the enzymes involved in purine synthesis [58].
Upregulation of the mitochondrial folate and serine/glycine enzymes is associated with increased sensitivity to methotrexate in different tumor types [59]. High levels of the enzymes participating in the mitochondrial branch of the folate cycle, serine hydroxymethyltransferase 2 (SHMT2), methylenetetrahydrofolate dehydrogenase 2 (MTHFD2), and monofunctional tetrahydrofolate synthase 1L (MTHFD1L), have been linked to rapid proliferation in the NCI-60 panel of cancer cell lines [60]. Serine hydroxymethyltransferase 2 (SHMT2) and MTHFD2 were indeed among the most overexpressed metabolic genes in nineteen different cancer types analyzed [61]. Increased levels of MTHFD2 are related to breast cancer cell migration and invasion [40] and show correlation with markers of poor prognosis [62]. Upregulation of SHMT2, MTHFD2, and ALDH1L2 (10-formyltetrahydrofolate dehydrogenase) were found to be associated with poor survival in colorectal cancer [63]. The enzymes of the mitochondrial serine/glycine metabolism are elevated in primary NSCLC tumor initiating cells, specifically, glycine decarboxylase (GLDC). High levels of GLDC are also identified in other primary tumors, especially ovarian and germ cell tumors [64].
These data support the role of mitochondria as important regulators of cell proliferation through the folate-serine/glycine pathway, and have led to the development of novel inhibitors targeting mitochondrial one carbon metabolism [65]. However, the high sequence homology of the mitochondrial enzymes MTHFD2 and SHMT2 with their cytosolic counterparts, MTHFD1 and SHMT1, complicates precision medicine approaches. Only two compounds inhibiting MTHFD2, LY345899 [66] and DS18561882 [67], have shown anticancer activity both in vitro and in vivo. Knockdown experiments of SHMT2 in colorectal cancer xenografts show total blockage of tumor development only when SHMT1 is also downregulated [68]. Many of the inhibitors developed target both SHMT1 and SHMT2 due to their high sequence identity. They include derivatives from herbicidal compounds called pyrazolopyrans, used as antimalarial drugs [69]. Optimization has generated several dual SHMT1/2 inhibitors, including SHIN1 [68] and SHIN2 [70]. While SHIN1 has poor stability and short half-life in vivo, SHIN2 potently inhibits T cell acute lymphoblastic leukemia xenograft growth, and shows synergistic effects with methotrexate [70]. In addition, several folate analogs inhibit SHMT, such as LTX, which may be further optimized [71, 72].
Fatty Acid β-Oxidation (FAO)
Fatty acids are energy rich nutrients, providing more than twice as much ATP as carbohydrates or proteins per gram of dry mass [73]. Fatty acid oxidation (FAO), also called β-oxidation, is a cyclic series of catabolic reactions performed by a trifunctional enzyme complex located in the mitochondrial matrix (Fig. 4 and Supplementary Box 8).
Glutaminase (GLS) converts glutamine to glutamate, which is further metabolized to α-KG by GDH. In turn, α-KG can be converted to citrate. Both α-KG and citrate can be exported to the cytosol for use as precursors for de novo fatty acid synthesis. Glutamate can also be metabolized by GPT2/GOT2 aminotransferases, producing alanine/aspartate and α-KG. Several inhibitors of glutamine metabolism have been described, including BPTES, CB-830, CB-893, and compounds 968 for GLS, EGCG and R162 for GDH and AOA, and cycloserine for GOT2 and GPT2, respectively. For β-oxidation (FAO), fatty acids need to be transferred to mitochondria by the action of CPT1/2. The most used inhibitors of FAO are etomoxir and teglicar (ST1326), both targeting CPT1. Moreover, perhexiline acts as a dual CPT1/2 inhibitor. Abbreviations: α-KG, α-ketoglutarate; GLS, glutaminase; GDH, glutamate dehydrogenase; GPT2, mitochondrial glutamate-pyruvate transaminase 2; GOT2, aspartate aminotransferase; EGCG, epigallocatechin-3-gallate; AOA, amino-oxyacetic acid; IMS, intermembrane mitochondrial space.
This pathway is a source for high levels of ATP and it is activated to sustain high proliferation and to cope with stress in many tumors [74]. Normally, cells displaying fatty acid synthesis (FAS) suppress FAO through inhibition of carnitine palmitoyltransferases (CPT1) by malonyl-CoA produced by acetyl carboxylase 1 or 2 (ACC1 and ACC2). While ACC1 is a cytoplasmic enzyme of de novo fatty acids synthesis, ACC2 is located in mitochondria, and is involved in inhibition of FAO by producing malonyl-CoA in the proximity of CPT1 [75]. However, in some cancers, elevated FAO also provides high ATP levels. This occurs in cells that simultaneously engage in FAS, through epigenetic mechanisms that allow a differential expression of ACC1 and ACC2, resulting in selective ACC2 downregulation [74].
The most common inhibitors of FAO target the rate-limiting CPT1 enzyme. CPT1A (the liver isoform) is upregulated in high-grade serous ovarian cancer (HGSOC) and in acute lymphoblastic leukemia, and correlates to poor overall survival [76]. Etomoxir, the most utilized CPT1 inhibitor, prevents tumor progression in a model of HGSOC [77]. Elevated levels of CPT1A are associated with poor prognosis in acute lymphoblastic leukemia [76]. SRC and MYC-driven FAO supports triple-negative breast cancer (TNBC) [78, 79]. Etomoxir blocks tumor growth and metastatic features of breast cancer patient-derived xenografts (PDXs) [78], in MYC-driven transgenic TNBC mouse models, and in MYC-overexpressing PDXs [79]. Notably, we found that FAO is the main energy source in MYCN-amplified neuroblastoma and that etomoxir reduces in vitro proliferation and tumor burden in vivo [80]. Etomoxir further induces cell death of glioblastoma cells in vitro [81] and delays tumor apparition and progression in a glioblastoma mouse model [82]. However, toxicity, off-target effects on ETC Complex I, and the high doses needed, limits its potential for clinical applications. This has led to the development of the more selective CPT1 inhibitor teglicar (ST1326) [83] and the dual CPT1-CPT2 inhibitor perhexilline [84]. 3-Ketoacyl-CoA thiolase, the final FAO enzyme, is inhibited by trimetazidine and ranolazine [85, 86], both approved for the treatment of angina pectoris [87]. However, no FAO inhibitory activity was observed either in cell lines, in primary cells, or in mice [88]. Thus, more specific, and less toxic FAO inhibitors are needed to exploit lipid oxidation as a precision medicine target in cancer.
Glutamine metabolism
Glutamine is the most abundant amino acid in human plasma and is the main form of nitrogen transportation between organs. Although considered a non-essential amino acid, it is required for the synthesis of other amino acids, proteins, nucleotides, glutathione, and is involved in the activation of mTORC1 [17]. Numerous cancer cells are considered “glutamine addicted” and use glutamine as an anaplerotic source for the TCA cycle (Supplementary Box 9).
The first glutamine analogs reported were 6-diazo-5-oxo-L-norleucine (L-DON), azaserine [89, 90], and acivicin [91] but they had limited clinical applications due to toxicity [92]. Genetic silencing or the inhibition of glutaminase (GLS) using the allosteric inhibitor BPTES [93] decreases proliferation, induces cell death, and reduces proliferation in cancer cell lines as well as tumor growth in vivo [94, 95]. CB-839 is another GLS inhibitor that underwent clinical trials for treatment of several hematological tumors, colorectal cancer, melanoma, ccRCC, NSCLC, TNBC, and others (Fig. 4) [96].
High glutamate dehydrogenase (GDH) leads to increased α-KG synthesis, inducing glutamine-derived carbon flux into the TCA cycle and promoting anaplerosis and energy production. In addition, elevated GDH levels contribute to increased fumarate,that in turn, binds and activates the glutathione peroxidase enzyme, enhancing ROS detoxification in myeloma, leukemia, breast, and lung cancer cell lines. Glioblastoma cells show a strong dependency on GDH [97] and it is also essential for ammonia recycling in breast cancer cells, as it supports the high demand for amino acid synthesis [98]. Two GDH inhibitors are epigallocatechin-3-gallate, with low specificity [99], and R162, which impairs ROS detoxification and reduces glioma growth in vitro and in vivo [100]. Additional inhibitors of GDH are hexachlorophene and bithionol [101], but their anticancer potential is unexplored.
Aspartate aminotransferase 2 (GOT2) promotes ATP production and ROS homeostasis, supporting pancreatic tumor growth in vivo [102]. Pancreatic ductal adenocarcinoma depends on both cytosolic (GOT1) and mitochondrial aspartate aminotransferases [103]. The GOT2 gene is repressed by the tumor suppressor BRCA1 in breast cancer cells, and related to poor survival in TNBC patients [104]. Despite the relevance of transaminases in cancer, only few inhibitors are available. Amino-oxyacetic acid, an inhibitor of GOT1/GOT2 and other transaminases, diminishes oxygen consumption, reduces the growth of breast cancer cells and xenografts [105], and induces senescence in pancreatic cancer cells [106]. However, this compound is not specific enough for use as an anticancer drug.
Mitochondrial glutamate-pyruvate transaminase 2 (GPT2) is upregulated upon glutamine deprivation or GLS inhibition with BPTES or CB-839 in a process that involves ROS production and ATF4 (activating transcription factor 4) [107]. The combination of BPTES with the GPT2 inhibitor cycloserine [108] shows synthetic lethality in multiple cancer cell lines [107]. In breast cancer, its high expression is correlated to the pathological grade, as it promotes tumorigenesis and stemness [109]. Given the roles of GDH, GOT2, and GPT2 in cancer cells, further efforts should be invested to develop specific and potent inhibitors for these enzymes.
Mitochondrial ROS
Reactive oxygen species (ROS) are intracellular oxygen species (mainly, O2•–, H2O2, and OH˙). Their levels are tightly regulated in the cell, as an appropriate balance sustains signal transduction while avoiding cellular damage. Mitochondria are a major source of ROS, as they can be produced along the ETC but also by the reverse electron transport (RET; Supplementary Box 10) and different enzymatic reactions (Fig. 5 and Supplementary Box 11) [110].
Electrons can leak from the ETC, especially from Complex I and III (marked with yellow stars), from the reverse electron transport chain (RET) or mitochondrial enzymes as proline dehydrogenase (PRODH), glycerophosphate dehydrogenase (GPDH/GPD2), mitochondrial dehydroorotate dehydrogenase (DHODH), mono-amino oxidase (MAO), 2-oxoglutarate dehydrogenase (OGDH), and pyruvate dehydrogenase (PDH). This electron leakage can result in the formation of superoxide radical (O2•–), which superoxide dismutases (SOD) convert it into hydrogen peroxide (H2O2). H2O2 will be processed into water (H2O) by the antioxidant systems. The glutathione system includes the glutathione (GSH) peroxidase (GPX1) and the glutathione disulfide (GSSG) reductase (GR). The peroxiredoxin-thioredoxin system alternates between reduced to oxidized peroxiredoxin (PRDX) and thioredoxin (TRX), and thioredoxin reductase (TrxR), which catalyzes the NADPH-dependent reduction of TRX.
Increased ROS levels are a common feature in cancer and can be pro-tumorigenic by enhancing survival, proliferation, migration, invasion, and genetic instability, but also anti-tumorigenic when the levels cross the threshold that leads to high oxidative stress and cell death. Therefore, tumor cells express oncogenes that potentiate ROS production, and, at the same time, increase their antioxidant systems or decrease OXPHOS. Thus, achieving optimal ROS levels is a driver of cancer, known as the “ROS rheostat theory” [111, 112].
Oncoproteins can mediate an increase in ROS by two mechanisms: (i) increased TCA cycle fueling and mitochondrial mass and (ii) destabilization of the ETC utilized by KRAS, MYC, PI3K-AKT-mTOR, and BCR/ABL [110]. KRAS and MYC induce nuclear factor erythroid 2-related factor 2 (NRF2), activating the expression of genes from the antioxidant pathways, such as SODs, glutathione peroxidase, glutathione reductase, peroxiredoxin, thioredoxin, and catalase. Tumor cells can also use glutamine for its antioxidant capacity, as it is the precursor of glutathione, generating NADPH through the malate pathway, necessary for detoxification reactions. Another process is the modification of cancer cell metabolism to optimize ROS. Highly proliferative cancer cells with glucose abundance are glycolytic to avoid ROS production while still producing energy. In OXPHOS-dependent tumors, elevated respiration and high ROS are compensated by expression of antioxidant enzymes [112, 113]. The increase in ROS also leads to activation of other signaling pathways related to tumorigenesis, such as PI3K, MAPK, and HIFs [114].
Several drugs, including inhibitors of the ETC, induce ROS through different mechanisms. Therefore, evaluating the ROS baseline levels of a tumor could be useful to determine the responsiveness to ROS-inducing agents. It could be combined with inhibitors of the compensatory mechanisms, i.e., glycolysis or the antioxidant proteins [113]. Inhibitors of the glutamine pathway impair the formation of glutathione, and thus dysregulate the antioxidant system. In addition, glycolysis can be suppressed by 2-deoxyglucose or dichloroacetate. The glutathione system can be inhibited with NOV-002, L-buthionine-S, R-sulfoximine, canfosfamide, or ezatiostat hydrochloride. Sulfasalazine, an inhibitor of the cysteine/glutamate antiporter XCT, showed efficacy in pancreatic and SCLC cells [113]. The thioredoxin system is inhibited by PX-12, SAHA, PMX464, auranofin, and motexafin gadolinium [115], the 2-Cys peroxiredoxins by conoidin A [116], and peroxiredoxin 6 by MJ33 [117]. NOV-002 is a glutathione disulfide mimetic that induces oxidative stress and has been used in clinical trials for breast, ovarian, and NSCLC [118]. PX-12 has enrolled clinical trials for refractory cancers [119] while ATN-224, a SOD1 inhibitor, is in clinical trials for prostate cancer [120].
Ketogenic diet and mitochondria
Ketogenic diet is high in fat and low in carbohydrates, normally in a 4:1 fat/non-fat ratio [121]. The use of fat as a main source of energy mimics the metabolic state of starvation and the body switches to FAO as the main energy source. Fat is catabolized to ketone bodies in mitochondria of hepatocytes, consisting of 3-β-hydroxybutyrate (βOHB), acetoacetate, and acetone (Supplementary Box 12) [122].
This diet was first used as an anticancer approach in two patients with malignant brain cancer that did not respond to radiation and chemotherapy. Ketone blood levels increased 20–30-fold and the patients showed improved outcomes [123,124,125]. Some studies have reported that cancer cells have lower levels of succinyl-CoA-ketoacid-CoA transferase (SCOT) [126], making them unable to use βOHB as energy fuel. The increase in ketones can impair cancer cell growth while maintaining the functions of healthy tissues [122, 127]. MYC represses 3-hydroxymethylglutaryl-CoA synthase (HMGCS2) in colon cancer [128] and decreased expression of HMGCS2 is found in ccRCC [129] and hepatocellular carcinoma [130], associated with worse patient outcome. Inactivation of hydroxymethylglutaryl-CoA lyase resulted in enhanced proliferation and metastasis of nasopharyngeal carcinoma [131].
Importantly, not all studies applying ketogenic diet to cancer show a clear effect, suggesting that the type of cancer, genomic profile, as well as time and characteristics of the diet result in different outcomes. A combination of drug treatment and ketogenic diet may be an attractive approach to potentiate therapeutic response, as demonstrated in multiple clinical trials [132]. An alternative is calorie restriction, which apart from lowering glucose in blood and insulin, also decreases lipid levels. In models of pancreatic ductal adenocarcinoma and NSCLC, calorie restriction but not ketogenic diet resulted in reduced tumor progression [133].
Mitochondrial metabolism and immunotherapy
Mitochondria have crucial roles in CD8+ T cell differentiation, maintenance, and their functional decline in the TME. The efficacy of immunotherapy could be increased in combination with mitochondria-modulating treatments. PD-1 blockade therapy-responsive tumors have higher basal respiration, maximal respiration, spare respiratory capacity, and ATP turnover. The reduction in mitochondrial activity in T cells may explain the escape from PD-1 blockade therapy in some tumors [134]. Bezafibrate, an agonist for peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC1α), has been shown to boost antitumor immunity by upregulating mitochondrial OXPHOS and inhibiting apoptosis in an MC38-bearing mouse tumor model treated with PD-1 blockade [135]. The 4-1BB costimulatory receptor expressed on activated T and natural killer cells, augments glycolysis upon induction, by promoting the expression of glucose transporters to support CD8+ T cell proliferation [136]. Moreover, in melanoma tumors with high oxidative metabolism, and low glycolysis, PD-1 blockage correlated with poor response in patients [137]. All these studies support the importance of combination strategies with mitochondrial metabolic inhibitors to combat resistance to immunotherapy.
Future perspectives
One hundred years ago, Otto Warburg proposed that altered metabolism is a characteristic of cancer cells. A decade ago, metabolic reprogramming was recognized as one of the hallmarks of cancer, as tumor cells need to maintain a high rate of macromolecular synthesis, essential for cell growth and division. During the last years, advances have highlighted the importance of OXPHOS, FAO, as well as glutamine addiction in cancer cells, setting the focus on mitochondrial metabolism. Many tumor types harbor mutations in rate-limiting enzymes regulating major mitochondrial metabolic pathways. Some of these alterations result in accumulation of oncometabolites acting as epigenetic regulators, or in the increase of ROS production, contributing to tumorigenesis. Importantly, the significant role of mitochondrial metabolism in tumorigenesis could potentially be exploited as a strategy for cancer therapy. The successful use of the anti-folates aminopterin and methotrexane in clinical practice, with remission of acute lymphocytic leukemia in children, provided evidence that targeting metabolism represents an effective therapeutical approach. Notably, several compounds inhibiting mitochondrial metabolic enzymes have been identified, some of which, like etomoxir and BPTES, showed promising effects in vitro. However, translation from preclinical experiments has been challenging, with only few compounds currently in early-stage clinical trials (Table 1). Next-generation technologies, including metabolic profiling and single-cell sequencing of human tumors, as well as metabolic tracing of tumors in mice will provide novel knowledge of the mechanisms regulating mitochondrial metabolism in different cancer types. Identification of the most appropriate strategy for each patient will result in more specific and less toxic treatments. Importantly, the combination of standard chemotherapeutic drugs, immunotherapy, or ketogenic diet with mitochondrial inhibitors may be an attractive, efficient approach for cancer cure. The future looks bright and holds promise for discovery of novel remedies for precision medicine.
Data availability
This review article does not present any new primary data.
References
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646–74.
Warburg O. The metabolism of carcinoma cells. J Cancer Res. 1925;9:148–63.
Warburg O. On the origin of cancer cells. Science. 1956;123:309–14.
Yu L, Chen X, Sun X, Wang L, Chen S. The glycolytic switch in tumors: how many players are involved? J Cancer. 2017;8:3430–40.
Israelsen WJ, Dayton TL, Davidson SM, Fiske BP, Hosios AM, Bellinger G, et al. PKM2 isoform-specific deletion reveals a differential requirement for pyruvate kinase in tumor cells. Cell. 2013;155:397.
Fantin VR, St-Pierre J, Leder P. Attenuation of LDH-A expression uncovers a link between glycolysis, mitochondrial physiology, and tumor maintenance. Cancer Cell. 2006;9:425–34.
Lussey-Lepoutre C, Hollinshead KER, Ludwig C, Menara M, Morin A, Castro-Vega LJ, et al. Loss of succinate dehydrogenase activity results in dependency on pyruvate carboxylation for cellular anabolism. Nat Commun. 2015;6:8784.
Mullen AR, Wheaton WW, Jin ES, Chen P-H, Sullivan LB, Cheng T, et al. Reductive carboxylation supports growth in tumor cells with defective mitochondria. Nature. 2012;481:385–8.
Vander Heiden MG, Chandel NS, Li XX, Schumacker PT, Colombini M, Thompson CB. Outer mitochondrial membrane permeability can regulate coupled respiration and cell survival. Proc Natl Acad Sci USA. 2000;97:4666–71.
Giacomello M, Pyakurel A, Glytsou C, Scorrano L. The cell biology of mitochondrial membrane dynamics. Nat Rev Mol Cell Biol. 2020;21:204–24.
Anderson RG, Ghiraldeli LP, Pardee TS. Mitochondria in cancer metabolism, an organelle whose time has come? Biochim Biophys Acta Rev cancer. 2018;1870:96–102.
Saha SK, Islam SMR, Abdullah-AL-Wadud M, Islam S, Ali F, Park KS. Multiomics analysis reveals that GLS and GLS2 differentially modulate the clinical outcomes of cancer. J Clin Med. 2019;8:355.
Israelsen WJ, Vander Heiden MG. Pyruvate kinase: function, regulation and role in cancer. Semin Cell Dev Biol. 2015;43:43–51.
Rai G, Urban DJ, Mott BT, Hu X, Yang S-M, Benavides GA, et al. Pyrazole-based lactate dehydrogenase inhibitors with optimized cell activity and pharmacokinetic properties. J Med Chem. 2020;63:10984–1011.
Boudreau A, Purkey HE, Hitz A, Robarge K, Peterson D, Labadie S, et al. Metabolic plasticity underpins innate and acquired resistance to LDHA inhibition. Nat Chem Biol. 2016;12:779–86.
Fan J, Kamphorst JJ, Mathew R, Chung MK, White E, Shlomi T, et al. Glutamine-driven oxidative phosphorylation is a major ATP source in transformed mammalian cells in both normoxia and hypoxia. Mol Syst Biol. 2013;9:712.
Wise DR, Thompson CB. Glutamine addiction: a new therapeutic target in cancer. Trends Biochem Sci. 2010;35:427–33.
Cappel DA, Deja S, Duarte JAG, Kucejova B, Iñigo M, Fletcher JA, et al. Pyruvate-carboxylase-mediated anaplerosis promotes antioxidant capacity by sustaining TCA cycle and redox metabolism in liver. Cell Metab. 2019;29:1291–1305.e8.
Astuti D, Latif F, Dallol A, Dahia PL, Douglas F, George E, et al. Gene mutations in the succinate dehydrogenase subunit SDHB cause susceptibility to familial pheochromocytoma and to familial paraganglioma. Am J Hum Genet. 2001;69:49–54.
Bayley J-P, Kunst HPM, Cascon A, Sampietro ML, Gaal J, Korpershoek E, et al. SDHAF2 mutations in familial and sporadic paraganglioma and phaeochromocytoma. Lancet Oncol. 2010;11:366–72.
Vanharanta S, Buchta M, McWhinney SR, Virta SK, Peçzkowska M, Morrison CD, et al. Early-onset renal cell carcinoma as a novel extraparaganglial component of SDHB-associated heritable paraganglioma. Am J Hum Genet. 2004;74:153–9.
Italiano A, Chen C-L, Sung Y-S, Singer S, DeMatteo RP, LaQuaglia MP, et al. SDHA loss of function mutations in a subset of young adult wild-type gastrointestinal stromal tumors. BMC Cancer. 2012;12:408.
Ni Y, Seballos S, Ganapathi S, Gurin D, Fletcher B, Ngeow J, et al. Germline and somatic SDHx alterations in apparently sporadic differentiated thyroid cancer. Endocr Relat Cancer. 2015;22:121–30.
Galera-Ruiz H, Gonzalez-Campora R, Rey-Barrera M, Rollón-Mayordomo A, Garcia-Escudero A, Fernández-Santos JM, et al. W43X SDHD mutation in sporadic head and neck paraganglioma. Anal Quant Cytol Histol. 2008;30:119–23.
Schimke RN, Collins DL, Stolle CA. Paraganglioma, neuroblastoma, and a SDHB mutation: resolution of a 30-year-old mystery. Am J Med Genet Part A. 2010;152A:1531–5.
Tomlinson IPM, Alam NA, Rowan AJ, Barclay E, Jaeger EEM, Kelsell D, et al. Germline mutations in FH predispose to dominantly inherited uterine fibroids, skin leiomyomata and papillary renal cell cancer. Nat Genet. 2002;30:406–10.
Carvajal-Carmona LG, Alam NA, Pollard PJ, Jones AM, Barclay E, Wortham N, et al. Adult leydig cell tumors of the testis caused by germline fumarate hydratase mutations. J Clin Endocrinol Metab. 2006;91:3071–5.
Gottlieb E, Tomlinson IPM. Mitochondrial tumour suppressors: a genetic and biochemical update. Nat Rev Cancer. 2005;5:857–66.
Mardis ER, Ding L, Dooling DJ, Larson DE, McLellan MD, Chen K, et al. Recurring mutations found by sequencing an acute myeloid leukemia genome. N Engl J Med. 2009;361:1058–66.
Yan H, Parsons DW, Jin G, McLendon R, Rasheed BA, Yuan W, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360:765–73.
Amary MF, Bacsi K, Maggiani F, Damato S, Halai D, Berisha F, et al. IDH1 and IDH2 mutations are frequent events in central chondrosarcoma and central and periosteal chondromas but not in other mesenchymal tumours. J Pathol. 2011;224:334–43.
Borger DR, Tanabe KK, Fan KC, Lopez HU, Fantin VR, Straley KS, et al. Frequent mutation of isocitrate dehydrogenase (IDH)1 and IDH2 in cholangiocarcinoma identified through broad-based tumor genotyping. Oncologist. 2012;17:72–9.
Rohle D, Popovici-Muller J, Palaskas N, Turcan S, Grommes C, Campos C, et al. An inhibitor of mutant IDH1 delays growth and promotes differentiation of glioma cells. Science. 2013;340:626–30.
Yen K, Travins J, Wang F, David MD, Artin E, Straley K, et al. AG-221, a first-in-class therapy targeting acute myeloid leukemia harboring oncogenic IDH2 mutations. Cancer Discov. 2017;7:478–93.
Medeiros BC, Fathi AT, DiNardo CD, Pollyea DA, Chan SM, Swords R. Isocitrate dehydrogenase mutations in myeloid malignancies. Leukemia. 2017;2:272–81.
Pardee TS, Lee K, Luddy J, Maturo C, Rodriguez R, Isom S, et al. A phase I study of the first-in-class antimitochondrial metabolism agent, CPI-613, in patients with advanced hematologic malignancies. Clin Cancer Res. 2014;20:5255–64.
Lycan TW, Pardee TS, Petty WJ, Bonomi M, Alistar A, Lamar ZS, et al. A phase II clinical trial of CPI-613 in patients with relapsed or refractory small cell lung carcinoma. PLoS One. 2016;11:e0164244.
Javadov S, Jang S, Chapa-Dubocq XR, Khuchua Z, Camara AK. Mitochondrial respiratory supercomplexes in mammalian cells: structural versus functional role. J Mol Med. 2021;99:57–73.
Tang JX, Thompson K, Taylor RW, Oláhová M. Mitochondrial OXPHOS biogenesis: co-regulation of protein synthesis, import, and assembly pathways. Int J Mol Sci. 2020;21:1–32.
Lehtinen L, Ketola K, Mäkelä R, Mpindi J-P, Viitala M, Kallioniemi O, et al. High-throughput RNAi screening for novel modulators of vimentin expression identifies MTHFD2 as a regulator of breast cancer cell migration and invasion. Oncotarget 2013;4:48–63.
Larman TC, DePalma SR, Hadjipanayis AG, Protopopov A, Zhang J, Gabriel SB, et al. Spectrum of somatic mitochondrial mutations in five cancers. Proc Natl Acad Sci USA. 2012;109:14087–91.
Hopkins JF, Sabelnykova VY, Weischenfeldt J, Simon R, Aguiar JA, Alkallas R, et al. Mitochondrial mutations drive prostate cancer aggression. Nat Commun. 2017;8:656.
Hsu C-C, Lee H-C, Wei Y-H. Mitochondrial DNA alterations and mitochondrial dysfunction in the progression of hepatocellular carcinoma. World J Gastroenterol. 2013;19:8880–6.
Farrar MC, Jacobs TF. Tamoxifen. In Treasure Island (FL); 2021.
De A, Kuppusamy G. Metformin in breast cancer: preclinical and clinical evidence. Curr Probl Cancer. 2020;44:100488.
Kamarudin MNA, Sarker MMR, Zhou J-R, Parhar I. Metformin in colorectal cancer: molecular mechanism, preclinical and clinical aspects. J Exp Clin Cancer Res. 2019;38:491.
Molina JR, Sun Y, Protopopova M, Gera S, Bandi M, Bristow C, et al. An inhibitor of oxidative phosphorylation exploits cancer vulnerability. Nat Med. 2018;24:1036–46.
Lin ZY, Yun QZ, Wu L, Zhang TW, Yao TZ. Pharmacological basis and new insights of deguelin concerning its anticancer effects. Pharmacol Res. 2021;105935.
Rohlena J, Dong L-F, Ralph SJ, Neuzil J. Anticancer drugs targeting the mitochondrial electron transport chain. Antioxid Redox Signal. 2011;15:2951–74.
Sassi N, Mattarei A, Azzolini M, Szabo’ I, Paradisi C, Zoratti M, et al. Cytotoxicity of mitochondria-targeted resveratrol derivatives: interactions with respiratory chain complexes and ATP synthase. Biochim Biophys Acta. 2014;1837:1781–9.
Nixon GL, Moss DM, Shone AE, Lalloo DG, Fisher N, O’Neill PM, et al. Antimalarial pharmacology and therapeutics of atovaquone. J Antimicrob Chemother. 2013;68:977–85.
Baskaran R, Lee J, Yang S-G. Clinical development of photodynamic agents and therapeutic applications. Biomater Res. 2018;22:25.
Clinicaltrials.gov/ct2/results?cond=&term=fenretinide&cntry=&state%0A=&city=&dist=; date 2021/11/04.
Shrestha R, Johnson E, Byrne FL. Exploring the therapeutic potential of mitochondrial uncouplers in cancer. Mol Metab. 2021;51:101222.
Ducker GS, Rabinowitz JD. One-carbon metabolism in health and disease. Cell Metab. 2017;25:27–42.
Tibbetts AS, Appling DR. Compartmentalization of mammalian folate-mediated one-carbon metabolism. Annu Rev Nutr. 2010;30:57–81.
Farber S, Diamond LK. Temporary remissions in acute leukemia in children produced by folic acid antagonist, 4-aminopteroyl-glutamic acid. N Engl J Med. 1948;238:787–93.
Huennekens FM. The methotrexate story: a paradigm for development of cancer chemotherapeutic agents. Adv Enzym Regul. 1994;34:397–419.
Vazquez A, Tedeschi PM, Bertino JR. Overexpression of the mitochondrial folate and glycine-serine pathway: a new determinant of methotrexate selectivity in tumors. Cancer Res. 2013;73:478–82.
Jain M, Nilsson R, Sharma S, Madhusudhan N, Kitami T, Souza AL, et al. Metabolite profiling identifies a key role for glycine in rapid cancer cell proliferation. Science 2012;336:1040–4.
Nilsson R, Jain M, Madhusudhan N, Sheppard NG, Strittmatter L, Kampf C, et al. Metabolic enzyme expression highlights a key role for MTHFD2 and the mitochondrial folate pathway in cancer. Nat Commun. 2014;5:3128.
Liu F, Liu Y, He C, Tao L, He X, Song H, et al. Increased MTHFD2 expression is associated with poor prognosis in breast cancer. Tumour Biol. 2014;35:8685–90.
Miyo M, Konno M, Colvin H, Nishida N, Koseki J, Kawamoto K, et al. The importance of mitochondrial folate enzymes in human colorectal cancer. Oncol Rep. 2017;37:417–25.
Zhang WC, Shyh-Chang N, Yang H, Rai A, Umashankar S, Ma S, et al. Glycine decarboxylase activity drives non-small cell lung cancer tumor-initiating cells and tumorigenesis. Cell 2012;148:259–72.
Dekhne AS, Hou Z, Gangjee A, Matherly LH. Therapeutic targeting of mitochondrial one-carbon metabolism in cancer. Mol Cancer Ther. 2020;19:2245–55.
Ju H-Q, Lu Y-X, Chen D-L, Zuo Z-X, Liu Z-X, Wu Q-N, et al. Modulation of redox homeostasis by inhibition of MTHFD2 in colorectal cancer: mechanisms and therapeutic implications. J Natl Cancer Inst. 2019;111:584–96.
Kawai J, Toki T, Ota M, Inoue H, Takata Y, Asahi T, et al. Discovery of a potent, selective, and orally available MTHFD2 Inhibitor (DS18561882) with in vivo antitumor activity. J Med Chem. 2019;62:10204–20.
Ducker GS, Ghergurovich JM, Mainolfi N, Suri V, Jeong SK, Hsin-Jung Li S, et al. Human SHMT inhibitors reveal defective glycine import as a targetable metabolic vulnerability of diffuse large B-cell lymphoma. Proc Natl Acad Sci USA. 2017;114:11404–9.
Witschel MC, Rottmann M, Schwab A, Leartsakulpanich U, Chitnumsub P, Seet M, et al. Inhibitors of plasmodial serine hydroxymethyltransferase (SHMT): cocrystal structures of pyrazolopyrans with potent blood- and liver-stage activities. J Med Chem. 2015;58:3117–30.
García-Cañaveras JC, Lancho O, Ducker GS, Ghergurovich JM, Xu X, da Silva-Diz V, et al. SHMT inhibition is effective and synergizes with methotrexate in T-cell acute lymphoblastic leukemia. Leukemia 2021;35:377–88.
Dekhne AS, Shah K, Ducker GS, Katinas JM, Wong-Roushar J, Nayeen MJ, et al. Novel pyrrolo[3,2-d]pyrimidine compounds target mitochondrial and cytosolic one-carbon metabolism with broad-spectrum antitumor efficacy. Mol Cancer Ther. 2019;18:1787–99.
Scaletti E, Jemth A-S, Helleday T, Stenmark P. Structural basis of inhibition of the human serine hydroxymethyltransferase SHMT2 by antifolate drugs. FEBS Lett. 2019;593:1863–73.
Carracedo A, Cantley LC, Pandolfi PP. Cancer metabolism: fatty acid oxidation in the limelight. Nat Rev Cancer 2013;13:227–32.
Corbet C, Pinto A, Martherus R, Santiago de Jesus JP, Polet F, Feron O. Acidosis drives the reprogramming of fatty acid metabolism in cancer cells through changes in mitochondrial and histone acetylation. Cell Metab. 2016;24:311–23.
Abu-Elheiga L, Brinkley WR, Zhong L, Chirala SS, Woldegiorgis G, Wakil SJ. The subcellular localization of acetyl-CoA carboxylase 2. Proc Natl Acad Sci USA. 2000;97:1444–9.
Shi J, Fu H, Jia Z, He K, Fu L, Wang W. High expression of CPT1A predicts adverse outcomes: a potential therapeutic target for acute myeloid leukemia. EBioMedicine 2016;14:55–64.
Sawyer BT, Qamar L, Yamamoto TM, McMellen A, Watson ZL, Richer JK, et al. Targeting fatty acid oxidation to promote anoikis and inhibit ovarian cancer progression. Mol Cancer Res. 2020;18:1088–98.
Park JH, Vithayathil S, Kumar S, Sung P-L, Dobrolecki LE, Putluri V, et al. Fatty acid oxidation-driven src links mitochondrial energy reprogramming and oncogenic properties in triple-negative breast cancer. Cell Rep. 2016;14:2154–65.
Camarda R, Zhou AY, Kohnz RA, Balakrishnan S, Mahieu C, Anderton B, et al. Inhibition of fatty acid oxidation as a therapy for MYC-overexpressing triple-negative breast cancer. Nat Med. 2016;22:427–32.
Oliynyk G, Ruiz-Pérez MV, Sainero-Alcolado L, Dzieran J, Zirath H, Gallart-Ayala H, et al. MYCN-enhanced oxidative and glycolytic metabolism reveals vulnerabilities for targeting neuroblastoma. iScience. 2019;21:188–204.
Pike LS, Smift AL, Croteau NJ, Ferrick DA, Wu M. Inhibition of fatty acid oxidation by etomoxir impairs NADPH production and increases reactive oxygen species resulting in ATP depletion and cell death in human glioblastoma cells. Biochim Biophys Acta. 2011;1807:726–34.
Lin H, Patel S, Affleck VS, Wilson I, Turnbull DM, Joshi AR, et al. Fatty acid oxidation is required for the respiration and proliferation of malignant glioma cells. Neuro Oncol. 2017;19:43–54.
Hutchison TL, Saeed A, Wolkowicz PE, McMillin JB, Brouillette WJ. Stereoselective synthesis of a conformationally defined cyclohexyl carnitine analogue that binds CPT-1 with high affinity. Bioorganic Med Chem. 1999;7:1505–11.
Killalea SM, Krum H. Systematic review of the efficacy and safety of perhexiline in the treatment of ischemic heart disease. Am J Cardiovasc Drugs. 2001;1:193–204.
Kantor PF, Lucien A, Kozak R, Lopaschuk GD. The antianginal drug trimetazidine shifts cardiac energy metabolism from fatty acid oxidation to glucose oxidation by inhibiting mitochondrial long-chain 3-ketoacyl coenzyme A thiolase. Circ Res. 2000;86:580–8.
Anderson JR, Khou S, Nawarskas JJ. Ranolazine: a potential new treatment for chronic stable angina. Heart Dis. 2001;3:263–9.
Balla C, Pavasini R, Ferrari R. Treatment of angina: where are we? Cardiology. 2018;140:52–67.
Ma Y, Wang W, Devarakonda T, Zhou H, Wang X-Y, Salloum FN, et al. Functional analysis of molecular and pharmacological modulators of mitochondrial fatty acid oxidation. Sci Rep. 2020;10:1450.
Coffey GL, Ehrlich J, Fisher MW, Hillegas AB, Kohberger DL, Machamer HE, et al. 6-Diazo-5-oxo-L-norleucine, a new tumor-inhibitory substance. I. Biologic studies. Antibiot Chemother. 1956;6:487–97.
Stock CC, Reilly HC, Buckley SM, Clarke DA, Rhoads CP. Azaserine, a new tumour-inhibitory substance; studies with Crocker mouse sarcoma 180. Nature 1954;173:71–2.
Jayaram HN, Cooney DA, Ryan JA, Neil G, Dion RL, Bono VH. L-[alphaS, 5S]-alpha-amino-3-chloro-4,5-dihydro-5-isoxazoleacetic acid (NSC-163501): a new amino acid antibiotic with the properties of an antagonist of L-glutamine. Cancer Chemother Rep. 1975;59:481–91.
Ahluwalia GS, Grem JL, Hao Z, Cooney DA. Metabolism and action of amino acid analog anti-cancer agents. Pharm Ther. 1990;46:243–71.
DeLaBarre B, Gross S, Fang C, Gao Y, Jha A, Jiang F, et al. Full-length human glutaminase in complex with an allosteric inhibitor. Biochemistry. 2011;50:10764–70.
Elgogary A, Xu Q, Poore B, Alt J, Zimmermann SC, Zhao L, et al. Combination therapy with BPTES nanoparticles and metformin targets the metabolic heterogeneity of pancreatic cancer. Proc Natl Acad Sci USA. 2016;113:E5328–36.
Seltzer MJ, Bennett BD, Joshi AD, Gao P, Thomas AG, Ferraris DV, et al. Inhibition of glutaminase preferentially slows growth of glioma cells with mutant IDH1. Cancer Res. 2010;70:8981–7.
Xu X, Meng Y, Li L, Xu P, Wang J, Li Z, et al. Overview of the development of glutaminase inhibitors: achievements and future directions. J Med Chem. 2019;62:1096–115.
Yang C, Sudderth J, Dang T, Bachoo RM, McDonald JG, DeBerardinis RJ. Glioblastoma cells require glutamate dehydrogenase to survive impairments of glucose metabolism or Akt signaling. Cancer Res. 2009;69:7986–93.
Spinelli JB, Yoon H, Ringel AE, Jeanfavre S, Clish CB, Haigis MC. Metabolic recycling of ammonia via glutamate dehydrogenase supports breast cancer biomass. Science. 2017;358:941–6.
Aggarwal V, Tuli HS, Tania M, Srivastava S, Ritzer EE, Pandey A, et al. Molecular mechanisms of action of epigallocatechin gallate in cancer: Recent trends and advancement. Semin Cancer Biol. 2022;80:256–75.
Jin L, Li D, Alesi GN, Fan J, Kang H-B, Lu Z, et al. Glutamate dehydrogenase 1 signals through antioxidant glutathione peroxidase 1 to regulate redox homeostasis and tumor growth. Cancer Cell. 2015;27:257–70.
Li M, Smith CJ, Walker MT, Smith TJ. Novel inhibitors complexed with glutamate dehydrogenase: allosteric regulation by control of protein dynamics. J Biol Chem. 2009;284:22988–3000.
Yang H, Zhou L, Shi Q, Zhao Y, Lin H, Zhang M, et al. SIRT3-dependent GOT2 acetylation status affects the malate-aspartate NADH shuttle activity and pancreatic tumor growth. EMBO J. 2015;34:1110–25.
Son J, Lyssiotis CA, Ying H, Wang X, Hua S, Ligorio M, et al. Glutamine supports pancreatic cancer growth through a KRAS-regulated metabolic pathway. Nature 2013;496:101–5.
Hong R, Zhang W, Xia X, Zhang K, Wang Y, Wu M, et al. Preventing BRCA1/ZBRK1 repressor complex binding to the GOT2 promoter results in accelerated aspartate biosynthesis and promotion of cell proliferation. Mol Oncol. 2019;13:959–77.
Thornburg JM, Nelson KK, Clem BF, Lane AN, Arumugam S, Simmons A, et al. Targeting aspartate aminotransferase in breast cancer. Breast Cancer Res. 2008;10:R84.
Yang S, Hwang S, Kim M, Seo SB, Lee J-H, Jeong SM. Mitochondrial glutamine metabolism via GOT2 supports pancreatic cancer growth through senescence inhibition. Cell Death Dis. 2018;9:55.
Kim M, Gwak J, Hwang S, Yang S, Jeong SM. Mitochondrial GPT2 plays a pivotal role in metabolic adaptation to the perturbation of mitochondrial glutamine metabolism. Oncogene. 2019;38:4729–38.
Wong DT, Fuller RW, Molloy BB. Inhibition of amino acid transaminases by L-cycloserine. Adv Enzym Regul. 1973;11:139–54.
Cao Y, Lin S-H, Wang Y, Chin YE, Kang L, Mi J. Glutamic pyruvate transaminase GPT2 promotes tumorigenesis of breast cancer cells by activating sonic hedgehog signaling. Theranostics. 2017;7:3021–33.
Raimondi V, Ciccarese F, Ciminale V. Oncogenic pathways and the electron transport chain: a dangeROS liaison. Br J Cancer. 2020;122:168–81.
Maryanovich M, Gross A. A ROS rheostat for cell fate regulation. Trends Cell Biol. 2013;23:129–34.
Jaramillo MC, Zhang DD. The emerging role of the Nrf2-Keap1 signaling pathway in cancer. Genes Dev. 2013;27:2179–91.
Perillo B, Di Donato M, Pezone A, Di Zazzo E, Giovannelli P, Galasso G, et al. ROS in cancer therapy: the bright side of the moon. Exp Mol Med. 2020;52:192–203.
Sullivan LB, Chandel NS. Mitochondrial reactive oxygen species and cancer. Cancer Metab. 2014;2:17.
Jia J-J, Geng W-S, Wang Z-Q, Chen L, Zeng X-S. The role of thioredoxin system in cancer: strategy for cancer therapy. Cancer Chemother Pharm. 2019;84:453–70.
Liu G, Botting CH, Evans KM, Walton JAG, Xu G, Slawin AMZ, et al. Optimisation of conoidin A, a peroxiredoxin inhibitor. ChemMedChem. 2010;5:41–5.
Jain MK, Tao WJ, Rogers J, Arenson C, Eibl H, Yu BZ. Active-site-directed specific competitive inhibitors of phospholipase A2: novel transition-state analogues. Biochemistry. 1991;30:10256–68.
Montero AJ, Jassem J. Cellular redox pathways as a therapeutic target in the treatment of cancer. Drugs. 2011;71:1385–96.
Ramanathan RK, Stephenson JJ, Weiss GJ, Pestano LA, Lowe A, Hiscox A, et al. A phase I trial of PX-12, a small-molecule inhibitor of thioredoxin-1, administered as a 72-hour infusion every 21 days in patients with advanced cancers refractory to standard therapy. Investig N Drugs. 2012;30:1591–6.
Clinicaltrials.gov/ct2/show/NCT00150995?term=ATN-224&draw=4&rank=5; date 2021/11/05.
Milder J, Patel M. Modulation of oxidative stress and mitochondrial function by the ketogenic diet. Epilepsy Res. 2012;100:295–303.
Vidali S, Aminzadeh S, Lambert B, Rutherford T, Sperl W, Kofler B, et al. Mitochondria: the ketogenic diet - a metabolism-based therapy. Int J Biochem Cell Biol. 2015;63:55–9.
Nebeling L, Lerner E. Implementing a ketogenic diet based on medium-chain triglyceride oil in pediatric patients with cancer. J Am Dietetic Assoc. 1995;95:693–7.
Nebeling L, Miraldi F, Shurin S, Lerner E. Effects of a ketogenic diet on tumor metabolism and nutritional status in pediatric oncology patients: two case reports. J Am Coll Nutr. 1995;14:202–8.
Seyfried TN, Mukherjee P. Targeting energy metabolism in brain cancer: review and hypothesis. Nutr Metab. 2005;2:1–9.
Skinner R, Trujillo A, Ma X, Beierle EA. Ketone bodies inhibit the viability of human neuroblastoma cells. J Pediatr Surg. 2009;44:212–6.
Poff AM, Ari C, Arnold P, Seyfried TN, D’Agostino DP. Ketone supplementation decreases tumor cell viability and prolongs survival of mice with metastatic cancer. Int J Cancer. 2014;135:1711–20.
Camarero N, Mascaró C, Mayordomo C, Vilardell F, Haro D, Marrero PF. Ketogenic HMGCS2 is a c-Myc target gene expressed in differentiated cells of human colonic epithelium and down-regulated in colon cancer. Mol Cancer Res. 2006;4:645–53.
Han P, Wang Y, Luo W, Lu Y, Zhou X, Yang Y, et al. Epigenetic inactivation of hydroxymethylglutaryl CoA synthase reduces ketogenesis and facilitates tumor cell motility in clear cell renal carcinoma. Pathol Res Pract. 2021;227:153622.
Ding R, Chen T, Zhang Y, Chen X, Zhuang L, Yang Z. HMGCS2 in metabolic pathways was associated with overall survival in hepatocellular carcinoma: a LASSO-derived study. Sci Prog. 2021;104:368504211031749.
Luo W, Qin L, Li B, Liao Z, Liang J, Xiao X, et al. Inactivation of HMGCL promotes proliferation and metastasis of nasopharyngeal carcinoma by suppressing oxidative stress. 7. Nanning, China: First Affiliated Hospital of Guangxi Medical University; 2017. p. 11954.
Talib WH, Mahmod AI, Kamal A, Rashid HM, Alashqar AMD, Khater S, et al. Ketogenic diet in cancer prevention and therapy: molecular targets and therapeutic opportunities. Curr Issues Mol Biol. 2021;43:558–89.
Lien EC, Westermark AM, Zhang Y, Yuan C, Li Z, Lau AN, et al. Low glycaemic diets alter lipid metabolism to influence tumour growth. Nature. 2021;599:302–7.
Kumar A, Chamoto K, Chowdhury PS, Honjo T. Tumors attenuating the mitochondrial activity in T cells escape from PD-1 blockade therapy. Elife. 2020;9:e52330.
Chowdhury PS, Chamoto K, Kumar A, Honjo T. PPAR-induced fatty acid oxidation in t cells increases the number of tumor-reactive CD8(+) T cells and facilitates Anti-PD-1 therapy. Cancer Immunol Res. 2018;6:1375–87.
Choi BK, Lee DY, Lee DG, Kim YH, Kim S-H, Oh HS, et al. 4-1BB signaling activates glucose and fatty acid metabolism to enhance CD8(+) T cell proliferation. Cell Mol Immunol. 2017;14:748–57.
Najjar YG, Menk AV, Sander C, Rao U, Karunamurthy A, Bhatia R, et al. Tumor cell oxidative metabolism as a barrier to PD-1 blockade immunotherapy in melanoma. JCI Insight. 2019;4:e124989.
Liu Y-J, Fan X-Y, Zhang D-D, Xia Y-Z, Hu Y-J, Jiang F-L, et al. Dual inhibition of pyruvate dehydrogenase complex and respiratory chain complex induces apoptosis by a mitochondria-targeted fluorescent organic arsenical in vitro and in vivo. ChemMedChem. 2020;15:552–8.
Lund KC, Wallace KB. Adenosine 3’,5’-cyclic monophosphate (cAMP)-dependent phosphoregulation of mitochondrial complex I is inhibited by nucleoside reverse transcriptase inhibitors. Toxicol Appl Pharm. 2008;226:94–106.
Carlini F, Ridolfi B, Molinari A, Parisi C, Bozzuto G, Toccacieli L, et al. The reverse transcription inhibitor abacavir shows anticancer activity in prostate cancer cell lines. PLoS One. 2010;5:e14221.
Kulkarni SS, Cantó C. The molecular targets of resveratrol. Biochim Biophys Acta. 2015;1852:1114–23.
Chandran K, Aggarwal D, Migrino RQ, Joseph J, McAllister D, Konorev EA, et al. Doxorubicin inactivates myocardial cytochrome c oxidase in rats: cardioprotection by Mito-Q. Biophys J. 2009;96:1388–98.
Thomas JS, El-Khoueiry AB, Maurer BJ, Groshen S, Pinski JK, Cobos E, et al. A phase I study of intravenous fenretinide (4-HPR) for patients with malignant solid tumors. Cancer Chemother Pharm. 2021;87:525–32.
Ratheiser K, Schneeweiss B, Waldhäusl W, Fasching P, Korn A, Nowotny P, et al. Inhibition by etomoxir of carnitine palmitoyltransferase I reduces hepatic glucose production and plasma lipids in non-insulin-dependent diabetes mellitus. Metabolism. 1991;40:1185–90.
Mozolewska P, Duzowska K, Pakiet A, Mika A, ŚledziŃski T. Inhibitors of fatty acid synthesis and oxidation as potential anticancer agents in colorectal cancer treatment. Anticancer Res. 2020;40:4843–56.
Giannessi F, Pessotto P, Tassoni E, Chiodi P, Conti R, De Angelis F, et al. Discovery of a long-chain carbamoyl aminocarnitine derivative, a reversible carnitine palmitoyltransferase inhibitor with antiketotic and antidiabetic activity. J Med Chem. 2003;46:303–9.
Ashrafian H, Horowitz JD, Frenneaux MP. Perhexiline. Cardiovasc Drug Rev. 2007;25:76–97.
Bentebibel A, Sebastián D, Herrero L, López-Viñas E, Serra D, Asins G, et al. Novel effect of C75 on carnitine palmitoyltransferase I activity and palmitate oxidation. Biochemistry. 2006;45:4339–50.
Thangavelu K, Pan CQ, Karlberg T, Balaji G, Uttamchandani M, Suresh V, et al. Structural basis for the allosteric inhibitory mechanism of human kidney-type glutaminase (KGA) and its regulation by Raf-Mek-Erk signaling in cancer cell metabolism. Proc Natl Acad Sci USA. 2012;109:7705–10.
Shukla K, Ferraris DV, Thomas AG, Stathis M, Duvall B, Delahanty G, et al. Design, synthesis, and pharmacological evaluation of bis-2-(5-phenylacetamido-1,2,4-thiadiazol-2-yl)ethyl sulfide 3 (BPTES) analogs as glutaminase inhibitors. J Med Chem. 2012;55:10551–63.
Xie C, Jin J, Bao X, Zhan W-H, Han T-Y, Gan M, et al. Inhibition of mitochondrial glutaminase activity reverses acquired erlotinib resistance in non-small cell lung cancer. Oncotarget. 2016;7:610–21.
Gross MI, Demo SD, Dennison JB, Chen L, Chernov-Rogan T, Goyal B, et al. Antitumor activity of the glutaminase inhibitor CB-839 in triple-negative breast cancer. Mol Cancer Ther. 2014;13:890–901.
Lee Y-Z, Yang C-W, Chang H-Y, Hsu H-Y, Chen I-S, Chang H-S, et al. Discovery of selective inhibitors of Glutaminase-2, which inhibit mTORC1, activate autophagy and inhibit proliferation in cancer cells. Oncotarget. 2014;5:6087–101.
Richens A, McEwan JR, Deybach JC, Mumford JP. Evidence for both in vivo and in vitro interaction between vigabatrin and alanine transaminase. Br J Clin Pharm. 1997;43:163–8.
Acknowledgements
We are grateful to Dr. Shuijie Li , Karolinska Institutet for fruitful discussions. Figures were created with BioRender. L.S.-A. was supported by Karolinska Institutet Research Funds, J.L.-P. by a postdoctoral scholarship from Radiumhemmet Research Funds Stockholm and Anna-Brita and Bo Castengren’s Memorial Fund, M.V.R.-P. by a postdoctoral position from the Swedish Childhood Cancer Foundation, Karolinska Institutet Research Funds, and Anna-Brita and Bo Castegren’s Memorial Fund, and M.A.-H. by grants from the Swedish Cancer Society, the Swedish Childhood Cancer Foundation, the Swedish Research Council, Radiumhemmet Research Funds, and Karolinska Institutet.
Funding
Open access funding provided by Karolinska Institutet.
Author information
Authors and Affiliations
Contributions
Outline, original draft, and Table preparation, L.S.-A., J.L.-P., and M.V.R.-P.; Figure preparation, L.S.-A. and J.L.-P; review and editing, L.S.-A., J.L.-P., and M.A.-H. All authors read and agreed on the final version of the review.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Edited by G. Melino.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sainero-Alcolado, L., Liaño-Pons, J., Ruiz-Pérez, M.V. et al. Targeting mitochondrial metabolism for precision medicine in cancer. Cell Death Differ 29, 1304–1317 (2022). https://doi.org/10.1038/s41418-022-01022-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41418-022-01022-y
- Springer Nature Limited
This article is cited by
-
The troglitazone derivative EP13 disrupts energy metabolism through respiratory chain complex I inhibition in breast cancer cells and potentiates the antiproliferative effect of glycolysis inhibitors
Cancer Cell International (2024)
-
Strategic disruption of cancer’s powerhouse: precise nanomedicine targeting of mitochondrial metabolism
Journal of Nanobiotechnology (2024)
-
SIRT5-mediated ME2 desuccinylation promotes cancer growth by enhancing mitochondrial respiration
Cell Death & Differentiation (2024)
-
Tumor biomarkers for diagnosis, prognosis and targeted therapy
Signal Transduction and Targeted Therapy (2024)
-
Drug repurposing for cancer therapy
Signal Transduction and Targeted Therapy (2024)