Abstract
Background
Industrial biomanufacturing of value-added products using CO2 as a carbon source is considered more sustainable, cost-effective and resource-efficient than using common carbohydrate feedstocks. Cupriavidus necator H16 is a representative H2-oxidizing lithoautotrophic bacterium that can be utilized to valorize CO2 into valuable chemicals and has recently gained much attention as a promising platform host for versatile C1-based biomanufacturing. Since this microbial platform is genetically tractable and has a high-flux carbon storage pathway, it has been engineered to produce a variety of valuable compounds from renewable carbon sources. In this study, the bacterium was engineered to produce resveratrol autotrophically using an artificial phenylpropanoid pathway.
Results
The heterologous genes involved in the resveratrol biosynthetic pathway—tyrosine ammonia lyase (TAL), 4-coumaroyl CoA ligase (4CL), and stilbene synthase (STS) —were implemented in C. necator H16. The overexpression of acetyl-CoA carboxylase (ACC), disruption of the PHB synthetic pathway, and an increase in the copy number of STS genes enhanced resveratrol production. In particular, the increased copies of VvSTS derived from Vitis vinifera resulted a 2-fold improvement in resveratrol synthesis from fructose. The final engineered CR-5 strain produced 1.9 mg/L of resveratrol from CO2 and tyrosine via lithoautotrophic fermentation.
Conclusions
To the best of our knowledge, this study is the first to describe the valorization of CO2 into polyphenolic compounds by engineering a phenylpropanoid pathway using the lithoautotrophic bacterium C. necator H16, demonstrating the potential of this strain a platform for sustainable chemical production.
Similar content being viewed by others
Background
The increasing concerns regarding global warming and the depletion of fossil fuel resources have prompted researchers to explore sustainable methods for chemical production from renewable carbon resources. Microbial fermentation has emerged as a promising approach, aided by the recent advancements in synthetic biology-guided metabolic engineering tools [1]. Polyphenolic compounds, including resveratrol, bisdemethoxycurcumin, and naringenin, have been extensively studied due to their diverse biological activities [2]. Resveratrol (trans-3,5,4′-transhydroxy-stilbene), a naturally occurring stilbene, has been widely used in various applications, including as a flavoring, fragrance, medicinal, and nutritional supplement [3, 4]. Commercially, these bioactive compounds are either extracted from plants such as grapes, berries, peanuts, and other vine plants in trace amounts or synthesized chemically [3, 5]. Owing to their health benefits and the increasing demand, the microbial production of plant-derived natural products has mainly been achieved by microbial metabolic engineering. Considering the number of benefits such as low production cost, high product purity, and sustainability, microbial fermentation process of resveratrol production provides a promising alternative to plant extraction or chemical synthesis [3, 6].
Resveratrol is synthesized in plants via the phenylpropanoid pathway, beginning with the synthesis of phenylpropanoids (such as coumaric and ferulic acids) from the aromatic amino acids phenylalanine and tyrosine [2, 7]. Phenylpropanoids are converted into various polyphenolic compounds via type III polyketide synthases (PKS). PKS include stilbene synthase for the synthesis of stilbenes such as resveratrol and chalcone synthase for the synthesis of flavonoids [7]. Recently, significant progress has been made in the de novo synthesis of polyphenolic compounds using engineered microorganisms, such as Escherichia coli and yeast [3].
Cupriavidus necator H16 (formerly Ralstonia eutropha H16), a well-studied H2-oxidizing lithoautotrophic bacterium, is capable of fixing CO2 via the Calvin–Benson–Bassham (CBB) cycle and storing large amounts of fixed carbon in the form of poly(3-hydroxybutyrate) (PHB) [1, 8]. Since this microbial platform is genetically tractable and has versatile metabolic capabilities, it has been engineered to produce a variety of valuable compounds from CO2 demonstrating its potential as an industrial workhorse [1, 9,10,11,12,13]. Moreover, C. necator H16 exhibits a faster autotrophic growth rate (doubling time = 4.2 h) than the photoautotrophic cyanobacteria (doubling time = 7–12 h) [14,15,16] and has been employed as a central biocatalyst in the microbial electrosynthetic systems that can use solar energy to convert CO2 into value added chemicals [13].
While C. necator H16 is considered a suitable candidate for autotrophic and electro-autotrophic chemical production, it has never been engineered for the production of polyphenolic compounds, such as resveratrol. We report here for the first time on lithoautotrophic production of resveratrol by engineered C. necator strains. The best-performing strains were initially screened under heterotrophic conditions, and then evaluated under lithoautotrophic conditions. An artificial phenylpropanoid pathway consisting of tyrosine ammonia lyase (TAL), 4-coumarate-coA ligase (4CL), and stilbene synthase (STS) was introduced into C. necator H16 with supplementation of L-tyrosine. Various metabolic strategies, including the overexpression of acetyl-CoA carboxylase (ACC), disruption of the PHB synthetic pathway, and an increase in the copy number of STS genes, were implemented to enhance resveratrol production. Finally, we produced 1.9 mg/L of resveratrol using CO2 as the sole carbon source via lithoautotrophic production of engineered C. necator strains.
Methods
Strains and plasmids
All strains and plasmids used in this study are listed in Table 1. Cupriavidus necator H16 (KCTC 22,469; Korean Collection for Type Cultures, Daejeon, South Korea) and PHB−4 (DSM 541, KACC 11,970; Korean Agricultural Culture Collection, Wanju, South Korea) were used as host strains for resveratrol production.
While ACC gene was amplified from the genomic DNA of Corynebacterium glutamicum ATCC 13,032, STS, TAL and 4CL genes were codon-optimized and synthesized by IDT KOREA (Additional file: Table S2). All genes were expressed in the pBBR1-MCS2 vector under the control of the arabinose-inducible araBAD promoter. The primers used to construct the recombinant plasmids are listed in Additional file: Table S1. Plasmid preparation was performed using Mini Exprep plasmid SV (Geneall, South Korea). A QIAquick gel extraction kit (Qiagen, Germany) was used for the gel purification of DNA fragments. The restriction enzyme-based cloning method, Gibson Assembly (New England Biolabs, Massachusetts, USA), or Hifi DNA Assembly (New England Biolabs, Massachusetts, USA) was used to assemble the recombinant plasmids. To transform the constructed plasmids into C. necator, bacterial conjugation using Escherichia coli S17-1 donor strain harboring the desired plasmid was performed [17].
Heterotrophic and lithoautotrophic cultures
Cupriavidus necator H16 was routinely grown in Luria-Bertani (LB) broth at 30 ℃ and 200 rpm. For heterotrophic resveratrol fermentation, C. necator H16 strains were aerobically grown in 100 mL flasks containing 40 mL minimal media (MM) with 10 g/L fructose, 1.5 g/L KH2PO4, 6.74 g/L Na2HPO4•7H2O, 1.0 g/L (NH4)2SO4, 80 mg/L MgSO4•7H2O, 0.56 mg/L NiSO4•7H2O, 0.4 mg/L ferric citrate, 1 mg/L CaSO4•2H2O, and 0.5 g/L NaHCO3 supplemented with 5 mM tyrosine. The heterologous gene expression in the recombinant strains was induced at 6 h by the addition of 0.2% (w/v) L-arabinose unless otherwise indicated. For lithoautotrophic fermentation, the preculture was cultivated in MM with 10 g/L fructose at 30 ℃ and 200 rpm for 24 h. Cultured cells were collected by centrifugation at 4,200 rpm for 10 min, washed with MM without fructose and then inoculated into MM supplemented with 5 mM tyrosine in serum bottles. The strains were incubated at 30 ℃ and 200 rpm with the initial OD600 of 1 and 5, and filled with a 150 kPa of mixture gas (H2:O2:CO2 = 70:20:10 or 78:2:10; Airkorea Corporation, South Korea). The gas mixture was pressurized into the headspace of the 157 mL-serum bottles containing 20 mL MM every 24 h. The expression of biosynthetic genes was induced by adding 0.2% (w/v) L-arabinose after 24 h of autotrophic culture. During the cultivation of recombinant strains harboring resveratrol synthetic plasmids, kanamycin was also supplemented at a final concentration of 200 µg/mL.
Genetic manipulation
For the preparation of the △phaCAB strain based on the sacB-knockout system, pJQ200mp18Km plasmids were constructed by amplifying homologies to the 500 bp regions upstream and downstream of the phaCAB operon and transferred into C. necator H16 via conjugation as described previously with slight modifications [15, 17]. After culture in low salt-LB broth (2.5 g/L NaCl) supplemented with 15% (w/v) sucrose and gentamicin, the deletion strains were screened by PCR with diagnostic primers (Additional file 1: Table S1).
Analytical procedures
For the analysis of resveratrol and p-coumaric acid, 2 ml of the supernatant was centrifuged at 4,200 rpm for 10 min and extracted with an equal volume of ethyl acetate by vortexing. After extraction, the ethyl acetate layer was transferred to a glass tube and evaporated. The remaining residue was then dissolved in methanol. The samples filtered through a 0.2 μm syringe filter were analyzed by high-performance liquid chromatography (HPLC; Agilent technology 1100 Infinity, CA, USA) equipped with a ZORBAX SB-C18 column (4.6 × 150 mm, 3.5 μm, Agilent technology, CA, USA) maintained at 30 °C using a mobile phase composed of 40% acetonitrile and 60% water at a flow rate of 0.6 ml/min. The PHB content was quantified as previously described by Kim et al. (2022) [8].
RNA sequencing analysis
For verification of p-coumaric acid utilization by C. necator H16, sequencing experiments were performed by E-biogen, Inc. (Seoul, South Korea). RNA-seq samples were prepared after 20 h of aerobic fermentation in a minimal medium containing 10 g/L fructose with or without 1 g/L p-coumaric acid. RNA-seq analysis was performed as described previously by Kim et al. (2022) [8].
Results and discussion
Construction of a lithoautotrophic platform for production of resveratrol from CO2
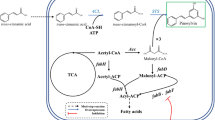
A lithoautotrophic microbial platform was designed to produce resveratrol from CO2 via a phenylpropanoid biosynthetic pathway (Fig. 1). As the starting point, we expressed tyrosine ammonia lyase (TAL), which converts L-tyrosine to p-coumaric acid. In previous studies, microbial production of resveratrol was achieved with the supplementation of the precursor p-coumaric acid or aromatic amino acids such as L-tyrosine [2, 18]. The aromatic amino acid tyrosine is formed via the shikimate pathway in plants and microorganisms [19]. In this study, 5 mM tyrosine was used as the starting precursor for resveratrol synthesis. Consequently, p-coumaroyl-CoA is formed from the conversion of p-coumaric acid by expressing 4-coumarate: coenzyme ligase (4CL). Stilbene synthase (STS) sequentially directs the condensation of one molecule of p-coumaroyl-CoA with three malonyl-CoA molecules to produce resveratrol [18] (Fig. 1).
Synthetic metabolic pathways for de novo biosynthesis of resveratrol from CO2 and tyrosine in engineered C. necator H16. An artificial phenylpropanoid biosynthetic pathway engineered for resveratrol biosynthesis are shown in the colored box. The abbreviations are as follows: TAL, tyrosine ammonia lyase; 4CL, 4-coumarate-CoA ligase; STS, stilbene synthase; ACC, acetyl-CoA carboxylase; 3-PGA, 3-phosphoglycerate; G3P, glyceraldehyde-3-phosphate; RuBP, ribulose biphosphate. The dashed lines indicates omitted reaction steps
To achieve resveratrol production from CO2, we constructed three recombinant plasmids carrying an STS gene from Vitis vinifera (VvSTS), a TAL gene from Flavobacterium johnsoniae (FjTAL), a 4CL gene from Arabidopsis thaliana (At4CL) and a ACC gene from Corynebacterium glutamicum (CgACC) under the control of arabinose-inducible araBAD promoter (Fig. 2A). To enhance the carbon flux of p-coumaroyl-CoA towards the resveratrol biosynthetic pathway, the recombinant plasmid containing a second copy of VvSTS was also constructed. Since the distance from the promoter affects the gene expression level [20], another VvSTS gene was placed in the last order to ensure the strong expression of FjTAL, At4CL and CgACC. The recombinant strains tested in this study are shown in Fig. 2B. First, the cell growth of C. necator H16 in the presence of resveratrol ranging from 0 to 50 mg/L was tested to evaluate the potential problems with the toxicity of resveratrol to cells (Additional file 1: Fig. S1). Notably, the presence of 50 mg/L resveratrol caused severe retardation of C. necator H16 cell growth.
Construction of recombinant C. necator strains. (A) Genetic map of a resveratrol biosynthetic plasmid containing a synthetic ribosome-binding site (RBS) and a nucleotide linker sequence inserted between each gene [20]. (B) List of engineered strains with different combinations of heterologous genes. The sources of heterologous genes are indicated: VvSTS, Vitis vinifera STS; FjTAL, Flavobacterium johnsoniae; At4CL, Arabidopsis thaliana 4CL; CgACC, Corynebacterium glutamicum ACC
Evaluation of heterotrophic resveratrol production by engineered strains
The engineered strains were evaluated for their resveratrol production capabilities, using fructose as the carbon source (Fig. 3). When the CR-1 strain carrying FjTAL, At4CL, and VvSTS genes was cultured in a minimal medium supplemented with 5 mM tyrosine, resveratrol synthesis was not detected (Fig. 3B). Since the biosynthesis of resveratrol requires three moles of malonyl-CoA, limited malonyl-CoA availability may be the decisive bottleneck in the native metabolism of C. necator H16. Most microorganisms generate malonyl-CoA solely from irreversible acetyl-CoA carboxylation catalyzed by acetyl-CoA carboxylases (ACCs), which is a key precursor of de novo fatty acid synthesis (FAS) [21]. Since the malonyl-CoA pool is highly controlled and consumed by FAS [21, 22], FAS is regarded as an undesired metabolic pathway in terms of resveratrol biosynthesis [22]. Therefore, we used two strategies to increase intracellular malonyl-CoA availability: inhibition of fatty acid synthesis and overexpression of acetyl-CoA carboxylase (ACC) (Fig. 3A).
To inhibit the synthesis of fatty acids that incorporate malonyl-CoA, 0.05 mM of the FAS inhibitor cerulenin was supplemented into the medium (Fig. 3B). The CR-1 strain supplemented with cerulenin produced 1.5 mg/L of resveratrol. This indicates that the limited availability of malonyl-CoA is a major bottleneck in resveratrol synthesis in C. necator H16. Cerulenin supplementation has been shown to enhance polyphenolic compound production in E. coli and C. glutamicum in prior studies [7, 23, 24]. However, it is expensive and can inhibit cell growth due to irreversible and non-selective FAS inhibition [22, 25,26,27]. To address this, gene downregulation strategies using antisense RNA or CRISPR interference have been used in previous studies for malonyl-CoA-derived compounds [28, 29].
Instead of reducing undesired malonyl-CoA consumption by adding cerulenin, we constructed a CR-2 strain overexpressing ACC derived from C. glutamicum to increase the intracellular malonyl-CoA pool. Since ACC of C. glutamicum comprises only two subunits (accBC and dtsR1) instead of a four-subunit protein complex of ACC derived from E. coli and others for catalytic activity [30], this enzyme was overexpressed in C. necator H16. The expression of heterologous ACC genes in C. necator H16 resulted in successful resveratrol synthesis, producing 3.5 mg/L (Fig. 3D).
To further channel more carbon flux from acetyl-CoA towards malonyl-CoA, we disrupted the PHB synthesis pathway. Using the sacB-based gene knock-out plasmid, the PHB non-producing strain ΔphaCAB was obtained with the deletion of the entire phaCAB operon encoding the essential enzymes for PHB synthesis (Table 1). We also employed a representative PHB-negative mutant, PHB−4 (DSM 541), which has a single nonsense mutation in the PHA synthase gene phaC [31]. Although PHB accumulation was not detected, both the PHB-negative PHB−4 and △CAB strains showed retarded cell growth in media containing fructose (10 g/L) as the carbon source (Additional file 1: Fig. S2). Since acetyl-CoA is not consumed in the PHB synthetic pathway, mutant cells might have altered cellular metabolism with the accumulation of metabolites, such as acetyl-CoA and pyruvate [31]. When C. necator H16, △CAB, and PHB−4 strains carried the pBAD-STS-TAL-4CL-ACC plasmid, the resulting strains CR-2, CR-3, and CR-4 produced 3.5, 4.1, and 3.5 mg/L of resveratrol, respectively (Fig. 3D). Although the cell growth rate of CR-3 was lower than that of CR-2, its resveratrol titer was obtained as 4.1 mg/L, 17% higher than that of CR-2. Disruption of the competing pathway by deleting the entire phaCAB operon effectively enhanced resveratrol synthesis. However, resveratrol production by the PHB−4 strain (CR-4) did not show any positive result and its growth rate was lower than that of CR-3. Therefore, the CR-3 strain with the highest resveratrol synthesis capability was selected for further engineering. Since cell growth and PHB synthesis in C. necator H16 depend on the nitrogen concentration with excess carbon available, the effect of nitrogen supplementation on resveratrol production was investigated. Also, the resveratrol production performances of the different strains with or without the PHB synthetic pathway were compared. While nitrogen limitation generally boosts PHB accumulation [32], the resveratrol synthesis was negatively affected under the nitrogen limitation condition (0.5 (NH4)2SO4-g/L). Although the CR-2 strain showed the enhanced cell growth at 24 h under nitrogen limitation (Fig. 3C), it led to the decreased resveratrol production. For the PHB-negative CR-3 and CR-4 strains, nitrogen limitation led to decreased cell growth, resulting in reduced resveratrol production (Fig. 3C, D). When the competing pathway was disrupted to avoid the depletion of acetyl-CoA, which is the precursor to PHB, in CR-3 and CR-4 strains, the redirected carbon flow towards acetyl-CoA under nitrogen limitation could not increase resveratrol synthesis. While the sufficient nitrogen supply is required for enhancing cell growth and resveratrol production, the > 2-fold enhancement of 1,3-butanediol production by engineered C. necator H16 was observed under nutrient-limiting conditions [15]. Despite decreased cell growth, the availability of 3-hydroxybutyryl-CoA precursor boosted by nitrogen limitation enabled the enhanced 1,3-butanediol production [15].
Resveratrol production using engineered strains under heterotrophic conditions. (A) Strategies for increased malonyl-CoA availability and resveratrol synthesis. The strategies used in this study including the overexpression of ACC, addition of cerulenin and disruption of PHB synthetic pathway are stated as red colors. The dashed lines indicates omitted reaction steps. The abbreviations are as follows: ACC, acetyl-CoA carboxylase; PEP, phosphoenolpyruvate; DHAP, 3-deoxy-D-arabinoheptulosanate-7-phosphate; FAS, fatty acid synthesis. (B) Resveratrol production using the CR-1 strain with or without cerulenin supplementation. (C) Cell growth and (D) resveratrol production using the CR-2, CR-3, and CR-4 strains. Cells were grown in either nitrogen-rich MM medium (solid lines, dark purple) containing 10 g/L fructose and 1 g/L (NH4)2SO4 or nitrogen-limiting MM (dashed lines, light purple) containing 10 g/L fructose and 0.5 g/L (NH4)2SO4. The MM medium was supplemented with 5 mM tyrosine for resveratrol synthesis. The resveratrol titer was measured at the end of 96 h-fermentation. Error bars represent the standard deviation from three biological replicates. Student’s two-tailed t-test was performed to determine the significance of differences (*, p < 0.05)
Additional STS copy increases the flux of p-coumaroyl CoA towards resveratrol biosynthesis
While malonyl-CoA availability was regulated by overexpression of ACC and disruption of the PHB synthetic pathway, yielding 4.1 mg/L of resveratrol, one molecule of p-coumaroyl-CoA via coumaric acid conversion by the action of 4CL was still required for resveratrol synthesis. Although high levels of p-coumaric acid are toxic to cells, the possibility of coumaric acid as a carbon source of C. necator H16 at low concentrations has been suggested in previous studies [33]. The catabolic mechanisms of lignin-derived aromatic compounds, including coumaric, ferulic, and cinnamic acids, have not been completely elucidated in C. necator H16. It has been suggested that p-coumarate is converted to 4-hydroxybenzoate and then fed into the TCA cycle [33]. As shown in Fig. 4A, the growth of C. necator H16 was observed in the presence of 3–5 mM p-coumaric acid as the sole carbon source, although a high concentration of p-coumaric acid (5 mM) led to a prolonged lag phase. Although the genes responsible for the conversion of p-coumaric acid to 4-hydroxybenzoate were not completely identified, cell growth on 4-hydroxybenzoate and the upregulation of the genes involved in the reactions starting from 4-hydroxybenzoate to succinyl-CoA were observed when the cells were cultured with supplementation of p-coumaric acid (Additional file 1: Fig. S3). This indicates that tyrosine supplemented in the medium as the precursor for resveratrol synthesis might be converted to primary metabolites in the engineered strains. As the activation of coumarate catabolic pathways in C. necator H16 causes the loss of p-coumaroyl CoA, an important precursor for resveratrol synthesis, we aimed to redirect the carbon flux of p-coumaroyl-CoA towards the resveratrol biosynthetic pathway by overexpressing two copies of VvSTS (Fig. 4B). The addition of a second copy of VvSTS in the CR-2 and CR-3 strains, resulting in CR-5 and CR-6 strains, increased resveratrol production from 3.5 to 4.1 mg/L to 6.4 and 6.9 mg/L, respectively (Fig. 5B). The final OD600 values at 96 h of CR-5 and CR-6 strains were not significantly different from those of CR-2 and CR-3 strains, respectively (Fig. 5A). The > 70% increase in resveratrol synthesis in the CR-5 and CR-6 strains demonstrated that increasing STS activity is important to make use of the available p-coumaroyl-CoA. With further efforts to understand the metabolism of aromatic compounds in C. necator H16, the identification, and deletion of key genes involved in the conversion of p-coumaroyl-CoA to 4-hydroxybenzoate could be an alternative strategy for increasing p-coumaroyl-CoA availability for resveratrol production. Incha et al. (2020) identified genes involved in the coumarate catabolic pathways of Pseudomonas putida KT2440 that could potentially impact p-coumaroyl-CoA-derived products. In engineered P. putida, deletion of the gene ech (enoyl-CoA hydrolase-lyase) in coumarate catabolism increased type III polyketide bisdemethoxycurcumin [34].
Microbial production of resveratrol has been reported in various microorganisms (Table 2). In particular, significant efforts in metabolic engineering toward high-level resveratrol production have been achieved in E. coli, C. glutamicum, Saccharomyces cerevisiae, and Yarrowia lipolytica [3]. While the first production of resveratrol with the titer of 1.45 µg/L was achieved in engineered yeast [35], a high level of resveratrol production reaching approximately 800 mg/L was found in engineered S. cerevisiae by optimized fed-batch fermentation [36]. With advancements in synthetic biology tools, there have been numerous efforts to regulate metabolic pathways and optimize resveratrol production; E. coli and C. glutamicum have yielded 300 and 110 mg/L of resveratrol titer, respectively [7, 18, 25, 36, 37]. Other bacterial hosts, such as Streptomyces venezuelae and Aspergillus niger have also been used to produce resveratrol by introducing a heterologous phenylpropanoid biosynthetic pathway [38, 39]. It was reported that engineered S. venezuela produced resveratrol for the first time, with a yield of just 0.4 mg/L [39]. In this study, we achieved 6.8 mg/L of resveratrol from fructose in engineered C. necator H16, which is the first time that a polyphenolic compound has been synthesized in this host. Taken together, the CR-6 strain constructed in this study represents a promising starting point for further engineering towards a more efficient resveratrol production.
Coumarate catabolic pathways in C. necator H16 causes the loss of p-coumaroyl CoA, an important precursor for resveratrol synthesis. (A) Cell growth of C. necator H16 strain on various aromatic compounds as the sole carbon source. PCA: p-coumaric acid, 4-HBA: 4-hydroxybenzoic acid. The initial optical density at 600 nm (OD600) was 0.2 at 600 nm and the initial p-coumaric acid and 4-hydroxybenzoic acid concentrations were 3 and 5 mM, respectively. (B) Metabolic engineering of C. necator H16 for re-directing malonyl-CoA flux towards resveratrol synthesis by addition of VvSTS
Effects of increasing the copy number of VvSTS and disrupting the PHB pathway on resveratrol production under heterotrophic conditions. (A) Cell growth curve and (B) resveratrol production in the CR-2, CR-3, CR-5, and CR-6 strains. The initial OD600 was 5 and the initial fructose concentration was 10 g/L. The MM medium was supplemented with 5 mM tyrosine for resveratrol synthesis. The resveratrol titer was measured at the end of 96 h-fermentation
Lithoautotrophic production of resveratrol from CO2
The final goal of this study was to produce resveratrol from CO2. For autotrophic production of resveratrol, the recombinant strains CR-2, CR-3, CR-5, and CR-6 were cultivated in serum bottles supplemented with CO2, H2, and O2 (Fig. 6). Although the cell growth rates of the CR-2 and CR-3 strains were significantly higher than those of the CR-5 and CR-6 strains with initial cell densities (OD600) of 1 (Fig. 6A), resveratrol synthesis was not observed in the CR-2 and CR-3 strains (Fig. 6B). Approximately 1.2 mg/L of resveratrol was produced from CO2 and tyrosine by the CR-5 and CR-6 strains carrying a second copy of VvSTS. However, differences in autotrophic performance between the CR-5 and CR-6 strains were not observed (Fig. 6B). Since the high cell density culture offers an efficient way to enhance the microbial fermentation productivities [45], the autotrophic cultures with the higher initial cell densities were performed. When the initial cell density was increased from 1 to 5, both the CR-5 and CR-6 strains produced slightly more resveratrol, yielding 1.4 and 1.5 mg/L, respectively (Fig. 6C and D). In addition, autotrophic fermentation under oxygen stress (H2:O2:CO2 = 88:2:10) was performed to promote resveratrol synthesis. Although C. necator H16 is typically cultured in a gas composition of H2:O2:CO2 = 70:20:10 for autotrophic growth, some previous works have reported that oxygen stress (< 3% O2) induced enhanced PHB production while restricting cell growth [46]. When oxygen stress (H2:O2:CO2 = 88:2:10) was induced in the CR-5 and CR-6 strains, resveratrol production increased from 1.4 to 1.5 mg/L to 1.8–1.9 mg/L.
Although C. necator H16 has been used as a host for the potential commercial production of polyhydroxyalkanoates (PHAs), it has been engineered to produce a variety of chemicals, such as isopropanol, acetoin, humulene, methyl ketone, (R)-1,3-butanediol, and alkene, under both heterotrophic and autotrophic conditions (Table 3) [1, 9, 10, 12, 15]. More recently, the CO2 conversion into sugars and value-added compounds by engineered C. necator strains using H2 as an energy source produced 470 mg/L trehalose, 250 mg/L glucose, and 1.7 mg/L lycopene [11, 13, 47]. This work is the first step towards the production of plant type III PKS-derived compounds in C. necator, but the synergistic combination of metabolic and fermentation optimization strategies for increasing productivity should be addressed in the future. With further studies on improving efficiencies of carbon fixation and resveratrol synthesis, a better understanding of carbon flux of CO2 in resveratrol-producing C. necator strains should allow us to enhance the viability of lithoautotrophic biochemical production. Due to the inhibitory effect of resveratrol on the cell growth of C. necator H16 (Additional file: Fig. S1), further metabolic engineering strategies for enhancing microbial tolerance towards resveratrol as well as the application of in situ product removal strategies are required to avoid the product toxicity limitations. Since the engineered C. necator strains still require the supplementation of exogenous tyrosine as a precursor, the metabolic engineering efforts on de novo tyrosine biosynthetic pathway is also needed to produce resveratrol solely from CO2.
Resveratrol production using engineered strains from CO2 and tyrosine. Lithoautotrophic fermentation profiles of the engineered strains for resveratrol production under different gas compositions of (A, B) H2:O2:CO2 = 70:20:10 vs. (C, D) H2:O2:CO2 = 88:2:10 (O2-limited) with different initial cell densities. The MM medium was supplemented with 5 mM tyrosine for resveratrol synthesis and the expression of resveratrol-biosynthetic genes was induced by adding 0.2% (w/v) L-arabinose after 24 h of autotrophic culture. The resveratrol titer was measured at the end of 144 h-fermentation
Conclusions
In this study, a microbial lithoautotrophic platform was developed by introducing an artificial resveratrol biosynthetic pathway into C. necator H16. Implementation of phenylpropanoid pathways consisting of tyrosine ammonia lyase (TAL), 4-coumarate-coA ligase (4CL), and stilbene synthase (STS) with supplementation of L-tyrosine in combination with disrupting PHB biosynthesis and enhancing carbon flux by increasing copies of STS enabled the strain to produce 1.9 mg/L of resveratrol from CO2 and tyrosine. Further intensive efforts on the metabolic engineering of phenylpropanoid and tyrosine-biosynthetic pathways will be necessary to realize the industrial production of resveratrol from CO2. The recombinant C. necator strain developed in this study is considered a promising starting strain for further engineering to produce polyphenolic compounds. With the continued engineering of C. necator H16, this strain will become a more attractive lithoautotrophic platform, providing a sustainable route for the valorization of CO2 to high-value natural products.
Data availability
All the data for this study are available within this published article and its additional files.
References
Marc J, Grousseau E, Lombard E, Sinskey AJ, Gorret N, Guillouet SE. Over expression of GroESL in Cupriavidus necator for heterotrophic and autotrophic isopropanol production. Metab Eng. 2017;42:74–84.
Noda S, Kondo A. Recent advances in microbial production of aromatic chemicals and derivatives. Trends Biotechnol. 2017;35:785–96.
Feng C, Chen J, Ye W, Liao K, Wang Z, Song X, Qiao M. Synthetic biology-driven microbial production of resveratrol: advances and perspectives. Front Bioeng Biotechnol. 2022;10:833920.
Lu Y, Shao D, Shi J, Huang Q, Yang H, Jin M. Strategies for enhancing resveratrol production and the expression of pathway enzymes. Appl Microbiol Biotechnol. 2016;100:7407–21.
Thapa SB, Pandey RP, Park YI, Kyung Sohng J. Biotechnological advances in resveratrol production and its chemical diversity. Molecules. 2019;24.
Sáez-Sáez J, Wang G, Marella ER, Sudarsan S, Cernuda Pastor M, Borodina I. Engineering the oleaginous yeast Yarrowia Lipolytica for high-level resveratrol production. Metab Eng. 2020;62:51–61.
Lim CG, Fowler ZL, Hueller T, Schaffer S, Koffas MA. High-yield resveratrol production in engineered Escherichia coli. Appl Environ Microbiol. 2011;77:3451–60.
Kim S, Jang YJ, Gong G, Lee S-M, Um Y, Kim KH, Ko JK. Engineering Cupriavidus necator H16 for enhanced lithoautotrophic poly(3-hydroxybutyrate) production from CO2. Microb Cell Fact. 2022;21:231.
Crépin L, Lombard E, Guillouet SE. Metabolic engineering of Cupriavidus necator for heterotrophic and autotrophic alka(e)ne production. Metab Eng. 2016;37:92–101.
Krieg T, Sydow A, Faust S, Huth I, Holtmann D. CO2 to terpenes: autotrophic and electroautotrophic α-humulene production with Cupriavidus necator. Angew Chem Int Ed Engl. 2018;57:1879–82.
Löwe H, Beentjes M, Pflüger-Grau K, Kremling A. Trehalose production by Cupriavidus necator from CO2 and hydrogen gas. Bioresour Technol. 2021;319:124169.
Müller J, MacEachran D, Burd H, Sathitsuksanoh N, Bi C, Yeh YC, Lee TS, Hillson NJ, Chhabra SR, Singer SW, Beller HR. Engineering of Ralstonia eutropha H16 for autotrophic and heterotrophic production of methyl ketones. Appl Environ Microbiol. 2013;79:4433–9.
Wu H, Pan H, Li Z, Liu T, Liu F, Xiu S, Wang J, Wang H, Hou Y, Yang B, Lei L, Lian J. Efficient production of lycopene from CO2 via microbial electrosynthesis. Chem Eng J. 2022;430:132943.
Bernstein HC, McClure RS, Hill EA, Markillie LM, Chrisler WB, Romine MF, McDermott JE, Posewitz MC, Bryant DA, Konopka AE, Fredrickson JK, Beliaev AS. Unlocking the constraints of cyanobacterial productivity: acclimations enabling ultrafast growth. mBio. 2016;7. https://doi.org/10.1128/mbio.00949.
Gascoyne JL, Bommareddy RR, Heeb S, Malys N. Engineering Cupriavidus necator H16 for the autotrophic production of (R)-1,3-butanediol. Metab Eng. 2021;67:262–76.
Lütte S, Pohlmann A, Zaychikov E, Schwartz E, Becher JR, Heumann H, Friedrich B. Autotrophic production of stable-isotope-labeled arginine in Ralstonia eutropha strain H16. Appl Environ Microbiol. 2012;78:7884–90.
Xiong B, Li Z, Liu L, Zhao D, Zhang X, Bi C. Genome editing of Ralstonia eutropha using an electroporation-based CRISPR-Cas9 technique. Biotechnol Biofuels. 2018;11:172.
Zhao Y, Wu BH, Liu ZN, Qiao J, Zhao GR. Combinatorial optimization of resveratrol production in engineered E. Coli. J Agric Food Chem. 2018;66:13444–53.
Averesch NJH, Krömer JO. Metabolic engineering of the shikimate pathway for production of aromatics and derived compounds-present and future strain construction strategies. Front Bioeng Biotechnol. 2018;6:32.
Grousseau E, Lu J, Gorret N, Guillouet SE, Sinskey AJ. Isopropanol production with engineered Cupriavidus necator as bioproduction platform. Appl Microbiol Biotechnol. 2014;98:4277–90.
Zha W, Rubin-Pitel SB, Shao Z, Zhao H. Improving cellular malonyl-CoA level in Escherichia coli via metabolic engineering. Metab Eng. 2009;11:192–8.
Milke L, Marienhagen J. Engineering intracellular malonyl-CoA availability in microbial hosts and its impact on polyketide and fatty acid synthesis. Appl Microbiol Biotechnol. 2020;104:6057–65.
Leonard E, Lim KH, Saw PN, Koffas MA. Engineering central metabolic pathways for high-level flavonoid production in Escherichia coli. Appl Environ Microbiol. 2007;73:3877–86.
Kallscheuer N, Vogt M, Stenzel A, Gätgens J, Bott M, Marienhagen J. Construction of a Corynebacterium glutamicum platform strain for the production of stilbenes and (2S)-flavanones. Metab Eng. 2016;38:47–55.
Milke L, Ferreira P, Kallscheuer N, Braga A, Vogt M, Kappelmann J, Oliveira J, Silva AR, Rocha I, Bott M, Noack S, Faria N, Marienhagen J. Modulation of the central carbon metabolism of Corynebacterium glutamicum improves malonyl-CoA availability and increases plant polyphenol synthesis. Biotechnol Bioeng. 2019;116:1380–91.
Price AC, Choi K-H, Heath RJ, Li Z, White SW, Rock CO. Inhibition of β-ketoacyl-acyl carrier protein synthases by thiolactomycin and cerulenin: structure and mechanism. J Biol Chem. 2001;276:6551–9.
Giner-Robles L, Lázaro B, de la Cruz F, Moncalián G. fabH deletion increases DHA production in Escherichia coli expressing pfa genes. Microb Cell Fact. 2018;17:88.
Wu J, Du G, Chen J, Zhou J. Enhancing flavonoid production by systematically tuning the central metabolic pathways based on a CRISPR interference system in Escherichia coli. Sci Rep. 2015;5:13477.
Yang Y, Lin Y, Li L, Linhardt RJ, Yan Y. Regulating malonyl-CoA metabolism via synthetic antisense RNAs for enhanced biosynthesis of natural products. Metab Eng. 2015;29:217–26.
Wang S, Jin X, Jiang W, Wang Q, Qi Q, Liang Q. The expression modulation of the key enzyme Acc for highly efficient 3-hydroxypropionic acid production. Front Microbiol. 2022;13:902848.
Raberg M, Voigt B, Hecker M, Steinbüchel A. A closer look on the polyhydroxybutyrate- (PHB-) negative phenotype of Ralstonia eutropha PHB-4. PLoS ONE. 2014;9:e95907.
Tian J, Sinskey AJ, Stubbe J. Kinetic studies of polyhydroxybutyrate granule formation in Wautersia eutropha H16 by transmission electron microscopy. J Bacteriol. 2005;187:3814–24.
Wang W, Yang S, Hunsinger GB, Pienkos PT, Johnson DK. Connecting lignin-degradation pathway with pre-treatment inhibitor sensitivity of Cupriavidus necator. Front Microbiol. 2014;5:247.
Incha MR, Thompson MG, Blake-Hedges JM, Liu Y, Pearson AN, Schmidt M, Gin JW, Petzold CJ, Deutschbauer AM, Keasling JD. Leveraging host metabolism for bisdemethoxycurcumin production in Pseudomonas putida. Metab Eng Commun. 2020;10:e00119.
Becker JV, Armstrong GO, van der Merwe MJ, Lambrechts MG, Vivier MA, Pretorius IS. Metabolic engineering of Saccharomyces cerevisiae for the synthesis of the wine-related antioxidant resveratrol. FEMS Yeast Res. 2003;4:79–85.
Li M, Schneider K, Kristensen M, Borodina I, Nielsen J. Engineering yeast for high-level production of stilbenoid antioxidants. Sci Rep. 2016;6:36827.
Wu J, Zhou P, Zhang X, Dong M. Efficient de novo synthesis of resveratrol by metabolically engineered Escherichia coli. J Ind Microbiol Biotechnol. 2017;44:1083–95.
Chong Y, Yan A, Yang X, Cai Y, Chen J. An optimum fermentation model established by genetic algorithm for biotransformation from crude polydatin to resveratrol. Appl Biochem Biotechnol. 2012;166:446–57.
Park SR, Yoon JA, Paik JH, Park JW, Jung WS, Ban Y-H, Kim EJ, Yoo YJ, Han AR, Yoon YJ. Engineering of plant-specific phenylpropanoids biosynthesis in Streptomyces venezuelae. J Biotechnol. 2009;141:181–8.
Ni J, Tao F, Wang Y, Yao F, Xu P. A photoautotrophic platform for the sustainable production of valuable plant natural products from CO2. Green Chem. 2016;18:3537–48.
Gaspar P, Dudnik A, Neves AR, Forster J. Engineering Lactococcus lactis for stilbene production. In: Presented at the 28th International Conference on Polyphenols. 2016.
Shin SY, Jung SM, Kim MD, Han NS, Seo JH. Production of resveratrol from tyrosine in metabolically engineered Saccharomyces cerevisiae. Enzyme Microb Technol. 2012;51:211–6.
Palmer CM, Miller KK, Nguyen A, Alper HS. Engineering 4-coumaroyl-CoA derived polyketide production in Yarrowia Lipolytica through a β-oxidation mediated strategy. Metab Eng. 2020;57:174–81.
Katsuyama Y, Funa N, Horinouchi S. Precursor-directed biosynthesis of stilbene methyl ethers in Escherichia coli. Biotechnol J. 2007;2:1286–93.
Westman JO, Franzén CJ. Current progress in high cell density yeast bioprocesses for bioethanol production. Biotechnol J. 2015;10:1185–95.
Tang R, Weng C, Peng X, Han Y. Metabolic engineering of Cupriavidus necator H16 for improved chemoautotrophic growth and PHB production under oxygen-limiting conditions. Metab Eng. 2020;61:11–23.
Wang X, Luo H, Wang Y, Wang Y, Tu T, Qin X, Su X, Huang H, Bai Y, Yao B, Zhang J. Direct conversion of carbon dioxide to glucose using metabolically engineered Cupriavidus necator. Bioresour Technol. 2022;362:127806.
Acknowledgements
Not applicable.
Funding
This research was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (Ministry of Science and ICT) (No. RS-2022-00156236) and the Korea Institute of Science and Technology (KIST) Institutional Programs (2E33291).
Author information
Authors and Affiliations
Contributions
YJJ carried out the experiments and drafted the manuscript. JKK was responsible for supervising the overall work and writing the final manuscript. YJL contributed to the autotrophic fermentation experiments. GG, SL, YU and KHK contributed to the discussion of the results and reviewed the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Jang, Y., Lee, Y.J., Gong, G. et al. Carbon dioxide valorization into resveratrol via lithoautotrophic fermentation using engineered Cupriavidus necator H16. Microb Cell Fact 23, 122 (2024). https://doi.org/10.1186/s12934-024-02398-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12934-024-02398-x