Abstract
Acetoin, a versatile platform chemical and popular food additive, poses a challenge to the biosafety strain Bacillus subtilis when produced in high concentrations due to its intrinsic toxicity. Incorporating the PHB synthesis pathway into Bacillus subtilis 168 has been shown to significantly enhance the strain’s acetoin tolerance. This study aims to elucidate the molecular mechanisms underlying the response of B. subtilis 168-phaCBA to acetoin stress, employing transcriptomic and metabolomic analyses. Acetoin stress induces fatty acid degradation and disrupts amino acid synthesis. In response, B. subtilis 168-phaCBA down-regulates genes associated with flagellum assembly and bacterial chemotaxis, while up-regulating genes related to the ABC transport system encoding amino acid transport proteins. Notably, genes coding for cysteine and d-methionine transport proteins (tcyB, tcyC and metQ) and the biotin transporter protein bioY, are up-regulated, enhancing cellular tolerance. Our findings highlight that the expression of phaCBA significantly increases the ratio of long-chain unsaturated fatty acids and modulates intracellular concentrations of amino acids, including l-tryptophan, l-tyrosine, l-leucine, l-threonine, l-methionine, l-glutamic acid, l-proline, d-phenylalanine, l-arginine, and membrane fatty acids, thereby imparting acetoin tolerance. Furthermore, the supplementation with specific exogenous amino acids (l-alanine, l-proline, l-cysteine, l-arginine, l-glutamic acid, and l-isoleucine) alleviates acetoin’s detrimental effects on the bacterium. Simultaneously, the introduction of phaCBA into the acetoin-producing strain BS03 addressed the issue of insufficient intracellular cofactors in the fermentation strain, resulting in the successful production of 70.14 g/L of acetoin through fed-batch fermentation. This study enhances our understanding of Bacillus’s cellular response to acetoin-induced stress and provides valuable insights for the development of acetoin-resistant Bacillus strains.
Similar content being viewed by others
Introduction
Acetoin (3-hydroxy-2-butanone) stands as a versatile platform chemical extensively employed in diverse industries such as food, cosmetics, and plant growth promotion [1, 2]. Its industrial production traditionally relies on environmentally unfriendly chemical synthesis, raising safety concerns when used as a food additive [3, 4]. Consequently, there is a growing interest in adopting more sustainable and safer microbial fermentation methods [5].
Various strains, obtained through natural screening and metabolic engineering modifications, have been explored for fermentative acetoin production, encompassing Bacillus subtilis, Bacillus licheniformis, Corynebacterium glutamicum, Saccharomyces cerevisiae, Escherichia coli, Candida glabrata and Clostridium acetobutylicum [6,7,8]. Among these, B. subtilis, a natural acetoin producer, distinguishes itself due to its biosafety profile and well-established genetic manipulation systems, making it the preferred strain for acetoin fermentation [9]. In comparison to expression systems such as Escherichia coli, Saccharomyces cerevisiae, and Corynebacterium glutamicum, the B. subtilis expression system is considered safer (GRAS, generally recognized as safe) for producing food additives like acetoin and tetramethylpyrazine [10]. Moreover, the rapid growth of B. subtilis, its robust industrial fermentation performance, and its ability to metabolize various sugars make it a promising option for the cost-effective acetoin production [11, 12]. In recent years, the application of synthetic biology strategies and effective tools has further enhanced B. subtilis’s capability to produce various chemicals and enzymes [13]. These competitive advantages position B. subtilis as an ideal host for acetoin production.
Previously, studies have underscored the toxicity of acetoin to microbial cells, including its inhibitory effects on Staphylococcus aureus cultures and Bacillus subtilis 168 growth [14, 15]. Overcoming this bottleneck in acetoin production necessitates a thorough understanding of the resistance factors and tolerance mechanisms of Bacillus to acetoin.
One promising strategy for enhancing strain resistance involves introducing the polyhydroxybutyrate (PHB) synthesis pathway in host bacteria. Studies by Hye-Rim Jung, Mathiyazhagan Narayanan, and Hun-Suk Song have demonstrated introducing PHB synthesis genes in various bacteria can increase tolerance to different stressors and enhance production [16,17,18]. PHB serves as a carbon and energy storage polymer within microbial cells, offering protection against UV stress, starvation, heat stress, and osmotic shock [19, 20]. Given that introduction of the PHB synthesis pathway in Escherichia coli has improved tolerance to organic solvents, this strategy holds promise for addressing acetoin tolerance [18].
In this study, we introduced the polyhydroxybutyrate (PHB) synthesis pathway into B. subtilis 168, resulting in a significant enhancement of acetoin tolerance, with the acetoin tolerance concentration reaching more than 80 g/L. To illuminate the underlying tolerance mechanisms, we employed transcriptomics and metabolomics to analyze relevant metabolic pathways and differentially expressed genes. Our analysis revealed that the introduction of the PHB synthesis gene increased the proportion of long-chain unsaturated fatty acids in the cell membrane of B. subtilis 168. Simultaneously, it elevated the intracellular concentration of specific amino acids associated with tolerance, as well as biotin concentration. To assess the impact of the improved tolerance of B. subtilis to acetoin on fermentation, we used B. subtilis 168 as the starting strain, knocked out genes related to the competitive pathway (ldhA) and the decomposition pathway of the acetoin synthesis pathway (acoA, bdhA), and integrated key enzymes of the acetoin synthesis pathway (alsSD, yodC), successfully constructing the acetoin production strain BS03. Furthermore, incorporating PHB synthase into the acetoin-producing strain BS03-phaCBA successfully addressed the issue of insufficient intracellular cofactors in the fermentation strain, ultimately resulting in the production of 70.14 g/L of acetoin through fed-batch fermentation.
Result
Impact of acetoin stress on the growth of B. subtilis 168-pMA5 and B. subtilis 168-phaCBA
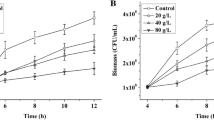
A comprehensive assessment was conducted to elucidate the influence of varying acetoin concentrations on the growth of B. subtilis 168-pMA5 and B. subtilis 168-phaCBA. Figure 1 illustrates that B. subtilis 168-phaCBA exhibited significantly elevated growth rates across all tested acetoin concentrations (0, 40, 60, and 80 g/L) in comparison to B. subtilis 168-pMA5. Notably, B. subtilis 168-phaCBA demonstrated a remarkable capacity to tolerate 80 g/L of acetoin, sustaining growth, while B. subtilis 168-pMA5 exhibited an inability to thrive under these conditions. To comprehensively investigate the metabolic and gene transcript responses of B. subtilis 168-phaCBA to acetoin stress, we conducted additional experiments assessing the impact of different acetoin concentrations (0, 20, 40, and 60 g/L) on the growth and sugar consumption during acetoin fermentation. Our findings revealed that acetoin supplementation impeded the growth of B. subtilis during acetoin fermentation. Notably, the utilization of glucose by the recombinant strain was significantly hindered when the acetoin concentration exceeded 40 g/L in the medium (as depicted in Fig. 2a, b). In pursuit of a comprehensive understanding of the transcriptional and metabolomic responses to acetoin stress, we focused on the severe acetoin stress group with a concentration of 60 g/L acetoin for subsequent analyses. This deliberate selection enabled a detailed exploration of the intricate regulatory mechanisms and metabolic shifts within B. subtilis 168-pMA5 and B. subtilis 168-phaCBA under high acetoin stress conditions.
Effects of acetoin stress on gene expression in B. subtilis 168-pMA5 and B. subtilis 168-phaCBA
To elucidate the tolerance mechanism of B. subtilis 168-phaCBA under acetoin stress, we examined the differences in gene expression between B. subtilis 168-pMA5 and B. subtilis 168-phaCBA under acetoin stress. We also compared the gene expression variations between B. subtilis 168-pMA5 and B. subtilis 168-phaCBA under acetoin stress. Differential gene expression was analyzed using edgeR software, focusing on genes with a False Discovery Rate (FDR) of less than 0.05 and an absolute log2 fold change (|log2FC|) greater than 1. The results indicated that under acetoin stress, B. subtilis 168-pMA5 showed 1774 significantly differentially expressed genes, with 1003 genes up-regulated and 771 genes down-regulated. Gene Ontology (GO) analysis, categorized these genes into three main groups: biological processes, cellular components, and molecular functions. In the biological process category, up-regulated and down-regulated genes were primarily associated with cellular processes, metabolic processes, and single-organism processes. In the cellular component category, the genes were concentrated in cells, cell parts, membranes, and membrane parts. In the molecular function category, the up-regulated and down-regulated genes were primarily involved in binding and catalytic activity (as illustrated in Fig. 3c).
Differentially expressed genes (DEGs) GO classification analysis and KEGG classification analysis of B. subtilis 168-pMA5 and B. subtilis 168-phaCBA under acetoin stress. a Identification of enriched DEGs by KEGG database in the 60 g/L acetoin treatment group (B. subtilis 168-pMA5) and 0 g/L acetoin control group (B. subtilis 168-pMA5). b KEGG enriched pathways of DEGs after acetoin stress between B. subtilis 168-pMA5 and B. subtilis 168-phaCBA. c GO analysis of DEGs in the 60 g/L acetoin treatment group (B. subtilis 168-pMA5) and 0 g/L acetoin control group (B. subtilis 168-pMA5). d GO analysis of DEGs after acetoin stress between B. subtilis 168-pMA5 and B. subtilis 168-phaCBA
Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis unveiled significant enrichment in metabolic pathways (Fig. 3a), These pathways encompassed biosynthesis of amino acids, oxidative phosphorylation, biosynthesis of secondary metabolites, amino acid metabolism (valine, leucine, isoleucine biosynthesis, cysteine, and methionine), C5-branched dibasic acid metabolism, fatty acid degradation, photosynthesis, TCA cycle, phenylalanine, tyrosine, and tryptophan biosynthesis, 2-oxo carboxylic acid metabolism, biosynthesis of siderophore group nonribosomal peptides, microbial metabolism in diverse environments, carbon metabolism, arginine and proline metabolism, two-component system, nicotinate and nicotinamide metabolism, carbon fixation in photosynthetic organisms, β-alanine metabolism, tryptophan metabolism, glycolysis/gluconeogenesis, biosynthesis of various antibiotics, among others (Additional file 1: Table S1).
Comparatively, B. subtilis 168-phaCBA exhibited 863 significantly different genes compared to the control strain B. subtilis 168-pMA5. Among these, 294 genes were significantly up-regulated, while 569 genes were significantly down-regulated (Fig. 3d). In the Biological Process category, both up-regulated and down-regulated genes in B. subtilis 168-phaCBA were mainly associated with cellular processes, localization, metabolic processes, and signal-organism processes. In the Cellular Component category, these genes were predominantly related to cells, cell parts, membranes, and membrane parts. In the Molecular Function category, both up-regulated and down-regulated genes were centered on binding and catalytic activity. KEGG enrichment analysis identified the 20 pathways with the highest enrichment degree (Fig. 3b). The significantly altered DEGs in B. subtilis 168-phaCBA were enriched in pathways such as flagellar assembly, phosphotransferase system (PTS), fructose and mannose metabolism, bacterial chemotaxis, phenylalanine, tyrosine, and tryptophan biosynthesis, biosynthesis of various antibiotics, photosynthesis, nitrogen metabolism, ABC transporters, biofilm formation, and alanine, aspartate, and glutamate metabolism (Additional file 1: Table S2).
Metabolomics analysis of intracellular metabolite changes in B. subtilis 168-pMA5 and B. subtilis 168-phaCBA under acetoin stress
To unravel the physiological changes associated with the heterologous expression of phaCBA in B. subtilis 168 under acetoin stress, we employed MetaboAnalyst to scrutinize the KEGG pathway enrichment of differential metabolites in two conditions: B. subtilis 168-pMA5 under 0 g/L and 60 g/L acetoin, and the comparison between B. subtilis 168-pMA5 and B. subtilis 168-phaCBA under 60 g/L acetoin stress (Fig. 4a, b). The scatter plot in Fig. 4a illustrates the impact factor, reflecting significant metabolic pathway changes in B. subtilis 168-pMA5 under severe acetoin stress. These changes encompassed pathways such as the biosynthesis of plant secondary metabolites, central carbon metabolism in cancer, biosynthesis of amino acids, biosynthesis of cofactors, and biosynthesis of alkaloids derived from histidine and purine, among others. Similarly, the metabolic pathway changes between B. subtilis 168-pMA5 and B. subtilis 168-phaCBA under severe acetoin stress were comparable, as shown in Fig. 4b. Clustered heat maps in Fig. 4c depict alterations in intracellular metabolites in B. subtilis 168-pMA5 (0 g/L and 60 g/L acetoin) and B. subtilis 168-phaCBA (60 g/L acetoin). These heat maps highlight significant changes in intracellular amino acid fractions, membrane fatty acid groups, and energy metabolism in both B. subtilis 168-pMA5 and B. subtilis 168-phaCBA under severe acetoin stress. Notably, when compared with B. subtilis 168-pMA5, B. subtilis 168-phaCBA exhibited a significant increase in the content of l-tryptophan, l-tyrosine, l-leucine, l-threonine, l-methionine, l-glutamic acid, l-proline, d-phenylalanine, and l-arginine in the intracellular amino acid components. The content of saturated fatty acids, such as myristic acid, hexadecanedioate, and tridecanoic acid, decreased significantly, while the content of unsaturated fatty acids, including oleic acid, palmitoleic acid, and undecanoic acid, increased. Additionally, the cofactors UDP, NDP, FMN, ADP, and AMP also significantly increased. These findings suggest that B. subtilis 168-phaCBA exhibited enhanced viability under severe acetoin stress.
Metabolic pathway analysis of significantly differential metabolites and cluster analysis of significantly differential metabolite contents after acetoin stress treatment. a Metabolic pathway analysis of significantly differential metabolites in the 60 g/L acetoin treatment group (B. subtilis 168-pMA5) and 0 g/L acetoin control group (B. subtilis 168-pMA5). b Metabolic pathway analysis of significantly differential metabolites in acetoin stress between B. subtilis 168-pMA5 and B. subtilis 168-phaCBA. c Heatmap obtained by hierarchical clustering analysis of differential metabolites between B. subtilis 168-pMA5 (0 g/L, 60 g/L acetoin stress) and B. subtilis 168-phaCBA (60 g/L acetoin stress)
Effect of acetoin on fatty acid composition of cell membranes in B. subtilis 168-pMA5 and B. subtilis 168-phaCBA
According to previous reports in the literature, microorganisms facing various unfavorable environmental stresses alter the fatty acid composition of cell membranes, thereby enhancing coping capacity. To further elucidate the tolerance mechanism of B. subtilis 168-phaCBA to high acetoin concentrations, we investigated the effects of acetoin (0 and 60 g/L) on the fatty acid composition of the cell membranes in both B. subtilis 168-pMA5 and B. subtilis 168-phaCBA. In the absence of acetoin stress, the contents of membrane fatty acid fractions in B. subtilis 168-pMA5 and B. subtilis 168-phaCBA were significantly different. Under acetoin stress, the proportions of saturated fatty acids C16:0 in the cell membrane fatty acids of B. subtilis 168-pMA5 underwent substantial changes, reducing from 80.06 to 11.62%. Meanwhile, the proportions of saturated fatty acids i-C15:0, C15:0, and C17:0 increased from 0.8%, 3.88%, and 1.04% to 10.67%, 35.57%, and 11.1%, respectively. The unsaturated fatty acid C22:1, did not change significantly. Simultaneously, the cell membrane fatty acid fractions in B. subtilis 168-phaCBA also exhibited alterations, with the most significant change observed in the unsaturated fatty acid C22:1, increasing from 12.72 to 26.06%.
The results, as presented in Table 1, revealed that in B. subtilis 168-phaCBA, the total unsaturated fatty acids (TUFA) and the ratio of unsaturated fatty acids to saturated fatty acids (U/S) gradually increased, while the total saturated fatty acids (TSFA) decreased with increasing acetoin concentration. In contrast, B. subtilis 168-pMA5 did not exhibit an increase in the content of unsaturated fatty acids or the U/S ratio with acetoin stress. Notably, the TUFA content in B. subtilis 168-phaCBA was 29.6% and 137.6% higher than that in B. subtilis 168-pMA5 in the presence of 0 and 60 g/L acetoin, respectively. Moreover, the U/S ratios in B. subtilis 168-phaCBA were 33.3% and 191.6% higher than those in B. subtilis 168-pMA5 at 0 and 60 g/L acetoin concentration, respectively.
Metabolic and transcriptional differences analysis
To delve into the distinctions between B. subtilis 168-pMA5 and B. subtilis 168-phaCBA, we pinpointed 23 differentially expressed genes (DEGs) concurrently regulated in both comparison groups out of the 43 differentially expressed metabolites (DEMs). The regulated levels of these DEGs are depicted in Fig. 5b. Notably, genes involved in dethiobiotin and biotin synthesis, such as bioY, bioB, bioD, bioW, bioI, bioF, and bioK, exhibited up-regulation in the B. subtilis 168-phaCBA group (Additional file 3: Table S4). Metabolites, including N-acetylornithine, guanine, d-phenylalanine, 5′-methythioadenosine, N-acetyl-d-glucosamine, l-proline, l-leucine, and l-histidine, demonstrated significant correlations with these biosynthetic genes. Conversely, a marked negative correlation was observed with metabolites like N-acetylmethionine and hexadecanedioate. Among the DEGs, the expression of tcyC (cystine ABC transporter) and mtnU (ketoglutaramate omega-amidase) increased by 1.3-fold and 1.75-fold, respectively. These genes are associated with the synthesis of l-cysteine and (6z)-Octadecenoic acid, contributing to Bacillus resistance. In contrast, there was a significant decrease in the expression of genes like nitric-oxide synthase (nos), methyl-tetrahydrofolate methyltransferase (yxjH), and glutamine synthetase (glnA). These genes were positively correlated with the synthesis of the saturated fatty acid hexadecanedioate and negatively correlated with the synthesis of l-proline, unsaturated fatty acid (6z)-Octadecenoic acid, and l-cysteine.
a Effect of exogenous addition of different amino acids (l-histidine, l-alanine, l-proline, l-valine, l-arginine, l-cysteine, l-leucine, l-glutamic acid, l-isoleucine, l-methionine) on the growth of B. subtilis 168 under acetoin stress. b Differential expression results for metabolites and related transcripts. Horizontal coordinates are associated metabolites, vertical coordinates are transcript names, red indicates positive correlation, and blue indicates negative correlation
To comprehend the impact of intracellular amino acids on B. subtilis under acetoin stress, we supplemented the acetoin stress medium with various amino acids. After 8 h of fermentation incubation, it was found that exogenous supplementation with l-alanine (OD600 = 4.41), l-arginine (OD600 = 4.21) and l-cysteine (OD600 = 4.37), significantly increased the biomass of B. subtilis 168 compared to other amino acids and the blank control CK-1 (OD600 = 3.90). Continued fermentation up to 10 h revealed that the biomass of B. subtilis was higher than the blank control CK-1 with exogenous supplementation of l-alanine, l-proline, l-arginine, l-cysteine, l-glutamic acid, and l-isoleucine. The results demonstrated that exogenous supplementation of l-alanine, l-proline, l-cysteine, l-arginine, l-glutamic acid, and l-isoleucine partially alleviated acetoin stress (Fig. 5a). Considering that the earlier release of acetoin stress inhibition during the growth of B. subtilis is more favorable for product synthesis, supplementation with l-alanine, l-arginine, and l-cysteine was the most effective.
Shake flask batch fermentation culture of recombinant strains
The aerobic fermentation results for the recombinant B. subtilis strains indicated that strain BS03 (B. subtilis 168ΔbdhAΔacoAΔLdhA, integrated expression of alsSD-yodC under PHapII at the amy locus) increased the acetoin concentration by 6.48% to approximately 34.3 g/L compared to BS03-phaCBA. Additionally, the concentration of the by-product 2,3-Butanediol decreased by 16.52% (Fig. 6a). To understand these differences, changes in intracellular cofactors (NAD+/NADH) in the recombinant strains were analyzed. The results showed that the concentrations of intracellular cofactors NAD+ and NADH were higher in BS03-phaCBA compared to BS03 throughout the entire fermentation cycle (Fig. 6b, c). This suggests that the expression of genes related to the metabolic pathway of acetoin was stronger in BS03-phaCBA. One possible reason for this observation is that the knockdown of lactate dehydrogenase (ldhA) and acetoin reductase (bdhA) led to NADH accumulation. Both of these enzymes are NADH-dependent, and acetoacetyl-CoA derived from Halomonas bluephagenesis TD01 has a significant preference for NADH, reducing the toxic effects of NADH accumulation on the cells [21, 22].
a Fermentation experiments with recombinant strains BS03 and BS03-phaCBA of acetoin **significance code: P < 0.01. b, c Effects of heterologous expression phaCBA on intracellular NADH and NAD+ concentrations. d Overview of the production of acetoin from glucose by B. subtilis engineered bacteria. Blue words represent genes expressed on plasmid pMA5. Green words represent genes integratively expressed. Purple words represent knockout genes
Fed-batch fermentation
To assess the acetoin production capacity of recombinant strain BS03-phaCBA, the fed-batch fermentation was conducted in a 7.5 L fermenter. As shown in Fig. 7, at 25 h of fermentation, the residual sugar content and acetoin concentration were 15.4 g/L and 50.66 g/L, respectively. During this period, the dissolved oxygen increased from 11.4 to 15.6% compared to 24 h. Generally, a high oxygen supply favors acetoin formation and decreases 2,3-butanediol final titer [23]. Therefore, after disposable batch feeding, the speed is increased to 800 rpm. The highest yield of 70.14 g/L was achieved at 34 h of fermentation.
Discussion
In our tolerance experiments, we observed significant growth inhibition of B. subtilis 168 when the acetoin concentration reached 40 g/L or higher. To gain insights into the genetic and metabolic changes occurring under acetoin stress (60 g/L), transcriptomics and metabolomics analyses were conducted, revealing alterations in 24 metabolic pathways. Some of these pathways play crucial roles in resisting acetoin stress and maintaining normal cellular functions.
Oxidative phosphorylation: Genes related to oxidative phosphorylation showed up-regulation, suggesting that B. subtilis requires enhanced oxidative phosphorylation and increased respiration to maintain intra- and extracellular homeostasis under acetoin stress (Additional file 2: Table S3) [24]. Photosynthesis and fatty acid degradation: Genes involved in photosynthesis and fatty acid degradation were significantly up-regulated. The induction of these operons may be related to glucose starvation, affecting glucose transport and utilization, leading to sugar starvation, spore formation, and increased expression of genes in the fatty acid degradation pathway [25]. Two-component system: Genes such as dnaA, kinC, and kapB, associated with the two-component system, showed increased expression. These genes are related to DNA replication and spore formation. The up-regulation of these genes reflects the strain’s stress response to the virulence of B. subtilis 168 under acetoin stress. Efflux Pumps and CAMP Repulsion: Genes associated with bacitracin efflux (bceA, vraD) and CAMP repulsion (e.g., dltA, dltB, dltC, dltD, dltE) exhibited a significant reduction in expression (Additional file 2: Table S3). Efflux pumps play a crucial role in transporting toxic substances out of the cell [26]. A high concentration of acetoin influx, coupled with reduced efflux pump activity, can lead to damage to macromolecules like proteins and DNA. This can inhibit cell growth or even cause cell lysis and death [27]. C5-Branched-dibasic acid metabolism: Genes associated with C5-branched dibasic acid metabolism, linked to acetoin synthesis, exhibited significant reduction, explaining the inability to sustain acetoin production at later fermentation stages. Valine, leucine, and isoleucine biosynthesis: Overall down-regulation of genes associated with valine, leucine, and isoleucine biosynthesis was observed. Branched-chain amino acids have been shown to play a role in maintaining cell membrane fluidity and integrity, possibly contributing to B. subtilis tolerance [28]. Overall, these findings provide insights into various molecular mechanisms and pathways involved B. subtilis 168’s response to acetoin stress. Understanding these mechanisms is crucial for developing strategies to improve acetoin tolerance and production in this strain.
In this study, the heterologous expression of phaCBA in B. subtilis 168 was found to significantly improve the tolerance to acetoin. Transcriptomic analyses identified key changes in gene expression contributing to this enhanced tolerance. Down-regulation of flagellar assembly and bacterial chemotaxis: A notable finding was the significant decrease in the expression of genes related to flagellar assembly and bacterial chemotaxis. Under stressful conditions, inhibiting flagellar assembly might be an energy-saving strategy, allowing the bacterium to allocate resources more efficiently to other survival mechanisms [29, 30]. Similarly, reduced chemotaxis activity helps the cell adapt to its environment and allocate resources more wisely under stressful conditions. This aligns with previous studies that have shown down-regulation of flagellar biosynthesis in response to stress, suggesting a conserved strategy among bacteria [31, 32]. Up-regulation of transporter proteins: Several transporter protein genes, such as molybdate (modA, modB), osmoprotectant (opuBC, opuBB, opuBA), biotin (bioY), and zinc/manganese/iron (II), were significantly up-regulated in the ABC transporters system (Additional file 3: Table S4). Biotin, in particular, is essential for some enzymes involved in fatty acid synthesis [33]. Its deficiency can lead to a reduced proportion of unsaturated fatty acids in cell membranes, which could impact the cell’s defenses against stressors [34, 35]. Studies have shown that the addition of biotin uptake genes can improve the growth rate and solvent yield in other bacterial strains, emphasizing the beneficial role of up-regulating biotin transporter protein genes for enhancing tolerance to acetoin in B. subtilis [36]. Understanding these changes in gene expression provides valuable insights into the mechanisms underlying acetoin tolerance improvement in B. subtilis 168 with heterologous expression of phaCBA. This knowledge is essential for the development of strategies aimed at further optimizing acetoin production and tolerance in this strain. Up-regulating biotin transporter protein genes is beneficial for enhancing B. subtilis’ tolerance to acetoin. Up-regulation of cystine and d-methionine transporter proteins: The observed up-regulation of transporter protein genes for cystine (tcyB, tcyC) and d-methionine (metQ) in the ABC transporters system suggests an important role in acetoin tolerance. Cysteine is known to play a role in essential cellular functions such as the assembly of iron-sulfur clusters, which can mitigate oxidative damage [37]. The positive effects of l-cysteine on reductases and peroxidases involved in scavenging reactive oxygen species (ROS) align with the notion that acetoin stress can increase intracellular ROS production, inducing damage and apoptosis [38, 39]. By enhancing the transport of cystine and d-methionine, the strain’s tolerance to acetoin was increased, potentially due to improved antioxidant defenses. Up-regulation of energy metabolism-related metabolites: Intracellular metabolomic analysis showed that the contents of ADP, ATP, FMN, and 3′-AMP in B. subtilis 168-phaCBA were significantly up-regulated compared to B. subtilis 168-pMA5 under acetoin stress (Fig. 4c). This suggests an enhanced energy metabolism in response to acetoin stress. Increased energy production is necessary to support the enhanced tolerance capacity of the strain. Fatty acid composition alterations and improved acetoin tolerance: The influence of acetoin stress on the fatty acid composition of microbial membranes is highlighted in the study. Under high concentrations of acetoin stress, the proportion of unsaturated fatty acids in B. subtilis increased, and the ratio of unsaturated to saturated fatty acids (U/S) was significantly higher in B. subtilis 168-phaCBA compared to B. subtilis 168-pMA5. This alteration, attributed to the expression of phaCBA, enhances the strain’s tolerance to acetoin by maintaining the fluidity and integrity of the cell membrane [40,41,42]. The increased content of unsaturated fatty acids aligns with findings in other studies where modification of membrane fatty acid fractions contributed to improved tolerance to various stresses [43]. These study findings indicate a connection between altering membrane fatty acid composition and structure and the enhancement of organic solvent tolerance. Enhanced tolerance through altered amino acids: The significant changes in intracellular amino acids, such as l-proline, l-glutamic acid, l-histidine, l-methionine, l-arginine, l-tyrosine, and l-leucine further contribute to the enhanced tolerance to acetoin in B. subtilis 168-phaCBA. The addition of exogenous amino acids, including l-alanine, l-proline, l-glutamic acid, l-arginine, and l-isoleucine, alleviated the stress imposed by acetoin. These amino acids have been shown to enhance stress tolerance in various organisms. For instance, arginine addition can protect yeast from ethanol damage by maintaining the integrity of the cell wall and cytoplasmic membranes [44]. Proline and glutamic acid accumulation can increase the tolerance of B. subtilis to butanol and maintain normal biological function under stress conditions [45]. Methionine can enhance the antioxidant stress capacity of the strain by converting it to cysteine [46, 47]. The review article by Saini et al. extensively discussed the amino acids linked to ethanol stress tolerance in yeast cells [48]. Among these, isoleucine, methionine, phenylalanine, proline, and tryptophan were found effective in mitigating the stress induced by ethanol. In our study, both isoleucine and proline demonstrated equal efficacy in alleviating the stress caused by acetoin. Combinatorial analysis of metabolites and gene expressions: The combinatorial analysis of significantly changed metabolites confirmed the relevance of certain metabolites and related gene expressions in enhancing B. subtilis’ ability to overcome acetoin stress. These include metabolites such as l-proline, l-cysteine, (6z)-Octadecenoic acid, and biotin, which were up-regulated. Conversely, the content of the saturated fatty acid hexadecanedioate and its expression were significantly down-regulated. The changes in metabolites and gene expressions collectively contribute to the enhanced tolerance of B. subtilis to acetoin stress. In conclusion, the combination of increased unsaturated fatty acids, altered amino acid profiles, and metabolite changes, in response to the heterologous expression of phaCBA, enhances the tolerance of B. subtilis 168 to acetoin stress. This study provides valuable insights into the mechanisms underlying stress tolerance in microbial strains and may have practical implications for industrial applications. Understanding these mechanisms could contribute to the development of more resilient microbial strains for various biotechnological processes.
Practical implications for industrial applications: The findings of this study hold practical implications for industrial applications, where enhancing strain tolerance to toxic products can significantly increase the fermentation yields of target products. Previous studies, such as those on E. coli overexpressing specific genes to overcome butanol tolerance, have demonstrated the effectiveness of enhancing strain tolerance for improved fermentation yields. The introduction of chaperones and chaperone-like proteins has also shown promise in enhancing tolerance to solvents and increasing the synthesis of specific products [49, 50]. In the case of B. subtilis 168-phaCBA, the study suggests that the heterologous expression of phaCBA can be a valuable strategy for improving acetoin tolerance and production. The construction of the engineered acetoin-producing bacterial strain BS03-phaCBA achieved an impressive acetoin yield of 70.14 g/L in fed-batch fermentation, highlighting its potential for biotechnological and industrial applications. Nevertheless, as evident from recent studies, there are critical avenues for refinement to enhance acetoin production in this strain. As identified in the literature, the synthesis of intracellular polyhydroxybutyrate (PHB) enhances acetoin tolerance but concurrently imposes a metabolic burden on the acetoin synthesis pathway. Consequently, a precise metabolic balance is imperative to maximize acetoin production while mitigating the burdens associated with PHB synthesis. Achieving this balance involves sophisticated genetic engineering to fine-tune metabolic pathways and enzyme activities. In conclusion, while our study has made significant strides in acetoin production, it also underscores the untapped potential for optimization. Further research and engineering efforts are warranted to unravel the full potential of acetoin production in B. subtilis 168-phaCBA, offering opportunities for advancements in biotechnological applications.
Conclusion
In summary, the introduction of phaCBA expression into B. subtilis 168 led to significant alterations in the cell membrane’s fatty acid composition and influenced the intracellular uptake and synthesis of amino acids and biotin. Consequently, this modification enhanced the strain’s tolerance to acetoin. Our findings underscore the importance of synthesizing long-chain unsaturated fatty acids, along with supplementing specific amino acids such as l-alanine, l-proline, l-glutamic acid, l-arginine, and l-isoleucine, in alleviating acetoin-induced stress. The metabolomic and transcriptomic analyses conducted in this study have revealed a diverse array of metabolites that contribute to acetoin tolerance. These insights pave the way for future investigations and the rational design of more resilient hosts for acetoin production. Overall, this study provides valuable contributions to the understanding of microbial responses to acetoin stress and sets the stage for further advancements in strain engineering for enhanced acetoin production. The identified strategies and mechanisms may have broader implications for improving stress tolerance in microbial hosts for various industrial applications.
Material and methods
Strains and plasmids
Escherichia coli DH5α was employed for plasmid amplification. B. subtilis 168 and BS03 (B. subtilis 168ΔbdhAΔacoAΔLdhA, integrated expression of alsSD-yodC under PHapII at the amy locus) were maintained in our laboratory. BS03-phaCBA was constructed in this study. The gene sequences of PHB synthesis pathway-related genes phaC (derived from Cupriavidus necator, GeneID: 57643537), phaB (derived from Halomonas bluephagenesis TD01, LOCUS: WP_009724067) and phaA (derived from Cupriavidus necator, GeneID: 57643536) was obtained from the National Center of Biotechnology Information (NCBI) database, and codon-optimized and synthesized de novo by GenScript Biotech Corporation. These genes were expressed with the plasmid pMA5 in both B. subtilis 168 and BS03-phaCBA. The recombinant plasmid was introduced into B. subtilis using the Spizizen method [51].
Medium and culture conditions
The recombinant strain was streaked on LB solid medium (10 g/L tryptone, 5 g/L yeast powder, 10 g/L NaCl, 20 g/L agar powder) and then incubated overnight in a 37 °C incubator. A loop of colonies from the LB solid medium was collected and inoculated into 50 mL/250 mL LB liquid medium, followed by incubation at 37 °C, with shaking at 160 rpm for 12 h to generate seed cultures. The seeds culture was then transferred at 5% (v/v) into 250 mL shake flasks containing 50 mL of fermentation medium (100 g/L glucose, 5 g/L yeast powder, 15 g/L corn steep powder, 2 g/L Urea, pH6.8). Microaerobic and aerobic fermentation were performed at 37 ℃ with shaking at 160 rpm and 220 rpm, respectively. The acetoin tolerance test involved the following steps. Recombinant strains, cultured under microaerobic conditions for 36 h, were centrifuged (6000 rpm, 10 min), washed three times with PBS (pH7.4), and inoculated into LB liquid medium containing various acetoin concentrations 0, 40, 60, and 80 g/L). Initial OD600 was maintained between 1.8 and 2.1, and the cultures were then incubated at 37 ℃ and 220 rpm for 10 h. To assess the impact of acetoin addition on bacterial growth during fermentation, recombinant strains were cultured in acetoin fermentation medium for 4 h at 37 ℃ and 160 rpm. Subsequently, acetoin was added to achieve final concentrations of 0 g/L, 20 g/L, 40 g/L, 60 g/L, and 80 g/L, respectively. Biomass measurements were taken at various time points up to the conclusion of the 84-h fermentation. All analytical reagents were purchased from Nanjing Wanqing Chemical Glass Wear & Instrument Co. Ltd.
Transcriptional analysis
Cells of both B. subtilis 168-pMA5 and B. subtilis 168-phaCBA were collected after 36 h of cultivation under conditions with and without 60 g/L acetoin. Collected cells were washed three times with sterile water, frozen in liquid nitrogen, and stored at − 80 ℃ for subsequent RNA-seq analyses. Total RNA was extracted using the TRIzol-based method (Life Technologies, CA, USA). RNA degradation and potential contamination were assessed by 1% agarose gels. RNA purity was determined by OD260/OD280, and OD260/OD230 ratios using the NanoPhotometer® spectrophotometer (IMPLEN, CA, USA). To enrich for mRNA and deplete rRNA, 1 µg of total RNA was processed using the Illumina MRZB12424 Ribo-Zero rRNA Removal Kit (Bacteria) (Illumina, San Diego, CA, USA). The cDNA, DNA, and small RNA libraries were subsequently subjected to sequencing on the Illumina platform by Genedenovo Biotechnology Co., Ltd (Guangzhou, China). To analyze gene expression levels, the data were normalized using the fragments Per Kilobase of Transcript per Million (FPKM) mapped reads method. Normalization accounts for differences in gene lengths and sequencing data quantity. The identification of differentially expressed genes (DEGs) was carried out using the edgeR package (http://www.r-project.org/), with fold changes ≥ 2 and a false discovery rate-adjusted P value (q value) < 0.05 as the criteria for significance DEGs were subsequently subjected to an enrichment analysis of Gene Ontology (GO) functions and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways. A threshold of q value < 0.05 was applied to identify significantly enriched functional categories and pathways.
LC–MS-based metabolomics analysis
Cells were collected after cultivating 36 h of cultivation under different acetoin concentrations (0 g/L, 60 g/L). For each sample, broth equivalent to 200 mg of cells was collected by centrifugation at 6000 rpm for 10 min at 4 °C. Collected pellets were immediately frozen using liquid nitrogen and stored at − 80 °C until further analysis. Metabolite extraction, liquid chromatography-mass spectrometry (LC–MS) analysis, annotation and data preprocessing were carried out by Shanghai Personal Biotechnology Co., Ltd (Shanghai, China). The LC analysis was performed on a Vanquish UHPLC System (Thermo Fisher Scientific, USA). Chromatography was conducted using an ACQUITY UPLC HSS T3 column (2.1 × 100 mm, 1.8 µm) (Waters, Milford, MA, USA) maintained at 40 ℃. The flow rate and injection volume were set at 0.3 mL/min and 2 μL, respectively. For LC-ESI (+)-MS analysis, the mobile phases consisted of (B2) 0.1% formic acid in acetonitrile (v/v) and (A2) 0.1% formic acid in water (v/v) [52]. Mass spectrometric detection was performed using an Orbitrap Exploris 120 mass spectrometer (Thermo Fisher Scientific, USA) with an ESI ion source. Simultaneous MS1 and MS/MS (Full MS-ddMS2 mode, data-dependent MS/MS) acquisition was employed [53]. Key parameters included sheath gas pressure (40 arb), aux gas flow (10 arb), spray voltage (3.50 kV and − 2.50 kV for ESI (+) and ESI (−), respectively), capillary temperature (325 °C), MS1 range (m/z 100–1000), MS1 resolving power (60,000 FWHM), number of data-dependent scans per cycle, MS/MS resolving power (15,000 FWHM), normalized collision energy (30%), and dynamic exclusion time (automatic). Differential metabolites were subjected to pathway analysis using MetaboAnalyst, combining results from powerful pathway enrichment analysis with pathway topology analysis [54]. The identified metabolites in metabolomics were then mapped to KEGG pathways for biological interpretation of higher-level systemic functions. Metabolites and corresponding pathways were visualized using the KEGG Mapper tool.
Membrane fatty acid analysis
Lipids from B. subtilis 168-pMA5 and B. subtilis 168-phaCBA cells were extracted and methylated following the MIDI method [55]. After freeze–drying, the tested bacterial strain was added to a screw-capped test tube. Then, 5 mL of 0.4 M KOH-CHO3H was added and saponified at 60 °C for 1 h. After cooling, 4 mL of HCl-CH3OH (V/V = 1:9) was added and the mixture was bathed at 60 °C for 20 min. Upon cooling, 3% NaCl (w/v) was added, followed by fatty acid extraction using n-hexane. Gas Chromatography–Mass Spectrometry (GC–MS) analyses were conducted using a 7890A GC and 5975C MSD (Agilent Technologies, Waldbronn, Germany) and samples were analyzed using a DB-5MS capillary column. Helium was used as the carrier gas at a flow rate of 1 mL/min, the injection volume was set to 1 μL, and the detector temperature was set to 260 °C. The injector temperature and column oven temperature were both set to 280 °C. Mass spectrometry conditions for GC–MS analysis included ionization in EI mode with an electron energy of 70 eV, ion source temperature of 230 °C, and quadrupole temperature of 150 °C [56].
Growth analysis of B. subtilis 168 with exogenous amino acid addition
Bacillus subtilis 168 cells were initially cultured at 37 °C for 12 h. Subsequently, they were inoculated into the LB liquid medium containing 40 g/L acetoin. The effects of various amino acids on cell growth were studied by adding these 0.5% (w/v) amino acids (l-histidine, l-alanine, l-proline, l-valine, l-arginine, l-cysteine, l-leucine, l-glutamic acid, l-isoleucine, l-methionine) to LB liquid medium containing 40 g/L acetoin. The control groups consisted of LB liquid medium with 40 g/L acetoin without amino acid supplementation (control group 1, CK-1) and LB liquid medium without the addition of acetoin and amino acids (control group 2, CK-2).
Fed-batch fermentation in 7.5-L fermenter
Fed-batch fermentations were conducted in a 7.5-L fermenter with a working volume of 3 L. The operational parameters included a pH of 6.5, a temperature of 37 °C, and a rotation speed of 600 rpm. The inoculum volume was set at 5% (v/v), and the aeration ratio was 2 vvm (air volume per culture volume per minute). The initial glucose concentration in the medium was 150 g/L. When the glucose level dropped below 20 g/L, a 200 mL feed medium, containing 500 g/L glucose and 60 g of yeast powder dissolved in 100 mL sterile water, was introduced into the fermenter. The rotation speed was increased from 600 to 800 rpm.
Analysis methods
Acetoin, acetic acid and 2,3-butanediol were quantified using liquid chromatography with an Agilent 1290 Infinity instrument (Agilent Technologies, Waldbronn, Germany). An Aminex HPX-87H column was used, with an injection volume of 20 μL. The mobile phase consisted of 5 mM H2SO4, and the flow rate was set at 0.6 mL/min. The column temperature was maintained at 65 °C, and a refractive index detector was employed [57]. Biomass was determined using a spectrophotometer (756S, China) at 600 nm. The cell dry weight was calculated using the empirical formula 1 OD600 = 0.352 DCW (g/L) [58]. Intracellular concentrations of NADH and NAD+ were determined using the Beyotime Biotechnology (Shanghai, China) NAD+/NADH Assay Kit (WST-8 method) following the manufacturer’s instructions [59].
Availability of data and materials
The obtained data will be available from the corresponding author upon reasonable request.
Abbreviations
- PHB:
-
Polyhydroxyalkanoates
- LC–MS:
-
Liquid chromatography mass spectrometry
- TUFA:
-
Total unsaturated fatty acids
- TSFA:
-
Total saturated fatty acids
- TPUFA:
-
Total polyunsaturated fatty acid
- KEGG:
-
Kyoto Encyclopedia of Genes and Genomes
- DEGs:
-
Differentially expressed genes
- DEMs:
-
Differentially expressed metabolites
- B. subtilis 168:
-
Bacillus subtilis 168
- PBS:
-
Phosphatic buffer solution
- OD:
-
Optical density
References
Roncal T, Caballero S, Díaz de Guereñu MDM, Rincón I, Prieto-Fernández S, Ochoa-Gómez JR. Efficient production of acetoin by fermentation using the newly isolated mutant strain Lactococcus lactis subsp. lactis CML B4. Process Biochem. 2017;58:35–41.
Meng W, Ma C, Xu P, Gao C. Biotechnological production of chiral acetoin. Trends Biotechnol. 2022;40(8):958–73.
Cui Z, Wang Z, Zheng M, Chen T. Advances in biological production of acetoin: a comprehensive overview. Crit Rev Biotechnol. 2022;42:1135–56.
Maina S, Prabhu AA, Vivek N, Vlysidis A, Koutinas A, Kumar V. Prospects on bio-based 2,3-butanediol and acetoin production: recent progress and advances. Biotechnol Adv. 2022;54: 107783.
Jia X, Peng X, Liu Y, Han Y. Conversion of cellulose and hemicellulose of biomass simultaneously to acetoin by thermophilic simultaneous saccharification and fermentation. Biotechnol Biofuels. 2017;10:232.
Xiao Z, Lu JR. Strategies for enhancing fermentative production of acetoin: a review. Biotechnol Adv. 2014;32:492–503.
Petrov K, Petrova P. Current advances in microbial production of acetoin and 2,3-butanediol by Bacillus spp. Fermentation. 2021;7:307.
Xu Q, Xie L, Li Y, Lin H, Sun S, Guan X, Hu K, Shen Y, Zhang L. Metabolic engineering of Escherichia coli for efficient production of (3R)-acetoin. J Chem Technol Biotechnol. 2015;90:93–100.
Gu Y, Xu X, Wu Y, Niu T, Liu Y, Li J, Du G, Liu L. Advances and prospects of Bacillus subtilis cellular factories: from rational design to industrial applications. Metab Eng. 2018;50:109–21.
de BoerSietske A, Diderichsen B. On the safety of Bacillus subtilis and B. amyloliquefaciens: a review. Appl Microbiol Biotechnol. 1991;36:1–4.
Park SA, Bhatia SK, Park HA, Kim SY, Sudheer PDVN, Yang Y-H, Choi K-Y. Bacillus subtilis as a robust host for biochemical production utilizing biomass. Crit Rev Biotechnol. 2021;41:827–48.
Zhang X-Z, Sathitsuksanoh N, Zhu Z, Percival Zhang YH. One-step production of lactate from cellulose as the sole carbon source without any other organic nutrient by recombinant cellulolytic Bacillus subtilis. Metab Eng. 2011;13:364–72.
Altenbuchner J. Editing of the Bacillus subtilis genome by the CRISPR-Cas9 system. Appl Environ Microbiol. 2016;82:5421–7.
Yang S-J, Dunman PM, Projan SJ, Bayles KW. Characterization of the Staphylococcus aureus CidR regulon: elucidation of a novel role for acetoin metabolism in cell death and lysis. Mol Microbiol. 2006;60:458–68.
Wang S, Hou Y, Chen X, Liu L. Kick-starting evolution efficiency with an autonomous evolution mutation system. Metab Eng. 2019;54:127–36.
Jung HR, Lee JH, Moon YM, Choi TR, Yang SY, Song HS, Park JY, Park YL, Bhatia SK, Gurav R, Ko BJ, Yang Y-H. Increased tolerance to furfural by introduction of polyhydroxybutyrate synthetic genes to Escherichia coli. J Microbiol Biotechnol. 2019;29:776–84.
Jung H-R, Choi T-R, Han YH, Park Y-L, Park JY, Song H-S, Yang S-Y, Bhatia SK, Gurav R, Park H, et al. Production of blue-colored polyhydroxybutyrate (PHB) by one-pot production and coextraction of indigo and PHB from recombinant Escherichia coli. Dyes Pigm. 2020;173: 107889.
Song H-S, Jeon J-M, Bhatia SK, Choi T-R, Lee SM, Park SL, Lee HS, Yoon J-J, Ahn J, Lee H, et al. Enhanced isobutanol production by co-production of polyhydroxybutyrate and cofactor engineering. J Biotechnol. 2020;320:66–73.
Chen GG-Q. Plastics from bacteria: natural functions and applications. Berlin: Springer Science & Business Media; 2009.
Wang Q, Yu H, Xia Y, Kang Z, Qi Q. Complete PHB mobilization in Escherichia coli enhances the stress tolerance: a potential biotechnological application. Microb Cell Fact. 2009;8:47.
Zhang X, Zhang R, Bao T, Rao Z, Yang T, Xu M, Xu Z, Li H, Yang S. The rebalanced pathway significantly enhances acetoin production by disruption of acetoin reductase gene and moderate-expression of a new water-forming NADH oxidase in Bacillus subtilis. Metab Eng. 2014;23:34–41.
Ling C, Qiao G-Q, Shuai B-W, Olavarria K, Yin J, Xiang R-J, Song K-N, Shen Y-H, Guo Y, Chen G-Q. Engineering NADH/NAD+ ratio in Halomonas bluephagenesis for enhanced production of polyhydroxyalkanoates (PHA). Metab Eng. 2018;49:275–86.
Li L, Wei X, Yu W, Wen Z, Chen S. Enhancement of acetoin production from Bacillus licheniformis by 2,3-butanediol conversion strategy: metabolic engineering and fermentation control. Process Biochem. 2017;57:35–42.
Wang N, Zhang P, Zhou X, Zheng J, Ma Y, Liu C, Wu T, Li H, Wang X, Wang H, et al. Isolation, identification, and characterization of an acid-tolerant Pichia kudriavzevii and exploration of its acetic acid tolerance mechanism. Fermentation. 2023;9(6):540.
Tojo S, Satomura T, Matsuoka H, Hirooka K, Fujita Y. Catabolite repression of the Bacillus subtilis FadR regulon, which is involved in fatty acid catabolism. J Bacteriol. 2011;193:2388–95.
Almeida RS, Freitas PR, Araújo ACJ, Alencar Menezes IR, Santos EL, Tintino SR, Moura TF, Filho JR, Ferreira VA, Silva ACA, et al. GC–MS profile and enhancement of antibiotic activity by the essential oil of Ocotea odorífera and safrole: inhibition of Staphylococcus aureus efflux pumps. Antibiotics. 2020;9(5):247.
Cesselin B, Henry C, Gruss A, Gloux K, Gaudu P. Mechanisms of acetoin toxicity and adaptive responses in an acetoin-producing species, Lactococcus lactis. Appl Environ Microbiol. 2021;87:e01079-01021.
Wang Y-F, Tian J, Ji Z-H, Song M-Y, Li H. Intracellular metabolic changes of Clostridium acetobutylicum and promotion to butanol tolerance during biobutanol fermentation. Int J Biochem Cell Biol. 2016;78:297–306.
He S, Cui Y, Dong R, Chang J, Cai H, Liu H, Shi X. Global transcriptomic analysis of ethanol tolerance response in Salmonella Enteritidis. Curr Res Food Sci. 2022;5:798–806.
Zhuang X-Y, Lo C-J. Construction and loss of bacterial flagellar filaments. Biomolecules. 2020;10:1528.
Tang Y, Wang Y, Yang Q, Zhang Y, Wu Y, Yang Y, Mei M, He M, Wang X, Yang S. Molecular mechanism of enhanced ethanol tolerance associated with hfq overexpression in Zymomonas mobilis. Front Bioeng Biotechnol. 2022;10:1098021.
Gonzalez R, Tao H, Purvis JE, York SW, Shanmugam KT, Ingram LO. Gene array-based identification of changes that contribute to ethanol tolerance in ethanologenic Escherichia coli: comparison of KO11 (parent) to LY01 (resistant mutant). Biotechnol Prog. 2003;19:612–23.
Evers MS, Roullier-Gall C, Morge C, Sparrow C, Gobert A, Vichi S, Alexandre H. Thiamine and biotin: relevance in the production of volatile and non-volatile compounds during Saccharomyces cerevisiae alcoholic fermentation in synthetic grape must. Foods. 2023;12:972.
Reyes LH, Almario MP, Kao KC. Genomic library screens for genes involved in n-butanol tolerance in Escherichia coli. PLoS ONE. 2011;6: e17678.
Gavin John J, Umbreit Wayne W. Effect of biotin on fatty acid distribution in Escherichia coli. J Bacteriol. 1965;89:437–43.
Yang Y, Lang N, Yang G, Yang S, Jiang W, Gu Y. Improving the performance of solventogenic clostridia by reinforcing the biotin synthetic pathway. Metab Eng. 2016;35:121–8.
Zhan Y, Xu H, Tan Hween T, Ho Ying S, Yang D, Chen S, Ow Dave S-W, Lv X, Wei F, Bi X, Chen S. Systematic adaptation of Bacillus licheniformis to 2-phenylethanol stress. Appl Environ Microbiol. 2023;89:e01568-01522.
Chandgude V, Välisalmi T, Linnekoski J, Granström T, Pratto B, Eerikäinen T, Jurgens G, Bankar S. Reducing agents assisted fed-batch fermentation to enhance ABE yields. Energy Convers Manag. 2021;227: 113627.
Li S, Liu L, Chen J. Mitochondrial fusion and fission are involved in stress tolerance of Candida glabrata. Bioresour Bioprocess. 2015;2:12.
Luo Q, Wu J, Wu M. Enhanced acetoin production by Bacillus amyloliquefaciens through improved acetoin tolerance. Process Biochem. 2014;49:1223–30.
Tian X, Yu Q, Yao D, Shao L, Liang Z, Jia F, Li X, Hui T, Dai R. New insights into the response of metabolome of Escherichia coli O157:H7 to ohmic heating. Front Microbiol. 2018;9:2936.
Zhang Y-M, Rock CO. Membrane lipid homeostasis in bacteria. Nat Rev Microbiol. 2008;6:222–33.
Tan Z, Yoon JM, Nielsen DR, Shanks JV, Jarboe LR. Membrane engineering via trans unsaturated fatty acids production improves Escherichia coli robustness and production of biorenewables. Metab Eng. 2016;35:105–13.
Cheng C, Zhang M, Xue C, Bai F, Zhao X. Development of stress tolerant Saccharomyces cerevisiae strains by metabolic engineering: new aspects from cell flocculation and zinc supplementation. J Biosci Bioeng. 2017;123:141–6.
Mahipant G, Paemanee A, Roytrakul S, Kato J, Vangnai AS. The significance of proline and glutamate on butanol chaotropic stress in Bacillus subtilis 168. Biotechnol Biofuels. 2017;10:122.
Hullo M-F, Auger S, Soutourina O, Barzu O, Yvon M, Danchin A, Martin-Verstraete I. Conversion of methionine to cysteine in Bacillus subtilis and its regulation. J Bacteriol. 2007;189:187–97.
Takagi H, Ohtsu I. l-Cysteine metabolism and fermentation in microorganisms. In: Yokota A, Ikeda M, editors. Amino acid fermentation. Tokyo: Springer Japan; 2017. p. 129–51.
Saini P, Beniwal A, Kokkiligadda A, Vij S. Response and tolerance of yeast to changing environmental stress during ethanol fermentation. Process Biochem. 2018;72:1–12.
Jang YS, Yang J, Kim JK, Kim TI, Park Y-C, Kim IJ, Kim KH. Adaptive laboratory evolution and transcriptomics-guided engineering of Escherichia coli for increased isobutanol tolerance. Biotechnol J. 2023;19:2300270.
Mezzina Mariela P, Álvarez Daniela S, Egoburo Diego E, Díaz Peña R, Nikel Pablo I, Pettinari MJ. A new player in the biorefineries field: Phasin PhaP enhances tolerance to solvents and boosts ethanol and 1,3-propanediol synthesis in Escherichia coli. Appl Environ Microbiol. 2017;83:e00662-00617.
Vojcic L, Despotovic D, Martinez R, Maurer K-H, Schwaneberg U. An efficient transformation method for Bacillus subtilis DB104. Appl Microbiol Biotechnol. 2012;94:487–93.
Zelena E, Dunn WB, Broadhurst D, Francis-McIntyre S, Carroll KM, Begley P, O’Hagan S, Knowles JD, Halsall A, Wilson ID, Kell DB. Development of a robust and repeatable UPLC−MS method for the long-term metabolomic study of human serum. Anal Chem. 2009;81:1357–64.
Want EJ, Masson P, Michopoulos F, Wilson ID, Theodoridis G, Plumb RS, Shockcor J, Loftus N, Holmes E, Nicholson JK. Global metabolic profiling of animal and human tissues via UPLC-MS. Nat Protoc. 2013;8:17–32.
Xia J, Wishart DS. Web-based inference of biological patterns, functions and pathways from metabolomic data using MetaboAnalyst. Nat Protoc. 2011;6:743–60.
Sasser M. Identification of bacteria by gas chromatography of cellular fatty acids. USFCC Newsl. 1990;20:1–6.
Li F, Zhu L. Surfactant-modified fatty acid composition of Citrobacter sp. SA01 and its effect on phenanthrene transmembrane transport. Chemosphere. 2014;107:58–64.
Li T, Liu P, Guo GG, Liu ZX, Zhong L, Guo LX, Chen C, Hao N, Ouyang PK. Production of acetoin and its derivative tetramethylpyrazine from okara hydrolysate with Bacillus subtilis. AMB Express. 2023;13:25.
Hu F, Liu Y, Lin J, Wang W, Li S. Efficient production of surfactin from xylose-rich corncob hydrolysate using genetically modified Bacillus subtilis 168. Appl Microbiol Biotechnol. 2020;104:4017–26.
Tesfay MA, Win X, Lin H, Liu Y, Li C, Lin J, Lin J. Efficient l-xylulose production using whole-cell biocatalyst with NAD+ regeneration system through co-expression of xylitol dehydrogenase and NADH oxidase in Escherichia coli. Biochem Eng J. 2021;175: 108137.
Acknowledgements
We are grateful to/thank Guangzhou Genedenovo Biotechnology Co., Ltd for assisting in sequencing and/or bioinformatics analysis.
Funding
This study was supported by the National Key Research and Development Program of China (2022YFC2104800), the Six Talent Peaks Project in Jiangsu Province (No. 2019-NY-058).
Author information
Authors and Affiliations
Contributions
TL: investigation, methodology, data curation, writing original draft. YQ and LZ: software. HL: investigation, validation. GG and ZL: collated the data and references. NH: resource, project administration. PO: conceptualization. All the authors read and approved the manuscript for submission.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file1: Table S1.
Pathway enrichment of B. subtilis 168-pMA5 under acetoin stress. Table S2. Pathway enrichment of B. subtilis 168-pMA5 and B. subtilis 168-phaCBA under acetoin stress.
Additional file 2: Table S3.
All the gene difference analysis results of B. subtilis 168-pMA5 under acetoin stress.
Additional file 3: Table S4.
All the gene difference analysis results of B. subtilis 168-pMA5 and B. subtilis 168-phaCBA under acetoin stress.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Li, T., Li, H., Zhong, L. et al. Analysis of heterologous expression of phaCBA promotes the acetoin stress response mechanism in Bacillus subtilis using transcriptomics and metabolomics approaches. Microb Cell Fact 23, 58 (2024). https://doi.org/10.1186/s12934-024-02334-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12934-024-02334-z