Abstract
Background
The most abundant human milk oligosaccharide in breast milk, 2′-fucosyllactose (2′-FL), has been approved as an additive to infant formula due to its multifarious nutraceutical and pharmaceutical functions in promoting neonate health. However, the low efficiency of de novo synthesis limits the cost-efficient bioproduction of 2′-FL.
Results
This study achieved 2′-FL de novo synthesis in a generally recognized as safe (GRAS) strain Bacillus subtilis. First, a de novo biosynthetic pathway for 2′-FL was introduced by expressing the manB, manC, gmd, wcaG, and futC genes from Escherichia coli and Helicobacter pylori in B. subtilis, resulting in 2′-FL production of 1.12 g/L. Subsequently, a 2′-FL titer of 2.57 g/L was obtained by reducing the competitive lactose consumption, increasing the regeneration of the cofactor guanosine-5′-triphosphate (GTP), and enhancing the supply of the precursor mannose-6-phosphate (M6P). By replacing the native promoter of endogenous manA gene (encoding M6P isomerase) with a constitutive promoter P7, the 2′-FL titer in shake flask reached 18.27 g/L. The finally engineered strain BS21 could produce 88.3 g/L 2′-FL with a yield of 0.61 g/g lactose in a 3-L bioreactor, without the addition of antibiotics and chemical inducers.
Conclusions
The efficient de novo synthesis of 2′-FL can be achieved by the engineered B. subtilis, paving the way for the large-scale bioproduction of 2′-FL titer in the future.
Similar content being viewed by others
Introduction
Studies on the intestinal microbiome in recent years have found that the composition of intestinal microorganisms impacted on human health [1,2,3]. Breast milk is an important food for infants; its special ingredients, such as oligosaccharides, antibodies, and vitamins, greatly influence the composition of the infant’s gut microbiome, thus affecting the infant’s health. Human milk oligosaccharides (HMOs) are the third most abundant solid substance in breast milk, following lactose and fat [4]. 2′-Fucosyllactose (2′-FL) is the most abundant HMOs, accounting for ~ 30% of the total HMOs [5, 6], and is composed of L-fucose, D-galactose, and D-glucose units. 2′-FL has gained much attention in recent years given its bioactive nature [5], which offers important health and economic benefits. Although indigestible to human infants [7], it plays a crucial role in developing the immune system, regulating the intestinal flora, and suppressing pathogenic infections [8,9,10,11,12]. These known bioactivities and their potential value make HMOs, especially 2′-FL, an attractive research target for preventing or treating diseases in infants and adults. To date, 2′-FL has been approved as a prebiotic for use in infant formula by the U.S. Food and Drug Administration, the European Food Safety Authority, and the Australian Therapeutic Goods Administration.
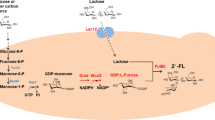
Currently, two promising routes are available to produce 2′-FL: chemical synthesis and biosynthesis [13, 14]. The chemical synthesis of 2′-FL is accomplished through multistep protection and deprotection reactions. This method is inefficient and uses toxic chemicals, rendering the chemical method unfit for common use [14]. The reaction steps for 2′-FL biosynthesis are simpler and more environmentally friendly than its chemical synthesis. The two known 2′-FL biosynthetic pathways are the salvage and de novo synthesis pathway. Both pathways are involved in lactose fucosylation (α-1,2-fucosyltransferase; FutC), the difference being in the synthesis pathway of GDP-L-fucose. The salvage pathway was first discovered in Bacteroides fragilis. It catalyzes GDP-L-fucose production from the substrate fucose by the fucokinase/GDP-L-fucose phosphorylase [15]. The de novo pathway is part of mannose metabolism, which starts from mannose-6-phosphate (M6P) and is catalyzed by four enzymes, namely, phosphomannomutase, mannose-1-phosphate guanylyltransferase, GDP-mannose-4, 6-dehydratase, and GDP-L-fucose synthase, to generate GDP-L-fucose (Fig. 1). The de novo pathway utilizes a low-cost carbon source, such as glucose and sucrose, instead of fucose; it is more economical and industrially applicable. Escherichia coli, Saccharomyces cerevisiae, and Corynebacterium glutamicum have been engineered to produce 2′-FL using the de novo pathway [16,17,18]. Besides the abovementioned microorganisms, Bacillus subtilis has also been selected as a host for producing functional nutraceuticals due to its generally recognized as safe (GRAS) status [19]. In previous studies, we have achieved the biosynthesis of 2′-FL in the engineered B. subtilis by using the salvage pathway [20]. However, there are few reports about the de novo biosynthesis of 2′-FL in the engineered B. subtilis strains.
Engineered B. subtilis strain for de novo synthesis of 2ʹ-FL using sucrose and lactose as carbon sources. Green arrows and genes indicate a heterogenous 2′-FL de novo biosynthetic pathway. Serial numbers represent the corresponding metabolic reactions. Abbreviations: F-1,6-P, fructose-1, 6-diphosphate; F6P, fructose-6-phosphate; G6P, glucose-6-phosphate; GDP-F, GDP-L-fucose; GDP-M, GDP-mannose; M1P, mannose-1-phosphate; PEP, phosphoenolpyruvate; sucrose-6P, sucrose-6-phosphate
In this study, we achieved 2′-FL de novo synthesis by introducing the de novo pathway of GDP-fucose and FutC in B. subtilis. First, B. subtilis was tested for 2′-FL tolerance to determine whether this host has the potential to produce high concentrations of 2′-FL. Then, the heterologous 2′-FL de novo synthesis pathway derived from E. coli and Helicobacter pylori was introduced in B. subtilis, and a 2′-FL titer of 1.12 g/L was obtained. Next, the supply of the precursors lactose and M6P and the cofactor (guanosine-5′-triphosphate, GTP) was increased, and the titer was increased by 129%. By optimizing the promoter of M6P isomerase, the 2′-FL titer of 18.27 g/L was obtained in the shake flask using sucrose and lactose as carbon sources. Finally, in a 3-L bioreactor, the 2′-FL titer of the finally engineered strain BS21 reached 88.3 g/L, the highest 2′-FL titer reported so far.
Methods
Plasmids, strains, and culture conditions
The strains used in this study are listed in Table 1. All plasmid constructions were performed using E. coli DH5α. The engineered BS strain (B. subtilis-PxylA-comK::comK) was used as a host strain for the heterologous synthesis of 2′-FL. Luria–Bertani (LB) medium (10 g/L tryptone, 5 g/L yeast extract and 10 g/L NaCl) was used to culture E. coli DH5α and the engineered B. subtilis. The minimal medium with the following composition was used: 17.1 g/L Na2HPO4·12H2O, 3 g/L KH2PO4, 1 g/L NH4Cl, 0.5 g/L NaCl, 0.0001 M CaCl2, 0.001 M MgSO4, 0.005 mM FeCl3, 1 mg/L MnSO4·4H2O, 1.7 mg/L ZnCl2, 0.43 mg/L CuCl2·2H2O, 0.6 mg/L CoCl2·6H2O, 0.6 mg/L Na2MoO4·2H2O. When needed, antibiotics were added as follows: kanamycin 50 μg/mL, chloromycetin 5 μg/mL, ampicillin 100 μg/mL. To cultivate B. subtilis or E. coli strains, a single colony was inoculated in a shake tube with LB medium, as described earlier, at 37 °C and 220 rpm for 8–10 h. A Competent Cell Preparation Kit (Takara Biomedical Technology, Beijing, China) was used to prepare E. coli DH5α competent cells. A previously reported method was followed for the preparation of B. subtilis competent cells [20]. To examine the tolerance of B. subtilis to 2′-FL, B. subtilis was cultured in 200 μL of LB media containing different concentrations of 2′-FL (0 g/L, 10 g/L, 20 g/L and 30 g/L) in 96-well plates (Corning 3603) at 37 °C and 750 rpm. The optical density (OD600) was determined at 12-h intervals.
Plasmid construction and DNA manipulation
All plasmids are listed in Additional file 1: Table S1, and the primers and promoters are listed in Additional file 1: Table S2. A CRISPR/Cpf1 genome editing approach was used to perform gene knockout or overexpression in B. subtilis, as reported previously [21]. First, the pcrF19NM plasmid was digested using BsaI. T4 DNA ligase was added to connect the crRNA fragment with linearized pcrF19NM plasmid to construct a gene-editing plasmid. In addition, the plasmid with gene-editing DNA fragments was constructed using the Seamless Cloning Kit (Beyotime Biotechnology, Shanghai, China) according to the manufacturer’s instructions. The plasmids of pHT-XCR6 and pcrF19NM-XXX (XXX is the name of the corresponding gene, such as manB, manC, gmd, wcaG, and futC) were sequentially transformed into B. subtilis, and DNA modification was performed. The validated positive colony was inoculated into the LB medium containing 0.005% sodium dodecyl sulfate (SDS) for plasmid curing. Finally, plasmid-free strains were obtained through antibiotic screening. The specific genome integration sites are as follows: manB gene was integrated between the yqiG and spo0A genes, and the manc gene was integrated between the yxkC and galE; the gmd gene was integrated between the ybcI and ybzH; the wcaG gene was integrated between the ybbU and alkA; and the futC gene was integrated between the rpsD and tyrS genes.
Analytical methods
The 2′-FL and xylose concentrations were analyzed using high-performance liquid chromatography (HPLC) system (Agilent Technologies 1260 Series) equipped with a Rezex ROA Organic Acid H + (8%) Column (Phenomenex, Torrance, CA, USA). The column and the refractive index detector temperature were set at 50 °C, and the mobile phase was 10 mM H2SO4 at a flow rate of 0.5 mL/min at 40 °C. The sucrose and lactose concentrations were analyzed using the HPLC system (Agilent Technologies 1260 Series) equipped with an XBridge BEH Amide Column (Waters, Milford, MA, USA). The column and the refractive index detector temperature were set at 35 °C, and the mobile phase was 75% acetonitrile at a flow rate of 1.0 mL/min at 35 °C. The liquid chromatography/mass spectrometry (LC/MS) matrix-assisted laser desorption/ionization time-of-flight system (Waters) was used to identify 2′-FL according to a previous study [22], and the mass range was 100–700 m/z. The relative transcription levels of the regulated genes were determined by quantitative real-time polymerase chain reaction (qRT-PCR) as described previously [23], and the rpsJ gene was used as the internal standard [24]. All experiments were independently carried out at least thrice.
Batch fermentation in shake flasks
During shake flask fermentation, the fermentation medium with the following composition was used: 6 g/L tryptone, 12 g/L yeast extract, 12.5 g/L K2HPO4·3H2O, 2.5 g/L KH2PO4, and 10 mL/L trace metal solution (composition: 4 g/L FeSO4·7H2O, 4 g/L CaCl2, 1 g/L MnSO4·H2O, 0.2 g/L NaMoO4·2H2O, 0.2 g/L ZnSO4·7H2O, 0.1 g/L AlCl3·6H2O, 0.1 g/L CuCl2·2H2O, and 0.05 g/L H3BO4). Sucrose and lactose were sterilized separately and added to the sterilized shake flask to a final concentration of 20 and 10 g/L (changed to 80 and 20 g/L after optimization), respectively. If the induction of the xylose promoter PxylA was required, 20 g/L xylose was added as an inducer. B. subtilis strains were cultivated on LB solid culture medium for 10–12 h at 37 °C, and a single colony was inoculated into 20 mL liquid LB medium in 250 mL shake flasks at 37 °C and 220 rpm for 10–12 h. The seed cultures were further inoculated into 30 mL of the fermentation medium at the rate of 10% in a 250 mL baffled flask at 37 °C and 220 rpm for 72 h, and three replicates were set for each strain. Sampling was performed every 12 h for OD600, 2′-FL, carbon source, and by-products measurements.
Fed-batch culture in 3-L bioreactor
The fermentation medium used for the fed-batch culture consisted of 24 g/L yeast extract, 24 g/L tryptone, 13.1 g/L K2HPO4·3H2O, 3 g/L KH2PO4, 6.7 g/L urea and 10 mL/L trace metal solution (composition: 4 g/L FeSO4·7H2O, 4 g/L CaCl2, 1 g/L MnSO4·H2O, 0.2 g/L NaMoO4·2H2O, 0.2 g/L ZnSO4·7H2O, 0.1 g/L AlCl3·6H2O, 0.1 g/L CuCl2·2H2O, and 0.05 g/L H3BO4). Seed culture was carried out in baffled 500 mL shake flasks containing 75 mL LB medium at 37 °C with shaking at 220 rpm for 12 h. The seed culture (75 mL) was inoculated into a 3-L bioreactor (T&J Bioengineering Co., Ltd., Shanghai, China) with an initial 1.5 L fermentation medium. The pH was kept at 6.0–6.5 by adding 29% NH3·H2O, and the temperature was maintained at 37 °C. The aeration rate and the agitation speed were 2.0 vvm and 1000 rpm, respectively. Sucrose and lactose were maintained at 10–30 g/L and 10–20 g/L by feeding concentrated sucrose (800 g/L) and lactose (300 g/L), respectively.
Statistical analysis
All data were the average of three independent studies with standard deviations. The * and ** indicate P < 0.05 and P < 0.01 relative to control, respectively.
Results and discussion
2′-FL tolerance of the B. subtilis strain
Previous studies indicated that 2′-FL had growth-inhibiting and adhesion-reducing effects on certain microorganisms [25, 26], and thereby the tolerance of B. subtilis to 2′-FL needs to be examined. B. subtilis was cultured in LB medium containing different concentrations of 2′-FL with 96-well plates. The maximum OD600 of all experimental groups added with 10, 20, and 30 g/L 2′-FL decreased by 9.62%, 10.40%, and 11.17%, respectively (Fig. 2A). Although the maximum OD600 was slightly lower than that in the control group without 2′-FL addition, it did not significantly decrease with higher 2′-FL concentrations. In order to identify whether the tolerance of B. subtilis to 2′-FL comes from its ability to utilize 2′-FL, B. subtilis was cultured in minimal medium with 2′-FL as the sole carbon source, and the minimal medium with glucose or sucrose as the sole carbon source was used as the control. As shown in Fig. 2B, B. subtilis cannot grow in the minimum medium with 2′-FL as the sole carbon source, while can grow normally in the presence of glucose or sucrose. These results suggested that B. subtilis could be used as a host for 2′-FL production.
2′-FL tolerance of B. subtilis. A Maximum OD600 of B. subtilis in different 2′-FL concentrations (96-well plate). All data were the average of three independent studies with standard deviations. *P < 0.05; *P < 0.01. B Cell growth of B. subtilis in minimal medium with 2′-FL as the sole carbon source. Glucose and sucrose were used as controls, respectively
2′-FL production by introducing the de novo pathway
GDP-L-fucose is a key precursor for 2′-FL biosynthesis [27]. The de novo pathway could utilize low-cost carbon sources (glucose, sucrose, and so on) to synthesize 2′-FL. To produce 2′-FL utilizing sucrose and lactose, the de novo pathway was constructed by introducing manB, manC, gmd, and wcaG genes from E. coli BL21 (DE3) for synthesizing GDP-L-fucose and the futC gene from H. pylori into B. subtilis 168 (Fig. 1). To express the heterologous genes efficiently in B. subtilis, the original promoters of manB, manC, gmd, wcaG, and futC genes were respectively replaced by constitutive promoter P43 and integrated into the genome of B. subtilis, yielding the strain BS0. The shake flask culture of BS0 was performed, while the extracellular 2′-FL and intracellular GDP-L-fucose cannot be detected, implying that one or more genes in the GDP-L-fucose synthesis pathway were not normally transcribed or translated. In addition, protein structure instability may also lead to the above results.
To explore the reasons for the above unexpected results, the plasmid pP43NMK was used to respectively express the manB, manC, gmd, and wcaG genes. Four strains (BS-MB, BS-MC, BS-GD, and BS-WG) were constructed to verify the expressions of phosphomannomutase (ManB; 50.19 kDa), mannose-1-phosphate guanylyltransferase (ManC; 53.81 kDa), GDP-mannose-4,6-dehydratase (Gmd; 42.05 kDa), and GDP-L-fucose synthase (WcaG; 36.15 kDa). SDS–polyacrylamide gel electrophoresis results showed that BS-MB, BS-GD, and BS-WG strains displayed single bands of approximate 51, 42, and 37 kDa, respectively (Additional file 1: Fig. S1), while the gel electrophoresis result of strain BS-MC was similar to the control group. The aforementioned results suggested that the target genes in strains BS-MB, BS-GD, and BS-WG were transcribed and translated but not the target gene in strain BS-MC.
The P43 promoter is a strong constitutive promoter used to express various heterologous genes in B. subtilis [28, 29]. The above results indicated that the manC gene was not expressed, and eventually 2′-FL cannot be synthesized normally. It was speculated that the expression strength of the P43 promoter was not appropriate to ManC. Therefore, the inducible promoter PxylA was used based on earlier reports to optimize the expression level of manC [30]. The accumulation of 1.12 g/L 2′-FL in the shake flask fermentation supernatant of strain BS2 (PxylA-manC) was achieved, whereas no 2′-FL was detected in the fermentation supernatant of the control strain BS (Fig. 3A). The LC/MS identification results are shown in Fig. 3B and C. Results showed that the inducible promoter PxylA enabled the transcription and translation of manC, and 2′-FL was eventually synthesized in B. subtilis.
Production of 2′-FL in the strain BS2. A 2′-FL titer and OD600 of 20 g/L xylose-induced strain BS2. (B and C) 2′-FL standard and BS2 strain shake flask fermentation supernatant were analyzed by HPLC and LC/MS, respectively. Cells were cultivated for 36 h at 37 °C and 220 rpm. All data were the average of three independent studies with standard deviations
Improving lactose utilization by knocking out β-galactosidase
As an important precursor for 2′-FL de novo synthesis, lactose is extremely important for the overproduction of 2′-FL. The lactose transportation in B. subtilis is dependent on the phosphotransferase system [31]. The β-galactosidase encoded by yesZ and ganA was able to hydrolyze lactose in B. subtilis [32, 33]. To test the lactose utilization ability of B. subtilis, B. subtilis was cultured in LB medium containing different concentrations of lactose (1.5, 5, 10, 25, 50, and 75 g/L). Figure 4A shows that B. subtilis consumed 70–90% of the lactose added in the medium. Among them, the experimental group with an initial lactose concentration of 75 g/L had the maximum residual lactose (~ 21.93 g/L) but with the consumption of > 50 g/L lactose. The final OD600 of B. subtilis gradually increased with the increase of lactose concentration, indicating that excess lactose was catabolized for cell growth (Fig. 4A). In addition, the effect of blocking the endogenous lactose metabolic pathway was tested by knocking out the yesZ gene, and results showed that yesZ deletion resulted in the cessation of lactose consumption in B. subtilis (Fig. 4A). According to the chemical reaction equation of 2′-FL synthesis, the stoichiometric ratio of lactose to 2′-FL was 1:1 (Fig. 1). Therefore, results indicated that the lactose transportation system of B. subtilis sufficed the needs of 2′-FL synthesis.
Increasing the supply of lactose, M6P and GTP. A The lactose utilization ability of wild-type B. subtilis and yesZ gene-deleted strain in 48-well plates (48 h). Symbol “ + ” means that this gene is present in the genome, and “ − ” indicates that this gene was deleted. B 2′-FL synthesis efficiency of strains with blocking lactose metabolism pathways was verified by fermentation. C Fermentation results of enhanced GTP supply experiments. D Fermentation results of enhanced precursor M6P supply experiments. All data were the average of three independent studies with standard deviations
Although the lactose transport capacity was demonstrated, endogenous lactose metabolic pathways reduced the effective supply of lactose in B. subtilis (Fig. 1). β-Galactosidase could catalyze lactose hydrolysis; two β-galactosidase genes (yesZ and ganA) were found in B. subtilis [34,35,36]. Next, the effect of blocking the lactose catabolic pathway by knocking out different genes on 2′-FL accumulation was investigated. The results of shake flask fermentation (Fig. 4B) showed that knockout of the yesZ or ganA gene alone and in combination increased the 2′-FL titer to 1.22, 1.22, and 1.31 g/L, respectively. An earlier study suggested that the endogenous lactose transport system in B. subtilis was insufficient for the availability of 2′-FL production [20]. Results confirmed that the endogenous lactose transport system of B. subtilis sufficed the 2′-FL production with sufficient lactose, but a large amount of lactose was used for cell growth. Therefore, two β-galactosidase genes needed to be knocked out to improve lactose availability in B. subtilis.
Enhancing 2′-FL production by engineering GTP and M6P supply
GTP and nicotinamide adenine dinucleotide phosphate were required as GDP donors and energy sources in the GDP-L-fucose de novo synthesis pathway (Fig. 1). 2′-FL production could be enhanced by increasing the GTP supply [20, 37, 38]. To increase GTP availability for optimal 2′-FL production, the B. subtilis ndk gene encoding a nucleoside-diphosphate kinase that converted GMP into GTP was respectively overexpressed by the original promoter (Pnative-ndk), inducible promoter (PxylA-ndk), and constitutive promoters (Phbs-ndk and P43-ndk). Shake flask fermentation results showed that the 2′-FL titer of strains BS7 (PxylA-ndk) and BS8 (Phbs-ndk) increased to 1.41 and 1.43 g/L, respectively, whereas the titers of the remaining strains decreased (Fig. 4C). Therefore, strain BS8 was selected for subsequent research.
The direct precursor of the de novo synthesis of GDP-L-fucose was M6P, which was catalyzed by the M6P isomerase encoded by manA and yvyI (putative) genes [39]. manA expression was regulated by mannose and a transcription activator (manR), and the endogenous promoter of manA was subjected to carbon catabolite repression [40]. To increase the supply of the precursor M6P, the original manA and yvyI were retained in the genome of strain BS8, and manA and yvyI expression cassettes harboring the original and strong promoters were introduced (Pnative-yvyI, P43-yvyI, Pnative-manA and P43-manA). Shake flask cultivation was performed for these strains with BS8 as a control. In Fig. 4D, the titers of the four strains (BS10–BS13) integrating the manA or yvyI gene increased to 1.59, 1.47, 1.84, and 1.75 g/L, respectively. Among these, with the Pnative-manA expression cassette, the titer increased most significantly up to 1.84 g/L. All gene insertions exhibited a positive effect on 2′-FL accumulation. Results indicated that the precursor M6P supply played an important role in 2′-FL production.
Noninducible 2′-FL production and fermentation optimization
As mentioned in the preceding sections, a 2′-FL-producing strain with an improved titer was obtained. The titer and OD600 curves during the fermentation process (Additional file 1: Fig. S2) showed that 2′-FL accumulated slowly in the later stages of fermentation. Considering that this engineered strain required xylose as an inducer for 2′-FL production, there could be two possible reasons for the reduction in productivity volume: (1) B. subtilis could use xylose as a carbon source for growth and metabolite synthesis [41]. Therefore, xylose depletion as an inducer might have led to the inhibition of 2′-FL synthesis. (2) With the prolongation of the fermentation time, the residual carbon source was gradually consumed, and the lack of carbon source resulted in a decrease in production volume. Therefore, carbon source and inducer supplemental fermentation were performed. Results showed that adding the inducer xylose alone considerably increased the titer by 78.59% (2.00 g/L), and supplementing the lactose or sucrose alone increased the 2′-FL titer by 27.91% (1.43 g/L) and 20.77% (1.35 g/L), respectively (Fig. 5A). Results demonstrated that adding the inducer dose was the most effective in increasing the titer. In Fig. 5A, the 2′-FL titer increased by 129.72% with the addition of lactose and sucrose by increasing the inducer dose, and 2.57 g/L 2′-FL was obtained in the shake flask.
Fermentation optimization and optimization of the expression level of the manC gene. A and B Synthesis efficiency of 2′-FL was verified when the inducer xylose or carbon source (sucrose or lactose) was supplemented during 24-h fermentation using the BS12 strain. Symbol “o” indicates initial fermentation conditions (xylose 20 g/L, lactose 10 g/L, and sucrose 20 g/L). Symbol “ + ” indicates that 20 g/L xylose, lactose 10 g/L, and sucrose 20 g/L were supplemented when fermentation reached 24-h, and “–” means none of the three sugars were added. C Seven different constitutive promoters (P1–P7) were used to express the manC gene. D Optimization of fermentation conditions for strain BS20. All carbon sources were added at the beginning of fermentation. Symbol “ − ” means no added xylose. All data were the average of three independent studies with standard deviations
Although xylose supplementation significantly increased the 2′-FL titer, the high cost was unsuitable for an industrial-level production economy. To avoid using xylose, seven constitutive promoters (P1–P7) from B. subtilis with gradually increasing expression levels were used to replace inducible promoter PxylA. The fermentation results shown in Fig. 5B indicated that the titers of the strains harboring the P1 to P6 promoters decreased by 5.47%, 7.63%, 8.33%, 6.63%, 4.67%, and 6.36%, respectively. The strain BS20 harboring the strongest promoter had the highest 2′-FL titer, reaching 2.88 g/L (Fig. 5B). The highest 2′-FL titer was obtained by optimizing the addition of sucrose and lactose. In Fig. 5C, 80g/L sucrose and 20 g/L lactose were added at the beginning of the fermentation process, and the 2′-FL titer reached 14.06 g/L in 72 h (Fig. 5C). Meanwhile, considering that xylose could be consumed by B. subtilis as a carbon source, the effect of xylose on the 2′-FL titer was tested. Results showed that adding xylose did not affect 2′-FL accumulation (Fig. 5C).
Enhancing 2′-FL production by engineering the transcriptional regulation of M6P isomerase
To further validate the 2′-FL production capacity of the engineered strain BS20, fermentation was performed in a 3-L bioreactor (Fig. 6A). Surprisingly, with the extension of fermentation time, the 2′-FL titer stopped increasing at 12 h, whereas the OD600 continued to increase, and a final 2′-FL titer of 36.09 g/L was obtained. All the exogenous genes in the 2′-FL de novo synthesis pathway was expressed with constitutive promoters, so there is no transcriptional repression for the expression of these genes. It was reported that, the endogenous manA gene (encoding the M6P isomerase) belongs to the mannose operon, and there is a phosphorylated transcription activator manR upstream of the mannose operon [40]. Therefore, we speculate that the stop of 2′-FL synthesis after 12 h may be due to the transcriptional inhibition of the endogenous manA gene.
Engineering manA transcriptional regulation system to promote continuous synthesis of 2′-FL. A Fed-batch fermentation of strain BS20 in a 3-L bioreactor. 2′-FL synthesis stopped after 12 h fermentation. B The original promoter of manA was replaced to promote continuous 2′-FL production. qRT-PCR results showed that the expression level of the manA gene controlled by the constitutive promoter at 24 h was 2.297 times higher than that at 12 h. All data were the average of three independent studies with standard deviations
Based on the above analysis, the original promoter (Pnative) of the manA gene of the BS20 strain was replaced with the constitutive promoter P7, generating the strain BS21. The cells of BS20 and BS21 cultured in shake flask for 12 and 24 h were collected, and the relative transcription level of the manA gene was analyzed by qRT-PCR (Fig. 6B). The results showed that the relative expression of manA at 24 h in the strain BS20 decreased to 0.664 times that at 12 h, whereas the relative expression of manA at 24 h in the strain BS21 increased to 2.297 times that at 12 h. Accordingly, the strain BS21 produced the highest 2′-FL titer of 18.27 g/L, which was 1.28-fold higher than that of the strain BS20 (14.29 g/L). This result suggested that the transcriptional regulation of manA hinders the continuous synthesis of 2′-FL in the later stage of fermentation, and the expression of manA gene with the constitutive promoter can solve this issue.
The production of 2′-FL by the finally engineered strain BS21 was performed in a 3-L bioreactor. The pH was kept at 6.0–6.5 by adding NH4OH; sucrose and lactose concentrations were maintained at 10–30 g/L and 10–20 g/L, respectively. Figure 7 shows that the strain BS21 could produce 88.3 g/L 2′-FL with a yield of 0.61 g/g lactose, representing the highest 2′-FL titer reported so far.
Conclusions
This study constructed the 2′-FL de novo synthesis pathway in B. subtilis and further improved the 2′-FL titer by increasing the supply of M6P, lactose and cofactor GTP. In addition, the native promoter of manA gene was replaced with the constitutive promoter P7, leading to the further increase of 2′-FL titer. In a 3-L bioreactor, the 2′-FL titer of the finally engineered strain BS21 reached 88.3 g/L with a yield up to 0.61 mol/mol lactose. In the future, the dynamic regulation of central carbon metabolism with genetic circuits can be performed to further improve the synthesis efficiency of 2′-FL.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding authors on reasonable request.
References
Blanton LV, Charbonneau MR, Salih T, Barratt MJ, Venkatesh S, Ilkaveya O, Subramanian S, Manary MJ, Trehan I, Jorgensen JM, et al. Gut bacteria that prevent growth impairments transmitted by microbiota from malnourished children. Science. 2016. https://doi.org/10.1126/science.aad3311.
Shamash M, Maurice CF. Phages in the infant gut: a framework for virome development during early life. ISME J. 2022;16:323–30.
Sudheer S, Gangwar P, Usmani Z, Sharma M, Sharma VK, Sana SS, Almeida F, Dubey NK, Singh DP, Dilbaghi N, et al. Shaping the gut microbiota by bioactive phytochemicals: an emerging approach for the prevention and treatment of human diseases. Biochimie. 2022;193:38–63.
Dogra SK, Martin FP, Donnicola D, Julita M, Berger B, Sprenger N. Human milk oligosaccharide-stimulated bifidobacterium species contribute to prevent later respiratory tract infections. Microorganisms. 2021;9:1939.
Vandenplas Y, Berger B, Carnielli V, Ksiazyk J, Lagström H, Sanchez Luna M, Migacheva N, Mosselmans J-M, Picaud J-C, Possner M, et al. Human milk oligosaccharides: 2′-Fucosyllactose (2′-FL) and Lacto-N-neotetraose (LNnT) in infant formula. Nutrients. 2018;10:1161.
Zhang W, Liu Z, Gong M, Li N, Lv X, Dong X, Liu Y, Li J, Du G, Liu L. Metabolic engineering of Escherichia coli for the production of Lacto-N-neotetraose (LNnT). Syst Microbiol and Biomanuf. 2021;1:11.
Ballard O, Morrow AL. Human milk composition: nutrients and bioactive factors. Pediatr Clin North Am. 2013;60:49–74.
Donovan SM, Comstock SS. Human milk oligosaccharides influence neonatal mucosal and systemic immunity. Ann Nutr Metab. 2016;69(Suppl 2):42–51.
Koromyslova A, Tripathi S, Morozov V, Schroten H, Hansman GS. Human norovirus inhibition by a human milk oligosaccharide. Virology. 2017;508:81–9.
Mohammad NS, Nazli R, Zafar H, Fatima S. Effects of lipid based multiple micronutrients supplement on the birth outcome of underweight pre-eclamptic women: a randomized clinical trial. Pak J Med Sci. 2022;38:219–26.
Wicinski M, Sawicka E, Gebalski J, Kubiak K, Malinowski B. Human milk oligosaccharides: Health benefits, potential applications in infant formulas, and pharmacology. Nutrients. 2020;12:266.
Ayechu-Muruzabal V, van Stigt AH, Mank M, Willemsen LEM, Stahl B, Garssen J, Van’t Land B. Diversity of Human milk oligosaccharides and effects on early life immune development. Front Pediatr. 2018;6:239.
Petschacher B, Nidetzky B. Biotechnological production of fucosylated human milk oligosaccharides: prokaryotic fucosyltransferases and their use in biocatalytic cascades or whole cell conversion systems. J Biotechnol. 2016;235:61–83.
Zhou W, Jiang H, Wang L, Liang X, Mao X. Biotechnological production of 2′-Fucosyllactose: a prevalent fucosylated Human milk oligosaccharide. ACS Synth Biol. 2021;10:447–58.
Coyne MJ, Reinap B, Lee MM, Comstock LE. Human symbionts use a host-like pathway for surface fucosylation. Science. 2005;307:1778–81.
Parschat K, Schreiber S, Wartenberg D, Engels B, Jennewein S. High-titer de novo biosynthesis of the predominant Human milk oligosaccharide 2′-fucosyllactose from sucrose in Escherichia coli. ACS Synth Biol. 2020;9:2784–96.
Lee JW, Kwak S, Liu JJ, Yu S, Yun EJ, Kim DH, Liu C, Kim KH, Jin YS. Enhanced 2′-Fucosyllactose production by engineered Saccharomyces cerevisiae using xylose as a co-substrate. Metab Eng. 2020;62:322–9.
Jin-Ho S, Young-Wook C, Hae-Yong J. Corynebacterium glutamicum for use in producing 2′-fucosyllactose. Xiamen: Seoul National University R&DB Foundation; AP Technologies Corp; 2020.
Zhang Q, Wu Y, Gong M, Zhang H, Liu Y, Lv X, Li J, Du G, Liu L. Production of proteins and commodity chemicals using engineered Bacillus subtilis platform strain. Essays Biochem. 2021;65:173–85.
Deng JY, Gu LY, Chen TC, Huang H, Yin XQ, Lv XQ, Liu YF, Li N, Liu ZM, Li JH, et al. Engineering the substrate transport and cofactor regeneration systems for enhancing 2′-fucosyllactose synthesis in Bacillus subtilis. ACS Synth Biol. 2019;8:2418–27.
Wu Y, Liu Y, Lv X, Li J, Du G, Liu L. CAMERS-B: CRISPR/Cpf1 assisted multiple-genes editing and regulation system for Bacillus subtilis. Biotechnol Bioeng. 2020;117:1817–25.
Yu S, Liu JJ, Yun EJ, Kwak S, Kim KH, Jin YS. Production of a human milk oligosaccharide 2′-fucosyllactose by metabolically engineered Saccharomyces cerevisiae. Microb Cell Fact. 2018;17:101.
Wu Y, Chen T, Liu Y, Lv X, Li J, Du G, Ledesma-Amaro R, Liu L. CRISPRi allows optimal temporal control of N-acetylglucosamine bioproduction by a dynamic coordination of glucose and xylose metabolism in Bacillus subtilis. Metab Eng. 2018;49:232–41.
Lu Z, Yang S, Yuan X, Shi Y, Ouyang L, Jiang S, Yi L, Zhang G. CRISPR-assisted multi-dimensional regulation for fine-tuning gene expression in Bacillus subtilis. Nucleic Acids Res. 2019;47: e40.
Salli K, Soderling E, Hirvonen J, Gursoy UK, Ouwehand AC. Influence of 2′-fucosyllactose and galacto-oligosaccharides on the growth and adhesion of Streptococcus mutans. Br J Nutr. 2020;124:824–31.
Weichert S, Jennewein S, Hüfner E, Weiss C, Borkowski J, Putze J, Schroten H. Bioengineered 2′-fucosyllactose and 3-fucosyllactose inhibit the adhesion of Pseudomonas aeruginosa and enteric pathogens to human intestinal and respiratory cell lines. Nutr Res. 2013;33:831–8.
Bode L, Contractor N, Barile D, Pohl N, Prudden AR, Boons GJ, Jin YS, Jennewein S. Overcoming the limited availability of human milk oligosaccharides: challenges and opportunities for research and application. Nutr Rev. 2016;74:635–44.
Wang PZ, Doi RH. Overlapping promoters transcribed by Bacillus subtilis sigma 55 and sigma 37 RNA polymerase holoenzymes during growth and stationary phases. J Biol Chem. 1984;259:8619–25.
Wang J, Wang W, Wang H, Yuan F, Xu Z, Yang K, Li Z, Chen Y, Fan K. Improvement of stress tolerance and riboflavin production of Bacillus subtilis by introduction of heat shock proteins from thermophilic bacillus strains. Appl Microbiol Biotechnol. 2019;103:4455–65.
Westbrook AW, Ren X, Oh J, Moo-Young M, Chou CP. Metabolic engineering to enhance heterologous production of hyaluronic acid in Bacillus subtilis. Metab Eng. 2018;47:401–13.
Reizer J, Bachem S, Reizer A, Arnaud M, Saier MH Jr, Stülke J. Novel phosphotransferase system genes revealed by genome analysis - the complete complement of PTS proteins encoded within the genome of Bacillus subtilis. Microbiology (Reading). 1999;145(Pt 12):3419–29.
Watzlawick H, Morabbi Heravi K, Altenbuchner J. Role of the ganSPQAB operon in degradation of galactan by Bacillus subtilis. J Bacteriol. 2016;198:2887–96.
Carneiro L, Yu L, Dupree P, Ward RJ. Characterization of a β-galactosidase from Bacillus subtilis with transgalactosylation activity. Int J Biol Macromol. 2018;120:279–87.
Borriss R, Danchin A, Harwood CR, Médigue C, Rocha EPC, Sekowska A, Vallenet D. Bacillus subtilis, the model Gram-positive bacterium: 20 years of annotation refinement. Microb Biotechnol. 2018;11:3–17.
Shipkowski S, Brenchley JE. Bioinformatic, genetic, and biochemical evidence that some glycoside hydrolase family 42 β-galactosidases are arabinogalactan type I oligomer hydrolases. Appl Environ Microbiol. 2006;72:7730–8.
Saqib S, Akram A, Halim SA, Tassaduq R. Sources of β-galactosidase and its applications in food industry. Biotech. 2017;7:79.
Ni Z, Wu J, Li Z, Yuan L, Wang Y, Chen X, Yao J. Enhanced bioproduction of fucosylated oligosaccharide 3-fucosyllactose in engineered Escherichia coli with an improved de novo pathway. Biosci Biotechnol Biochem. 2021;85:1772–81.
Choi YH, Park BS, Seo JH, Kim BG. Biosynthesis of the human milk oligosaccharide 3-fucosyllactose in metabolically engineered Escherichia coli via the salvage pathway through increasing GTP synthesis and β-galactosidase modification. Biotechnol Bioeng. 2019;116:3324–32.
Morabbi Heravi K, Manzoor I, Watzlawick H, de Jong A, Kuipers OP, Altenbuchner J. Phosphosugar stress in Bacillus subtilis: Intracellular accumulation of mannose 6-phosphate derepressed the glcR-phoC operon from repression by GlcR. J Bacteriol. 2019;201:e00732.
Sun T, Altenbuchner J. Characterization of a mannose utilization system in Bacillus subtilis. J Bacteriol. 2010;192:2128–39.
Hu F, Liu Y, Lin J, Wang W, Li S. Efficient production of surfactin from xylose-rich corncob hydrolysate using genetically modified Bacillus subtilis 168. Appl Microbiol Biotechnol. 2020;104:4017–26.
Acknowledgements
Facility support from the Science Center for Future Foods at the Jiangnan University is acknowledged.
Funding
This work was financially supported by the National Natural Science Foundation of China (31930085, 31870069), the National Key Research and Development Program of China (2018YFA0900504, 2018YFA0900300), and the Fundamental Research Funds for the Central Universities (SKLF-ZZB-202106, JUSRP121010).
Author information
Authors and Affiliations
Contributions
Long Liu conceived, supervised and coordinated the project. Quanwei Zhang, Yonglian Zhu, and Linfeng Xu executed related research. Quanwei Zhang conceived drafted the first manuscript which was later revised and corrected by Xueqin Lv, Yanfeng Liu, Jianghua Li and Guocheng Du. Ziyang Huang, Zhenmin Liu, and Hongzhi Xia gave us some advice from the perspective of industrialization. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Competing interests
The authors declare no competing financial interest.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
12934_2022_1838_MOESM1_ESM.docx
Additional file 1: Table S1. Plasmids used in this study. Table S2. Primers and promoters used in this study. Figure S1. The SDS-PAGE analysis of cell extracts from single-gene expression strain. Figure S2. Production of 2′-FL in the shake flakes by the engineered strain BS2.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhang, Q., Liu, Z., Xia, H. et al. Engineered Bacillus subtilis for the de novo production of 2′-fucosyllactose. Microb Cell Fact 21, 110 (2022). https://doi.org/10.1186/s12934-022-01838-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12934-022-01838-w