Abstract
Background
Rhodococcus jostii RHA1 and other actinobacteria accumulate triglycerides (TAG) under nutrient starvation. This property has an important biotechnological potential in the production of sustainable oils.
Results
To gain insight into the metabolic pathways involved in TAG accumulation, we analysed the transcriptome of R jostii RHA1 under nutrient-limiting conditions. We correlate these physiological conditions with significant changes in cell physiology. The main consequence was a global switch from catabolic to anabolic pathways. Interestingly, the Entner-Doudoroff (ED) pathway was upregulated in detriment of the glycolysis or pentose phosphate pathways. ED induction was independent of the carbon source (either gluconate or glucose). Some of the diacylglycerol acyltransferase genes involved in the last step of the Kennedy pathway were also upregulated. A common feature of the promoter region of most upregulated genes was the presence of a consensus binding sequence for the cAMP-dependent CRP regulator.
Conclusion
This is the first experimental observation of an ED shift under nutrient starvation conditions. Knowledge of this switch could help in the design of metabolomic approaches to optimize carbon derivation for single cell oil production.
Similar content being viewed by others
Background
Microbial triglycerides, called single cell oils (SCO), have biotechnological potential in the production of sustainable oils for their use either as biodiesel or as commodity oils. Biodiesel is produced by transesterification of triacylglycerides with short-chain alcohols (mainly methanol). Vegetable oils and animal fats such as soybean oil, rapeseed oil, palm oil or waste cooking oils are used as feedstocks for biodiesel production [1]. However, this strategy has been criticized for being a non-sustainable process since it leads to a reduction in edible oil feedstocks [2]. Production of biodiesel using SCO is considered as a promising alternative solution [3]. SCO produce high quality biodiesel esters according to currently existing standards [4, 5]. SCO are appropriate for their use as a biodiesel source since the producing microorganisms can grow using a variety of substrates, show rapid life cycles and can be easily modified by genetic engineering.
Several microorganisms, including bacteria, yeasts, molds and microalgae, can be considered as oleaginous microorganisms [6]. Regarding bacteria, the accumulation of the neutral lipids triacylglycerols (TAGs), wax esters (WEs) and polyhydroxyalkanoates (PHAs) has been reported. The main purpose of this accumulation is to store carbon and energy under growth-limiting conditions. While PHAs are synthesized in a wide variety of bacteria [7], the accumulation of triacylglycerols (TAGs) has only been described for a few bacteria belonging to the proteobacteria and actinobacteria groups (for a review see [8]). Acinetobacter [9] Mycobacterium [10], Streptomyces [11] or Rhodococcus [12] are such examples. Accumulation of TAGs is remarkably high in the actinobacteria Rhodococcus and Gordonia, which accumulate up to 80% of the cellular dry weight in the form of neutral lipids with maximal TAG production of 88.9 and 57.8 mg/l, respectively [13].
Rhodococcus are aerobic, non-sporulating soil bacteria, with unique enzymatic activities used for several environmental and biotechnological processes [14]. Rhodococcus strains are industrially used for large-scale production of acrylamide and acrylic acid as well as for the production of bioactive steroid compounds and fossil fuel biodesulfurization [15]. Moreover, Rhodococcus are able to degrade contaminant hydrophobic natural compounds and xenobiotics. R. jostii RHA1 has been shown to convert lignocellulose into different phenolic compounds [16] while it also has the potential to use this waste material for the production of valuable oils [17].
Due to its capability for degrading hydrocarbons, R. jostii RHA1 is one of the best studied Rhodococcus species in the terms of biotechnological applications [18–20]. Moreover, high TAG accumulating capability has been reported [21] and its genomic sequence is available [22].
In this article we decipher the metabolic changes associated to nutrient starvation conditions that influence TAG accumulation.
Methods
Bacterial strain and growth conditions
Rhodococcus jostii strain RHA1 was grown aerobically at 30 °C in Streptomyces medium, Fluka (Rich Medium, RM, 4.0 g/l glucose, 4.0 g/l Yeast extract, and 10.0 g/l Malt extract). After 48 h, 25 ml of R. jostii cells in RM were collected by centrifugation, washed with mineral salts medium M9 (Minimal Medium, MM, [23], 95 mM Na2HPO4, 44 mM KH2PO4, 17 mM NaCl, 0.1 mM CaCl2 and 2 mM MgSO4) containing 20% w/v sodium gluconate (MMGln) or 20% w/v glucose (MMGls) as the sole carbon sources and transfer into 25 ml of MMGln or MMGls. The concentration of ammonium chloride in MM was reduced to 10 mM to enhance lipid accumulation.
Extraction and analysis of lipids
Pelleted cells were extracted with hexane/isopropanol (3:1 v/v). An aliquot of the whole cell extract was analyzed by thin layer chromatography (TLC) on silica gel plates (Merck) applying n-hexane/diethyl ether/acetic acid (80:20:1, v/v/v) as a solvent system. Lipid fractions were revealed using iodine vapour. Trioleine and oleic acid (Merck) were used as standards.
RNA extraction
RNA was extracted from RM and MM-grown cells originally harvested from 3 ml of culture. Total RNA isolation involved vortexing of the pellet with 6 ml of RNA Protect (QIAGEN) followed by centrifugation. The pellet was thereafter lysed using 280 μl of lysis buffer (10% Zwittergent (Calbiochem), 15 mg/ml Lysozime (Sigma) and 20 mg/ml Proteinase K (Roche) in TE buffer). Total RNA was purified with RNeasy mini kit (QIAGEN, Valencia, CA) combined with DNase I (QIAGEN) according to the manufacturer’s instructions. The quantity and quality of RNA were assessed using a NanoDrop ND-1000 spectrophotometer (NanoDrop Technology, Rockland, DE) and Experion Automated Electrophoresis using the RNA StdSens Analysis Kit (Bio Rad).
mRNA enrichment
Removal of 16S and 23S rRNA from total RNA was performed using MicrobExpress™ Bacterial mRNA Purification Kit (Ambion) according to the manufacturer’s protocol with the exception that no more than 5 μg total RNA was treated per enrichment reaction. Each RNA sample was divided into multiple aliquots of ≤5 μg RNA and separate enrichment reactions were performed for each sample. Enriched mRNA samples were pooled and run on the 2100 Bioanalzyer (Agilent) to confirm reduction of 16S and 23S rRNA prior to preparation of cDNA fragment libraries.
Preparation of cDNA fragment libraries
Ambion RNA fragmentation reagents were used to generate 60–200 nucleotide RNA fragments with an input of 100 ng of mRNA. Following precipitation of fragmented RNA, first strand cDNA synthesis was performed using random N6 primers and Superscript II Reverse Transcriptase, followed by second strand cDNA synthesis using RNaseH and DNA pol I (Invitrogen, CA). Double stranded cDNA was purified using Qiaquick PCR spin columns according to the manufacturer’s protocol (Qiagen).
RNA-Seq using the Illumina genome analyzer
The Illumina Genomic DNA Sample Prep kit (Illumina, Inc., San Diego, CA) was used according to the manufacturer’s protocol to process double-stranded cDNA for RNA-Seq. This process included end repair, A-tailing, adapter ligation, size selection, and pre-amplification. Amplified material was loaded onto independent flow cells. Sequencing was carried out by running 36 cycles on the Illumina Genome Analyzer IIx. The quality of the RNA-Seq reads was analyzed by assessing the relationship between the quality score and error probability. These analyses were performed on Illumina RNA-Seq quality scores that were converted to phred format (http://www.phrap.com/phred/).
Computational methods
To filter genes with low signal/noise ratio we built 3 subsets of each condition taking randomly 70% of the total sequenced reads for each subset. The alignment was performed by Bowtie [24] against the R. jostii RHA1 reference genomes of the chromosome and three endogenous plasmids (Genome Reviews CP000431-4_GR). Gene expression was determined by Samtools [25], Artemis [26] and home-made perl scripts. We represent gene expression as reads per kilobase (RPK) and the data was normalized by quantiles according to [27]. Statistical analysis was performed by DESeq package [28] and R software.
Quantitative real-time RT-PCR (qRT-PCR)
cDNA was generated from 1.5 µg of total RNA using the iScript kit (BioRad) according to manufacturer’s instructions. 1 µl of the cDNA template was then used in quantitative real-time PCR reactions using iQ SUYBRE Green Supermix (BioRad) and a iCycler iQ5(BioRad). Primers were designed using Primer3 (http://primer3.sourceforge.net). The cycle of threshold (Ct) was determined for each reaction using the iQ5 Optical System Software 2.0 (BioRad). All qRT-PCR reactions were done in triplicate.
KDPG aldolase activity assay
KDPG aldolase activity was quantified by a lactate dehydrogenase (LDH) coupled assay where the production of pyruvate is related to the NADH consumption, as described in [29]. 2 ml of R. jostii RHA1 RM or MMGls cultures were harvested and resuspended in 1 ml of buffer TrisHCl 100 mM pH 7.5, NaCl 300 mM, EDTA 1 mM, DTT 1 mM and PMSF 1 mM. The cells were lysed using 0.2 mm silica beads and a Fast Prep-24 system (MP Biomedicals) for 3 cycles of 60 s and centrifuged at 100,000g for 25 min at 4 °C. 150 μl aliquots of the resulting RM or MMGls total extracts were then treated with 1 μl of LDH (5 U/μL), 0.70 μl of NADH (50 mM) and 1 μl of KDPG (50 mM). Decrease in NADH absorbance at 340 nm was measured in quartz microcuvettes (150 μl) in a UV-1603 spectrophotometer (Shimadzu) for 5 min. Total protein concentration was determined by Bradford assays using BSA as standard. KDGP activity was calculated as moles of NADH consumed per mg of total protein per second (mol/s/mg).
Results and discussion
Culture conditions for R. jostii RHA1, TAGs accumulation and RNA-Seq analysis
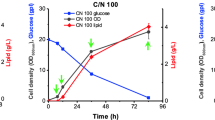
R. jostii RHA1 is able to transform a diverse range of organic substrates into large quantities of TAGs [21]. The best conditions for TAG accumulation in R. opacus occur when gluconate is used as carbon source in a nitrogen-limited medium [30]. We have checked TAG accumulation over time in R. jostii RHA1 cells transferred to M9 medium with 10 mM ammonium chloride and sodium gluconate (20% w/v) as carbon source (MMGln medium, Fig. 1). While TAG accumulation was already detected upon 4 h in MMGln (Fig. 1), no TAG accumulation was observed at any time in a complex rich-nutrient medium (RM). TAGs were also accumulated in an M9 medium with 20 mM ammonium chloride (MMN) and even when MMN was enriched with 0.2% casamino acids (data not shown). Thus, for comparative analysis of the R. jostii transcriptome under conditions that lead or do not lead to TAG accumulation, RNA-Seq analyses were performed on two RNA samples collected from R.jostii RHA1 strain incubated either 24 h in RM medium (exponential phase) or 4 h in MMGln after 48 h in RM medium. cDNA was generated from mRNA-enriched total RNA preparations from each strain and sequenced using the Illumina Genome Analyzer IIx as described in Methods, to yield a total number of 9,611,145 reads for MMGln and 14,330,620 reads for RM (Table 1).
TLC analysis of the crude organic extracts obtained from the R. jostii RHA1 cultures used for RNA-Seq. Cells were grown in RM or MMGln media prepared as described in "Methods" section. Lipids were extracted and separated by TLC on silica gel plates, solvent extract: hexane/2-isopropane acid (3:1 v/v). Lane 1 control trioleine; 2 control oleic acid; 3 Cells grown 4 h in MMGln; 4 Cells grown 8 h in MMGln; 5 Cells grown 24 h in RM. R jostii isolated TAGs are shown by a black arrow
For comparative analysis of the R. jostii transcriptome under conditions that lead or do not lead to TAG accumulation, reads per kilobase (RPK) were calculated for each of the 9145 annotated R jostii genes [22] and normalized for each condition as described in “Methods” section (Additional file 1: Table S1). After data processing, we observed 701 upregulated genes (twofold or greater, MMGln vs RM) and 538 downregulated genes (twofold or greater, MMGln vs RM) (Table 2; Fig. 2a). Whereas the percentage of chromosomal upregulated and downregulated genes was similar (6.3 vs 6.8%), the percentage of plasmid upregulated genes was much higher than the percentage of downregulated genes (13.3 vs. 2.0% in pRHL1, 11.7 vs. 4.4% in pRHL2 and 11.4 vs. 0.9% in pRHL3) (Table 2). Predominant gene upregulation is a common feature of different bacterial stress conditions where a quick response to environmental changes is needed [31]. It is also apparent that, for the whole genome, genes showing high induction predominate over genes showing high repression (Fig. 2b). 42 genes showed eightfold or higher upregulation, while only 8 genes showed eightfold or higher downregulation (Additional file 1: Table S1).
Comparative analysis of R. jostii RHA1 transcriptome under nutrient-rich and nutrient-limiting (TAG accumulating) conditions
For an overview of the metabolic changes that occurred after nutrient deprivation maintaining the carbon source excess, we identified the KEGG pathways [32] corresponding to the up- or downregulated genes. For some functional categories (i.e., oxidative phosphorylation, pentose phosphate, ABC transporters, fatty acid metabolism), upregulated genes predominate (Fig. 3). In contrast, for other categories (i.e., amino acids metabolism and inositol phosphate metabolism), downregulated genes predominate. To better understand the global effects of nutrient deprivation, we looked at specific pathways rather than to functional categories. Downregulation is the rule in several metabolic activities, both catabolic and biosynthetic, as well as in the turnover of macromolecules. Key assimilatory pathways were repressed (Phosphate and sulphate assimilation, synthesis of glutamine synthetase, synthesis of C1-carriers). DNA duplication machinery and several biosynthetic pathways (i.e., pyrimidine, peptidoglycan) were also repressed. With respect to the catabolic pathways, repression occurred in: (i) degradation of several alternative carbon sources and (ii) sugar transport via phosphotransferase system (PTS). Turnover by RNA degradation was also repressed. These downregulated pathways can be interpreted as a result of cells stopping metabolic activities that lead to cell proliferation as a consequence of nutrient starvation.
Other alterations in gene expression can be directly correlated to specific starvation conditions: excess of the carbon source or depletion of the nitrogen source. Hence, significant alterations of metabolic pathways are related to nitrogen starvation: (i) amino acid catabolism is repressed and (ii) reactions that might render free ammonia from organic compounds are induced (i.e., formamidase and ethanolamine ammonia lyase). Finally, a set of metabolic activities are induced as a consequence of the fact that nutrient-starved cells can still incorporate the carbon source leading, for instance, to the synthesis of TAGs. In fact, induction of glycerol-3P-acyltransferase, fatty acid synthesis, acyl-carrier protein and biotin biosynthetic enzymes was observed. The transcriptome analysis of R. opacus PD630 under TAG accumulating conditions has been recently reported [33]. 3 h after cells were transferred to a minimal medium (MSM3) similar to our MMGln medium, 21.15% of the genes were upregulated >2-fold and 9.36% downregulated >2-fold. Globally, genes related to biogenesis were upregulated while genes involved in energy production or carbohydrate metabolism were downregulated. 4273 R. jostii RHA1 homologous genes have been found in R. opacus PD630 chromosome. Most of the upregulated genes in R. jostii MMGln are also upregulated in R. opacus MSM3 (Additional file 1: Table S3), thus confirming the metabolic shift observed for R. jostii under TAG accumulating conditions.
Genes of the Entner-Doudoroff (ED) pathway are highly upregulated
Switching metabolism to the synthesis of TAGs not only requires the upregulation of enzymes specifically involved in the corresponding biosynthetic pathways, but also the upregulation of the corresponding pathways that generate the appropriate building blocks, ATP and reducing power [34]. One of the main functional categories presenting upregulated genes that were activated when R. jostii cells were grown in MMGln was the pentose phosphate pathway (Fig. 3). However, a detailed analysis of the specific genes of this functional category that are upregulated showed them to belong to the ED catabolic pathway. The ED pathway is, in addition to the Embden-Meyerhof-Parnas (EMP) and pentose phosphate pathways, one of three pathways that process 6-carbon sugars [35, 36]. The first step in the ED pathway is the formation of gluconate-6-phosphate by oxidation of glucose-6-phosphate or phosphorylation of gluconate. Then, the 6-phosphogluconate dehydratase catalyzes the dehydration of 6-phosphogluconate to produce KDPG. Finally, the cleavage of KDPG catalysed by the KDPG aldolase yields pyruvate and glyceraldehyde-3-phosphate. Electrons drawn in reactions catalysed by the glucose-6P-dehydrogenase are transferred to NADP+. According to the RNA-Seq transcriptomic analysis, every gene coding for the different enzymes of the ED pathway was highly upregulated in the MMGln conditions (Fig. 4; Table 3).
Consistently, genes involved in ED pathway were also found amongst the genes upregulated in the TAG accumulating medium in R. opacus PD630 (Additional file 1: Table S3).
For RNA-Seq transcriptomic analysis, we used gluconate as a carbon source in MMGln because gluconate led to the highest level of TAG accumulation in R. opacus [30]. Therefore, induction of the ED pathway could be the consequence of the use of gluconate as the sole carbon source and not of a general mechanism for TAG accumulation under nutrient-deprived conditions. To solve this question, we tested whether the presence of glucose in MMGls also induces TAG accumulation and the ED pathway in R. jostii. TAG accumulation in MM containing either glucose or gluconate as carbon source was evaluated by fluorescence measurements using red nile and the Victor-3 fluorometer system (Perkin Elmer). We observed that glucose was also able to induce TAG accumulation in R jostii, but to a lower extent than gluconate (data not shown). Two likely hypotheses to explain this are: (i) only gluconate is able to induce the ED pathway and glucose is metabolized to TAG by the EM pathway, or (ii) glucose is also metabolized by the ED pathway but with a slightly lower yield, because glucose has to be transformed first to gluconate.
To check if glucose was also able to activate the ED pathway under nutrient-limiting conditions, we used RT-qPCR to measure the expression of the most upregulated genes involved in the ED pathway. The expression of these genes was compared in RM and in MM with gluconate or glucose as carbon source. As shown in Table 4, the three selected genes (ro2369: glucose-6-phosphate 1-dehydrogenase, ro02367: KHG/KDPG aldolase, and ro02362: gluconokinase) were again highly upregulated when gluconate was used as carbon source in the nutrient-limited medium. Interestingly, similar upregulation was observed when the MM contained glucose instead of gluconate. Thus, the ED is also activated with glucose as carbon source supporting that the activation is due to the metabolic stress and not due to the use of gluconate as carbon source. We have selected the gene ro00588 (cold shock protein) as control or housekeeping gene. Expression of this gene led to a 1.008 fold change (MMGln vs RM) in RNA-Seq and it was also almost unaffected in any of the three used media in the RT-qPCR experiment (Table 4).
We have also analysed the enzymatic activity of the KHG/KDPG aldolase in crude extracts of R. jostii RHA1 grown on MMGls or RM as described in Methods. In accordance with the transcriptomic results, KDPG aldolase activity (Additional file 2: Figure S1) was 8.75 times higher in MMGls (3.5 nmol/s/mg) than in RM (0.4 nmol/s/mg).
Catabolism of the carbon source (either glucose or gluconate) by the ED pathway renders two moles of pyruvate per mole of carbon source. One mole of ATP is generated also. However, generation of reduced coenzymes depends on the carbon source. Whereas catabolism of 1 mol of glucose by the ED pathway generates 1 mol NADPH and 1 mol NADH, catabolism of gluconate generates only 1 mol NADH (see below).
Energy and redox metabolism in R. jostii RHA1 cells grown in MMGln
More than 30 genes that code for proteins of the oxidative phosphorylation process are upregulated and none of these genes is downregulated (Fig. 3). More specifically, the upregulated genes mainly code for subunits of the complex I or NADH dehydrogenase, while the genes of the F1-ATPase remain unchanged. Hence, respiratory activity may provide part of the ATP required for TAG biosynthesis.
The highest transcriptional repression was observed for the ro03923 gene coding for a NADPH dehydrogenase (Table 5). Oxidation of glucose to pyruvate by the EMP has a net yield of 2 ATP and 2 NADH per molecule of glucose. In contrast, if the ED pathway is used, the net yield is 1 ATP, 1 NADH and 1 NADPH per molecule of glucose. It should be pointed out here that if, instead of glucose, gluconate is oxidized by the ED pathway, the net yield should be 1 ATP and 1 NADH per molecule of gluconate (see Fig. 4). According to [37], the synthesis of fatty acids requires stoichiometric amounts of ATP and acetyl-CoA, NADPH and NADH for each C2 addition. Considering that catabolism of gluconate to pyruvate by the ED pathway renders NADH and not NADPH, there is a requirement for this latter reduced coenzyme for TAG biosynthesis. This may explain the downregulation of the NADPH dehydrogenase (ro03923, 0.06x).
Different metabolic pathways lead to acetyl-CoA generation from pyruvate. Pyruvate dehydrogenase, partially repressed, may account for the conversion of a fraction of the total pyruvate available to acetyl-CoA. Induction of other enzymes, such as acetyl-CoA synthase (8 homologs in RHA1 like ro04332 and ro11190, 6.9× and 5.9× upregulated, respectively) (Additional file 1: Table S1), that can generate acetyl-CoA from acetate without a requirement for NAD+ suggests that a fraction of the available pyruvate could be converted to acetyl-CoA by enzymes that do not generate NADH.
Induction of the Kennedy pathway for TAG accumulation
The glyceraldehyde-3-phosphate generated by the ED enzyme KDPG aldolase could be used for pyruvate formation, but also for conversion to dihydroxyacetone-phosphate by a reaction catalyzed by the triose-phosphate isomerase enzyme (TpiA). Then, the dihydroxyacetone-phosphate intermediate may be converted into glycerol-3-phosphate by a NAD(P)-dependent glycerol-3-phosphate dehydrogenase enzyme (GpsA). Glycerol-3-phosphate is later sequentially acylated, after removing the phosphate group, to form TAG (Kennedy pathway). Interestingly, the genes tpiA (ro07179, 1.76×) and gpsA (ro06505, 1.78×) were both upregulated to some extent by cells during cultivation in nutrient starvation conditions. Moreover, genes involved in the de novo fatty acid biosynthesis were also upregulated. An acetyl-CoA carboxylase enzyme (ACC) coded by ro04222 (2.36×) was significantly induced in starved cells. ACC catalyzes the formation of malonyl-CoA molecules, which are used for fatty acid biosynthesis by the enzymatic complex known as fatty acid synthase I (FAS-I). FAS-I, a unique, large protein with different catalytic activities, is responsible for fatty acid biosynthesis in rhodococci, which are used for phospholipids and TAG synthesis. FAS-I coded by ro01426 (2.81×) was highly upregulated in cells under nutrient starvation conditions. Although the genes coding for several enzymes of the Kennedy pathway were not significantly upregulated in MMGln, some of the diacylglycerol acyltransferase genes were indeed upregulated (Fig. 5). The acyltransferase enzymes involved in the upper reactions of the Kennedy pathway were slightly upregulated in MMGln, such as ro05648 (GPAT) 1.99×, ro01115 (AGPAT) 1.67×, and ro05647 (AGPAT) 1.70× (Fig. 5 and Additional file 1: Table S1). Wax ester synthase/acyl coenzyme A:diacylglycerol acyltransferases (WS/DGATs) are key bacterial enzymes that catalyze the final step of TAG biosynthesis (acylation of DAG intermediates). Fourteen WS/DGAT genes were identified in R. jostii [21]. The WS/DGAT genes ro05356 (Atf8) and ro02966 (Atf7) were upregulated almost sixfold and fourfold, respectively. Indeed, atf8 transcripts were also the most abundant WS/DGAT transcripts during RHA1 grow on benzoate under nitrogen-limiting conditions, being this enzyme determinant for TAG accumulation [16]. Moreover, the genes ro01601 (Atf6) and ro05649 (Atf9) were expressed 2 times more in MMGln than in RM. These four WS/DGAT enzymes are expected to be specifically involved in the TAG synthesis. Finally, ro02104 (tadA), another gene described to be involved in TAG accumulation, was upregulated 3.7 times in MMGln (Additional file 1: Table S1). TadA is a predicted apolipoprotein associated with lipid droplets in R. jostii RHA1 [38] and R. opacus PD630 [33]. TadA mutant was described to accumulate 30–40% less TAG than the parental R. opacus PD630 strain [39]. This protein may mediate lipid body formation in TAG-accumulating rhodococcal cells with a similar structural role than apolipoproteins in eukaryotes [39].
Putative CRP binding sites are present in the highly expressed genes
Alternative sigma factors such as sigma54 are widely used in bacteria as a quick response to cope with environmental changes such as nutrient deprivation. To find if these alternative factors are being used for the upregulation of the R. jostii genes in MMGln, the program BPROM (http://www.softberry.com/) for the recognition of sigma70 promoters was used with the 150 bp immediately upstream from each ORF start. A putative sigma70 binding site was found in most upregulated genes. Hence, regulatory element(s) alternative to sigma70 subunit must be responsible for the transcriptional activation of the R. jostii genes in MMGln. These element(s) should target conserved binding sites in some of the altered genes.
The identification and localization of conserved sequences within the upstream regions of the upregulated genes was performed by the MEME Suite [40]. The consensus sequence 5′-GTGANNTGNGTCAC-3′ was found in almost every promoter region of the 40 highest upregulated genes, as shown in Additional file 1: Table S2 and Fig. 6a. This conserved sequence is identical to the cAMP Receptor Protein (CRP) consensus binding site found either in E. coli (5′-tGTGANNNNNNTCACa-3′, [41]) or Pseudomonas aeruginosa (5′-ANWWTGNGAWNYAGWTCACAT-3′ [42]. Moreover, the protein coded by ro04321 is 90% identical (Fig. 6b) to the corresponding CRP protein in Mycobacterium tuberculosis [43]. Structural modelling by Phyre 2 [44] of the putative R. jostii CRP correctly predicts a CRP fold with 223 residues (92%) modelled at >90% accuracy.
a Conserved sequences found by using the meme program within the 11 most upregulated R. jostii promoters in MMGln. The consensus sequence is also shown. b Alignment of the R. jostii putative CRP sequence (YP_704269) with the CRP sequences of E. coli (PDB 1O3Q), P. Aeruginosa (PDB 2OZ6) and M. tuberculosis (PDB 3D0S)
Bacterial CRPs are transcription factors that respond to cAMP by binding at target promoters when cAMP concentration increases. 254 CRP-binding sites have been found in E. coli, regulating at least 378 promoters [41]. In R. jostii, 371 putative CRP binding sites have been found (Additional file 1: Table S2). Thus, there is a CRP binding site per, approximately, each 25 genes. However, the density increases significantly up to 1 site per 4 genes in the genes that we identified as highly upregulated (eightfold or greater) when Rhodococcus cells grow in MMGln. Specifically, in all the promoters controlling genes involved in the ED pathway there is at least one CRP binding site. Most of these promoters are divergent promoters and both of the controlled operons are upregulated. Moreover, CRP binding sites have also been found in the promoter regions of the two main upregulated WS/DGAT genes (ro05356 and ro02966), but not in the promoter regions of the other WS/DGAT genes. Strikingly, the promoter regions of the most upregulated operons in R. opacus PD630 also contain a CRP putative binding sequence (Additional file 1: Table S3).
In E. coli, gluconate was shown to lower both CRP and cAMP to nearly the same extent as glucose [45]. Hence, it is likely that in R. jostii, the predicted cAMP increase, rather than being related to the carbon source, is related to the stress generated by depletion of nutrients.
We also searched for the presence of a CRP binding site in the upstream regulatory region of the orthologs of the 40 Rhodococcus genes in other microorganisms using the MEME Suite (Additional file 1: Table S4). According to the results, it seems that the CRP mediated activation of the ED pathway is only conserved in R. opacus, also an oleogenic rhodococci. CRP binding sites were also found in the promoter regions of a few genes in the other two Rhodococcus genomes analyzed (R. equi and R. erythropolis). However, no consensus CRP binding sequence was found in the promoter regions of the orthologous genes in Escherichia coli or Pseudomonas putida. We have also searched without success for CRP binding sites in similar operons of non-oleaginous organisms containing WS/DGAT enzymes, such as Mycobacterium tuberculosis, Acinetobacter baumanii or Marinobacter aquaolei. Thus, it seems the upregulation of these R. jostii genes by CRP is related to the TAG accumulation.
Conclusions
Different microorganisms are able to accumulate TAGs or other neutral lipids to serve as carbon and energy sources during starvation. One of these microorganisms is R. jostii strain RHA1. Transcriptomic analysis of R. jostii RHA1 under conditions that lead or do not lead to TAG accumulation allowed us to identify the metabolic pathways that are relevant for oxidation of the carbon source, biosynthesis and TAG accumulation under nutrient-deprivation.
Two interesting results arose from our work. First, under nutrient-deprivation, Rhodococcus metabolizes carbohydrates such as glucose or gluconate by the Entner-Doudoroff pathway. Up- or downregulation of other key enzymes (i.e., pyruvate dehydrogenase, acetyl CoA synthetase, NADH oxidase), provides the ATP, reducing equivalents and building blocks for TAG synthesis. Second, the metabolic shift is likely driven by an increase in cAMP concentration that activates the expression of several operons via CRP.
Both observations could help in engineering metabolic modifications to improve TAG yield for biotechnological applications.
References
Charpe TW, Rathod VK. Biodiesel production using waste frying oil. Waste Manag. 2011;31:85–90.
Hawley C. Criticism mounts against biofuels. BusinessWeek: Europe [Internet]. 2008. http://www.businessweek.com/globalbiz/content/jan2008/gb20080124_071995.htm. Accessed 24 Oct 2011.
Shi S, Valle-Rodríguez JO, Siewers V, Nielsen J. Prospects for microbial biodiesel production. Biotechnol J. 2011;6:277–85.
Ratledge C, Cohen Z. Microbial and algal oils: do they have a future for biodiesel or as commodity oils? Lipid Technol. 2008;20:155–60.
Vicente G, Bautista LF, Gutiérrez FJ, Rodríguez R, Martínez V, Rodríguez-Frómeta RA, et al. Direct transformation of fungal biomass from submerged cultures into biodiesel. Energy Fuels. 2010;24:3173–8.
Cohen Z, Ratledge C. Single cell oils: microbial and algal oils. 2nd ed. Champaign: AOCS Publishing; 2010.
Steinbüchel A, Hustede E, Liebergesell M, Pieper U, Timm A, Valentin H. Molecular basis for biosynthesis and accumulation of polyhydroxyalkanoic acids in bacteria. FEMS Microbiol Lett. 1992;103:217–30.
Alvarez HM, Steinbüchel A. Triacylglycerols in prokaryotic microorganisms. Appl Microbiol Biotechnol. 2002;60:367–76.
Kalscheuer R, Steinbüchel A. A novel bifunctional wax ester synthase/acyl-CoA:diacylglycerol acyltransferase mediates wax ester and triacylglycerol biosynthesis in Acinetobacter calcoaceticus ADP1. J Biol Chem. 2003;278:8075–82.
Daniel J, Deb C, Dubey VS, Sirakova TD, Abomoelak B, Morbidoni HR, et al. Induction of a novel class of diacylglycerol acyltransferases and triacylglycerol accumulation in Mycobacterium tuberculosis as it goes into a dormancy-like state in culture. J Bacteriol. 2004;186:5017–30.
Kaddor C, Biermann K, Kalscheuer R, Steinbüchel A. Analysis of neutral lipid biosynthesis in Streptomyces avermitilis MA-4680 and characterization of an acyltransferase involved herein. Appl Microbiol Biotechnol. 2009;84:143–55.
Alvarez HM, Kalscheuer R, Steinbüchel A. Accumulation and mobilization of storage lipids by Rhodococcus opacus PD630 and Rhodococcus ruber NCIMB 40126. Appl Microbiol Biotechnol. 2000;54:218–23.
Gouda MK, Omar SH, Aouad LM. Single cell oil production by Gordonia sp. DG using agro-industrial wastes. World J Microbiol Biotechnol. 2008;24:1703–11.
Bell KS, Philp JC, Aw DWJ, Christofi N. A review: the genus Rhodococcus. J Appl Microbiol. 1998;85:195–210.
van der Geize R, Dijkhuizen L. Harnessing the catabolic diversity of rhodococci for environmental and biotechnological applications. Curr Opin Microbiol. 2004;7:255–61.
Ahmad M, Taylor CR, Pink D, Burton K, Eastwood D, Bending GD, et al. Development of novel assays for lignin degradation: comparative analysis of bacterial and fungal lignin degraders. Mol BioSyst. 2010;6:815–21.
Alvarez HM. Biotechnological Production and Significance of Triacylglycerols and Wax Esters. In: Timmis KN, editor. Handbook of Hydrocarbon and Lipid Microbiology [Internet]. Springer Berlin Heidelberg; 2010. p. 2995–3002. http://link.springer.com/referenceworkentry/10.1007/978-3-540-77587-4_222. Accessed 7 Feb 2017.
Seto M, Kimbara K, Shimura M, Hatta T, Fukuda M, Yano K. A novel transformation of polychlorinated biphenyls by Rhodococcus sp. strain RHA1. Appl Environ Microbiol. 1995;61:3353–8.
Navarro-Llorens JM, Patrauchan MA, Stewart GR, Davies JE, Eltis LD, Mohn WW. Phenylacetate catabolism in Rhodococcus sp. strain RHA1: a central pathway for degradation of aromatic compounds. J Bacteriol. 2005;187:4497–504.
Patrauchan MA, Florizone C, Dosanjh M, Mohn WW, Davies J, Eltis LD. Catabolism of benzoate and phthalate in Rhodococcus sp. strain RHA1: redundancies and convergence. J Bacteriol. 2005;187:4050–63.
Hernández M, Mohn W, Martínez E, Rost E, Alvarez A, Alvarez H. Biosynthesis of storage compounds by Rhodococcus jostii RHA1 and global identification of genes involved in their metabolism. BMC Genom. 2008;9:600.
McLeod MP, Warren RL, Hsiao WWL, Araki N, Myhre M, Fernandes C, et al. The complete genome of Rhodococcus sp. RHA1 provides insights into a catabolic powerhouse. Proc Natl Acad Sci USA. 2006;103:15582–7.
Sambrook J. Molecular cloning: a laboratory manual, Third Edition. 3rd ed. New york: Cold Spring Harbor Laboratory Press; 2001.
Langmead B, Trapnell C, Pop M, Salzberg S. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009;10:R25.
Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, et al. The sequence alignment/map format and SAMtools. Bioinformatics. 2009;25:2078–9.
Rutherford K, Parkhill J, Crook J, Horsnell T, Rice P, Rajandream MA, et al. Artemis: sequence visualization and annotation. Bioinformatics. 2000;16:944–5.
Bullard J, Purdom E, Hansen K, Dudoit S. Evaluation of statistical methods for normalization and differential expression in mRNA-Seq experiments. BMC Bioinform. 2010;11:94.
Anders S, Huber W. Differential expression analysis for sequence count data. Genome Biol. 2010;11:R106.
Chen X, Schreiber K, Appel J, Makowka A, Fähnrich B, Roettger M, et al. The Entner-Doudoroff pathway is an overlooked glycolytic route in cyanobacteria and plants. Proc Natl Acad Sci USA. 2016;113:5441–6.
Alvarez HM, Mayer F, Fabritius D, Steinbüchel A. Formation of intracytoplasmic lipid inclusions by Rhodococcus opacus strain PD630. Arch Microbiol. 1996;165:377–86.
Wall ME, Hlavacek WS, Savageau MA. Design of gene circuits: lessons from bacteria. Nat Rev Genet. 2004;5:34–42.
Kanehisa M, Goto S, Sato Y, Furumichi M, Tanabe M. KEGG for integration and interpretation of large-scale molecular data sets. Nucleic Acids Res. 2011;40:D109–14.
Chen Y, Ding Y, Yang L, Yu J, Liu G, Wang X, et al. Integrated omics study delineates the dynamics of lipid droplets in Rhodococcus opacus PD630. Nucleic Acids Res. 2014;42:1052–64.
Dávila Costa JS, Herrero OM, Alvarez HM, Leichert L. Label-free and redox proteomic analyses of the triacylglycerol-accumulating Rhodococcus jostii RHA1. Microbiology (Reading, Engl.). 2015;161:593–610.
Conway T. The Entner-Doudoroff pathway: history, physiology and molecular biology. FEMS Microbiol Lett. 1992;103:1–27.
Peekhaus N, Conway T. What’s for dinner?: Entner-Doudoroff metabolism in Escherichia coli. J Bacteriol. 1998;180:3495–502.
Alvarez H. Biology of Rhodococcus. Berlin, Heidelberg: Springer; 2010.
Ding Y, Yang L, Zhang S, Wang Y, Du Y, Pu J, et al. Identification of the major functional proteins of prokaryotic lipid droplets. J Lipid Res. 2012;53:399–411.
MacEachran DP, Prophete ME, Sinskey AJ. The Rhodococcus opacus PD630 heparin-binding hemagglutinin homolog TadA mediates lipid body formation. Appl Environ Microbiol. 2010;76:7217–25.
Bailey TL, Boden M, Buske FA, Frith M, Grant CE, Clementi L, et al. MEME SUITE: tools for motif discovery and searching. Nucleic Acids Res. 2009;37:W202–8.
Shimada T, Fujita N, Yamamoto K, Ishihama A. Novel roles of cAMP receptor protein (CRP) in regulation of transport and metabolism of carbon sources. PLoS ONE. 2011;6:e20081.
Kanack KJ, Runyen-Janecky LJ, Ferrell EP, Suh S-J, West SEH. Characterization of DNA-binding specificity and analysis of binding sites of the Pseudomonas aeruginosa global regulator, Vfr, a homologue of the Escherichia coli cAMP receptor protein. Microbiology (Reading, Engl.). 2006;152:3485–96.
Stapleton M, Haq I, Hunt DM, Arnvig KB, Artymiuk PJ, Buxton RS, et al. Mycobacterium tuberculosis cAMP receptor protein (Rv3676) differs from the Escherichia coli paradigm in Its cAMP binding and DNA binding properties and transcription activation properties. J Biol Chem. 2010;285:7016–27.
Kelley LA, Sternberg MJE. Protein structure prediction on the Web: a case study using the Phyre server. Nat Protoc. 2009;4:363–71.
Hogema BM, Arents JC, Inada T, Aiba H, Van Dam K, Postma PW. Catabolite repression by glucose 6-phosphate, gluconate and lactose in Escherichia coli. Mol Microbiol. 1997;24:857–67.
Authors’ contributions
AJ analysed data and wrote the article, JAV performed the experiments, VF carried out the bioinformatics analysis, BL performed the experiments, FC wrote the article, HMA analysed data and wrote the article and GM designed research, analysed data and wrote the article. All authors read and approved the final manuscript.
Acknowledgements
We are grateful to Dr. Juan Maria Garcia-Lobo and Dr. Maria Cruz Rodriguez for RNA-Seq analysis performed in the massive sequencing service at the IBBTEC. We thank Dr. Lindsay Eltis for the gift of the strain R. jostii RHA1.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
All relevant data are presented in the main paper and Additional file 1.
Funding
This work was financed by Grants BIO2010-14809 from the Spanish Ministry of Science and Innovation and BFU2014-55534-C2-2-P from the Spanish Ministry of Economy and Competitiveness to GM. H.M. Alvarez is a career investigator of the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Argentina.
Author information
Authors and Affiliations
Corresponding author
Additional files
12934_2017_651_MOESM1_ESM.xlsx
Additional file 1: Table S1. RPK values of all genes. Table S2. CRP binding sequences in R. jostii. Table S3. R. jostii RHA1comparison to R. opacus PD630. Table S4. CRP binding sequences in rhodoccoci.
12934_2017_651_MOESM2_ESM.tif
Additional file 2: Figure S1. (A) Kinetic determination of KDPG aldolase activity in MMGls and RM. (B). KDPG aldolase activity calculated from the kinetic curves. Error bars show the standard deviation from three independent experiments.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Juarez, A., Villa, J.A., Lanza, V.F. et al. Nutrient starvation leading to triglyceride accumulation activates the Entner Doudoroff pathway in Rhodococcus jostii RHA1. Microb Cell Fact 16, 35 (2017). https://doi.org/10.1186/s12934-017-0651-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12934-017-0651-7