Abstract
Methanococcus maripaludis is a rapidly growing, fully sequenced, genetically tractable model organism among hydrogenotrophic methanogens. It has the ability to convert CO2 and H2 into a useful cleaner energy fuel (CH4). In fact, this conversion enhances in the presence of free nitrogen as the sole nitrogen source due to prolonged cell growth. Given the global importance of GHG emissions and climate change, diazotrophy can be attractive for carbon capture and utilization applications from appropriately treated flue gases, where surplus hydrogen is available from renewable electricity sources. In addition, M. maripaludis can be engineered to produce other useful products such as terpenoids, hydrogen, methanol, etc. M. maripaludis with its unique abilities has the potential to be a workhorse like Escherichia coli and S. cerevisiae for fundamental and experimental biotechnology studies. More than 100 experimental studies have explored different specific aspects of the biochemistry and genetics of CO2 and N2 fixation by M. maripaludis. Its genome-scale metabolic model (iMM518) also exists to study genetic perturbations and complex biological interactions. However, a comprehensive review describing its cell structure, metabolic processes, and methanogenesis is still lacking in the literature. This review fills this crucial gap. Specifically, it integrates distributed information from the literature to provide a complete and detailed view for metabolic processes such as acetyl-CoA synthesis, pyruvate synthesis, glycolysis/gluconeogenesis, reductive tricarboxylic acid (RTCA) cycle, non-oxidative pentose phosphate pathway (NOPPP), nitrogen metabolism, amino acid metabolism, and nucleotide biosynthesis. It discusses energy production via methanogenesis and its relation to metabolism. Furthermore, it reviews taxonomy, cell structure, culture/storage conditions, molecular biology tools, genome-scale models, and potential industrial and environmental applications. Through the discussion, it develops new insights and hypotheses from experimental and modeling observations, and identifies opportunities for further research and applications.
Similar content being viewed by others
Background
Methanococci are non-pathogenic, strictly anaerobic, hydrogenotrophic archaebacteria isolated from marine environments. Some are mesophilic, and others are thermophilic or hyperthermophilic [1]. The mesophilic methanococci are divided into four species: Methanococcus maripaludis, M. vannielii, M. voltae, and M. aeolicus [2]. In this article, we focus on M. maripaludis, whose type strain M. maripaludis JJ was isolated from salt marsh sediments in South Carolina [3]. Thenceforth, numerous strains have been isolated from estuarine sites in South Carolina, Georgia, and Florida [4]. Table 1 lists the characteristics of five fully sequenced strains (S2, C5, C6, C7 and X1).
M. maripaludis is a fast growing mesophilic microbe with a doubling time of 2 h and optimum growth temperature at 38 °C. It reduces CO2 to methane via a modified Wood-Ljungdahl pathway, also known as Wolfe cycle [5]. Unlike other microorganisms that need complex carbon substrates such as pentoses, hexoses, alcohols, and their derivatives for their growth, M. maripaludis can use a simple substrate such as CO2 as the sole carbon source, and N2 as the sole nitrogen source [6]. However, it needs H2 or formate (HCOOH) for energy [1, 7, 8]. In other words, given a renewable source of H2, M. maripaludis has the potential to capture and convert the main source of global climate concerns, namely the CO2 emissions, into a useful fuel (methane).
Over the years, M. maripaludis S2 has become a well-studied model organism in the literature [9, 10] with well-developed genetic tools. However, in spite of more than 100 publications exploring its genetics and biochemistry, a comprehensive review of its metabolic processes is missing in the literature. This article presents a holistic and integrated view of its metabolic processes, and suggests some potential applications for this promising organism. In this article, we divide the entire metabolism of M. maripaludis into eight subsystems. We discuss six of them in detail in the main text, but defer, for the sake of brevity, the remaining two along with other relevant topics such as taxonomy and cultivation to Additional file 1. For each subsystem, we describe the key steps and their salient features, and identify the existing gaps in the metabolism. Then, we discuss molecular biology tools for manipulating the genome of M. maripaludis, and some limited systems biology work. Finally, we highlight potential applications for M. maripaludis.
Methanococcus maripaludis—cell structure
Figure 1 shows a simplistic view of the M. maripaludis cell. It is a weakly-motile coccus of 0.9–1.3 µm diameter [3]. This non-spore forming mesophile grows best between 20 and 45 °C at a pH ranging from 6.5 to 8.0 [11]. As in Fig. 1, its cell wall is a single, electron-dense, proteinaceous S-layer lacking peptidoglycan molecules. The S-layer proteins and flagellins have been discussed in the literature [12, 13]. Its cell wall lyses rapidly in low concentrations of detergents [3] and low-osmolarity buffers [14], which makes the isolation of DNA easier. Despite the differences in the presence of some amino acids, the primary structure of S-layer proteins shows a high degree of identity (38–45 %) with other microbes [15]. Ether lipids recovered from M. maripaludis mainly include glycolipids [14.2 mg ether lipid/g dry cell weight (DCW)] and polar lipids (0.4 mg ether lipid/g DCW) [16]. The motility in methanococci is due to the presence of flagella, however strong attachments by M. maripaludis to various surfaces require both flagella and pili [17]. Although the specific roles of the pili in M. mariplaudis are still unknown [18], if archaeal pili are similar to their bacterial counterparts, then they could be involved in functions related to cell-to-cell twitching, motility, attachment, biofilm formation, etc.
Figure 2 provides a comprehensive and consolidated picture of the metabolic system of M. maripaludis. As shown, it has eight major subsystems: methanogenesis, reductive tricarboxylic acid (RTCA) cycle, non-oxidative pentose phosphate pathway (NOPPP), glucose/glycogen metabolism, nitrogen metabolism, amino acid metabolism, and nucleotide metabolism. Methanogenesis, or the reduction of CO2 to methane, being its only pathway for energy generation, forms the foundation for its survival and growth [19, 20]. In other words, both methanogenesis and cell growth compete for the carbon source. The remaining seven subsystems provide the essential precursors for cell growth via two key intermediates: acetyl CoA and pyruvate. We now discuss the first six subsystems in detail.
Simplistic overview of eight major metabolic subsystems in M. maripaludis. HCOOH formate, [CO] enzyme-bound CO, Methyl-THMPT methyl-tetrahydromethanopterin, Acetyl-CoA acetyl coenzyme A, TCA tricarboxylic acid cycle, NOPPP non-oxidative pentose phosphate pathway, PRPP 5-Phosphoribosyl diphosphate, G3P glyceraldehyde-3-phosphate, F6P fructose-6-phosphate
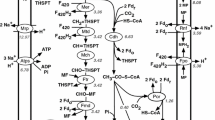
Methanogenesis
Methanogenesis (Fig. 3) is the biological production of methane via the reduction or disproportionation of relatively simpler carbon substrates such as CO2, formate, acetate, and methanol as follows.
While formate, acetate, and methanol can oxidize/reduce by themselves, CO2 needs an electron donor such as H2 [3], formate [21], or electricity [22]. In M. maripaludis, methanogenesis occurs via the reduction of CO2 with H2/formate/electricity, or the disproportionation of formate. Lohner et al. [22] demonstrated H2-independent electromethanogenesis from CO2 in both wild-type M. maripaludis strain S2 and hydrogenase mutant strain MM1284. The mutant strain under the same conditions showed a factor of 10 lower methane production rates as compared to the wild-type strain S2 [22]. However, their attempts to prove biomass growth were inconclusive.
Energy producing pathway in M. maripaludis. F420 coenzyme F420, Vhu/Vhc F420 non-reducing hydrogenase, Fru/Frc F420 reducing hydrogenase, Fdh formate dehydrogenase, Hdr heterodisulfide reductase, Fwd/Fmd tungsten/molybdenum containing formylmethanofuran dehydrogenase, EchA energy-converting hydrogenase A, EchB energy-converting hydrogenase B, Fd(ox) oxidized ferredoxin, Fd (rd) reduced ferredoxin, Ftr formyltransferase, THMPT tetrahydromethanopterin, Mch methyleneTHMPT cyclohydrolase, Mtd methyleneTHMPT dehydrogenase, Hmd 5,10-methenylTHMPT hydrogenase, Mer methyleneTHMPT reductase, Mtr methyltransferase, Mcr methyl-COM reductase, HS-COM coenzyme M (2-mercaptoethanesulfonate), Methyl-S-COM 2-(Methylthio)coenzymeM, SH-CoB thio-coenzyme B, COM-S-S-COB coenzyme M 7-mercaptoheptanoylthreonine-phosphate heterodisulfide
The formate-dependent methanogenesis involves an additional endergonic step where formate is oxidized to CO2 via formate dehydrogenase with a simultaneous reduction of coenzyme F420 [23]. The reduced coenzyme F420 serves as the electron carrier for two intermediary steps in methanogenesis, but H2 is probably not an intermediate [21, 24]. As shown in Fig. 3, the resulting CO2 feeds into the first step of methanogenesis.
A recent study [8] showed the effects of H2 and formate limitation/excess on growth yield and regulation of methanogenesis using a continuous culture of M. maripaludis. They concluded that the growth yield (g DCW/mol CH4) decreased remarkably with excess H2 or formate. While they speculated energy spilling or dissipation to be a possible cause, the exact cause is still unclear.
While M. maripaludis can also assimilate other carbon substrates, such as acetate and pyruvate, they are not physiologically relevant for methane production [25, 26]. No methane production from acetate (17), and extremely low methane from pyruvate [26] (only 1–4 % compared to that for H2) have been reported.
Mechanism
The structures and functions of the cofactors and coenzymes involved in methanogenesis are listed in Table 2. The first step in methanogenesis is the reduction of CO2. It involves the simultaneous oxidation of low-potential reduced ferredoxins and capture of CO2 by methanofuran (MFR) to form formyl-MFR (ΔG0 = 0 kJ/mol) [27]. These extremely low- potential ferredoxins could come from two pools [28]. One is EchA that not only uses one H2, but also consumes proton-motive force (PMF) to generate ferredoxins. This accounts for only 4 % of the reduced ferredoxins as shown in a ∆5H2ase mutant [24]. The second and the major pool is Vhu/Hdr bifurcation complex that consumes two H2 and generates one pair of relatively high potential electrons to reduce CoB-S-S-CoM and another pair of extremely low potential electrons to reduce the ferredoxins. The formyl group from formyl-MFR is then transferred to THMPT (ΔG0 = −5 kJ/mol) to form formyl-THMPT, and the latter is then dehydrated to methenyl-THMPT (ΔG0 = −5 kJ/mol) [29]. In the next two steps, the reduced F420 gets oxidized by supplying electrons to reduce methenyl-THMPT to methylene-THMPT (ΔG0 = +6 kJ/mol) and methylene-THMPT to methyl-THMPT (ΔG0 = −6 kJ/mol) [30]. These reactions are fully reversible, as evidenced by their near-zero free energy changes. The oxidized F420 is then reduced (ΔG0 = −11 kJ/mol) in the presence of H2 [27]. Next, the methyl group from methyl-THMPT is transferred to coenzyme M (HS-CoM) in an exergonic step (ΔG0 = −30 kJ/mol) coupled with 2Na+ translocation by a membrane-bound enzyme complex [31]. This reaction builds up an electrochemical Na+ gradient, which drives energy production via ATP synthase [27]. The final step of methanogenesis is the reductive demethylation of methyl-S-CoM to methane and CoM-S-S-CoB (ΔG0 = −30 kJ/mol). Subsequently, this CoM-S-S-CoB gets reduced with the help of H2 to form HS-CoM and HS-CoB (ΔG0 = −39 kJ/mol) [32]. This reduction of CoM-S-S-CoB mediates via an electron bifurcation mechanism [27]. This step along with the earlier step involving the Na+ translocation supplies the major energy demand of M. maripaludis.
Hydrogenases
The key to the survival of M. maripaludis on CO2 is its ability to take up external H2 and generate electrons from \({\text{H}}_{ 2} \, \to {\text{ 2H}}^{ + } + {\text{ 2e}}^{ - }\) with the help of seven hydrogenases (Fig. 3). These are Fru, Frc, Vhu, Vhc, Hmd, EchA, and EchB, which can be categorized in different manners. The first five are cytoplasmic and the last two are membrane-bound [33]. Fru and Frc use cofactor F420 [34]; Vhu and Vhc use ferredoxin and CoM/CoB [35]; Hmd uses direct H2 [34]; and EchA and EchB use ferredoxins as electron carriers [24]. M. maripaludis needs four pairs of electrons to reduce one mole of CO2 to methane. Fru/Frc can supply two pairs, Vhu/Vhc can supply two pairs, Hmd can supply one pair, and EchA/EchB can supply one pair each.
Of the above, Fru/Frc and Vhu/Vhc play a major role in H2 uptake. Fru and Frc reduce two molecules of coenzyme F420 with the help of two H2 [34]. One F420 (rd) gets oxidized by reducing methenyl-THMPT and the other by reducing methylene-THMPT. Vhu and Vhc facilitate the flow of electrons from H2 to heterodisulfide reductase (Hdr) complex [35], which in turn catalyzes the reductions of CoM/CoB and ferredoxins via an electron bifurcation mechanism [32]. These reduced ferredoxins are the major electron suppliers during the first step of methanogenesis.
Hmd uses H2 to reduce methenyl-THMPT to methylene-THMPT [34] without any carrier. As shown in Fig. 3, Mtd also catalyzes the same reaction, but with the help of reduced F420 as an electron carrier. Hendrickson et al. [34] demonstrated that during the growth on H2 and CO2, Hmd is not essential in the presence of active Fru/Frc, but is essential otherwise. In contrast, the ΔFru, ΔFrc, and ΔHmd mutants grew normally during formate-dependent growth, proving that formate acted as the electron donor.
EchA generates a small portion of low-potential reduced ferredoxins required for the first step of methanogenesis. Its role in M. maripaludis is anaplerotic, because it is required only under certain conditions such as (1) to replenish the intermediates of methanogenesis cycle, and (2) imperfect coupling during electron bifurcation [24]. Lie et al. [24] showed this by eliminating all nonessential pathways of H2 metabolism and using formate as the sole electron donor. In this case, both Hdr complex and EchA independently provided the electrons for growth.
In contrast, EchB supplies electrons to anabolic oxidoreductases for the synthesis of precursors such as pyruvate and acetyl CoA [33, 36]. EchB mutants affect the autotrophic growth severely, but it is unclear how they still survive. When conditions limit growth, anabolic CO2 fixation is unimportant, but methanogenesis continues. Under such a scenario, EchA is essential, but EchB could be detrimental [24].
During formate-dependent growth, the H2 required for the essential anaplerotic (EchA) and anabolic (EchB) functions is produced from formate. This H2 production can occur via two pathways as demonstrated by Lupa et al. [23] in M. maripaludis. One involves Fdh1-Fru/Frc, and the other involves Fdh1-Mtd-Hmd. Of these two, the former seems to be predominant (~90 %), as the deletion of either Fdh1 or Fru/Frc reduced H2 production rates severely [23].
Energy generation/conservation
In most organisms, electron movement along the cell membrane is the key to energy transduction. Substrate oxidation releases electrons that move along the membrane-bound cytochrome carriers and extrudes protons out of the cell to generate a potential gradient. The potential difference drives the protons back into the cell, while at the same time synthesizing ATP from ADP and Pi via ATP synthase [37]. Hydrogenotrophic methanogens such as M. maripaludis lack such an electron transport chain [38]. In place of cytochrome carriers, M. maripaludis uses methyl-THMPT: HS-COM methyltransferase (Mtr), the only membrane-bound enzyme complex in the core methanogenic pathway, to extrude Na+/H+ out of the cell [27, 31]. This creates a Na+/H+ ion motive force (positive outside), which on their translocations into the cell generate ATP via an A1A0-type ATP synthase [39]. However, a direct experimental evidence specifically for Na+ or H+ gradient does not exist in the literature. To conserve ATP, M. maripaludis uses reduced ferredoxins as low-potential electron carriers for the highly endergonic reduction of CO2 to formyl-MFR. As discussed earlier, these ferredoxins are supplied predominantly by the Hdr complex [32] and supplemented by EchA.
The genome sequence of M. maripaludis indicates the presence of membrane-bound A1A0-type ATPases (chimeric ATP synthases) instead of the F1F0-type ATPases found in Bacteria and Eukarya [40, 41]. The catalytic unit of the A1A0-type ATPase is structurally homologous to the V-type ATPase and functionally homologous to the F1F0-type ATPase [42]. But, the membrane-embedded motors in the A1A0-type ATP synthases are exceptional due to their novel functions and structural features [43].
Acetyl-CoA synthesis
M. maripaludis can synthesize acetyl-CoA from either CO2 or acetate [7, 25]. The CO2-based synthesis occurs with the help of carbon monoxide decarbonylase/acetyl-CoA synthase complex (CODH/ACS) [7]. Sequencing studies have confirmed the existence of CODH/ACS in a single cluster (MMP0980-MMP0985) [41]. During the CO2-based synthesis, methyl-THMPT, an intermediate of methanogenesis, contributes the methyl carbon of acetyl-CoA, while the CO generated from the reduction of CO2 in the presence of reduced ferredoxins by CODH, contributes the carboxyl carbon [7]. The acetate-based synthesis is accomplished by AMP-forming acetate CoA ligase (MMP0148, acsA) in M. maripaludis [25]. Shieh et al. [25] showed that M. maripaludis can assimilate up to 60 % of its cellular carbon from exogenous acetate. A sequencing study [41] also showed the presence of ADP-forming acetyl-CoA synthetase gene (MMP0253, acd) in M. maripaludis, which catalyzes acetate formation and ATP synthesis from acetyl CoA, ADP and Pi. However, no literature study has experimentally demonstrated the biosynthesis of free acetate by M. maripaludis.
Pyruvate synthesis
Pyruvate is the entry point into glycolysis, citric acid cycle, and amino acid metabolism. Acetyl-CoA is converted to pyruvate through pyruvate:ferredoxin oxidoreductases (PORs) [34, 44, 45] as follows:
This is reversible in that PORs also catalyze pyruvate oxidation to acetyl-CoA in the absence of H2 [26]. However, pyruvate oxidizes very slowly in M. maripaludis and PORs appear to function mainly in the anabolic directions during growth [44].
The PORs containing five polypeptides in M. maripaludis are encoded by one gene cluster (porABCDEF). Of these, porEF is unique to M. maripaludis, because the N-terminal sequences of the first four polypeptides (porABCD) are similar to those in other Archaea [45]. The importance of porEF in M. maripaludis was highlighted by Lin et al. [46]. They showed that porEF mutants of M. maripaludis JJ grew extremely slowly and pyruvate-dependent methanogenesis was completely inhibited. Interestingly, porF mutant failed to restore growth, but restored methanogenesis to wild-type levels. In contrast, porE mutant restored growth partially, but did not restore methanogenesis. This indicates that porF serves as an electron donor to PORs.
Pyruvate is also a precursor for alanine biosynthesis via alanine dehydrogenase (MMP1513, ald) [47]. The same enzyme catalyzes the reverse reaction also, i.e. alanine to ammonia and pyruvate, in M. maripaludis. In addition, alanine transaminase that catalyzes the conversion of alanine to pyruvate in several organisms including Pyrococcus furiosus, Escherichia coli, Mus musculus, and Homo sapiens, may also exist in M. maripaludis. Our inference is based on a BLASTp search with the protein sequences of M. maripaludis. Our search located proteins with high similarity (e-value = 7e−63) to the alanine transaminase from P. furiosus. M. maripaludis uptakes alanine with the help of alanine permease and alanine racemase. While the former transports both l-alanine and d-alanine into the cell, the latter is essential for converting d-alanine to l-alanine [47–49], because alanine dehydrogenase is specific for l-alanine only.
Glycolysis/gluconeogenesis and glycogenolysis/glyconeogenesis
M. maripaludis does not assimilate carbohydrates such as pentoses and hexoses, as it lacks the required transporters [41]. However, it has all the enzymes and cofactors required for glycolysis/gluconeogenesis and glycogenolysis/glyconeogenesis with some unique features. In fact, studies have shown that methanococci such as M. maripaludis synthesize and store glycogen as a reserve metabolite, and use it for methane generation in the absence of exogenous substrates [50]. The bifunctional activity of ADP-dependent phosphofructokinase (PFK)/glucokinase (GK) has been demonstrated experimentally in both M. jannaschii [51] and M. maripaludis [52]. Castro-Fernandez et al. [52] measured the activities of glucose phosphorylation versus dephosphorylation. They unexpectedly observed that the latter was two-folds more efficient than the former. Based on these observations, they indicated that M. maripaludis can catalyze d-glucose formation, and suggested a possibility of methane production from glycogen or d-glucose during starvation in M. maripaludis.
Unlike non-methanogenic Archaea that use ED pathway, M. maripaludis [50] uses a modified Embden-Meyerhof-Parnas (EMP) pathway with some unique features. These features include the reduction of ferredoxins instead of NAD (e.g. PORs and GAPOR) [53], ADP-dependent kinases [51], zero or very low ATP yields [54], highly divergent phosphoglucose isomerase [55], and phosphoglycerate mutase [56]. Of the eight enzymatic steps (5-13) from pyruvate to glucose-6-phosphate (Fig. 4), five are reversible and catalyzed by the same enzyme, while the rest are irreversible (5, 9, and 12). However, even for the three irreversible steps, reverse steps are catalyzed by alternative enzymes [phosphenolpyruvate synthase (PPS), glyceraldehyde-3-phosphate dehydrogenase (GAPDH), and fructose-bisphosphatase (FBP)]. In other words, all the steps leading to glucose-6-phosphate from pyruvate are reversible in principle. Glycogen in M. maripaludis is then synthesized by converting 1) glucose-6-phosphate to glucose-1-phosphate via phosphoglucomutase (MMP1372), 2) glucose-1-phosphate to UDP-glucose via UTP-glucose-1-phosphate uridylyltransferase (MMP1091), and 3) the growing polymeric chain of UDP-glucose to glycogen via glycogen synthase (MMP1294) with the release of UDP molecule [41]. On the other hand, glycogen in M. maripaludis degrades to glucose-6-phosphate by converting (1) glycogen to glucose-1-phosphate via glycogen phosphorylase (MMP1220), and (2) glucose-1-phosphate to glucose-6-phosphate via glucose phosphomutase (MMP1372) [41, 50]. The low activity of PFK in comparison to FBP in M. maripaludis suggests that glyconeogenesis is the predominant function of EMP pathway in M. maripaludis pointing to the storage of glycogen as a reserve material [50]. This predominance of the anabolic direction is further confirmed by the high activities of the reversible hexose phosphate conversions (via glucose phosphomutase, glucose-6-phosphate isomerase, and fructose-bisphosphate aldolase) and triose phosphate conversions for pentose biosynthesis (via enolase, 2, 3-bisphosphoglycerate mutase, and glyceraldehyde-3-phosphate dehydrogenase). Yu et al. [50] further showed that glycogen content increased from 0.11 % ± 0.05 % DCW (A660 ≤ 0.5) to 0.34 ± 0.19 % DCW (A6601.0–1.6) during growth, while glycogen consumption depended on the substrate for methanogenesis.
Glycolysis/gluconeogenesis and glycogenolysis/glyconeogenesis in M. maripaludis with associated ORFs. The solid lines show the presence of enzymes in M. maripaludis and dotted lines show their absence. The enzymes corresponding to the various reaction numbers are: 1. carbon-monoxide dehydrogenase (1.2.7.4, codh & porEF); 2. acetyl CoA decarbonylase (2.1.1.245, acds); 3. pyruvate:ferredoxin oxidoreductase/synthase (1.2.7.1, porABCD); 4. acetyl CoA synthetase (AMP forming) (6.2.1.1, acsA); 5. phosphenolpyruvate kinase (2.7.1.40, pyk); 6. enolase (4.2.1.11, eno); 7. 2,3-bisphosphoglycerate mutase (5.4.2.12, pgm); 8. phosphoglycerate kinase (2.7.2.3, pgk); 9. glyceraldehyde-3-phosphate dehydrogenase (1.2.1.59, gapdh); 10. triosephosphate isomerase (5.3.1.1, tpi); 11. fructose-bisphosphate aldolase (4.1.2.13. fbp); 12. phosphofructokinase (2.7.1.147, pfk); 13. glucose-6-phosphate isomerase (5.3.1.9, pgi); 14. Phosphoglucomutase (5.4.2.8, pgm); 15. glycogen phosphorylase (2.4.1.1, glgP); 16. pyruvate carboxylase (6.4.1.1, pycB); 25. phosphoenolpyruvate carboxylase; 26. phosphoenolpyruvate synthase (2.7.9.2, ppsA); 27. NADP-dependent glyceraldehyde-3-phosphate dehydrogenase (1.2.1.9, gapn) OR ferredoxin-dependent glyceraldehyde-3-phosphate dehydrogenase (1.2.7.6, gapor); 28. fructose-bisphosphatase (3.1.3.11, fbp); 29. ADP-specific phosphofructokinase (2.7.1.147, pfk); 30. Transketoloase (2.2.1.1, tkl); 35. Acetate CoA synthetase (ADP-forming) (6.2.1.13, acd); 36. UTP-glucose-1-phosphate uridylyltransferase (2.7.7.9); 37. starch synthase (2.4.1.21, glgA)
Given the key roles of glycolysis/gluconeogenesis in M. maripaludis, it is critical to understand their regulation. In general, a pathway can be regulated by (1) substrate availability, (2) up- or down-regulating enzyme activities for rate-limiting steps, (3) allosteric regulation of enzymes, and (4) covalent modifications such as phosphorylations of substrates. Essentially, the enzymes catalyzing the irreversible steps are most suited for regulation [57]. In most Archaea, nonphosphorylating NADP+-dependent glyceraldehyde-3-phosphate (G3P) dehydrogenase (GAPN), phosphorylating glyceraldehyde-3-phosphate dehydrogenase (GAPDH), and glyceraldehyde-3-phosphate ferredoxin oxidoreductase (GAPOR) act as the regulatory points in glycolysis [58–60]. The genome sequence of M. maripaludis codes for all three genes, namely GAPN (MMP1487), GAPDH (MMP0325), and GAPOR (MMP0945) [41]. GAPOR catalyzes ferredoxin-dependent G3P oxidation, GAPN catalyzes NADP-dependent G3P oxidation, and GAPDH catalyzes G3P synthesis. Based on the activity, transcriptomic, and flux balance analyses in M. maripaludis, Park et al. [61] showed that GAPOR is a post-transcriptionally regulated enzyme that is completely inhibited by the presence of 1 µM ATP, and (unlike GAPN) is most likely involved only under non-optimal growth conditions.
Yu et al. [50] mentioned pH-dependent PFK (optimum pH = 6.0) as an important regulatory enzyme in M. maripaludis. The activation and inhibition of PFK was found to be dependent on the presence/absence of various substrates such as ADP, AMP, Pi, cAMP, and citrate. Yu et al. [50] also reported that full activity of pyruvate kinase, another key enzyme in glycolysis, depended on Mn2+. In contrast to Mn2+, Fe2+ showed 70 % activity, and Mg2+ showed 20 % activity of pyruvate kinase, while Zn2+, Cu2+, Co2+, and Ni2+ showed zero activity. The activity of phosphoglycerate mutase was unaffected by Mg2+ and AMP, and depended on the presence of reduced dithiothreitol, cysteine hydrochloride, and glutathione.
Tri-carboxylic acid (TCA) cycle
TCA cycle plays an important role in generating electron carriers such as NADH & FAD for energy production [62]. Most aerobes have an oxidative TCA cycle to oxidize complex carbon molecules, such as sugars, to CO2 and H2O to generate energy [62]. However, most anaerobes have RTCA cycles to reduce CO2 and H2O to synthesize carbon compounds. Methanogens being anaerobes also have RTCA cycles. Furthermore, their TCA cycles are incomplete, as they lack several steps and enzymes [63]. M. maripaludis in particular lacks phosphoenolpyruvate carboxykinase, citrate synthase, aconitate, and isocitrate dehydrogenase [25, 64]. The missing steps in M. maripaludis are shown as dashed lines in Fig. 4. As shown in Fig. 4, pyruvate is the entry metabolite in M. maripaludis for TCA cycle. In the absence of phosphenolpyruvate carboxylase (PPC), M. maripaludis converts pyruvate to oxaloacetate via pyruvate carboxylase (PYC). Oxaloacetate is then reduced to 2-oxoglutarate via a series of intermediates (Malate, Fumarate, Succinate, Succinyl CoA) in the TCA cycle as shown in Fig. 5. Hendrickson et al. [41] reported the complete genome sequence of M. maripaludis and noted that 2-oxoglutarate oxidoreductase, the last enzyme in the TCA cycle, has four subunits (MMP0003, MMP1315, MMP1316, and MMP1687) that are not contiguous. This is in contrast to PORs that are also oxidoreductases, but have contiguous subunits (MMP1502-MMP1507).
Reductive tricarboxylic acid cycle in M. maripaludis with associated ORFs. The solid lines show the presence of enzymes in M. maripaludis and dotted lines show their absence. The enzymes corresponding to the various reaction numbers are: 16. pyruvate carboxylase (6.4.1.1, pycB); 17. malate dehydrogenase (1.1.1.37, mdh) 18. fumarate hydratase (4.2.1.2, fumA); 19. succinate dehydrogenase/fumarate reductase (1.3.5.4/1.3.4.1, sdhA); 20. succinyl-CoA synthetase (6.2.1.5, sucC & sucD); 21. 2-oxoglutarate oxidoreductase (1.2.7.3, korABDG); 22. citrate synthase; 23. aconitate; 24. isocitrate dehydrogenase
Regulation of TCA cycle in M. maripaludis in particular, and Archaea in general, is poorly understood. However, 2-oxoglutarate plays an important role in nitrogen regulation [65]. In M. maripaludis, NrpR protein represses nitrogen fixation in ammonia-rich conditions by binding to the nif promoters [66]. In the absence of ammonia, 2-oxoglutarate is unable to synthesize glutamate, hence its level increases. High levels of 2-oxoglutarate act as the inducer and prevent binding of NrpR to nif promoters, resulting in the activation of nitrogen fixation and glutamine synthetase to bring down 2-oxoglutarate levels.
The TCA regulation in Methanobacterium thermoautotrophicum, another methanogen with an incomplete reductive cycle, can shed some light on the regulation in M. maripaludis. As reported by Eyzaguirre et al. [30] for M. thermoautotrophicum, M. maripaludis may also exhibit unidirectional synthesis of phosphenolpyruvate via phosphenolpyruvate synthetase (ppsA). The activity of this enzyme may be inhibited by AMP, ADP, and 2-oxoglutarate. Similarly, PYC, the ATP-dependent enzyme responsible for pyruvate carboxylation in M. maripaludis, may exhibit anabolic function as reported by Mukhopadhyay et al. [30] in M. thermoautotrophicum. Furthermore, its activity may depend on biotin, ATP, Mg2+ (or Mn2+, Co2+), pyruvate, and bicarbonates; and it may be inhibited by ADP and 2-oxoglutarate.
Pentose phosphate pathway (PPP)
PPP is essential for the syntheses of nucleotides and nucleic acids in M. maripaludis. Glyceraldehyde-3-phosphate and fructose-6-phosphate synthesized during glycolysis/gluconeogenesis form the feeds to PPP and produce xylulose-5-phosphate and erythrose-4-phosphate (E4P) via transketolase (TKL) in the first step. Yu et al. [50] proposed a NOPPP in M. maripaludis (Fig. 6). They suggested the presence of this pathway based on the zero activities of oxidative enzymes [glucose-6-phosphate dehydrogenase and 6-phosphogluconate dehydrogenase] and high activities of non-oxidative enzymes [transketolase (MMP1113, MMP1115), transaldolase (MMP1308), ribose-5-phosphate 3-epimerase (MMP1114), and ribulose-5-phosphate isomerase (MMP1189)] [41, 50] . Tumbula et al. [67] supported this observation by ruling out oxidative PPP based on the labelling patterns of riboses after supplementing the medium with [2-13C] acetate. They argued that E4P cannot be the precursor for aromatic amino acids (AroAAs), if NOPPP is its only route. Therefore, they conjectured an alternative route (carboxylation of a triose such as dihydroxyacetone phosphate) for E4P. Porat et al. [68] on the other hand showed that E4P is not a precursor for AroAAs in M. maripaludis. They proposed two alternative routes for the syntheses of AroAAs based on the presence of dehydroquinate dehydratase. The details of these routes are provided in the Additional file 1. NOPPP is mainly regulated by substrate availability [69, 70]. However, no such regulation has been shown yet in M. maripaludis.
Non-oxidative pentose phosphate pathway in M. maripaludis with associated ORFs. The enzymes corresponding to the various reaction numbers are: 30. Transketoloase (2.2.1.1, tkl); 31. ribulose-phosphate 3-epimerase (5.1.3.1, rpe); 32. ribose-5-phosphate isomerase (5.3.1.6); 33. transketoloase (2.2.1.1, tkt); 34. translaldolase (2.2.1.2, tal)
Nitrogen metabolism
M. maripaludis can utilize three nitrogen sources: ammonia, alanine, and dinitrogen (free N2) with ammonia being the most preferred source for growth [47, 71] .
Ammonia assimilation occurs in M. maripaludis via glutamine synthetase (encoded by glnA) which makes glutamine from glutamate and ammonia [72]. Glutamine then serves as the precursor for protein synthesis. Cohen-Kupiec et al. [72] observed that glnA mutants are unable to grow even in the presence of exogenous glutamine and alanine indicating the essentiality of glnA and absence of glutamine transporters.
As discussed before, alanine uptake occurs in M. maripaludis via alanine racemase and alanine permease [47]. Moore et al. [47] confirmed that alanine converts to pyruvate and ammonia by alanine dehydrogenase.
Free N2 fixation or diazotrophy in methanogens including M. maripaludis is well established and extensively reviewed in the literature [73–75]. A comparison of four nitrogen-fixing hydrogenotrophic methanococci (M. formicicus, M. maripaludis, M. aeolicus, and M. thermolithotrophicus) is given in Table 3 [71, 76–78]. Blank et al. [79] showed diazotrophy in wild-type M. maripaludis and its four mutants using transposon insertion mutagenesis. Kessler et al. [73] characterized the nif gene cluster in M. maripaludis based on a sequence analysis. Six nif genes (nifH, D, K, E, N, and X) and two homologues of bacterial nitrogen sensor-regulator glnB (i & ii) exist between nifH and nifD in a single operon in M. maripaludis (Fig. 7a). Although the conserved order of nif genes resembles that in Bacteria, the presence of single operons and two homologues between nif genes is unique to Archaea.
As shown in Fig. 7b, diazotrophy is effected by multiprotein nitrogenase complex comprising an Fe protein and a MoFe protein [80]. In the presence of N2, the oxidized Fe protein reduces by taking electrons from reduced ferredoxins. The reduced Fe protein then oxidizes in the presence of ATP and reduces the MoFe protein. The MoFe protein donates the electrons to N2 and reduces it to ammonia in three successive steps: nitrogen to diamine to hydrazine to two ammonia molecules and one H2. These reductive steps require electrons from reduced ferredoxins, and their relatively high-energy demand makes N2 fixation unfavorable in M. maripaludis. Therefore, the cells are less likely to activate this fixation, when ammonia or alanine is available [48, 81].
Regulation
Cohen-Kupiec et al. [72] hypothesized that both nif and glnA are regulated by the same nucleotide binding sequences (Fig. 7c) residing in the nif promoter region of M. maripaludis. Lie et al. [82] confirmed this by isolating NrpR protein. NrpR represses both N2 fixation and glnA expression by binding to the aforementioned nitrogen operator sequences (nifOR). While NrpR represses nif transcription fully in the presence of ammonia, it represses it partially in the presence of alanine, and represses fully in the presence of free N2. As discussed before, NrpR in turn is regulated by 2-oxoglutarate levels in the TCA cycle [65]. As shown in Fig. 7c, the binding of NrpR to nifOR weakens during nitrogen deficiency, allowing 2-oxoglutarate to induce nif transcription. Experiments have also demonstrated that cell growth with alanine was only marginally lower than with ammonia, while significantly reduced with free nitrogen [48].
glnB proteins (i & ii), encoded within the nif gene cluster as shown in Fig. 7a, play a key role in nitrogen sensing and regulation in M. maripaludis [81]. glnB + strain lost nitrogenase activity within an hour of ammonia addition [83], while glnB mutant did not. This indicates that the glnB proteins switch off the nitrogenase activity in the presence of ammonia. Kessler et al. [71] reported the importance of molybdenum-dependent nitrogenases during nitrogen fixation in M. maripaludis.
The above six subsections along with amino acid and nucleotide metabolisms described in the Additional file 1 complete our picture for the metabolic processes of M. maripaludis. In spite of the extensive literature on this organism, we could identify at least a few gaps in our current understanding, which offer opportunities for further research. While a comprehensive picture for the metabolic network and its individual components is useful for the research community, we need to note that all these subsystems reside in a single cell, interact with each other in a complex manner, and result in various cellular phenotypes. Furthermore, the picture so far has been largely qualitative and experimental. As recent research [42, 84–86] has demonstrated the importance of synergizing the experimental with the computational, the qualitative with the quantitative, and the elemental with the systemic, we now complement our picture with a quantitative and systems biology perspective. This is essential for fruitful applications of M. maripaludis. However, very little work exists on the systems biology of M. maripaludis.
Molecular biology tools
The 1.6 Mb long M. maripaludis genome covers 1722 protein-coding genes with unique hydrogenases [34]. Hendrickson et al. [41] have reported its complete genome sequence and ORF functionalities [87]. Genetic tools are available for manipulating its fully sequenced genome via selectable markers [88], shuttle vectors [89], integrative plasmids and gene replacements [90], and markerless mutagenesis [47].
Selectable markers
It is difficult to identify antibiotic resistant markers in methanogens due to different ribosome structures and the absence of peptidoglycans in their cell walls. Puromycin resistance in M. maripaludis was reported by transforming it with pKAS100 and pKAS102 plasmids [89]. To aid vector transformation, an optimized polyethylene glycol (PEG) method was proposed [91]. This method increased transformation frequency by an order of four to five (2 × 105 transformants/µg of insertion vector) as compared to the natural transformation method. Subsequent methylation of plasmid with PstI methylase increased transformations at least four-folds, thus approaching those obtained for E. coli. Addition of divalent cations inhibit transformation [91]. Neomycin is the second selectable marker reported for M. maripaludis [88], for which aminoglycoside phosphotransferase genes APH3’I and APH3’II were cloned under the control of Methanococcus voltae methyl reductase promoter. 500–1000 µg/ml of neomycin delayed the growth of M. maripaludis, and 1000 µg/ml inhibited it completely. Kanamycin and geneticin are non-inhibitory for M. maripaludis [92, 93].
Shuttle vectors
Tumbula et al. [9] constructed a shuttle vector pDLT44 for M. maripaludis JJ using plasmid pURB500 (from M. maripaludis C5) and pMEB.2 (E. coli vector containing a methanococcal puromycin resistance marker). This shuttle vector was found to be stable in E. coli under ampicillin selection. This was the first report of a plasmid replicated independently in a methanogen, which can be manipulated in E. coli. Although pURB500 was originated from a methanococcus, it did not replicate in M. voltae. Another study [94] reported expression shuttle and integrative vectors for M. maripaludis using histone promoter (P hmvA ) and multiple cloning sites from M. voltae for overexpressing ilvBN and ppsA. These expression vectors may be useful for studying the physiology and biochemistry of M. maripaludis. However, their transformation efficiencies vary from one strain to another [10]. For instance, M. maripaludis S2 showed much lower efficiency than M. maripaludis JJ, but it can be improved by manipulating a shuttle vector. Walters et al. [10] showed that a significantly smaller shuttle vector pAW42 was sufficient to maintain in M. maripaludis S2 and provided 7000-fold increase in transformation efficiency for pURB500-based vectors.
Integrative plasmid and gene replacement
Stathopoulos et al. [95] constructed an integration vector pIJA03-cysS for M. maripaludis to determine the essentiality of cysS gene coding for cysteinyl-tRNA synthetase. They successfully replaced cysS by constructing a pBD1 vector by using another plasmid pPH21310. Several other mutants of M. maripaludis have also been constructed using the techniques of integrative plasmids and gene replacement. For example, acetate auxotrophs were isolated by random insertional mutagenesis in the wild type M. maripaludis with the help of pWDK104 [96]. Using transposon insertion mutagenesis, mutations were made in and around nifH gene to study nitrogen fixing abilities of four transformants. In another study of transposon insertion mutagenesis, an 8-kb region corresponding to the nif gene cluster was confirmed for nitrogen fixation [73].
Markerless mutagenesis
Moore [47] demonstrated markerless mutagenesis in M. maripaludis to show the roles of genes with an unusual ability to use d-alanine or l-alanine. They used a negative selection based system with hpt and upt genes encoding for hypoxanthine and uracil phosphoribosyl transferases present in M. maripaludis. The Hpt system was used to produce markerless in-frame deletion mutations in three genes (ald, alr, and agcS) coding for alanine dehydrogenase, alanine racemase, and alanine permease. hpt was used together with upt to restore the function of wild type ald.
Systems biology
In recent years, systems biology models have been developed for many microbes [97], plants [98], and animals [99] to understand, analyze, and quantify the extent and impact of intracellular interactions and genetic perturbations [100]. However, no such model existed for M. maripaludis, until Goyal et al. [101] reported the first constraint-based genome-scale metabolic model (iMM518). The model comprised of 570 reactions, 556 metabolites, and 518 genes (30 % ORF coverage) across 52 pathways. The details are available in [101]. The only other model [102] available in the literature is for a coculture of M. maripaludis and D. vulgaris, developed for studying methane production from lactate, and understanding the syntrophic association between the two. Unlike iMM518, this model is limited to the reactions in central metabolism only.
Goyal et al. [101] studied the essentialities of genes and reactions in M. maripaludis. Of the 518 genes in their model, 278 proved essential and 240 non-essential. 282 of the 570 reactions proved essential for cell growth. In a previous study, Sarmiento et al. [87] had experimentally analyzed the gene functions in M. maripaludis using whole-genome libraries of Tn5 transposon mutant. 34 of the metabolic genes deemed essential for growth by Sarmiento et al. [87] were also in the list of essential genes by Goyal et al. using iMM518.
Goyal et al. [101] compared the effectiveness of various carbon, hydrogen, and nitrogen sources on cell growth and methane production. Both their model and our recent experiments (unpublished work) suggest that M. maripaludis cannot grow on formate alone (in the absence of CO2). iMM518 also shows that it cannot grow on acetate alone, but no experiments have proven this. Lupa et al. [23] have reported growth on formate under N2/CO2 and Wood et al. [103] have reported growth on formate under H2/CO2. iMM518 predicts both. This suggests that both H2 and formate can act as electron donors, while CO2 is the sole carbon substrate. Between H2 and formate, H2 is a better hydrogen source (or electron donor) as growth on CO2/H2 is higher than CO2/formate.
Based on iMM518, ammonia is better than N2 as a nitrogen source for growth, but worse for methane production. Thus, free N2 is more advantageous for methane production than ammonia with the added benefit of reduced biomass. The reason seems to be the additional ATPs required by nitrogenases for fixing N2 to ammonia first before cellular assimilation. Since methanogenesis is the only energy producing pathway in M. maripaludis, the additional energy is supplied by enhanced methanogenesis.
iMM518 enabled Goyal et al. [101] to identify the best gene combinations whose deletions would maximize methane production in M. maripaludis. Some of their identified targets for single and multiple gene deletions are adkA (MMP1031), acd (MMP0253), mdh (MMP0645), acd (MMP0253) & mdh (MMP0645), adkA (MMP1031) & mdh (MMP0645), and acd (MMP0253) & cimA (MMP1018) & mdh (MMP0645).
In addition to the above, iMM518 also successfully predicted several experimentally observed phenotypes such as the roles of leuA that encodes the first enzyme for leucine biosynthesis, porE and/or porF whose deletions affect the growth and oxidation of pyruvate, and nif and glnA expressions during nitrogen availability. With its quantitative power and useful insights into the metabolic processes, such a validated model can help tremendously in reengineering M. maripaludis for desired ends.
A recent experimental study [104] measured extracellular fluxes of CO2, H2, and CH4 in M. maripaludis during growth on CO2 as the sole carbon substrate. Using these fluxes and a systems biology model (in this case, iMM518), the study also proposed a procedure for estimating maintenance energy parameters (growth associated maintenance, GAM and non-growth associated maintenance, NGAM).
Potential applications
Methanogens play a key role in the global carbon cycle by reducing atmospheric CO2 [27]. Their unique characteristics in general, and those of M. maripaludis in particular, offer potential for applications in wastewater treatment, carbon capture and utilization, value-added chemicals production, GTL (Gas to Liquid) applications, and methane production from renewable hydrogen or via electromethanogenesis. Although M. maripaludis has not been used in an industrial setup so far, its attractive features offer much potential for these applications. We now briefly discuss the existing work on each potential application, and assess the promise of M. maripaludis.
Wastewater treatment
Tabatabaei et al. [105] has summarized the characteristics of methanogenic populations used in wastewater treatment. The production of biogas from the anaerobic degradation of waste relies on a symbiotic relationship between syntrophic bacteria (Syntrophomas, Synthrophospora, and Syntrophobacter) and methanogens [106]. The former convert acid-phase products into acetates and H2 for use by the latter. New evidence [107–109] also suggests the possibility of direct electron transfer through nanowires/electrically conductive pili between the two. Without the methanogens removing the acetates and H2, acetogenesis cannot proceed [110].
Of all the methanococci, M. maripaludis has many advantages such as autotrophic growth, short doubling time, N2-fixation, and stimulation of growth by acetate/amino acids. Compared to mesophilic methanococci (e.g. M. vannielli, M. voltae, and M. aeolicus), M. maripaludis seems a better choice for a pure culture. M. vannielli has a long doubling time of 8 h, M. voltae does not show autotrophy and requires both acetate and amino acids for its growth, while M. aeolicus requires a higher temperature of ~46 °C for optimum growth. The genera such as methanocaldococcus, methanotorris, and methanothermococcus are thermophilic and require even higher temperatures (65–85 °C) for optimum growth. Thus, M. maripaludis has an advantage over other methanococci for maintaining a low partial pressure of H2 during wastewater treatment at low temperatures.
Carbon capture and conversion
The flue gas exhausts from power plants typically have 3–15 % CO2 in majority N2. Global CO2 emissions are the major cause for global warming and climate change [111]. While the world’s leading nations have committed to reduce CO2 emissions in the future, the possibility of sequestration has been losing favor due to various geological and societal reasons. In such a scenario, the interest in converting CO2 to useful products or fuels is increasing. Given the availability of renewable H2 from wind, solar, nuclear, etc., M. maripaludis with its ability to uptake CO2 in the presence of N2 offers a potential route to capture and convert CO2 simultaneously from CO2 emissions to a useful fuel such as methane [83]. M. maripaludis in a microbe consortium with other methanogens such as M. aeolicus [77], M. thermolithotrophicus [76], M. formicicus [78] can be in principle used as to capture and convert CO2 from power and chemical plant emissions. Given that all methanogens are anaerobic, such a possibility does demand some pretreatment of the flue gas emissions that typically contain some residual oxygen. Further studies are clearly needed to test the feasibility and economics of such an application at large scale.
Methane from renewable energy
The main challenge in using methanogens for large-scale biomethane production is the need for H2. The only viable source for this H2 is a renewable energy source such as solar, tidal, nuclear, or wind. Formate and H2 are being studied [112] as potential options for energy storage to temporally balance the availability of energy with the use of electricity. M. maripaludis along with other methanogens offers the potential for converting formate and H2 along with CO2 into useful fuels such as methane and methanol.
The other possibility is to convert surplus renewable electricity directly into methane via electrochemical methanogenesis. This is already established for a mixed-culture of methanogenic microbes comprising Methanobacterium sp. (>93 %) and Methanobrevibacter (~5 %) [113]. Recently, Lohner et al. [22] also demonstrated the uptake of electrons by a hydrogenase-mutant of M. maripaludis, although methane production relative to the wild type was only 1/10 as discussed before. However, these studies do point to the potential of methanogens such as M. maripaludis as biocatalysts for the electrochemical conversion of CO2.
Hydrogen production
H2 production by various aerobic and anaerobic microbes has been reported in the literature [114, 115]. While genetically engineered E. coli strains can produce 1.7 µmol/mg DCW min to 4.2 µmol/mg DCW min [116] of H2 from formate during growth on glucose, the wild-type M. maripaludis S2 can readily produce 1.4 µmol/mg DCW min from formate with only CO2 [23]. Thus, scope exists to enhance this production rate further by reengineering M. maripaludis.
Other applications
Several applications remain unexplored for M. maripaludis in spite of its unique advantages. For instance, it can be studied for the production of high value-added pharmaceuticals, vitamins, amino acids, corrinoids, and terpenoids. Our recent studies (unpublished work) with iMM518 suggests that geraniol, a useful flavoring agent [117], can be produced by up-regulating hmgA and/or idi1 in M. maripaludis. The biotransformation of 2,4,5-trinitrotoluene, a priority pollutant, and metabolic conversion of 5-methylfurfurals and 2-methylfufurals (formed during the concentration of aqueous wastes in the paper and pulp industries) to furfurals have been studied with Methanococcus spp. (strain B) [118, 119]. Given the availability of ready genetic tools, such biotransformations can also be explored with M. maripaludis. Other possible applications include the production of liquid biofuels such as methanol, butanol, etc., as being explored [120] by some research groups around the world.
Conclusions
M. maripaludis is a model methanogen with some unique metabolic features (e.g. methanogenesis from simple carbon substrates such as CO2, glycolysis via modified EMP pathway, conversion of carbon to a fuel rather than accumulation into biomass, whole cell biocatalysis, diazotrophy, synthesis of all 22 amino acids, and RTCA) that other common workhorses such as E. coli and yeasts do not. This paper presented an integrated and comprehensive review of its metabolic processes, which is missing in the literature in spite of extensive fundamental research on specific aspects of its biochemistry and genetics. We classified and described the salient features of its eight major subsystems and integrated them into a holistic schematic. Our review suggests that further efforts are required towards understanding regulation, acetate biosynthesis, amino acid synthesis, electron transport chain, glycolysis, value-added chemicals/fuels production, metabolism under stress, carbon capture and utilization applications, and systems biology models. Recent tools for next generation sequencing and -omics, and their integration with systems biology models can deepen our understanding of M. maripaludis at the molecular level and promote further research into this interesting microbe. We hope that this review will benefit both modelers and experimentalists to familiarize themselves with M. maripaludis as an opportunistic methanogen and pursue further studies on our identified gaps within its biochemical pathways and applications.
Abbreviations
- AroAAs:
-
aromatic amino acids
- CODH/ACS:
-
carbon monoxide decarbonylase/acetyl-CoA synthase complex
- CO2 :
-
carbon dioxide
- CoM:
-
coenzyme M
- DCW:
-
dry cell weight
- EMP:
-
Embden-Meyerhof-Parnas
- E4P:
-
Erythrose-4-phosphate
- HCOOH:
-
formate
- FBP:
-
fructose-bisphosphatase
- GTL:
-
gas to liquid
- GK:
-
glucokinase
- G3P:
-
glyceraldehyde-3-phosphate
- GAPDH:
-
G3P dehydrogenase
- GAPOR:
-
G3P ferredoxin oxidoreductase
- GAM:
-
growth associated maintenance
- H2 :
-
molecular hydrogen
- H2O:
-
water
- HDR:
-
heterodisulfide reductase
- CH4 :
-
methane
- MFR:
-
methanofuran
- MTR:
-
methyltransferase
- NGAM:
-
non-growth associated maintenance
- NOPPP:
-
non-oxidative pentose phosphate pathway
- Fd(ox):
-
oxidized ferredoxin
- PPS:
-
phosphenolpyruvate synthase
- PFK:
-
phosphofructokinase
- PEG:
-
polyethylene glycol
- PMF:
-
proton-motive force
- PYC:
-
pyruvate carboxylase
- PORs:
-
pyruvate:ferredoxin oxidoreductases
- Fd(rd):
-
reduced ferredoxin
- RTCA:
-
reductive tricarboxylic acid cycle
- TKL:
-
transketolase
References
Liu Y. Methanococcales. Handbook of hydrocarbon and lipid microbiology. Berlin, Heidelberg: Springer; 2010. pp 573–581.
Keswani J, Orkand S, Premachandran U, Mandelco L, Franklin M, Whitman W. Phylogeny and taxonomy of mesophilic Methanococcus spp. and comparison of rRNA, DNA hybridization, and phenotypic methods. Int J Syst Bacteriol. 1996;46:727–35.
Jones WJ, Paynter MJB, Gupta R. Characterization of Methanococcus maripaludis sp. nov., a new methanogen isolated from salt marsh sediment. Arch Microbiol. 1983;135:91–7.
Whitman WB, Shieh J, Sohn S, Caras DS, Premachandran U. Isolation and characterization of 22 mesophilic methanococci. Syst Appl Microbiol. 1986;7:235–40.
Escalante-Semerena J, Rinehart K, Wolfe R. Tetrahydromethanopterin, a carbon carrier in methanogenesis. J Biol Chem. 1984;259:9447–55.
Zellner G, Winter J. Secondary alcohols as hydrogen donors for CO2 reduction by methanogens. FEMS Microbiol Lett. 1987;44:323–8.
Shieh J, Whitman WB. Autotrophic acetyl coenzyme A biosynthesis in Methanococcus maripaludis. J Bacteriol. 1988;170:3072–9.
Costa KC, Yoon SH, Pan M, Burn JA, Baliga NS, Leigh JA. Effects of H2 and formate on growth yield and regulation of methanogenesis in Methanococcus maripaludis. J Bacteriol. 2013;195:1456–62.
Tumbula DL, Bowen TL, Whitman WB. Characterization of pURB500 from the archaeon Methanococcus maripaludis and construction of a shuttle vector. J Bacteriol. 1997;179:2976–86.
Walters AD, Smith SE, Chong JP. Shuttle vector system for Methanococcus maripaludis with improved transformation efficiency. Appl Environ Microbiol. 2011;77:2549–51.
Dworkin M, Falkow S. The prokaryotes: archaea. Bacteria: firmicutes, actinomycetes, vol 3. Berlin: Springer; 2006.
Jarrell KF, Koval SF. Ultrastructure and biochemistry of Methanococcus voltae. Crit Rev Microbiol. 1989;17:53–87.
Jarrell KF, Jones GM, Kandiba L, Nair DB, Eichler J. S-layer glycoproteins and flagellins: reporters of archaeal posttranslational modifications. Archaea. 2010;2010:13.
Jones W, Donnelly M, Wolfe R. Evidence of a common pathway of carbon dioxide reduction to methane in methanogens. J Bacteriol. 1985;163:126–31.
Akca E, Claus H, Schultz N, Karbach G, Schlott B, Debaerdemaeker T, Declercq JP, Konig H. Genes and derived amino acid sequences of S-layer proteins from mesophilic, thermophilic, and extremely thermophilic methanococci. Extremophiles. 2002;6:351–8.
Hedrick DB, Guckert J, White D. Archaebacterial ether lipid diversity analyzed by supercritical fluid chromatography: integration with a bacterial lipid protocol. J Lipid Res. 1991;32:659–66.
Jarrell KF, Stark M, Nair DB, Chong JP. Flagella and pili are both necessary for efficient attachment of Methanococcus maripaludis to surfaces. FEMS Microbiol Lett. 2011;319:44–50.
Ng SY, Wu J, Nair DB, Logan SM, Robotham A, Tessier L, Kelly JF, Uchida K, Aizawa S, Jarrell KF. Genetic and mass spectrometry analyses of the unusual type IV-like pili of the archaeon Methanococcus maripaludis. J Bacteriol. 2011;193:804–14.
Liu Y, Whitman WB. Metabolic, phylogenetic, and ecological diversity of the methanogenic archaea. Ann N Y Acad Sci. 2008;1125:171–89.
Ferry JG. Bioenergetics of methanogenesis. In: Ferry JG, editor. Methanogenesis—ecology, physiology, biochemistry and genetics. New York: Chapman and Hall; 1994. p. 536.
Costa KC, Lie TJ, Jacobs MA, Leigh JA. H2-independent growth of the hydrogenotrophic methanogen Methanococcus maripaludis. MBio. 2013;4:62.
Lohner ST, Deutzmann JS, Logan BE, Leigh J, Spormann AM. Hydrogenase-independent uptake and metabolism of electrons by the archaeon Methanococcus maripaludis. ISME J. 2014;8:1673–81.
Lupa B, Hendrickson EL, Leigh JA, Whitman WB. Formate-dependent H2 production by the mesophilic methanogen Methanococcus maripaludis. Appl Environ Microbiol. 2008;74:6584–90.
Lie TJ, Costa KC, Lupa B, Korpole S, Whitman WB, Leigh JA. Essential anaplerotic role for the energy-converting hydrogenase Eha in hydrogenotrophic methanogenesis. Proc Natl Acad Sci USA. 2012;109:15473–8.
Shieh J, Whitman WB. Pathway of acetate assimilation in autotrophic and heterotrophic methanococci. J Bacteriol. 1987;169:5327–9.
Yang Y-L, Ladapo J, Whitman WB. Pyruvate oxidation by Methanococcus spp. Arch Microbiol. 1992;158:271–5.
Thauer RK, Kaster AK, Seedorf H, Buckel W, Hedderich R. Methanogenic archaea: ecologically relevant differences in energy conservation. Nat Rev Microbiol. 2008;6:579–91.
Thauer RK, Kaster A-K, Goenrich M, Schick M, Hiromoto T, Shima S. Hydrogenases from methanogenic archaea, nickel, a novel cofactor, and H2 storage. Annu Rev Biochem. 2010;79:507–36.
Donnelly M, Wolfe R. The role of formylmethanofuran: tetrahydromethanopterin formyltransferase in methanogenesis from carbon dioxide. J Biol Chem. 1986;261:16653–9.
Mukhopadhyay BSS, Wolfe RS. Purification, regulation, and molecular and biochemical characterization of pyruvate carboxylase from Methanobacterium thermoautotrophicum strain ΔH. J Biol Chem. 1998;273:5155–66.
Kengen SMDP, Duits EF, Keltjens JT, van der Drift C, Vogels GD. Isolation of a 5-hydroxybenzimidazolyl cobamide-containing enzyme involved in the methyltetrahydromethanopterin: coenzyme M methyltransferase reaction in Methanobacterium thermoautotrophicum. Biochim Biophys Acta. 1992;1118:249–60.
Kaster A-K, Moll J, Parey K, Thauer RK. Coupling of ferredoxin and heterodisulfide reduction via electron bifurcation in hydrogenotrophic methanogenic archaea. Proc Natl Acad Sci. 2011;108:2981–6.
Major TA, Liu Y, Whitman WB. Characterization of energy-conserving hydrogenase B in Methanococcus maripaludis. J Bacteriol. 2010;192:4022–30.
Hendrickson EL, Leigh JA. Roles of coenzyme F420-reducing hydrogenases and hydrogen-and F420-dependent methylenetetrahydromethanopterin dehydrogenases in reduction of F420 and production of hydrogen during methanogenesis. J Bacteriol. 2008;190:4818–21.
Costa KCLT, Xia Q, Leigh JA. VhuD facilitates electron flow from H2 or formate to heterodisulfide reductase in Methanococcus maripaludis. J Bacteriol. 2013;195:6160–5.
Porat I, Kim W, Hendrickson EL, Xia Q, Zhang Y, Wang T, Taub F, Moore BC, Anderson IJ, Hackett M, et al. Disruption of the operon encoding Ehb hydrogenase limits anabolic CO2 assimilation in the archaeon Methanococcus maripaludis. J Bacteriol. 2006;188:1373–80.
Mitchell P. Chemiosmotic coupling in energy transduction: a logical development of biochemical knowledge. Berlin: Springer; 1974. p. 55.
Kühn W, Fiebig K, Hippe H, Mah RA, Huser BA, Gottschalk G. Distribution of cytochromes in methanogenic bacteria. FEMS Microbiol Lett. 1983;20:407–10.
Muller V, Blaut M, Heise R, Winner C, Gottschalk G. Sodium bioenergetics in methanogens and acetogens. FEMS Microbiol Lett. 1990;87:373–6.
Bull AT, Bunch AW, Robinson GK. Biocatalysts for clean industrial products and processes. Curr Opin Microbiol. 1999;2:246–51.
Hendrickson EL, Kaul R, Zhou Y, Bovee D, Chapman P, Chung J, Conway de Macario E, Dodsworth JA, Gillett W, Graham DE, et al. Complete genome sequence of the genetically tractable hydrogenotrophic methanogen Methanococcus maripaludis. J Bacteriol. 2004;186:6956–69.
Park JH, Lee KH, Kim TY, Lee SY. Metabolic engineering of Escherichia coli for the production of l-valine based on transcriptome analysis and in silico gene knockout simulation. Proc Natl Acad Sci. 2007;104:7797–802.
Lewalter K, Müller V. Bioenergetics of archaea: ancient energy conserving mechanisms developed in the early history of life. Biochimica Biophys Acta. 2006;1757:437–45.
Yang YL, Glushka JN, Whitman WB. Intracellular pyruvate flux in the methane-producing archaeon Methanococcus maripaludis. Arch Microbiol. 2002;178:493–8.
Lin WC, Yang YL, Whitman WB. The anabolic pyruvate oxidoreductase from Methanococcus maripaludis. Arch Microbiol. 2003;179:444–56.
Lin W, Whitman WB. The importance of porE and porF in the anabolic pyruvate oxidoreductase of Methanococcus maripaludis. Arch Microbiol. 2004;181:68–73.
Moore BC, Leigh JA. Markerless mutagenesis in Methanococcus maripaludis demonstrates roles for alanine dehydrogenase, alanine racemase, and alanine permease. J Bacteriol. 2005;187:972–9.
Lie TJ, Leigh JA. Regulatory response of methanococcus maripaludis to alanine, an intermediate nitrogen source. J Bacteriol. 2002;184:5301–6.
Whitman WB, Sohn S, Kuk S, Xing R. Role of amino acids and vitamins in nutrition of mesophilic Methanococcus spp. Appl Environ Microbiol. 1987;53:2373–8.
Yu J-P, Ladapo J, Whitman WB. Pathway of glycogen metabolism in Methanococcus maripaludis. J Bacteriol. 1993;176:325–32.
Sakuraba H, Yoshioka I, Koga S, Takahashi M, Kitahama Y, Satomura T, Kawakami R, Ohshima T. ADP-dependent glucokinase/phosphofructokinase, a novel bifunctional enzyme from the hyperthermophilic archaeon Methanococcus jannaschii. J Biol Chem. 2002;277:12495–8.
Castro-Fernandez V, Bravo-Moraga F, Herrera-Morande A, Guixe V. Bifunctional ADP-dependent phosphofructokinase/glucokinase activity in the order Methanococcales–biochemical characterization of the mesophilic enzyme from Methanococcus maripaludis. FEBS J. 2014;281:2017–29.
Verhees C, Kengen S, Tuininga J, Schut G, Adams M, de VOS W, Van Der Oost J. The unique features of glycolytic pathways in Archaea. Biochem J. 2003;375:231–46.
Siebers B, Schönheit P. Unusual pathways and enzymes of central carbohydrate metabolism in Archaea. Curr Opin Microbiol. 2005;8:695–705.
Verhees CH, Huynen MA, Ward DE, Schiltz E, de Vos WM, van der Oost J. The Phosphoglucose isomerase from the hyperthermophilic archaeon Pyrococcus furiosus is a unique glycolytic enzyme that belongs to the cupin superfamily. J Biol Chem. 2001;276:40926–32.
Graham DE, Xu H, White RH. A divergent archaeal member of the alkaline phosphatase binuclear metalloenzyme superfamily has phosphoglycerate mutase activity. FEBS Lett. 2002;517:190–4.
Berg JM, Tymoczko JL, Stryer L. The glycolytic pathway is tightly controlled. 2002.
Brunner NA, Siebers B, Hensel R. Role of two different glyceraldehyde-3-phosphate dehydrogenases in controlling the reversible Embden–Meyerhof–Parnas pathway in Thermoproteus tenax: regulation on protein and transcript level. Extremophiles. 2001;5:101–9.
van der Oost J, Schut G, Kengen SM, Hagen WR, Thomm M, de Vos WM. The Ferredoxin-dependent conversion of glyceraldehyde-3-phosphate in the hyperthermophilic archaeon Pyrococcus furiosus represents a novel site of glycolytic regulation. J Biol Chem. 1998;273:28149–54.
Ettema TJ, Ahmed H, Geerling AC, van der Oost J, Siebers B. The non-phosphorylating glyceraldehyde-3-phosphate dehydrogenase (GAPN) of Sulfolobus solfataricus: a key-enzyme of the semi-phosphorylative branch of the Entner-Doudoroff pathway. Extremophiles. 2008;12:75–88.
Park MO, Mizutani T, Jones PR. Glyceraldehyde-3-phosphate ferredoxin oxidoreductase from Methanococcus maripaludis. J Bacteriol. 2007;189:7281–9.
Fernie AR, Carrari F, Sweetlove LJ. Respiratory metabolism: glycolysis, the TCA cycle and mitochondrial electron transport. Curr Opin Plant Biol. 2004;7:254–61.
Simpson PG, Whitman WB. Anabolic pathways in methanogens. In Methanogenesis. Berlin: Springer; 1993. p. 445–472.
Ladapo J, Whitman WB. Method for isolation of auxotrophs in the methanogenic archaebacteria: role of the acetyl-CoA pathway of autotrophic CO2 fixation in Methanococcus maripaludis. Proc Natl Acad Sci. 1990;87:5598–602.
Lie TJ, Wood GE, Leigh JA. Regulation of nif expression in Methanococcus maripaludis: roles of the euryarchaeal repressor NrpR, 2-oxoglutarate, and two operators. J Biol Chem. 2005;280:5236–41.
Dodsworth JA, Cady NC, Leigh JA. 2-Oxoglutarate and the PII homologues NifI1 and NifI2 regulate nitrogenase activity in cell extracts of Methanococcus maripaludis. Mol Microbiol. 2005;56:1527–38.
Tumbula DL, Teng Q, Bartlett MG, Whitman WB. Ribose biosynthesis and evidence for an alternative first step in the common aromatic amino acid pathway in Methanococcus maripaludis. J Bacteriol. 1997;179:6010–3.
Porat I, Waters BW, Teng Q, Whitman WB. Two biosynthetic pathways for aromatic amino acids in the archaeon Methanococcus maripaludis. J Bacteriol. 2004;186:4940–50.
Harmen JG, vande W, Stan JJ, Brouns OJ. Pentose metabolism in Archaea. In: Blum P, editor. Archaea: new models for prokaryotic biology. Poole: Caister Academic Press; 2002. p. 71–95.
Berg JMTJ, Stryer L. Biochemistry. International edition. WH Freeman & Company Limited; 2006.
Kessler PS, McLarnan J, Leigh JA. Nitrogenase phylogeny and the molybdenum dependence of nitrogen fixation in Methanococcus maripaludis. J Bacteriol. 1997;179:541–3.
Cohen-Kupiec R. Marx Ca, Leigh J: Function and regulation of glnA in the methanogenic archaeon Methanococcus maripaludis. J Bacteriol. 1999;18:256–61.
Kessler PS, Blank C, Leigh JA. The nif gene operon of the methanogenic archaeon Methanococcus maripaludis. J Bacteriol. 1998;180:1504–11.
Nishizawa M, Miyazaki J, Makabe A, Koba K, Takai K. Physiological and isotopic characteristics of nitrogen fixation by hyperthermophilic methanogens: key insights into nitrogen anabolism of the microbial communities in Archean hydrothermal systems. Geochim Cosmochim Acta. 2014;138:117–35.
Thakur S, Bothra AK, Sen A. Exploring the genomes of symbiotic diazotrophs with relevance to biological nitrogen fixation. In: Agricultural bioinformatics. Berlin: Springer; 2014. p. 235–257.
Belay N, Sparling R, Choi B-S, Roberts M, Roberts J, Daniels L. Physiological and 15N-NMR analysis of molecular nitrogen fixation by Methanococcus thermolithotrophicus, Methanobacterium bryantii and Methanospirillum hungatei. Biochim Biophys Acta. 1988;971:233–45.
Kendall MM, Liu Y, Sieprawska-Lupa M, Stetter KO, Whitman WB, Boone DR. Methanococcus aeolicus sp. nov., a mesophilic, methanogenic archaeon from shallow and deep marine sediments. Int J Syst Evol Microbiol. 2006;56:1525–9.
Takai K, Nealson KH, Horikoshi K. Methanotorris formicicus sp. nov., a novel extremely thermophilic, methane-producing archaeon isolated from a black smoker chimney in the Central Indian Ridge. Int J Syst Evol Microbiol. 2004;54:1095–100.
Blank CE, Kessler PS, Leigh JA. Genetics in methanogens: transposon insertion mutagenesis of a Methanococcus maripaludis nifH gene. J Bacteriol. 1995;177:5773–7.
Dodge E. Carbon dioxide can be a resource rather than a waste product. 2014.
Kessler PS, Daniel C, Leigh JA. Ammonia switch-off of nitrogen fixation in the methanogenic archaeon Methanococcus maripaludis: mechanistic features and requirement for the novel GlnB homologues, Nif I(1) and NifI(2). J Bacteriol. 2001;183:882–9.
Lie TJ, Leigh JA. A novel repressor of nif and glnA expression in the methanogenic archaeon Methanococcus maripaludis. Mol Microbiol. 2002;47:235–46.
Kessler PS, Leigh JA. Genetics of nitrogen regulation in Methanococcus maripaludis. Genetics. 1999;152:1343–51.
Stephanopoulos G, Aristidou AA, Nielsen J. Metabolic engineering: principles and methodologies. Cambridge: Academic Press; 1998.
Satish Kumar V, Ferry JG, Maranas CD. Metabolic reconstruction of the archaeon methanogen Methanosarcina Acetivorans. BMC Syst Biol. 2011;5:28.
Park JH, Lee SY. Towards systems metabolic engineering of microorganisms for amino acid production. Curr Opin Biotechnol. 2008;19:454–60.
Sarmiento F, Mrázek J, Whitman WB. Genome-scale analysis of gene function in the hydrogenotrophic methanogenic archaeon Methanococcus maripaludis. Proc Natl Acad Sci. 2013;110:4726–31.
Argyle JL, Tumbula DL, Leigh JA. Neomycin resistance as a selectable marker in Methanococcus maripaludis. Appl Environ Microbiol. 1996;62:4233–7.
Sandbeck KA, Leigh JA. Recovery of an integration shuttle vector from tandem repeats in Methanococcus maripaludis. Appl Environ Microbiol. 1991;57:2762–3.
Lie TJ, Leigh JA. Genetic screen for regulatory mutations in Methanococcus maripaludis and its use in identification of induction-deficient mutants of the euryarchaeal repressor NrpR. Appl Environ Microbiol. 2007;73:6595–600.
Tumbula DL, Makula RA, Whitman WB. Transformation of Methanococcus maripaludis and identification of a Pst I-like restriction system. FEMS Microbiol Lett. 1994;121:309–14.
GIBCO B. Product catalogue and reference guide, life technologies Inc.
Weisburg WG, Tanner RS. Aminoglycoside sensitivity of archaebacteria. FEMS Microbiol Lett. 1982;14:307–10.
Gardner WL, Whitman W. Expression vectors for Methanococcus maripaludis overexpression of acetohydroxyacid synthase and β-galactosidase. Genet Soc Am. 1999;152:1439–47.
Stathopoulos C, Kim W, Li T, Anderson I, Deutsch B, Palioura S, Whitman W, Soll D. Cysteinyl-tRNA synthetase is not essential for viability of the archaeon Methanococcus maripaludis. Proc Natl Acad Sci USA. 2001;98:14292–7.
Kim W, Whitman WB. Isolation of acetate auxotrophs of the methane-producing archaeon Methanococcus maripaludis by random insertional mutagenesis. Genetics. 1999;152:1429–37.
Aggarwal S, Karimi IA, Lee DY. Reconstruction of a genome-scale metabolic network of Rhodococcus erythropolis for desulfurization studies. Mol Biosyst. 2011;7:3122–31.
Saha R, Suthers PF, Maranas CD. Zea mays iRS1563: a comprehensive genome-scale metabolic reconstruction of maize metabolism. PLoS ONE. 2011;6:e21784.
Selvarasu S, Karimi IA, Ghim G-H, Lee D-Y. Genome-scale modeling and in silico analysis of mouse cell metabolic network. Mol BioSyst. 2010;6:152–61.
Henry CS, DeJongh M, Best AA, Frybarger PM, Linsay B, Stevens RL. High-throughput generation, optimization and analysis of genome-scale metabolic models. Nat Biotechnol. 2010;28:977–82.
Goyal N, Widiastuti H, Karimi IA, Zhou Z. Genome-scale metabolic model of Methanococcus maripaludis S2 for CO2 capture and conversion to methane. Mol BioSyst. 2014;10:1043–54.
Stolyar S, VanDien S, Hillesland KL, Pinel N, Lie TJ, Leigh JA, Stahl DA. Metabolic modeling of a mutualistic microbial community. Mol Syst Biol. 2007;3:92.
Wood GE, Haydock AK, Leigh JA. Function and regulation of the formate dehydrogenase genes of the methanogenic archaeon Methanococcus maripaludis. J Bacteriol. 2003;185:2548–54.
Goyal N, Padhiary M, Karimi IA, Zhou Z. Flux measurements and maintenance energy for carbon dioxide utilization by Methanococcus maripaludis. Microb Cell Fact. 2015;14:1.
Tabatabaei M, Rahim RA, Abdullah N, Wright ADG, Shirai Y, Sakai K, Sulaiman A, Hassan MA. Importance of the methanogenic archaea populations in anaerobic wastewater treatments. Process Biochem. 2010;45:1214–25.
AliShah F, Mahmood Q, MaroofShah M, Pervez A, AhmadAsad S. Microbial ecology of anaerobic digesters: the key players of anaerobiosis. Sci World J. 2014;2014:183752.
Morita M, Malvankar NS, Franks AE, Summers ZM, Giloteaux L, Rotaru AE, Rotaru C, Lovley DR. Potential for direct interspecies electron transfer in methanogenic wastewater digester aggregates. MBio. 2011;2:e00159.
Summers ZM, Fogarty HE, Leang C, Franks AE, Malvankar NS, Lovley DR. Direct exchange of electrons within aggregates of an evolved syntrophic coculture of anaerobic bacteria. Science. 2010;330:1413–5.
AmeliaElena R, MallaáShrestha P, Fanghua L, Minita S, Devesh S, Mallory E, Karsten Z, Colin W, Kelly P, Nevin DR. A new model for electron flow during anaerobic digestion: direct interspecies electron transfer to Methanosaeta for the reduction of carbon dioxide to methane. Energy Environ Sci. 2014;7:408–15.
Song C. An overview of new approaches to deep desulfurization for ultra-clean gasoline, diesel fuel and jet fuel. Catal Today. 2003;86:211–63.
Aaron D, Tsouris C. Separation of CO2 from flue gas: a review. Sep Sci Technol. 2005;40:321–48.
Wu K, Birgersson E, Kim B, Kenis PJ, Karimi IA. Modeling and experimental validation of electrochemical reduction of CO2 to CO in a microfluidic cell. J Electrochem Soc. 2015;162:F23–32.
Marshall CW, Ross DE, Fichot EB, Norman RS, May HD. Electrosynthesis of commodity chemicals by an autotrophic microbial community. Appl Environ Microbiol. 2012;78:8412–20.
Nandi R, Sengupta S. Microbial production of hydrogen: an overview. Crit Rev Microbiol. 1998;24:61–84.
Kuhn M, Steinbüchel A, Schlegel HG. Hydrogen evolution by strictly aerobic hydrogen bacteria under anaerobic conditions. J Bacteriol. 1984;159:633–9.
Yoshida A, Nishimura T, Kawaguchi H, Inui M, Yukawa H. Enhanced hydrogen production from formic acid by formate hydrogen lyase-overexpressing Escherichia coli strains. Appl Environ Microbiol. 2005;71:6762–8.
Modification of Methanococcus maripaludis for production of geraniol. http://2012.igem.org/files/presentation/UGA-Georgia.pdf.
Boopathy R. Methanogenic transformation of methylfurfural compounds to furfural. Appl Environ Microbiol. 1996;62:3483–5.
Boopathy R, Kulpa CF. Biotransformation of 2, 4, 6-trinitrotoluene (TNT) by a Methanococcus sp. (strain B) isolated from a lake sediment. Can J Microbiol. 1994;40:273–8.
Engineering Methanococcus maripaludis for production of biofuels. http://faculty.washington.edu/leighj/research.html.
Copeland A, Lucas S, Lapidus A, Barry K, Glavina del Rio T, Dalin E, Tice H, Pitluck S, Chertkov O, Brettin T, Bruce D, Han C, Detter JC, Schmutz J, Larimer FL, Hauser L, Kyrpides N, Mikhailova N, SieprawskaLupa M, WhitmanWB, Richardson P. Complete genome sequence of Methanococcus maripaludis. Unpublished: US DOE Joint Genome Institute; 2007.
Mori K, Tsurumaru H, Harayama S. Iron corrosion activity of anaerobic hydrogen-consuming microorganisms isolated from oil facilities. J Biosci Bioeng. 2010;110:426–30.
Wang X, Greenfield P, Li D, Hendry P, Volk H, Sutherland TD. Complete genome sequence of a nonculturable Methanococcus maripaludis strain extracted in a metagenomic survey of petroleum reservoir fluids. J Bacteriol. 2011;193:5595.
White RH. Structural diversity among methanofurans from different methanogenic bacteria. J Bacteriol. 1988;170:4594–7.
Wolfe RS. Unusual coenzymes of methanogenesis. Trends Biochem Sci. 1985;10:396–9.
Noll KM, Rinehart KL, Tanner RS, Wolfe RS. Structure of component B (7-mercaptoheptanoylthreonine phosphate) of the methylcoenzyme M methylreductase system of Methanobacterium thermoautotrophicum. Proc Natl Acad Sci. 1986;83:4238–42.
Taylor CD, Wolfe RS. Structure and methylation of coenzyme M (HSCH2CH2SO3). J Biol Chem. 1974;249:4879–85.
Färber G, Keller W, Kratky C, Jaun B, Pfaltz A, Spinner C, Kobelt A, Eschenmoser A. Coenzyme F430 from methanogenic bacteria: complete assignment of configuration based on an X-Ray analysis of 12, 13-Diepi-F430 pentamethyl ester and on NMR spectroscopy. Helv Chim Acta. 1991;74:697–716.
Whitman W, Jeanthon C. Methanococcales. In: The Prokaryotes. New York: Springer; 2006. p. 257–73.
Huber H, Thomm M, König H, Thies G, Stetter KO. Methanococcus thermolithotrophicus, a novel thermophilic lithotrophic methanogen. Arch Microbiol. 1982;132:47–50.
Authors’ contributions
NG wrote the manuscript, IAK and ZZ edited the manuscript. All authors read and approved the final manuscript.
Acknowledgements
The authors would like to acknowledge all the anonymous referees for their helpful insights and suggestions.
Availability of data and materials
The datasets supporting the conclusions of this article are included within the article and its Additional file.
Competing interests
The authors declare that they have no competing interests.
Funding
Financial support for this work was provided by the National University of Singapore through a graduate research scholarship to Miss Nishu Goyal, and strategic funds under Grants R279-000-361-133/731 and R261-508-001-646/733.
Author information
Authors and Affiliations
Corresponding authors
Additional file
12934_2016_500_MOESM1_ESM.docx
Additional file 1. Taxonomy, cell structure, cultivation, amino acid metabolism, nucleotide biosynthesis, and molecular biology tools for M. maripaludis.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Goyal, N., Zhou, Z. & Karimi, I.A. Metabolic processes of Methanococcus maripaludis and potential applications. Microb Cell Fact 15, 107 (2016). https://doi.org/10.1186/s12934-016-0500-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12934-016-0500-0