Abstract
Background
Diabetic kidney disease (DKD) is associated with a higher risk of cardiovascular disease (CVD). Pentoxifylline (PTF), a nonselective phosphodiesterase inhibitor with anti-inflammatory, antiproliferative, and antifibrotic actions, has demonstrated renal benefits in both clinical trials and meta-analyses. The present work aimed to study the effects of PTF on the progression of subclinical atherosclerosis (SA) in a population of patients with diabetes and moderate to severe chronic kidney disease (CKD).
Methods
In this open-label, randomized controlled, prospective single-center pilot study the evolution of carotid intima-media thickness (CIMT) and ankle-brachial index (ABI) were determined in 102 patients with type 2 diabetes mellitus and CKD assigned to PTF, aspirin or control groups during 18 months. We also determined the variations in the levels of inflammatory markers and Klotho (KL), a protein involved in maintaining cardiovascular health, and their relationship with the progression of SA.
Results
Patients treated with PTF presented a better evolution of CIMT, increased KL mRNA levels in peripheral blood cells (PBCs) and reduced the inflammatory state. The progression of CIMT values was inversely related to variations in KL both in serum and mRNA expression levels in PBCs. Multiple regression analysis demonstrated that PTF treatment and variations in mRNA KL expression in PBCs, together with changes in HDL, were significant determinants for the progression of CIMT (adjusted R2 = 0.24, P < 0.001) independently of traditional risk factors. Moreover, both variables constituted protective factors against a worst progression of CIMT [OR: 0.103 (P = 0.001) and 0.001 (P = 0.005), respectively].
Conclusions
PTF reduced SA progression assessed by CIMT variation, a beneficial effect related to KL gene expression in PBCs.
Trial registration
The study protocol code is PTF-AA-TR-2009 and the trial was registered on the European Union Drug Regulating Authorities Clinical Trials (EudraCT #2009–016595–77). The validation date was 2010-03-09.
Similar content being viewed by others
Background
Diabetic mellitus (DM) is one of the leading causes of chronic kidney disease (CKD) and end-stage renal disease (ESRD) worldwide [1]. DM and CKD are recognized risk factors for cardiovascular disease (CVD), and the presence of both synergistically increases cardiovascular risk in patients with diabetic kidney disease (DKD) [2]. In these patients, traditional and non-traditional risk factors for atherosclerosis coexist, contributing to the excess of mortality. Non-traditional risk factors related to CKD include alterations in mineral metabolism and chronic inflammation [3, 4]. This scenario favors subclinical atherosclerosis (SA) to be more prevalent in people with CKD and DM, with a higher plaque load and faster progression. [5]. Furthermore, SA burden has been described as the most powerful predictor of cardiovascular events in this population [6]. Therefore, early detection of SA burden but also new, effective and safe therapeutic approaches starting at the earliest stages in patients with DKD are needed to prevent atheromatous disease and reduce cardiovascular events.
Pentoxifylline (PTF) is a methylxanthine derivative non-selective phosphodiesterase inhibitor currently indicated for peripheral arterial disease that exhibits anti-inflammatory, antiproliferative, and antifibrotic actions [7]. Moreover, clinical trials and meta-analyses have demonstrated renal protection secondary to anti-inflammatory and antiproteinuric effects in patients with DM and CKD treated with PTF when added to renin-angiotensin system (RAS) blockade [7].
The anti-aging protein αKlotho (hereinafter Klotho, KL) is an important regulator of mineral metabolism expressed predominantly in the kidneys but also, to a lesser extent, in the parathyroid glands, choroid plexus, vascular tissue, and peripheral blood cells (PBCs) [8,9,10]. Two forms of KL can be found: a single-pass transmembrane protein and a soluble form generated from the proteolytic cleavage of the extracellular domain of the former. Soluble KL levels in blood and urine decrease from the early stages of CKD, being a characteristic finding of renal involvement in DM since renal KL expression is markedly decreased in DKD compared to other forms of kidney disease [11]. Furthermore, clinical studies suggest that this reduction is associated with the prevalence and severity of CVD [10, 12,13,14] and all-cause mortality [15], being related to markers of vascular dysfunction and the incidence of atherosclerosis [10, 16,17,18,19]. We have recently conducted a cross-sectional case-control study showing independent associations between reduced serum and PBCs expression levels of KL and the presence of SA in patients with CKD [19]. A post hoc analysis of the Pentoxifylline for Renoprotection in Diabetic Nephropathy (PREDIAN) trial revealed that administration of PTF to patients with type 2 DM (T2DM) and CKD stages 3–4 significantly increased KL concentrations in both serum and urine [20, 21].
In this pilot randomized controlled trial, we have explored the potential benefits of PTF therapy on the progression of SA in patients with DKD. We also determined variations in the expression levels of KL and inflammatory cytokines in serum and PBCs.
Methods
Patients and study design
Single-center, open-label, randomized and prospective pilot study conducted at the University Hospital Nuestra Señora de Candelaria (Santa Cruz de Tenerife, Spain). Between May and November 2010, a total of 222 patients with T2DM and CKD stage 3 without clinical CVD were considered for initial enrollment. During this 7-month period, no significant variations were observed in the background therapies used in any of the final study groups. Patients who finally entered the study were randomly assigned to a control group, a PTF group (1200 mg/day), or to a group treated with aspirin (100 mg/day). The dose and frequency of oral PTF were 600 mg extended-release tablets twice daily with meals. Patients were asked to begin treatment by taking a single tablet after dinner. After four weeks, one tablet was administered every 12 h, thus reaching the recommended daily dose. The duration of the study was 18 months. Inclusion criteria: CKD patients in stage 3 [estimated glomerular filtration rate (eGFR) 30–59 ml/min/1.73 m2 according to the Modification of Diet in Renal Disease Study-4 (MDRD-4) equation]; older than 18 years; therapy with RAS blockers and statins; and no history of clinical atherosclerotic CVD. Exclusion criteria: ankle-brachial index (ABI) values ≥ 1.3; history of heart failure; chronic inflammatory, immunologic, or tumoral disease; positive serology to hepatitis B, hepatitis C, or HIV; acute inflammatory or infectious intercurrent episodes in the previous month; institutionalization; receipt of immunotherapy or immunosuppressive treatment; previous therapy with PTF; and inability or unwillingness to provide informed consent. The study protocol was approved by the Institutional Ethics Committee of the University Hospital Nuestra Señora de Candelaria and complied with ethical standards of the Declaration of Helsinki. Written informed consent was obtained from all participants. Participants medication-adherence and evolution were followed up with clinical visits every 3 or 6 months.
Vascular and biochemical assessments
The primary outcome was SA progression, which was assessed by measuring ABI and carotid intima-media thickness (CIMT) at inclusion and at the end of the study. The ABI was calculated using a portable pulse detector (Ultrasonic Mini Doppler ES-100VX; Hayashi Denki Co., Ltd., Kawasaki, Japan) with an 8 mHz probe. CIMT measurement was performed by a single reader in a blinded fashion by ultrasonography of the carotid arteries employing a high-resolution ultrasound (Philips ATL 5000 HDI; Royal Philips Electronics, Amsterdam, The Netherlands) equipped with a 6–13 MHz linear array transducer. According to the Guidelines for the management of arterial hypertension released by the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and the European Society of Cardiology (ESC) [22], we defined patients with SA as those having an ABI < 0.9 and/or CIMT ≥ 0.9 mm.
Secondary outcomes included the progression of CKD and the variations in the levels of inflammatory parameters and KL. Blood samples were drawn in the morning after an 8- to 12- overnight fasting at baseline and at the end of the study. Serum samples were retrieved, aliquoted, and immediately frozen at − 80ºC. Routine biochemical parameters were measured using standard methods. The serum levels of the cytokines tumor necrosis factor α (TNFα) and interleukin (IL) 10 were measured by ELISA methods (Quantikine®, R&D Systems, Abingdon, UK). Minimum detectable concentrations were 0.10 pg/mL and 0.09 pg/mL, respectively. Intra- and inter-assay coefficients of variability were < 10.8%. High-sensitivity serum C-reactive protein (hsCRP) was measured by a high-sensitivity particle enhanced immunoturbidimetric fully automated assay (Roche Diagnostics GmbH, Mannheim, Germany) in a Cobas 6000 analyzer with a sensitivity of 0.3 mg/L and intra- and inter-assay coefficients of variation of 1.6% and 8.4%, respectively. Concentrations of serum KL protein were measured by a solid phase sandwich ELISA using the human soluble αKL assay kit (Immuno-Biological Laboratories, Takasaki, Japan) according to manufacturer’s instructions. The assay sensitivity was 6.15 pg/mL and the intra- and inter-assay coefficients of variation were 2.7–3.5% and 2.9–11.4%, respectively.
Gene expression analysis in PBCs
Whole blood samples (2.5 mL) were collected in PAXgene blood RNA tubes (BD, Franklin Lakes, NJ) at the same time as serum samples. Total RNA was isolated using a PAXgene blood RNA kit (Qiagen, Valencia, CA) according to manufacturer’s specifications, quantified using a Thermo Scientific NanoDrop Lite Spectrophotometer (Thermo Fisher Scientific, MA, USA) and retrotranscribed by using a High-Capacity RNA-to-cDNA kit (Thermo Fisher Scientific). Transcripts of KL gene, TNF, IL10, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as constitutive gene, were measured by real-time TaqMan quantitative PCR (qRT-PCR) with TaqMan Fast Universal PCR master mix (Thermo Fisher Scientific) in a 7500 Fast Real-Time PCR System (Thermo Fisher Scientific). TaqMan gene expression assays for each transcript were: Hs00183100_m1 [KL], Hs00174128_ml [TNF], Hs0961622_m1 [IL10], and Hs99999905_m1 [GAPDH]. The level of target mRNA was estimated by relative quantification using the comparative method (2−ΔΔCt) by normalizing to GAPDH expression. Quantification of each sample was tested in triplicate and expressed as arbitrary units (a.u.).
Statistical analysis
Sample size calculation was based on previous studies reporting a mean CIMT of 0.68 ± 0.15 mm in patients with CKD. The number of patients needed to detect a 15% CIMT difference between groups over 18 months with longitudinal measurements, with a power of 90% and a 5% level of significance, and allowing a dropout percentage of 10%, was 96 (32 subjects in each group).
Continuous variables were checked for the normal distribution assumption using the Kolmogorov–Smirnov statistic. Normally and non-normally distributed variables are expressed as mean ± standard deviation (SD) or as median and interquartile range (IQR), respectively. Categorical data are presented as absolute values and percentages. Differences between groups or after treatment were analyzed using Fisher´s exact test, Student’s t-test, the Mann-Whitney U-test, or Wilcoxon signed rank test as appropriate. Spearman rank correlation was used to determine correlations between changes in SA markers and variations in inflammatory parameters or KL. A stepwise multiple regression analysis was performed to determine the independent association between changes in potential predictive variables and variations in ABI and CIMT. Tolerance and variance inflation factor (VIF) were analyzed in order to exclude collinearity. Finally, multiple logistic regression was performed to evaluate whether PTF therapy and changes in KL and inflammatory cytokines were independent predictors of changes in CIMT values above the median. Statistical analyses were conducted with SPSS software version 25 (IBM Corp. Armonk, NY, USA). A value of P < 0.05 was considered to be statistically significant.
Results
Study population
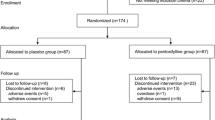
Figure 1 shows the flow of participants through the trial. A total of 222 patients were initially screened for eligibility. Of them, 8 refused to participate, 74 did not meet the inclusion criteria and 32 met the exclusion criteria. Therefore, 108 patients were randomized and 102 completed the study protocol.
Clinical data
Baseline characteristics of participants are shown in Table 1 and in supplementary material (Table S1, respectively. Demographic, clinical, and biochemical characteristics and concomitant treatments were balanced between the 3 study groups, with similar proportions of patients treated with antihypertensive, antidiabetic, and antiplatelet medications. A total of 102 patients were randomly assigned to the control group or one of the two treatment groups. All participants were white with a mean age of 67.3 ± 7.9 years, a similar distribution by sex (male, 49%), hypertension (92.2%), dyslipidemia (74.5%), and smoking habits (34.5%). MedianeGFR was 38.6 (IQR, 35.5–43.9)ml/min/1.73m2, with CKD stages 3a (76.5%) and 3b (23.5%) similarly distributed between groups. The median values for ABI and CIMT were 0.95 (IQR, 0.88–1.06) and 0.75 (IQR, 0.7–0.82) mm, respectively. Fifty-six patients (54.9%) had macroalbuminuria (urinary albumin-to-creatinine ratio [UACR] > 300 mg/g). SA was present in 42 patients (41.2%), with no differences in distribution among groups. Likewise, the three groups presented similar levels of HbA1c, fasting glucose, hs-CRP, and serum and PBCs-mRNA levels of TNFα, IL10 and KL.
BP, blood pressure; BMI, body mass index; ABI, ankle-brachial index; CIMT, carotid intima-media thickness; SA, subclinical atherosclerosis; eGFR, estimated glomerular filtrate rate; UACR, urinary albumin-to-creatinine ratio; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; hs-CRP, high sensitivity C-reactive protein; TNFα, tumor necrosis factor; IL, interleukin; KL, Klotho; ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin II receptor antagonist; CCB, calcium channel blockers
Evolution of SA, biochemical data, and gene expression in PBCs
After 18 months, the three groups experienced significant increments in CIMT values respect to baseline (Table 2). Control group increased CIMT by 0.015 mm (95% CI, 0.008 to 0.024; P < 0.01), the PTF group by 0.006 mm (95% CI, −0.001 to 0.007; P < 0.01), and aspirin group by 0.014 mm (95% CI, 0.004 to 0.019; P < 0.01) resulting in percent variations of 1.63%, 0.58% and 1.48%, respectively (Fig. 2A). Thus, the increase in CIMT was significantly lower in the PTF group than in the control and aspirin groups (P < 0.001). Only patients in the PTF group showed significant variation in ABI values at the end of the study respect to baseline, with an increment of 0.08 (95% CI, −0.03 to 0.11; P < 0.01). These variations represented a 7.11% increase of ABI values and a 17.6% reduction in the incidence of patients with ABI values < 0.9 in the PTF group. The differences in the variation of ABI values among groups did not reach statistical significance (P = 0.093).
Percent variations from baseline for CIMT (A), serum levels of inflammatory parameters and circulating KL (B), mRNA expression levels of cytokines and KL in PBCs (C), and variations in eGFR (D). Bars and ranges represent mean values and SEM. CIMT, carotid intima-media thickness; PTF, pentoxifylline; hs-CRP, high sensitivity C-reactive protein; TNFα, tumor necrosis factor; IL, interleukin; KL, Klotho; eGFR, estimated glomerular filtrate rate P values: ƒ, < 0.001 vs. control group; #, < 0.001 vs. PTF group; α, < 0.01 vs. control group; &, < 0.05 vs. control group
At the end of the study, hs-PCR levels increased in the control group from 5.23 ± 2.14 mg/L to 6.05 ± 2.62 mg/L (P < 0.01); conversely, it was reduced from 5.25 ± 2.45 mg/L to 4.61 ± 2.31 mg/L in the PTF group (P < 0.001), with no differences in the aspirin group (Table 2 and Supplementary Table S2). This resulted in a mean percent difference between control and PTF groups at 18th month for hs-PCR of 28.5% P < 0.001) in favor to the first (Fig. 2B). TNFα levels were also reduced from 15.3 pg/mL (IQR, 12.5–17.5) to 13.3 pg/mL (IQR, 12.4–16.3) (P < 0.01) in those patients treated with PTF, with no significant changes in the control or in the aspirin groups. This resulted in a mean percentage difference for TNFα changes with respect to the PTF group of 6.5% (95% CI, 0.83–12.1%; P < 0.01) and 5.63% % (95% CI, 0.49–10.8%; P < 0.01) in favor of the control group and the aspirin groups, respectively (Fig. 2B). Similarly, IL10 only increased significantly in the PTF group: from 31.8 pg/mL (IQR, 24.6–40 pg/mL) to 38.2 pg/mL (IQR, 29.2–48 pg/mL) (P < 0.001). Serum KL levels were significantly reduced in control and in aspirin groups (−3.29% [95% CI, −7.09 to 0.51] and − 2.4% [95% CI, −4.8% to −0.001%], respectively; P < 0.05 for both) (Fig. 2B). Conversely, patients in the PTF group presented a significant increment in soluble KL levels by 2.1% (95% CI, 0.7–4.14%; P < 0.05). A trend was observed for the difference in serum KL variatons between control and PTF groups at the end of the study: 5.4% (95% CI, 1.14–9.64%; P = 0.057) in favor to PTF group (Fig. 2B). PBCs KL gene expression significantly increased 0.47 a.u. (95% CI, 0.11 to 1.05; P < 0.01) in patients receiving PTF and decreased − 0.18 (95% CI, −0.39 to 0.07; P < 0.05) in the control and − 0.09 (95% CI, −0.19 to −0.01; P < 0.01) in the aspirin groups. Therefore, PBCs KL gene expression increased by 28.2% in the PTF group, whereas decreased by −5.27% and − 9.54% in the control and the aspirin groups, respectively (Fig. 2C).
After 18 months, the eGFR decreased by a mean ± SEM of 3.45 ± 0.47 and 3.58 ± 0.43 ml/min/1.73m2 in the control and aspirin groups, respectively. By contrast, eGFR only decreased 1.93 ± 0.33 ml/min/1.73m2 in patients treated with PTF (Fig. 2D). This resulted in significant mean differences respect to the control and the aspirin groups of 1.52 ml/min/1.73m2 (95% CI, 0.37 to 2.67 ml/min/1.73m2; P < 0.01) and 1.65 ml/min/1.73m2 (95% CI, −2.75 to −0.55 ml/min/1.73m2; P < 0.01), respectively, in favor to PTF in both cases. Regarding albuminuria, UACR experienced a mean percent decrease of 9.6% in the PTF group, whereas it increased by 3.6% and 12.6% in the control and aspirin groups, respectively. Three patients (8.8%) progressed from stage 3b to stage 4 in the control group versus 2 patients (5.9%) in the PTF group.
Correlations and multivariate analysis
Variations in CIMT were inversely correlated with changes in HDL-C concentrations (r = 0.25, P = 0.012), in serum and PBCs-mRNA levels of KL (r = −0.302, P = 0.002; r = −0.441, P < 0.001, respectively), as well as with variations in eGFR (r = −0.217, P = 0.028) (Table 3). Variations in ABI did not correlate with changes in none of the parameters included in the analysis. Among inflammatory markers, only variations in hs-CRP presented significant associations with markers of SA, being positively related with CIMT (r = 0.195, P = 0.049).
Multiple forward stepwise regression analysis was performed with changes in CIMT as the dependent variable. Results showed that variations in PBCs mRNA KL levels and PTF therapy, together with variations in HDL levels, were significantly associated with the evolution of CIMT (adjusted R2 = 0.24, P < 0.001) (Table 4). Tolerance and VIF values were higher than 0.60 and lower than 1.5 for all variables in any of the analysis.
In the multivariate logistic regression modelling (Table 5), delta CIMT ≥ 0.01 mm, which corresponds to the median value of the CIMT variations, was used as the dependent variable to define SA progression. Traditional risk factors for atherosclerosis (sex, baseline hypertension, smoking, DM, and dyslipidemia, together with variations in BMI, systolic BP, T-cholesterol and HbA1c) were entered as covariates (model 1), with additional models in which markers of renal function (eGFR and macroalbuminuria) (model 2), and inflammatory cytokines (model 3) were added. Results of this analyses showed that PTF treatment and variations in KL mRNA levels in PBCs were covariates associated with the progression of CIMT.
Discussion
Treatment with PTF for 18 months led to a slowdown in the rate of SA progression among patients with T2DM and stage 3 CKD. This beneficial effect was reflected in a nearly threefold smaller percentage increase in CIMT in patients receiving PTF compared with patients in the control and aspirin groups (0.58% vs. 1.63% and 1.48%, respectively). The better SA outcome in the PTF group was accompanied by increased KL mRNA expression in PBCs and reduced systemic levels of the inflammatory markers hs-CRP and TNFα. Moreover, patients in the PTF group also presented a lower decline in eGFR, with a significant mean difference of 1.52 ml/min/1.73m2 and 1.65 ml/min/1.73m2 with respect to control and aspirin groups, respectively, and a tendency to a significant antialbuminuric effect. The beneficial effect of PTF on CIMT progression was related to variations in KL levels independently of other cardiovascular risk factors. In deep, changes in both KL protein and PBCs KL mRNA levels were significantly related with the progression of CIMT. Specifically, both determinations inversely correlated with CIMT. Moreover, PTF therapy and mRNA KL expression in PBCs were found to be independent determinants for the variation in CIMT values, and protective factors against a worst progression of carotid SA.
Our results point to PTF as a kidney and vascular protective therapy in patients with T2DM and moderate to severe CKD. Whereas intermittent claudication is the main indication for PTF therapy, several studies suggests that this drug may have a broader role to play in the promotion of kidney and cardiovascular health. The PREDIAN trial [20] is the most important randomized clinical trial (RCT) evaluating the kidney protective effects of PTF in patients with T2DM and CKD stages 3–4 and residual albuminuria despite RAS blockade. Similar to the results of the present work, patients randomized to PTF in the PREDIAN trial had a smaller decline in eGFR (3.9%) and a higher reduction in albuminuria (12.5%) as compared to patients assigned to the control group (eGFR decreased by 9% and albuminuria increased by 4.4%; P < 0.01 and P < 0.001 between groups, respectively).
From the cardiovascular perspective, studies with PTF are scarce. Experimental works show that oral administration of PTF (40 mg/kg) to rabbits fed with a cholesterol-enriched diet to induce atherosclerotic plaque decreased the area of aortic atherosclerotic plaque by 38% without changes in serum lipids [23]. In the clinical scenario, two 6-months RCTs evaluated the effects of PTF in patients experiencing transient ischemic attacks (TIAs) compared with a control group receiving aspirin + dipyridamole [24, 25]. During the follow-up, proportion of recurrent TIAs was 14% in the PTF group, and 24.1% in the group receiving aspirin + dipyridamole. However, to our knowledge, none of these studies evaluated the progression of SA. In our study, we employed two methods widely used for assessing the presence of SA. ABI is considered as an indirect indicator of general atherosclerosis and an independent predictor of cardiovascular and all-cause mortality in CKD patients [26, 27]. CIMT is a widely accepted direct marker of atherosclerotic disease independently associated with increased cardiovascular risk, impaired kidney function, and long-term mortality in different stages of CKD [22, 27, 28].
Improved outcomes of PTF may be related with its anti-inflammatory effects. Clinical trials evaluating the anti-inflammatory properties of this drug report considerable modulating effects on the production of inflammatory cytokines and adhesion molecules in patients with acute coronary syndrome (ACS) and atherosclerosis [29,30,31]. In line with the results of our present work, administration of PTF (1200 mg/day) to patients with ACS reduced both hs‑CRP and TNFα levels [29, 30]. In CKD patients, where inflammation is prevalent and constitutes a major cardiovascular risk factor, the anti-inflammatory effects of PTF have been also determined [20, 32, 33]. In the PREDIAN trial, inflammatory markers were reduced in the PTF group; specifically, urine TNFα decreased by a 10.6%, with no changes in the control group [20].
The regulation of KL expression in CKD after exposure to PTF has been explored in clinical and experimental studies. In a post-hoc analysis of the PREDIAN trial, serum (5.9%; P < 0.05) and urine (9.3%; P < 0.001) KL levels increased after 12 months of PTF treatment; by contrast, KL decreased by 0.2% in serum and by 3.9% in urine in control subjects [21]. The effects of PTF on KL expression in kidney tubular cells were also determined, finding a direct dose-dependent increase of KL protein and mRNA levels after treatment [21].Experimental and clinical studies suggest that both reduced serum an PBCs-mRNA levels of KL might play a role in the pathogenesis of CVD [10, 12,13,14,15,16,17,18,19, 34]. However, there are few works on the relationship between KL and SA markers. Polymorphisms of KL have been associated with increased CIMT in hypertensive patients [35] and with the risk of early-onset occult coronary artery disease [36]. In CKD patients, decreased soluble KL levels are associated with increased brachial-ankle pulse wave velocity (adjusted OR = 0.60; 95% CI,0.39 to 0.98, P = 0.0075) [16]. We recently observed an association between reduced KL levels in serum and mRNA expression in PBCs with the presence of SA in CKD patients, being directly related with ABI and inversely with CIMT (P < 0.0001 for both) [19].
Macrophages, monocytes, lymphocytes, and other PBCs play a central role in the development of the inflammatory response associated with the atherogenic process. Therefore, the modulation of these cells constitutes an interesting approach for developing new therapeutic strategies in atherosclerosis. Marked reductions in KL expression in PBCs has been related with aging and with the development of pathologies with an inflammatory component including atherosclerosis [19, 37,38,39]. In a cross-sectional case-control study recently published by our group, which included patients with atherosclerosis and a control group of cadaveric organ donors without a history of CVD, we found significantly lower gene expression of KL in PBCs in the first group (56.4% difference, P < 0.001) anda higher methylation of the promoter region of KL (34.1 ± 4.1% vs. 14.6 ± 3.4%, P < 0.01) [10]. Although the intimate mechanisms of action deserve further investigation, the up-regulation of KL in these cells could antagonize the progression of atherosclerotic lesions by exerting anti-inflammatory effects, suppressing the stress response of the Golgi apparatus and the endoplasmic reticulum, reducing the levels of oxidant radicals, and preserving the immune function in senescent monocytes [40, 41, 42, 43]. All these mechanisms play key roles in the inflammatory response exerted by PBCs in the atherosclerotic process and, therefore, make KL expression in these cells an interesting target in such scenario.
Although our study provides novel information about the potential benefits of PTF on the progression of SA in patients with T2DM and CKD, we acknowledge several limitations. First, this was a single‑center study, and therefore, generalizability and reproducibility will require further validation. Second, the study was not designed in a double-blinded fashion, so the open-label design may have inherent bias. In addition, because this study was an independent clinical trial, a placebo was not used in the control group as a result of limited resources. Nevertheless, the main study outcomes were performed blinded to the study group allocation of patients. Third, although the sample size needed to detect differences was calculated, we recognize that the small sample size is a limitation of this study. Finally, serum concentrations of vitamin D, fibroblast growth factor-23, and parathyroid hormone -factors related to KL and calcium/phosphate metabolism, with potential impact on atherosclerosis- were not measured in our study, and therefore a possible influence on the relationship between KL and CVD cannot be completely ruled out.
Conclusions
In conclusion, PTF administration to patients with T2DM and CKD stage 3 reduced the progression of SA evaluated by the determination of CIMT. This beneficial effect was related to a stimulation of KL mRNA expression in PBCs. Furthermore, PTF treatment was associated with a slowing of the decline in eGFR. Whether PTF administration is an appropriate clinical approach to reduce the impact of atherosclerosis in patients with T2DM and CKD and if this is achieved through a KL-preserving effect is an intriguing question that requires further study.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding authors on reasonable request.
Abbreviations
- DM:
-
Diabetes mellitus
- T2DM:
-
Type 2 diabetes mellitus
- CKD:
-
Chronic kidney disease
- ESRD:
-
End-stage renal disease
- DKD:
-
Diabetic kidney disease
- PBCs:
-
Peripheral blood cells
- CVD:
-
Cardiovascular disease
- PTF:
-
Pentoxifylline
- PBCs:
-
Peripheral blood cells
- RAS:
-
Renin-angiotensin system
- BP:
-
Blood pressure
- ABI:
-
Ankle-brachial index
- CIMT:
-
Carotid intima-media thickness
- SA:
-
Subclinical atherosclerosis
- eGFR:
-
Estimated glomerular filtrate rate
- UACR:
-
Urinary albumin-to-creatinine ratio
- HDL-C:
-
High-density lipoprotein cholesterol
- LDL-C:
-
Low-density lipoprotein cholesterol
- hs-CRP:
-
High sensitivity C-reactive protein
- TNFα:
-
Tumor necrosis factor
- IL:
-
Interleukin
- KL:
-
Klotho
- GAPDH:
-
Glyceraldehyde-3-phosphate dehydrogenase
- VIF:
-
Variation inflation factor
- TIAs:
-
Transient ischemic attacks
- ACS:
-
Acute coronary syndrome
References
Tuttle KR, Bakris GL, Bilous RW, Chiang JL, de Boer IH, Goldstein-Fuchs J, Hirsch IB, Kalantar-Zadeh K, Narva AS, Navaneethan SD, Neumiller JJ, Patel UD, Ratner RE, Whaley-Connell AT, Molitch ME. Diabetic kidney disease: a report from an ADA Consensus Conference. Diabetes Care. 2014;37(10):2864–83.
Jankowski J, Floege J, Fliser D, Böhm M, Marx N. Cardiovascular Disease in chronic kidney disease: pathophysiological insights and therapeutic options. Circulation. 2021;143(11):1157–72.
Pálsson R, Patel UD. Cardiovascular complications of diabetic kidney disease. Adv Chronic Kidney Dis. 2014;21(3):273–80.
Rayego-Mateos S, Rodrigues-Diez RR, Fernandez-Fernandez B, Mora-Fernández C, Marchant V, Donate-Correa J, Navarro-González JF, Ortiz A, Ruiz-Ortega M. Targeting inflammation to treat diabetic kidney disease: the road to 2030. Kidney Int. 2023;103(2):282–96.
Palanca A, Castelblanco E, Perpiñán H, Betriu A, Soldevila B, Valdivielso JM, Bermúdez M, Duran X, Fernández E, Puig-Domingo M, Groop PH, Alonso N, Mauricio D. Prevalence and progression of subclinical atherosclerosis in individuals with 2 chronic kidney disease and diabetes. Atherosclerosis. 2018;276:50–7.
Palanca A, Castelblanco E, Betriu À, Perpiñán H, Soldevila B, Valdivielso JM, Bermúdez-Lopez M, Puig-Jové C, Puig-Domingo M, Groop PH, Fernández E, Alonso N, Mauricio D. Subclinical atherosclerosis burden predicts cardiovascular events in individuals with diabetes and chronic kidney disease. Cardiovasc Diabetol. 2019;18(1):93.
Donate-Correa J, Tagua VG, Ferri C, et al. Pentoxifylline for Renal Protection in Diabetic kidney Disease. A model of old drugs for New Horizons. J Clin Med. 2019;8(3):287.
Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51.
Donate-Correa J, Mora-Fernández C, Martínez-Sanz R, Muros-de-Fuentes M, Pérez H, Meneses-Pérez B, et al. Expression of FGF23/KLOTHO system in human vascular tissue. Int J Cardiol. 2013;165:179–83.
Martín-Núñez E, Pérez-Castro A, Tagua VG, Hernández-Carballo C, Ferri C, Pérez-Delgado N, et al. Klotho expression in peripheral blood circulating cells is associated with vascular and systemic inflammation in atherosclerotic vascular disease. Sci Rep. 2022;12:8422.
Asai O, Nakatani K, Tanaka T, Sakan H, Imura A, Yoshimoto S, et al. Decreased renal α-Klotho expression in early diabetic nephropathy in humans and mice and its possible role in urinary calcium excretion. Kidney Int. 2012;81(6):539–47.
Navarro-González JF, Donate-Correa J, Muros de Fuentes M, Pérez-Hernández H, Martínez-Sanz R. Mora-Fernández C. reduced Klotho is associated with the presence and severity of coronary artery disease. Heart. 2014;100:34–40.
Semba RD, Cappola AR, Sun K, Bandinelli S, Dalal M, Crasto C, et al. Plasma klotho and cardiovascular disease in adults. J Am Geriatr Soc. 2011;59:1596–601.
Pan HC, Chou KM, Lee CC, Yang NI, Sun CY. Circulating Klotho levels can predict long-term macrovascular outcomes in type 2 diabetic patients. Atherosclerosis. 2018;276:83–90.
Semba RD, Cappola AR, Sun K, Bandinelli S, Dalal M, Crasto C, et al. Plasma Klotho and mortality risk in older community-dwelling adults. J Gerontol Biol Sci Med Sci. 2011;66:794–800.
Kitagawa M, Sugiyama H, Morinaga H, Inoue T, Takiue K, Ogawa A, et al. A decreased level of serum soluble Klotho is an independent biomarker associated with arterial stiffness in patients with chronic kidney disease. PLoS ONE. 2013;8:e56695.
Kim HJ, Kang E, Oh YK, Kim YH, Han SH, Yoo TH, et al. The association between soluble klotho and cardiovascular parameters in chronic kidney disease: results from the KNOW-CKD study. BMC Nephrol. 2018;19:51.
Keles N, Caliskan M, Dogan B, Keles NN, Kalcik M, Aksu F, et al. Low serum level of Klotho is an early predictor of atherosclerosis. Tohoku J Exp Med. 2015;237:17–23.
Donate-Correa J, Ferri CM, Martin-Nuñez E, Perez-Delgado N, et al. Klotho as a biomarker of subclinical atherosclerosis in patients with moderate to severe chronic kidney disease. Sci Rep. 2021;11(1):15877.
Navarro-González JF, Mora-Fernández C, Muros de Fuentes M, et al. Effect of pentoxifylline on renal function and urinary albumin excretion in patients with diabetic kidney disease: the PREDIAN trial. J Am Soc Nephrol. 2015;26:220–9.
Navarro-González JF, Sánchez-Niño MD, Donate-Correa J, Martín-Núñez E, et al. Effects of Pentoxifylline on Soluble Klotho concentrations and renal tubular cell expression in Diabetic kidney disease. Diabetes Care. 2018;41(8):1817–20.
Benedetto FA, Mallamaci F, Tripepi G, Zoccali C. Prognostic value of ultrasonographic measurement of carotid intima media thickness in dialysis patients. J Am Soc Nephrol. 2001;12:2458–64.
Prasad K, Lee P. Suppression of hypercholesterolemic atherosclerosis by pentoxifylline and its mechanism. Atherosclerosis. 2007;192:313–22.
Herskovits E, Famulari A, Tamaroff L, et al. Preventive treatment of cerebral transient ischemia: comparative randomized trial of pentoxifylline versus conventional antiaggregants. Eur Neurol. 1985;24:73–81.
Herskovits E, Famulari A, Tamaroff L, et al. Comparative study of pentoxifylline vs antiaggregants in patients with transient ischaemic attacks. Acta Neurol Scand Suppl. 1989;127:31–5.
Chen J et al. Ankle brachial index and subsequent cardiovascular disease risk in patients with chronic kidney disease. J Am Heart Assoc. 2016;5, e003339.
Gracia M, Betriu A, Martinez-Alonso M, Arroyo D, Abajo M, Fernández E, Valdivielso JM, Investigators NEFRONA. Predictors of subclinical atheromatosis progression over 2 years in patients with different stages of CKD. Clin J Am Soc Nephrol. 2016;11:287–96.
Szeto CC, Chow KM, Woo KS, Chook P, Ching-Ha Kwan B, Leung CB, Kam-Tao Li P. Carotid intima media thickness predicts cardiovascular diseases in Chinese predialysis patients with chronic kidney disease. J Am Soc Nephrol. 2017;18:1966–72.
Fernandes JL, Dias de Oliveira RT, Mamoni RL, Coelho OR, Nicolau JC, Blotta MHSL, Serrano CV. Pentoxifylline reduces pro-inflammatory and increases anti-inflammatory activity in patients with coronary artery disease–a randomized placebo-controlled study. Atherosclerosis. 2008;196:434–42.
Brie DM, Mornos C, Brie DA, Luca CT, Petrescu L, Boruga M. Potential role for pentoxifylline as an anti-inflammatory drug for patients with acute coronary syndrome. Exp Ther Med. 2022;23:378.
Mohammadpour AH, Falsoleiman H, Shamsara J, Abadi GA, Rasooli R, Ramezani M. Pentoxifylline decreases serum level of adhesion molecules in atherosclerosis patients. Iran Biomed J. 2014;18:23–7.
Goicoechea M, Garcia de Vinuesa S, Quiroga B, et al. Effects of pentoxifylline on inflammatory parameters in chronic kidney disease patients: a ran-domized trial. J Nephrol. 2012;25:969–75.
Chen YM, Lin SL, Chiang WC, Wu KD, Tsai TJ. Pentoxifylline ameliorates proteinuria through suppression of renal monocyte chemoattractant protein-1 in patients with proteinuric primary glomerular diseases. Kidney Int. 2006;69:1410–5.
Memmos E, Sarafidis P, Pateinakis P, Tsiantoulas A, Faitatzidou D, Giamalis P, et al. Soluble Klotho is associated with mortality and cardiovascular events in hemodialysis. BMC Nephrol. 2019;20:217.
Oguro R, Kamide K, Kokubo Y, Shimaoka I, Congrains A, Horio T, et al. Association of carotid atherosclerosis with genetic polymorphisms of the klotho gene in patients with hypertension. Geriatr Gerontol Int. 2010;10:311–8.
Arking DE, Becker DM, Yanek LR, Fallin D, Judge DP, Moy TF, et al. KLOTHO allele status and the risk of early-onset occult coronary artery disease. Am J Hum Genet. 2003;72:1154–61.
Li L, Wang Y, Gao W, Yuan C, Zhang S, Zhou H, et al. Klotho reduction in alveolar macrophages contributes to cigarette smoke extract-induced inflammation in chronic obstructive pulmonary disease. J Biol Chem. 2015;290:27890–900.
Witkowski JM, Soroczyńska-Cybula M, Bryl E, Smoleńska Z, Jóźwik AJ. Klotho - a common link in physiological and rheumatoid arthritis-related aging of human CD4 + lymphocytes. Immunol. 2007;178:771–7.
Karami M, Mehrabi F, Allameh A, Kakhki MP, Amiri M, Aleagha MSE. Klotho gene expression decreases in peripheral blood mononuclear cells (PBMCs) of patients with relapsing-remitting multiple sclerosis. J Neurol Sci. 2017;381:305–7.
Bi F, Chen F, Li Y, Wei A, Cao W. Klotho preservation by Rhein promotes toll-like receptor 4 proteolysis and attenuates lipopolysaccharide-induced acute kidney injury. J Mol Med (Berl). 2018;96:915–27.
Lv J, Chen J, Wang M, Yan F. Klotho alleviates indoxyl sulfate-induced heart failure and kidney damage by promoting M2 macrophage polarization. Aging. 2020;12:9139–50.
Mytych J, Sołek P, Będzińska A, Rusinek K, Warzybok A, Tabęcka-Łonczyńska A, et al. Towards age-related anti-inflammatory therapy: Klotho suppresses activation of ER and golgi stress response in senescent monocytes. Cells. 2020;9:261.
Mytych J, Wos I, Solek P, Koziorowski M. Protective role of klotho protein on epithelial cells upon co-culture with activated or senescent monocytes. Exp Cell Res. 2017;350:358–67.
Acknowledgements
Not applicable.
Funding
This work was supported by Instituto de Salud Carlos III, Fondo Europeo de Desarrollo Regional, “Una manera de hacer Europa” (PI21/01037, PI16/00024, PI19/00035, RD16/0009/0022, RD21/0005/0013), Ministerio de Sanidad (Ref. TRA-182), and Fundación Canaria Instituto de Investigación Sanitaria de Canarias (FIISC) (PIFIISC21/08). JDC is recipient of a contract from Miguel Servet Programme (CP20/00122) of the Instituto de Salud Carlos III (ISCIII). CF is a recipient of a research contract from Agencia Canaria de Investigación, Innovación y Sociedad de la Información (ACIISI), Consejería de Economía, Industria, Comercio y Conocimiento, Gobierno de Canarias (TESIS2018010110), co-funded by Fondo Social Europeo (FSE) Programa Operativo Integrado de Canarias 2014–2020, Eje 3 Tema Prioritario 74 (85%). AGL is recipient of a contract from Agencia Canaria de Investigación Innovación y Sociedad de la Información del Gobierno de Canarias (ACIISI) (TESIS2021010045).
Author information
Authors and Affiliations
Contributions
J.F.N.-G., and C.M.-F. equally contributed to the study concept and design. J.D.-C., A.G.-L., C.F., and N.P.-D. processed the blood samples and performed ELISA and qPCR measurements. J.F.N.-G., J.D.-C., C.F., and C.M.-F. analyzed and interpreted the data. J.F.N.-G. and J.D.-C. performed the statistical analysis and wrote the manuscript. J.F.N.-G., J.D.-C., C.F., N.P.-D., A.G.-L., and C.M.-F. approved the final version of the manuscript for publication.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The trial was approved by the Institutional Review Board at the Hospital Universitario Nuestra Señora de Candelaria. The study protocol code is PTF-AA-TR-2009 and the trial was registered on the European Union Drug Regulating Authorities Clinical Trials (EudraCT #2009–016595– 77). The validation date was 2010-03-09.
Consent for publication
All authors approve the manuscript and give their consent for submission and publication.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Carmen Mora-Fernández and Juan F. Navarro-González these authors share senior authorship.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Donate-Correa, J., Ferri, C.M., Mora-Fernández, C. et al. Pentoxifylline ameliorates subclinical atherosclerosis progression in patients with type 2 diabetes and chronic kidney disease: a randomized pilot trial. Cardiovasc Diabetol 23, 314 (2024). https://doi.org/10.1186/s12933-024-02393-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12933-024-02393-x