Abstract
Introduction
Several studies have reported that pravastatin can mitigate the progression of kidney disease, but limited evidence exists regarding its effects on kidney function in Asian patients. This multicenter prospective observational study aimed to assess the effect of pravastatin on kidney function in Korean patients with dyslipidemia and type 2 diabetes mellitus (T2DM) in clinical practice.
Methods
This 48-week prospective multicenter study included 2604 of 2997 eligible patients with dyslipidemia and T2DM who had available estimated glomerular filtration rate (eGFR) measurements. The primary endpoint was eGFR percent change at week 24 from baseline. We also assessed secondary endpoints, which included percent changes in eGFR at weeks 12 and 48 from baseline, as well as changes in eGFR, metabolic profiles (lipid and glycemic levels) at 12, 24, and 48 weeks from baseline, and safety.
Results
We noted a significant improvement in eGFR, with mean percent changes of 2.5%, 2.5%, and 3.0% at 12, 24, and 48 weeks, respectively (all adjusted p < 0.05). The eGFR percent changes significantly increased in subgroups with baseline eGFR 30–90 mL/min/1.73 m2, glycated hemoglobin (HbA1c) ≥ 7 at baseline, no hypertension history, T2DM duration > 5 years, or previous statin therapy. Lipid profiles were improved and remained stable throughout the study, and interestingly, fasting glucose and HbA1c were improved at 24 weeks.
Conclusion
Our findings suggest that pravastatin may have potential benefits for improving eGFR in Korean patients with dyslipidemia and T2DM. This could make it a preferable treatment option for patients with reduced kidney function.
Trial Registration Number
NCT05107063 submitted October 27, 2021.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Although statins have been reported to attenuate kidney disease progression, differences in the effects of statins on kidney disease influence statin selection for patients with kidney dysfunction |
Previous studies suggest that pravastatin may improve kidney function in patients with moderate-to-severe kidney disease, but the evidence remains limited, emphasizing the need for a multicenter prospective observational study to confirm the effects of pravastatin on kidney function in Korean patients with type 2 diabetes mellitus (T2DM) |
This multicenter prospective observational study in Korean patients with T2DM aimed to investigate kidney function changes following pravastatin administration. Additionally, alterations in lipid profile, glycemic levels, and safety were observed |
What was learned from the study? |
After pravastatin administration, the mean percent change in estimated glomerular filtration rate (eGFR) at 24 weeks was 2.6 ± 27.7% in the effectiveness set and 3.9 ± 30.3% in the full eGFR set, demonstrating statistical significance (p < 0.05), and the rate of change remained statistically significant even after adjusting for potential confounding factors in kidney function |
These findings suggest that pravastatin may have potential benefits for improving eGFR in Korean patients with dyslipidemia and T2DM. However, long-term randomized controlled studies are required to confirm and validate our findings |
Introduction
Statins [3-hydroxy-3-methylglutaryl coenzyme A reductase (HMG-CoA) inhibitors] are used as first-line treatment for patients with dyslipidemia, aiding in the prevention and management of cardiovascular diseases (CVD) [1, 2]. In addition to their benefits in CVD, statins have been reported to attenuate kidney disease progression [3,4,5,6,7]. A meta-analysis of 57 randomized clinical trials (RCTs) found that statin users have slower estimated glomerular filtration rate (eGFR) decline and reduced proteinuria compared to non-users [3]. Furthermore, two meta-analyses reported that statin treatment reduces the risk of CVD in patients without chronic kidney disease (CKD) progression. However, there is limited evidence regarding patients on dialysis [4, 5]. A recent study involving US veterans aged 65 and older with stage 3 or 4 chronic kidney disease found that initiating statin therapy was associated with a significantly lower risk of all-cause mortality [8]. Therefore, guidelines have been developed recommending statin therapy for patients with type 1 diabetes mellitus (T1DM) or type 2 diabetes mellitus (T2DM) and CKD [9].
Previous studies have reported differences in the effects of statins on kidney disease progression, and these differences affect the choice of statins for patients with kidney dysfunction [7, 10, 11]. For example, the renal effects in patients with progressive renal disease (PLANET) trial concluded that rosuvastatin did not benefit kidney function, whereas atorvastatin reduced proteinuria and slowed kidney function deterioration [7]. The heart and renal protection (SHARP) trial showed that dual therapy of simvastatin and ezetimibe did not slow kidney disease progression in a large population of patients with CKD over a 4.8-year follow-up duration [10]. Another RCT found that the addition of pitavastatin to diet therapy had no additive beneficial effects on kidney function compared with diet therapy in patients with CKD who also had dyslipidemia and albuminuria [11].
Some previous studies have reported that pravastatin improved kidney function in patients with moderate-to-severe kidney disease [12, 13]. A post-hoc analysis of three RCTs conducted in the USA, UK, Scotland, and Australia reported that pravastatin was associated with a 34% decrease in the rate of kidney function loss. Moreover, a retrospective longitudinal cohort study on Japanese patients with T2DM reported that pravastatin showed superiority over other statins in preserving kidney function [13]; however, this study was performed on a relatively small cohort at a single center in Japan, and studies regarding the impact of pravastatin on kidney function within Asian populations have not yet been conducted.
As there is limited evidence concerning the impact of pravastatin on kidney function in Asian patients, we conducted a multicenter prospective observational study to evaluate its effects on kidney function among Korean patients with T2DM. Additionally, we observed changes in lipid profile, glycemic levels, and the safety profile following pravastatin administration.
Methods
Study Design and Population
A total of 50 investigators from 43 institutions in Korea conducted a multicenter prospective observational study between January 1, 2016 and April 19, 2018. The study enrolled adult patients aged 19 years and older diagnosed with dyslipidemia (defined as low-density lipoprotein-cholesterol [LDL-C] levels ≥ 100 mg/dL) and T2DM, for whom pravastatin was indicated as per routine clinical practice. T2DM diagnosis followed the criteria outlined in the American Diabetes Association guidelines or the use of antidiabetic medications during the eligibility assessment [14, 15]. Patients who had not taken pravastatin within the 4 weeks before the enrollment date were enrolled in the study—additionally, patients who had been using statins other than pravastatin on the enrollment date switched to pravastatin. The investigators determined pravastatin doses (5, 10, 20, or 40 mg once daily orally) based on the usual standard of practice.
Patients were excluded from the study if they met any of the following criteria: hypersensitivity or a history of hypersensitivity to pravastatin; pregnancy, breastfeeding, or women of childbearing potential; active liver disease or elevation of transaminase levels (aspartate aminotransferase [AST] or alanine aminotransferase [ALT]) ≥ three times the upper limit of normal; end-stage kidney disease requiring dialysis; cholestasis; myopathy; hypercholesterolemia due to hyperalphalipoproteinemia accompanied by elevated high-density lipoprotein-cholesterol (HDL-C) levels; or genetic conditions such as galactose intolerance, lactase deficiency, or glucose-galactose malabsorption.
Baseline assessments included demographic characteristics, physical examination, medical history, underlying diseases, eGFR, fasting plasma glucose (FPG) and glycated hemoglobin (HbA1c) levels, lipid profiles, and laboratory investigations (AST, ALT, and creatine kinase [CK]). The previous use of medications at baseline was defined as the usage of medications within the 4 weeks prior to the enrollment date. Hypertension was defined by a systolic blood pressure ≥ 140 mmHg, or a diastolic blood pressure ≥ 90 mmHg, or use of antihypertensive medications at baseline [16, 17].
Patients were monitored for a minimum of 24 weeks, with the option to extend the follow-up period to 48 weeks. Scheduled visits were planned at 12 weeks (optional), 24 weeks, and 48 weeks (optional). During these follow-up visits, concurrent medications such as antidiabetic and antihypertensive drugs and usage of statins were assessed. Additionally, adverse events (AEs) and laboratory data from blood and urine samples were also collected at each follow-up visit.
The study protocol and informed consent forms were approved by the institutional review board (IRB) of each participating institution, including Seoul National University Bundang Hospital, Republic of Korea: study no. B-1511-322-305 (detailed for each institution in Table S1). The study was registered with Clinicaltrials.gov (NCT05107063). The study was conducted in accordance with the principles of the Declaration of Helsinki 1964 and its later amendments, as well as the rules of each IRB. All patients voluntarily provided written informed consent before enrollment in the study. Data were de-identified to protect the privacy of the participating patients and to be fully compliant with locally applicable regulations.
Outcome Measures
The Modification of Diet in Renal Disease (MDRD) equation was used to calculate eGFR as follows [18]: eGFR (mL/min/1.73 m2) = 186 × (serum creatinine (Scr))−1.154 × age−0.203 × (0.742 if female) × (1.212 if African American).
The Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) was also used to calculate eGFR as follows [18]: eGFR (mL/min/1.73 m2) = 141 × min(sCr/κ, 1)α × max(sCr/κ, 1)−1.209 × 0.993age × 1.018 (if female) × 1.159 (if African American), where κ is 0.7 for women and 0.9 for men, α is − 0.329 for women and − 0.411 for men, min indicates the minimum of sCr/κ or 1, and max indicates the maximum of sCr/κ or 1.
The primary endpoint at 24 weeks was percent change in eGFR from baseline. The percent change was calculated at each visit using the following equation: Percent change at follow-up visit = (measurement at a follow-up visit/measurement at baseline − 1 ) × 100.
Secondary endpoints were as follows: percent changes in eGFR at 12 and 48 weeks from baseline; change in eGFR at 12, 24, and 48 weeks from baseline; and percent changes and changes in lipid profile (total cholesterol [TC], LDL-C, triglyceride [TG], and HDL-C) and glycemic levels (FPG and HbA1c).
To assess the safety profile of pravastatin, both AEs and relevant laboratory parameters, such as ALT, AST, and CK levels, were collected and analyzed. The investigators conducted an assessment to determine if the observed laboratory parameters indicated clinically significant abnormalities. Additionally, all AEs were assessed to ascertain their seriousness and potential association with pravastatin treatment. Serious adverse events (SAEs) were categorized using the following criteria: adverse events that resulted in mortality; events deemed life-threatening; events necessitating initial or extended hospitalization; events leading to persistent or significant disability or incapacity or substantially disrupting normal life functions; and events identified by the investigator as congenital anomalies or birth defects. The investigator assessed the causality of reported AEs and classified adverse events as certain, probable/likely, possible, conditional/unclassified, or unassessable/unclassifiable, which were then regarded as adverse drug reactions (ADRs).
Statistical Analysis
The safety set comprised patients who remained enrolled after excluding those who withdrew consent to participate in the study or had missed the initial pravastatin prescription record. The effectiveness set comprised patients from the safety set, excluding those who did not complete the study or meet the inclusion/exclusion criteria after the enrollment date, discontinued pravastatin, switched to another statin, had missing baseline eGFR measurement, or had missed all eGFR measurements during the follow-up period. The full eGFR set comprised patients from the effectiveness set with eGFR measurements at every follow-up interval (12, 24, and 48 weeks).
Demographics and clinical characteristics were summarized using descriptive statistics for patients in the effectiveness set. Continuous variables are presented using descriptive statistics such as mean and standard deviation (SD), whereas categorical variables are presented using frequencies or percentages (%).
The primary and secondary endpoints, including eGFR percent changes, were assessed for both the effectiveness and the full eGFR sets. Additionally, the secondary endpoints were assessed within the effectiveness set, focusing on percent changes and changes in lipid levels and glycemic levels. For comparison between two groups of primary and secondary endpoints (measurements at 12, 24, or 48 weeks vs baseline), the Shapiro–Wilk test was used to determine the normality of data distribution. Then, paired t tests were performed for continuous variables with normal distribution, whereas the Wilcoxon signed-rank test was performed for continuous variables without normal distribution. We also analyzed covariance (ANCOVA) to address potential confounders that could affect kidney function, including age, gender, duration of T2DM, baseline FPG, and HbA1c levels, changes in FPG and HbA1c levels during follow-up visits compared to baseline, and the presence of a history of hypertension. To address the influence of medications that could potentially affect kidney function, we conducted sensitivity analyses on patients who had not taken angiotensin receptor blockers (ARBs), angiotensin-converting enzyme inhibitors (ACEis), diuretics (DUs), or sodium-glucose cotransporter 2 (SGLT2) inhibitors from baseline to 24 weeks within the effectiveness set.
Subgroup analyses were conducted to investigate percent changes and changes in eGFR based on age group, gender, baseline eGFR and HbA1c, medical history of hypertension, T2DM duration, and previous statin treatments. The baseline eGFR was classified into four ranges as follows: eGFR ≥ 90, 60–89, 30–59, or < 30 mL/min/1.73 m2, referred to as CKD category G1, G2, G3, and G4/5, based on the 2012 Kidney Disease: Improving Global Outcomes (KDIGO) Clinical Practice Guidelines for CKD [19].
Furthermore, subgroup analyses were additionally conducted to assess metabolic profile changes in lipid and glycemic levels based on baseline eGFR and the changes in glycemic levels based on the modifying antidiabetic drugs during the follow-up period.
Safety analysis was conducted using the safety set. The number and incidence of AEs were assessed. Furthermore, the number and incidence of SAEs and ADRs were assessed. All AEs were coded using MedDRA version 18.1.
All statistical analyses were conducted using SAS ver. 9.4 (SAS Institute, Cary, NC, USA), and the level of significance was set at 5% (P < 0.05).
Results
Patient Disposition and Baseline Characteristics
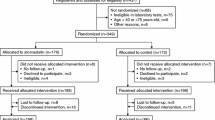
A total of 2997 patients were enrolled during the study period. During the follow-up period, 15 patients withdrew their consent, and another 10 were excluded because of missing initial pravastatin prescription records. Consequently, 2972 patients (99.2%) remained and were included in the safety set. Furthermore, 368 patients were excluded from the study because of various reasons: lost to follow-up, failure to meet eligibility criteria post-enrollment, discontinuation of pravastatin use, switching to other statins, missing baseline eGFR measurements, or missing all eGFR measurements during follow-up visits. Consequently, the effectiveness set comprised 2604 patients, accounting for 86.9% of the enrolled patients. A total of 1368 patients (45.7%) had all the records of eGFR measurements at baseline and follow-up visits and constituted the full eGFR set (Fig. 1).
The mean age of patients included in the effectiveness set was 61.6 years, and 41.9% (n = 1091) of the participants were aged ≥ 65 years. The mean duration of diagnosis of T2DM was 8.4 ± 7.9 years, and the mean FPG and HbA1c levels were 141.9 ± 46.2 mg/dL and 7.4 ± 3.6%, respectively. The mean eGFR was 78.4 ± 30.9 mL/min/1.73 m2, with 29.5% (n = 767) of patients with eGFR ≤ 59 mL/min/1.73 m2 (Table 1).
Effect on Kidney Function
In the effectiveness set, the mean percent change in eGFR at 24 weeks compared to the baseline, calculated using the MDRD equation and CKD-EPI, was 2.6 ± 27.7% and 2.1 ± 24.8%, respectively. Meanwhile, in the full eGFR set, these changes were 3.9 ± 30.3% and 3.8 ± 27.3%, respectively. In both sets and equations, eGFR showed a statistically significant increase (all p < 0.05 calculated by Wilcoxon signed-rank test) (Fig. 2 and Table S2). Additionally, the percent change in eGFR remained statistically significant after adjusting for potential confounding factors in kidney function (all p < 0.05 calculated by ANCOVA) (Fig. 2 and Table S2). In the sensitivity analysis conducted on patients who were not taking any medications that could affect kidney function, the percent change in eGFR still significantly improved at 24 weeks compared to baseline (p < 0.05 as calculated by ANCOVA) (Table S3). The mean eGFR increased over time, but the change was not statistically significant (all p > 0.05 calculated by ANCOVA) (Table S4).
Time course of percent changes in estimated glomerular filtration rate (mL/min/1.73 m2) from the baseline to each follow-up visit in a effectiveness set and b full eGFR set. eGFR, estimated glomerular filtration rate; FPG, fasting plasma glucose; HbA1c, glycosylated hemoglobin. Values are presented as the mean ± standard deviation. The effectiveness set comprised patients with baseline eGFR measurement and at least one eGFR measurement at 12, 24, and 48 weeks. The full eGFR set comprised patients with baseline eGFR measurement and eGFR measurements at all 12, 24, and 48 weeks. ap values were calculated using the Wilcoxon signed-rank test: *, **, ***p vs baseline, < 0.05, < 0.01, < 0.0001. bp values were adjusted by age, gender, duration of diabetes mellitus, baseline HbA1c and FPG, changes in HbA1c and FPG during follow-up visits from the baseline, the history of hypertension, and previous statin treatment using the analysis of covariance (ANCOVA): *, **, ***p vs baseline, < 0.05, < 0.01, < 0.0001
In the subgroup analysis, percent changes in eGFR were significantly increased in men and elderly patients aged ≥ 65 years (all adjusted p < 0.05 calculated by ANCOVA) (Table 2). Significant improvements in percent changes of eGFR were also observed in patients with a baseline HbA1c level ≥ 7 or DM duration > 5 years, as well as those without hypertension, who switched previous statin treatments, or had baseline eGFR 30–89 mL/min/1.73 m2 (all adjusted p < 0.05 calculated by ANCOVA) (Table 2). No interaction was observed between percent changes in eGFR and age group and gender (p for interaction > 0.05) (Table 2). In contrast, there was significant interaction between the percent change in eGFR and several subgroups, including baseline HbA1c level, medical history of hypertension, DM duration, baseline eGFR, and previous statin treatments (p for interaction < 0.05) (Table 2).
Effect on Metabolic Profiles of Lipid and Glucose
At 24 weeks, a significant reduction in TC and LDL-C levels was observed (all p < 0.05), with mean LDL-C levels < 100 mg/dL. In contrast, no significant changes were noted in TG and HDL-C levels (all p > 0.05). The mean reduction in TC, LDL-C, and TG levels from baseline was 12.7 ± 42.3 mg/dL, 16.2 ± 35.8 mg/dL, 1.8 ± 100.8 mg/dL, and HDL-C increased by an average of 0.3 ± 10.0 mg/dL (Table 3). Statistically significant reductions in TC and LDL-C levels were also observed in the subgroup analysis, irrespective of the participants’ baseline eGFR (all p < 0.05 and p for interaction > 0.05) (Table S5).
A significant reduction was observed in FPG and HbA1c levels compared with the baseline measurements, with reductions from baseline to 24 weeks at − 3.9 ± 47.0 mg/dL and − 0.3 ± 3.8%, respectively (all p < 0.05) (Table 3). These enhancements in glycemic control were similarly observed in subgroups other than participants with baseline eGFR < 30 mL/min/1.73 m2 (Table S6). Additionally, significant improvements in FPG and HbA1c levels were also observed in patients without modification of antidiabetic medications, i.e., who maintained concurrent diabetic medications throughout the follow-up period, when glycemic levels were compared at baseline and 24 weeks (all p < 0.05) (Fig. 3 and Table S7).
Subgroup analysis of changes of a fasting plasma glucose and b glycated hemoglobin from baseline with and without modification of antidiabetic drug regime during the follow-up period in the effectiveness set. FPG fasting plasma glucose, HbA1c glycated hemoglobin. Values are presented as the mean ± standard deviation. p values were calculated using the Wilcoxon signed-rank test: *, **, *** p vs baseline, < 0.05, < 0.01, < 0.0001
Adverse Events and Laboratory Investigations
AEs were reported in 8.55% (n = 254) of patients. The only AE reported at a rate of ≥ 0.5% was nasopharyngitis (0.5%; n = 15). ADRs were observed in 0.94% (n = 28) of patients. The most frequent ADR was rash (n = 4), followed by pruritus, myalgia, and hypoglycemia, all of which were reported in two patients. The rate of SAEs was 1.51% (n = 45), with hyperglycemia being the most common (0.10%; n = 3) (Table 4). Laboratory investigations found abnormal changes in AST and ALT levels in only six patients; however, all cases were assessed as unlikely to be related to pravastatin therapy by the principal investigator at the site where the abnormal changes were reported (Table 4).
Discussion
In this study, we investigated the effect of pravastatin on kidney function in Korean patients with dyslipidemia and T2DM. We found that pravastatin therapy could significantly improve the eGFR in patients with T2DM and dyslipidemia during the 48-week study period. In terms of changes in lipid profiles, the study showed significant decreases in both TC and LDL levels in patients, with LDL consistently staying below 100 throughout the follow-up period. A significant reduction in FPG and HbA1c levels was also observed at all visits. Pravastatin was generally well tolerated, and the AEs were within the range of previously reported safety profiles [20].
The mechanisms underlying the beneficial effects of pravastatin on kidney function have not been clearly elucidated. Nonetheless, two hypotheses could potentially explain the advantageous impacts of pravastatin on kidney function. First, pravastatin may not affect the synthesis of coenzyme Q10 (CoQ10), thus preventing kidney dysfunction. HMG-CoA inhibition reduces cholesterol synthesis in the liver; however, the production of fundamental substances such as coenzyme Q10 is also reduced [21, 22]. CoQ10 is an antioxidant that prevents the development of CKD by reducing oxidative stress in animal studies. Consequently, inhibition of CoQ10 synthesis may impair kidney function [23, 24]. However, pravastatin barely reduces CoQ10 synthesis because its lower lipophilicity does not allow pravastatin to move across the cellular membrane; thus, CoQ10 is localized in the hydrophobic portions of the cellular membrane [25]. Second, pravastatin may improve kidney function in patients with diabetic kidney disease by enhancing glucose metabolism. Several studies have reported that pravastatin can increase insulin sensitivity by enhancing the secretion of adiponectin [26, 27]. Moreover, according to the West of Scotland Coronary Prevention Study (WOSCOPS), pravastatin can reduce the risk of incident diabetes by 30% compared with that noted with a placebo [28]. These distinctive effects of pravastatin may be advantageous for kidney function, particularly in patients with T2DM.
Several studies have suggested the potential benefits of pravastatin on kidney function, with a meta-analysis of 33 RCTs indicating its positive effect on maintaining a high eGFR [29, 30]. In alignment with previous studies, the present study observed that kidney function improved following the administration of pravastatin. This improvement remains significant even after adjusting for confounders that could influence kidney function, such as duration of T2DM, changes in glycemic levels, and a medical history of hypertension. Patients not using concurrent medications known to affect kidney function, such as ARBs, ACEis, DUs, and SGLT2 inhibitors, also showed significant improvements in percent change in eGFR from baseline to 24 weeks. These results suggest that pravastatin could be beneficial in treating patients with dyslipidemia who have reduced kidney function. Nevertheless, this study overlooked confounding factors like blood pressure (BP) that could influence kidney function. Therefore, further investigations should incorporate BP measurements to confirm the effects of pravastatin on kidney function.
We observed variable pravastatin-induced effects on kidney function contingent on the baseline eGFR levels. In patients with a baseline eGFR of 30–89 mL/min/1.73 m2, we found a significant elevation in eGFR following pravastatin treatment. Conversely, patients with a baseline eGFR of ≥90 mL/min/1.73 m2 experienced a substantial reduction in eGFR. In these patients with high baseline eGFR measurements, a reduction in eGFR might indicate an improvement in kidney function. This is because the nephrons typically undergo structural and functional adaptations during the initial stages of CKD (baseline eGFR of ≥ 90 mL/min/1.73 m2), leading to an atypically high eGFR due to hyperfiltration. Furthermore, increased glomerular hydraulic pressure and the movement of ultrafiltrates and large molecules across the capillary walls can accelerate nephron damage [31]. Thus, a reduction in eGFR could signify a decrease in hyperfiltration, potentially mitigating the kidney burden in patients with baseline eGFR of ≥ 90 mL/min/1.73 m2.
The mean eGFR in subgroups DM duration > 5 years and baseline HbA1c level ≥ 7% improved significantly during the study period. Subgroups with DM duration ≤ 5 years and baseline HbA1c < 7% showed an increasing trend in eGFR; however, these results were not statistically significant. These subgroups are considered in an early stage of DM, and it is possible that most patients with eGFR ≥ 90 mL/min/1.73 m2 were included. Therefore, the effect of pravastatin on eGFR may have been inconspicuous owing to the relatively mild conditions in these subgroups.
Subgroup analyses found that the statin-naïve group did not exhibit a significant change, whereas the statin-switching group showed an improvement in eGFR. These findings suggest that pravastatin may have a lesser impact on reducing kidney function than other statins. Consistency was observed in another longitudinal cohort study from Japan, where pravastatin showed superiority over rosuvastatin, atorvastatin, and pitavastatin in maintaining kidney function among patients with T2DM [13]. Consequently, the results suggest that using pravastatin may be more beneficial in preserving kidney function than other statins for patients with T2DM and dyslipidemia.
The current guidelines recommend aiming for LDL-C levels < 100 mg/dL as the therapeutic goal in patients with DM, except those in the very high risk group [32]. During the study period, the mean LDL-C level in patients who received pravastatin remained below 100 mg/dL. These findings support the lipid-lowering effects of pravastatin in the general population with T2DM.
Our study showed improvements in glycemic levels among patients undergoing assessment of changes in glycemic levels throughout the follow-up period. Additionally, in a subgroup analysis to assess the change in glycemic levels with and without modification in antidiabetic medications throughout the follow-up period, significant improvements were observed in patients who maintain their antidiabetic medications. The findings align with previous studies assessing the association between pravastatin and glycemic levels. In a retrospective study assessing the effects of statins on change in the FPG level in individuals without diabetes in Korea, it was found that pravastatin was not associated with increasing FPG levels [33]. A meta-analysis assessing the glycemic control effects of statins similarly indicated that pravastatin did not increase HbA1c levels. Interestingly, this previous study also showed that pravastatin was associated with a statistically significant decrease of 0.33 in HOMA-IR [34]. Combining our results with previous studies, it may be prudent to explore pravastatin as a possible option for patients with dyslipidemia and T2DM who are concerned about worsening glycemic levels.
Pravastatin was safe and well tolerated in this study. AEs occurred in 8.55% of the safety population, with nasopharyngitis as the only AE reported in more than 0.5% of patients. Muscle symptom is the most common adverse drug reaction of statin therapy, and approximately 1–10% of patients suffer from muscle symptoms after statin treatment [35]. The incidence of musculoskeletal-related AEs was relatively lower in our study than in previous studies [36]. Moreover, only six patients (0.2%, 6 out of 2972 in the safety set) showed abnormal levels of AST and ALT, and investigators at each site determined these cases were unrelated to pravastatin.
This study has some limitations. First, this was a multicenter prospective observational study with no control group and was not a randomized controlled study. Thus, under-reporting was unavoidable owing to the inherent study design, leading to potential bias. Additionally, routine care-related factors (such as the use of concomitant drugs) were left to the discretion of the individual physician in charge. Furthermore, the study’s reliance on patient-reported questionnaire responses precluded the verification of consistent pravastatin adherence as claimed by the patients. These could have been a confounder that affected the results, but it could not be controlled for in this study. Second, only eGFR and no other parameters, including urine or blood proteins and organ imaging, were evaluated to assess kidney function. This may have limited the accuracy of the assessment or detection of other clinically relevant changes. Third, the follow-up period was relatively short. A longer follow-up duration would have enabled a clear evaluation of the impact of pravastatin on kidney function. Finally, generalizability remains problematic and contentious because this study only showed the results of data collected from enrolled patients.
Nevertheless, very few studies have been conducted involving approximately 3000 patients with DM and dyslipidemia to identify the impact of pravastatin on kidney function in actual clinical practice. The findings of this study provide real-world evidence of the beneficial effects of pravastatin in patients with comorbid diseases, such as T2DM and dyslipidemia, who require constant monitoring of their kidney functions.
Conclusion
This multicenter prospective observational study investigation found that pravastatin significantly improved eGFR in patients with T2DM and dyslipidemia, thus supporting its beneficial effects on kidney function. Significant improvements were also found in the lipid profiles, with no worsening in glycemic levels observed. Our findings suggest that pravastatin could be a viable and safe treatment choice for patients with T2DM and dyslipidemia.
Data Availability
The data underlying this article will be shared upon reasonable request to the corresponding author and after obtaining permission from the relevant medical ethical committees.
References
Mach F, Baigent C, Catapano AL, et al. ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk: the Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS). Eur Heart J. 2019;41:111–88. https://doi.org/10.1093/eurheartj/ehz455
Visseren FLJ, Mach F, Smulders YM, et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J. 2021;42:3227–337. https://doi.org/10.1093/eurheartj/ehab484.
Su X, Zhang L, Lv J, et al. Effect of statins on kidney disease outcomes: a systematic review and meta-analysis. Am J Kidney Dis. 2016;67:881–92. https://doi.org/10.1053/j.ajkd.2016.01.016.
Hou W, Lv J, Perkovic V, et al. Effect of statin therapy on cardiovascular and renal outcomes in patients with chronic kidney disease: a systematic review and meta-analysis. Eur Heart J. 2013;34:1807–17. https://doi.org/10.1093/eurheartj/eht065.
Cholesterol Treatment Trialists’ (CTT) Collaboration, Herrington WG, Emberson J, et al. Impact of renal function on the effects of LDL cholesterol lowering with statin-based regimens: a meta-analysis of individual participant data from 28 randomised trials. Lancet Diabetes Endocrinol. 2016;4:829–39. https://doi.org/10.1016/S2213-8587(16)30156-5.
Zhang Z, Wu P, Zhang J, Wang S, Zhang G. The effect of statins on microalbuminuria, proteinuria, progression of kidney function, and all-cause mortality in patients with non-end stage chronic kidney disease: a meta-analysis. Pharmacol Res. 2016;105:74–83. https://doi.org/10.1016/j.phrs.2016.01.005.
de Zeeuw D, Anzalone DA, Cain VA, et al. Renal effects of atorvastatin and rosuvastatin in patients with diabetes who have progressive renal disease (PLANET I): a randomised clinical trial. Lancet Diabetes Endocrinol. 2015;3:181–90. https://doi.org/10.1016/S2213-8587(14)70246-3.
Barayev O, Hawley CE, Wellman H, et al. Statins, mortality, and major adverse cardiovascular events among US veterans with chronic kidney disease. JAMA Netw Open. 2023;6:e2346373. https://doi.org/10.1001/jamanetworkopen.2023.46373.
de Boer IH, Khunti K, Sadusky T, et al. Diabetes management in chronic kidney disease: a consensus report by the American Diabetes Association (ADA) and Kidney Disease: Improving Global Outcomes (KDIGO). Diabetes Care. 2022;45:3075–90. https://doi.org/10.2337/dci22-0027.
Haynes R, Lewis D, Emberson J, et al. Effects of lowering LDL cholesterol on progression of kidney disease. J Am Soc Nephrol. 2014;25:1825–33. https://doi.org/10.1681/ASN.2013090965.
Ohsawa M, Tamura K, Wakui H, et al. Effects of pitavastatin add-on therapy on chronic kidney disease with albuminuria and dyslipidemia. Lipids Health Dis. 2015;14:161. https://doi.org/10.1186/s12944-015-0164-5.
Tonelli M, Isles C, Craven T, et al. Effect of pravastatin on rate of kidney function loss in people with or at risk for coronary disease. Circulation. 2005;112:171–8. https://doi.org/10.1161/CIRCULATIONAHA.104.517565.
Hanai K, Babazono T, Takemura S, et al. Comparative effects of statins on the kidney function in patients with type 2 diabetes. J Atheroscler Thromb. 2015;22:618–27. https://doi.org/10.5551/jat.26823.
American Diabetes Association. Erratum. Classification and diagnosis of diabetes. Sec. 2. In Standards of Medical Care in Diabetes—2016. Diabetes Care. 2016;39(Suppl 1):S13–22. https://doi.org/10.2337/dc16-er09.
American Diabetes Association. Erratum. Diagnosis and classification of diabetes: Standards of care in diabetes—2024. Diabetes Care. 2023;47:S20–42. https://doi.org/10.2337/dc24-S002.
Chobanian AV, Bakris GL, Black HR, et al. Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. National heart, lung, and blood institute; national high blood pressure education program coordinating committee. seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension. 2003;42:1206–52. https://doi.org/10.1161/01.HYP.0000107251.49515.c2.
Kim HL, Lee EM, Ahn SY, et al. The 2022 focused update of the 2018 Korean Hypertension Society Guidelines for the management of hypertension. Clin Hypertens. 2023;29:11. https://doi.org/10.1186/s40885-023-00234-9.
Jo JY, Ryu SA, Kim JI, et al. Comparison of five glomerular filtration rate estimating equations as predictors of acute kidney injury after cardiovascular surgery. Sci Rep. 2019;9:11072. https://doi.org/10.1038/s41598-019-47559-w.
Levin A, Stevens PE. Summary of KDIGO 2012 CKD Guideline: behind the scenes, need for guidance, and a framework for moving forward. Kidney Int. 2014;85:49–61. https://doi.org/10.1038/ki.2013.444.
Haria M, McTavish D. Pravastatin. A reappraisal of its pharmacological properties and clinical effectiveness in the management of coronary heart disease. Drugs. 1997;53:299–336. https://doi.org/10.2165/00003495-199753020-00008.
van Vliet AK, van Thiel GC, Huisman RH, Moshage H, Yap SH, Cohen LH. Different effects of 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors on sterol synthesis in various human cell types. Biochim Biophys Acta. 1995;1254:105–11. https://doi.org/10.1016/0005-2760(94)00176-y.
Ichihara K, Satoh K. Disparity between angiographic regression and clinical event rates with hydrophobic statins. Lancet. 2002;359:2195–8. https://doi.org/10.1016/S0140-6736(02)09098-0.
Sourris KC, Harcourt BE, Tang PH, et al. Ubiquinone (coenzyme Q10) prevents renal mitochondrial dysfunction in an experimental model of type 2 diabetes. Free Radic Biol Med. 2012;52:716–23. https://doi.org/10.1016/j.freeradbiomed.2011.11.017.
Persson MF, Franzén S, Catrina SB, et al. Coenzyme Q10 prevents GDP-sensitive mitochondrial uncoupling, glomerular hyperfiltration and proteinuria in kidneys from db/db mice as a model of type 2 diabetes. Diabetologia. 2012;55:1535–43. https://doi.org/10.1007/s00125-012-2469-5.
Kang MH, Kim W, Kim JS, et al. Hydrophilic versus lipophilic statin treatments in patients with renal impairment after acute myocardial infarction. J Am Heart Assoc. 2022;11:e024649. https://doi.org/10.1161/JAHA.121.024649.
Koh KK, Sakuma I, Quon MJ. Differential metabolic effects of distinct statins. Atherosclerosis. 2011;215:1–8. https://doi.org/10.1016/j.atherosclerosis.2010.10.036.
Marcoff L, Thompson PD. The role of coenzyme Q10 in statin-associated myopathy: a systematic review. J Am Coll Cardiol. 2007;49:2231–7. https://doi.org/10.1016/j.jacc.2007.02.049.
Freeman DJ, Norrie J, Sattar N, et al. Pravastatin and the development of diabetes mellitus: evidence for a protective treatment effect in the West of Scotland Coronary Prevention Study. Circulation. 2001;103:357–62. https://doi.org/10.1161/01.cir.103.3.357.
Nakamura H, Mizuno K, Ohashi Y, et al. Pravastatin and cardiovascular risk in moderate chronic kidney disease. Atherosclerosis. 2009;206:512–7. https://doi.org/10.1016/j.atherosclerosis.2009.03.031.
Zhao L, Li S, Gao Y. Efficacy of statins on renal function in patients with chronic kidney disease: a systematic review and meta-analysis. Ren Fail. 2021;43:718–28. https://doi.org/10.1080/0886022X.2021.1915799.
Tonneijck L, Muskiet MH, Smits MM, et al. Glomerular hyperfiltration in diabetes: mechanisms, clinical significance, and treatment. J Am Soc Nephrol. 2017;28:1023–39. https://doi.org/10.1681/ASN.2016060666.
Hur KY, Moon MK, Park JS, et al. 2021 Clinical practice guidelines for diabetes mellitus of the Korean Diabetes Association. Diabetes Metab J. 2021;45:461–81. https://doi.org/10.4093/dmj.2021.0156.
Kim J, Lee HS, Lee KY. Effect of statins on fasting glucose in non-diabetic individuals: nationwide population-based health examination in Korea. Cardiovasc Diabetol. 2018;17:155. https://doi.org/10.1186/s12933-018-0799-4.
Alvarez-Jimenez L, Morales-Palomo F, Moreno-Cabañas A, et al. Effects of statin therapy on glycemic control and insulin resistance: a systematic review and meta-analysis. Eur J Pharmacol. 2023;947:175672. https://doi.org/10.1016/j.ejphar.2023.175672.
Ramkumar S, Raghunath A, Raghunath S. Statin therapy: review of safety and potential side effects. Acta Cardiol Sin. 2016;32:631–9. https://doi.org/10.6515/acs20160611a.
Hoffman KB, Kraus C, Dimbil M, Golomb BA. A survey of the FDA’s AERS database regarding muscle and tendon adverse events linked to the statin drug class. PLoS ONE. 2012;7:e42866. https://doi.org/10.1371/journal.pone.0042866.
Acknowledgements
We would like to sincerely thank all the patients who generously participated in this study. Your valuable contributions have been crucial to the success of our research, and we are deeply appreciative of your involvement. The authors greatly acknowledge the contribution of all the investigators and research coordinators at each participating institution: Ki Young Lee (Gachon University Gil Medical Center), Dong-Young Lee (Veterans Health Service Medical Center), Seung Jin Han (Ajou University Hospital), Hyung Jong Kim (Cha University Bundang Medical Center), Sung Chang Chung (Dongkang Medical Center), Won Jun Kim (Gangneung Asan Medical Center), Mi-Seon Shin (Hanil General Hospital), Yun Hyi Ku (Korea Cancer Center Hospital), Young Sik Choi (Kosin University Gospel Hospital), Tae Ho Kim (Seoul Medical Center), Hyeong Kyu Park (Soon Chun Hyang University Hospital), Hyung Wook Kim (Suwon St. Vincent’s Hospital), Hyo Jeong Kim (Nowon Eulji Medical Center), Doo Man Kim (Hallym University Kangdong Sacred Heart Hospital), In Kyung Jeong (Kyung Hee University Hospital at Gangdong), Jae Hyuk Lee (Myongji Hospital), Han Seok Choi (Dongguk University Ilsan Hospital), Byung Chul Shin (Chosun University Hospital), Ji A Seo (Korea University Ansan Hospital), Yeong Hoon Kim (Inje University Busan Paik Hospital), Bo Hyun Kim (Pusan National University Hospital), Hyung-Gyun Oh (Gwangju Veterans Hospital), Mi Yeon Kang (St. Carollo General Hospital), Jung-Min Lee (Eunpyeong St. Mary’s hospital), Eui Dal Jung (Daegu Catholic University Medical Center), Hyeong Cheon Park (Gangnam Severance Hospital), Sang Heon Song (Pusan National University Hospital), Yoon-Goo Kim (Samsung Medical Center), Dong-Jun Kim (Inje University Ilsan Paik Hospital), Seok Hui Kang (Yeungnam University Medical Center), Jae Won Yang (Wonju Severance Christian Hospital), Hyo-Wook Gil (Soonchunhyang University Cheonan Hospital).
Medical Writing, Editorial, and Other Assistance
The authors also thank Dream CIS Inc. for statistical analysis assistance, funded by Daiichi Sankyo Korea Co., Ltd., Seoul, Korea. We also would like to thank Editage (http://www.editage.co.kr) and Ji-Hwan Bae for editing the manuscript.
Funding
Open Access funding enabled and organized by Seoul National University. The sponsorship for this study and publication fee for this article were funded by Daiichi Sankyo Korea Co., Ltd., Seoul, Korea.
Author information
Authors and Affiliations
Contributions
Hae Jin Kim, Kyu Yeon Hur, Yong-Ho Lee, Jin Taek Kim, Yong-Kyu Lee, Ki-Hyun Baek, Euy Jin Choi, Won Min Hwang, Ki Tae Bang, Jung Soo Lim, Yun Jae Chung, Sung Rae Jo, Joon Seok Oh, Soon Hee Lee, Seung-Hyun Ko, and Sung Hee Choi participated in the enrollment process and performed clinical follow-up and data acquisition. Hae Jin Kim, Kyu Yeon Hur, Yong-Ho Lee, and Sung Hee Choi contributed to the study conception, design, and data interpretation. Hae Jin Kim and Kyu Yeon Hur wrote the first draft and revised the manuscript. Sung Hee Choi contributed to the review and critical revision of the manuscript. All authors reviewed and approved the final manuscript draft submitted for publication and agree to be accountable for all aspects of the work, ensuring the accuracy and integrity of the publication.
Corresponding author
Ethics declarations
Conflict of interest
Hae Jin Kim, Kyu Yeon Hur, Yong-Ho Lee, Jin Taek Kim, Yong-Kyu Lee, Ki-Hyun Baek, Euy Jin Choi, Won Min Hwang, Ki Tae Bang, Jung Soo Lim, Yun Jae Chung, Sung Rae Jo, Joon Seok Oh, Soon Hee Lee, Seung-Hyun Ko, and Sung Hee Choi declare that they have no competing interests.
Ethical approval
The study protocol and informed consent forms were approved by the institutional review board (IRB) of each participating institution, including Seoul National University Bundang Hospital, Republic of Korea: study No. B-1511-322-305 (detailed for each institution in Table S1). The study was registered with Clinicaltrials.gov (NCT05107063). The study was conducted in accordance with the principles of the Declaration of Helsinki 1964 and its later amendments, as well as the rules of each IRB. All patients voluntarily provided written informed consent before enrollment in the study. Data were de-identified to protect the privacy of the participating patients and to be fully compliant with locally applicable regulations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Kim, H.J., Hur, K.Y., Lee, YH. et al. Effect of Pravastatin on Kidney Function in Patients with Dyslipidemia and Type 2 Diabetes Mellitus: A Multicenter Prospective Observational Study. Adv Ther (2024). https://doi.org/10.1007/s12325-024-02862-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12325-024-02862-5